Effects of Manure Removal Frequencies and Deodorants on Ammonia and GHG Concentrations in Livestock House
Abstract
:1. Introduction
2. Materials and Methods
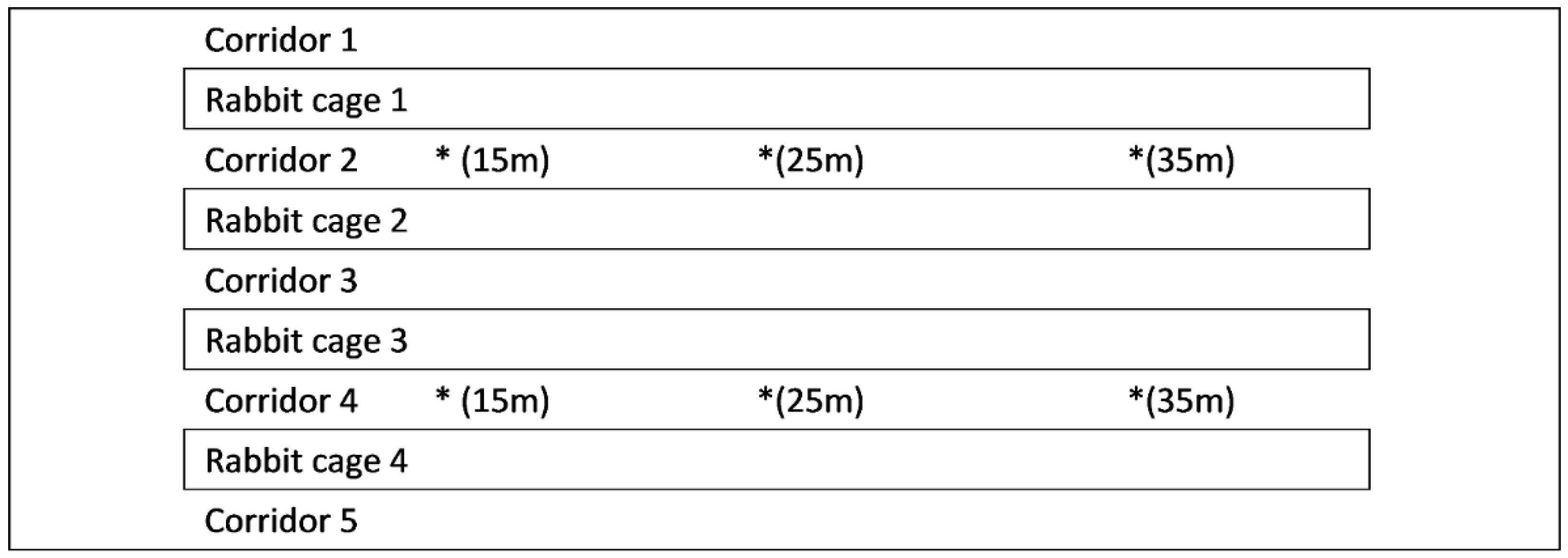
2.1. Experimental Room and Ventilation Management
2.2. Experimental Design
2.2.1. Experiment 1: Effects of Feces-Cleaning Frequency on Ammonia and Greenhouse Gas Concentrations
2.2.2. Experiment 2: Effects of Microbial Deodorant and ZnMNP Deodorant on Ammonia and Greenhouse Gas Concentrations
2.2.3. Ammonia, Greenhouse Gases, Temperature, and Relative Humidity Determination Methods
2.3. Statistical Analysis
3. Results
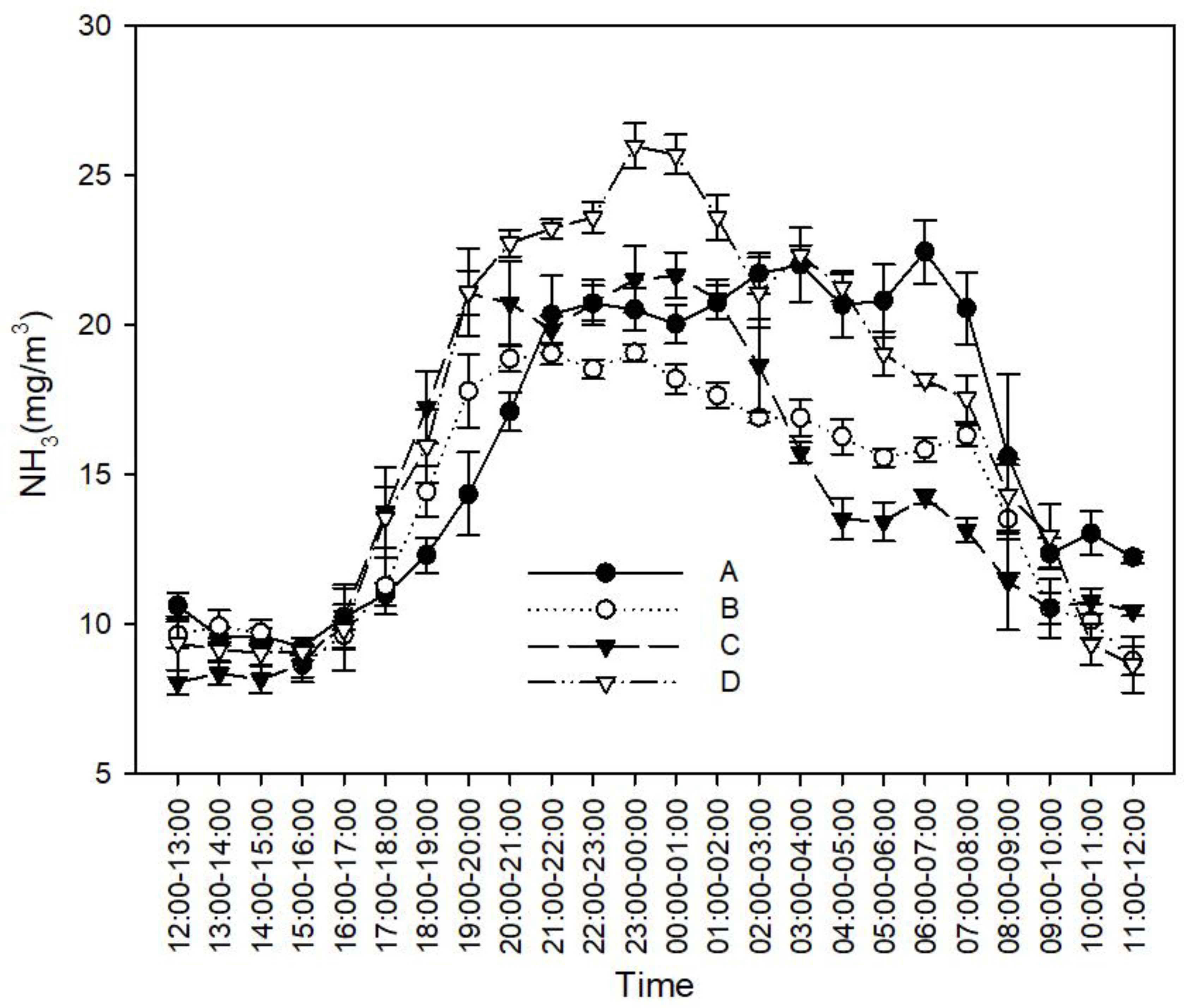
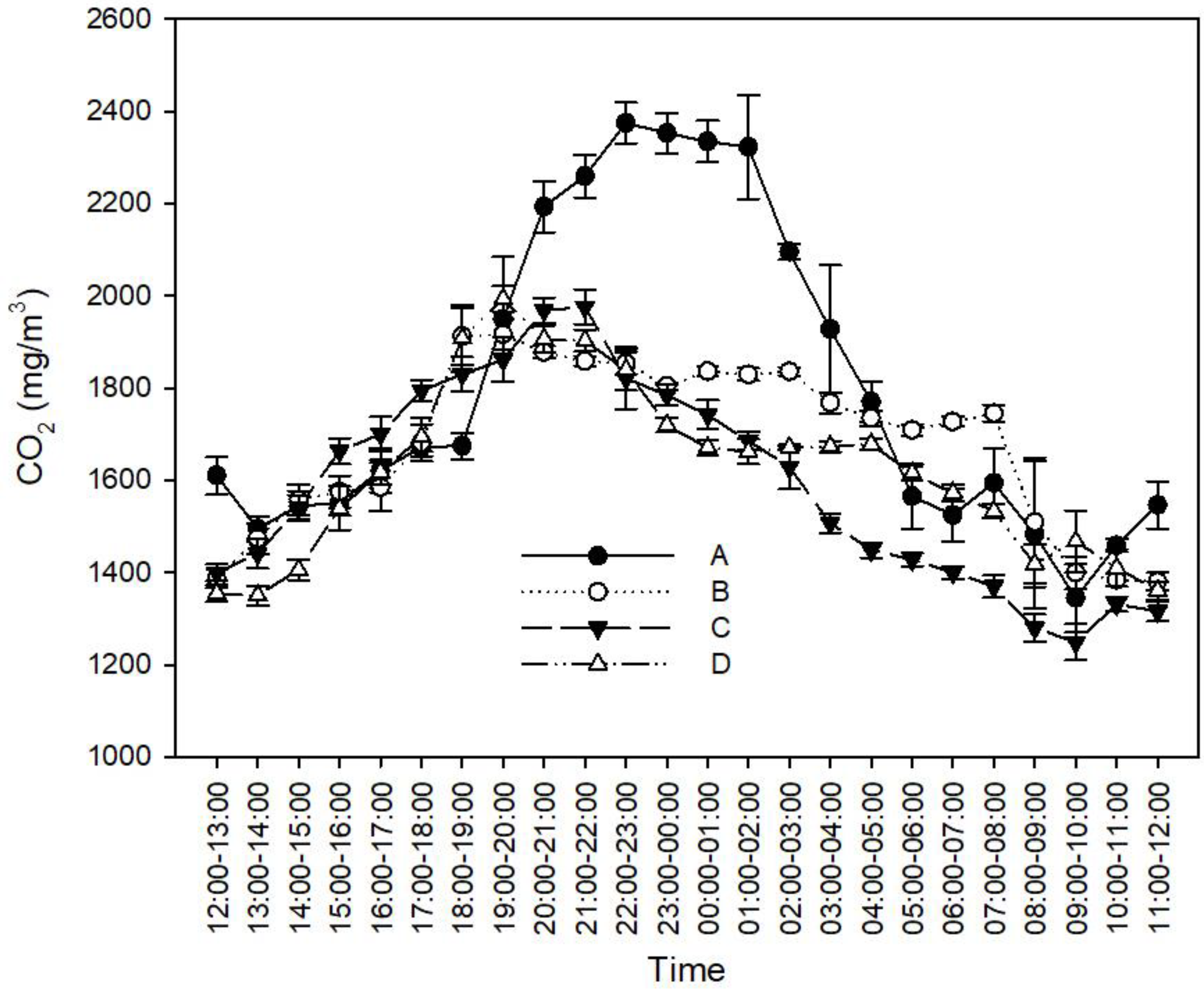
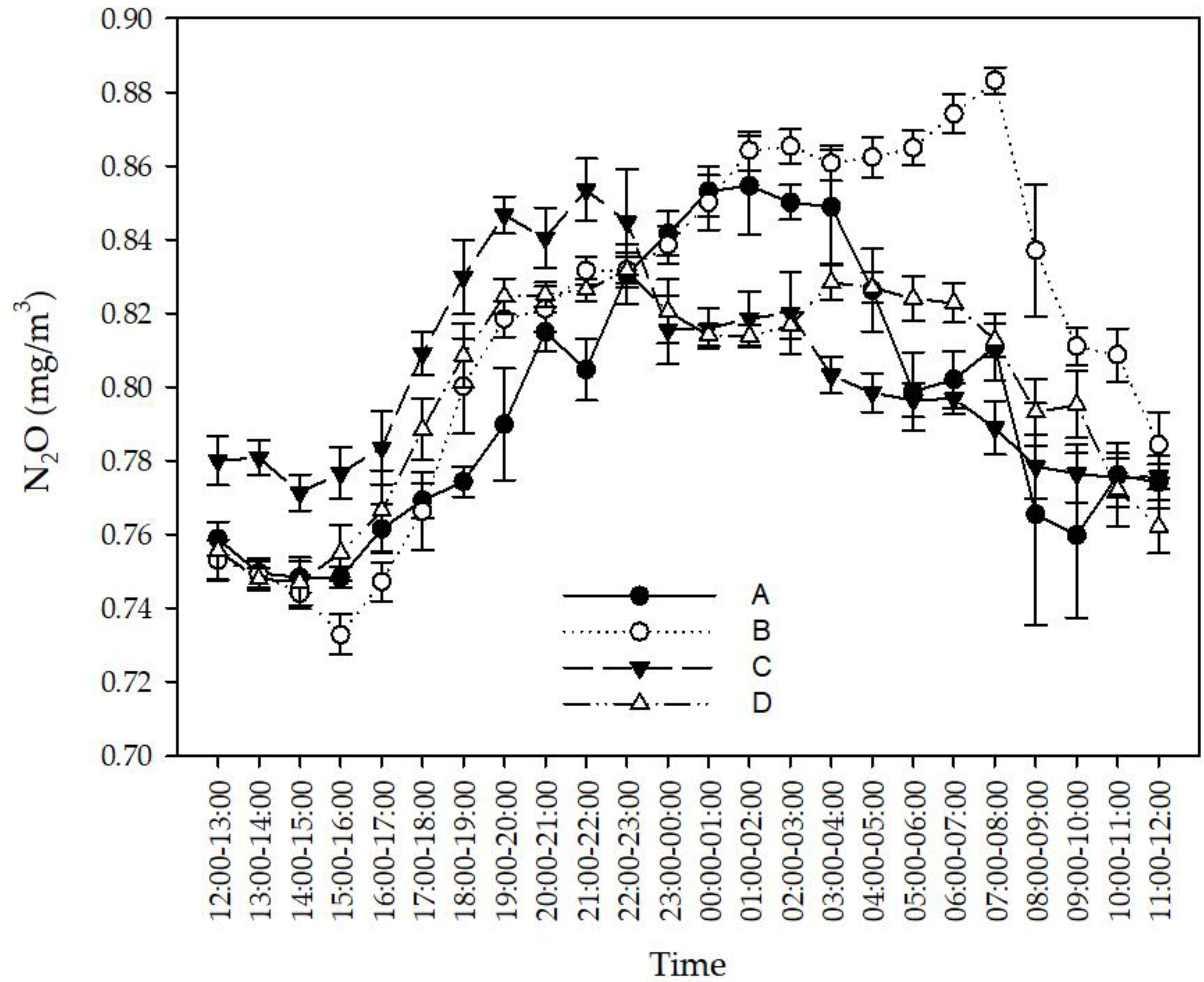
3.1. Effects of Feces-Cleaning Frequency on Ammonia and GHG Concentration in Rabbit Houses
3.1.1. Effects of Feces-Cleaning Frequency on NH3 Concentration
3.1.2. Effects of the Feces-Cleaning Frequency on the CO2 Concentration in the Rabbit House
3.1.3. Effects of Feces-Cleaning Frequency on the N2O Concentration in the Rabbit House
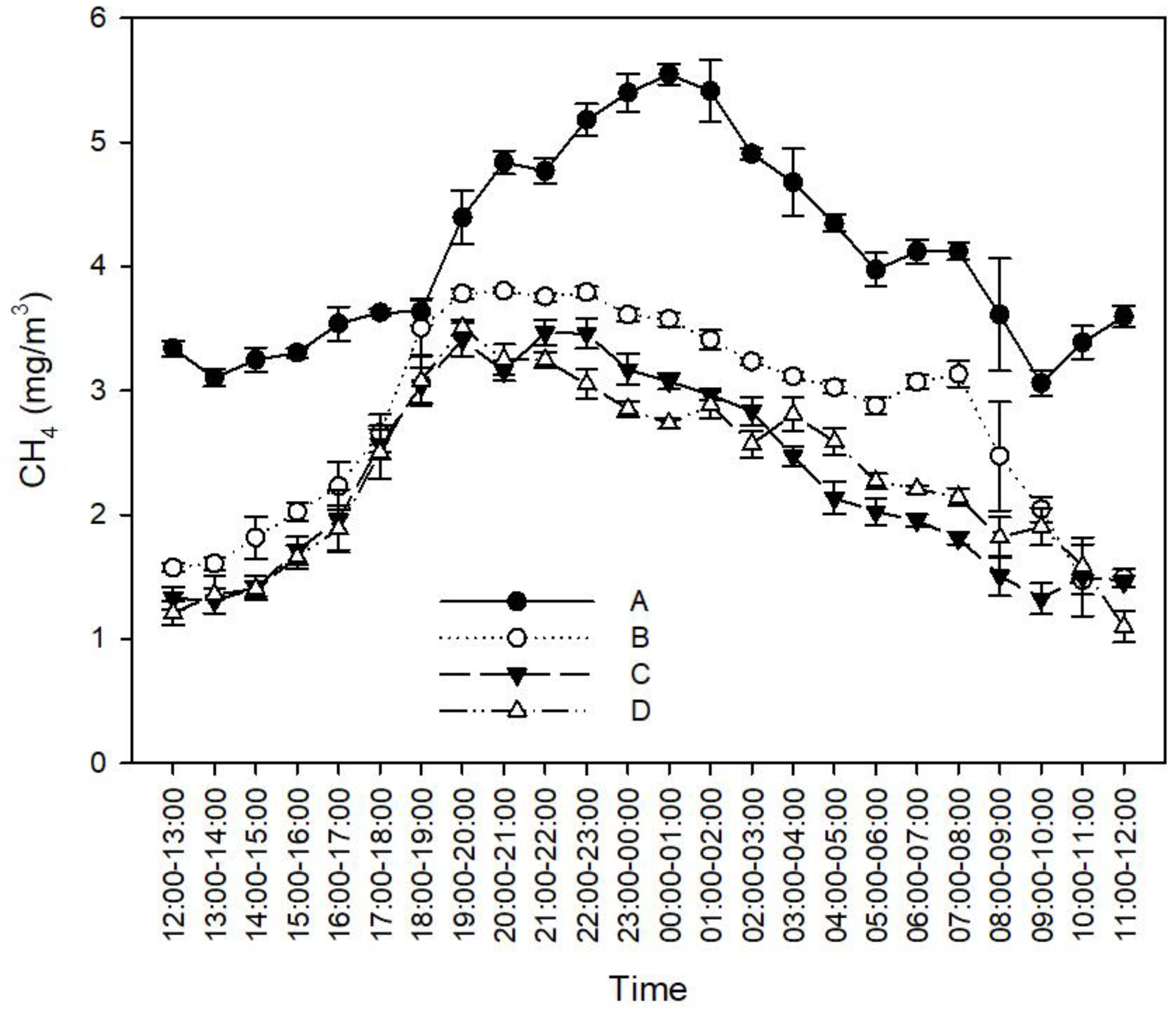
3.1.4. Effect of Feces-Cleaning Frequency on CH4 Concentration in the Rabbit House
3.2. Effects of Microbial Deodorant on NH3 and Greenhouse Gases in the Rabbit House
3.3. Effect of VenaZn Deodorant on NH3 and Greenhouse Gases in the Rabbit House
4. Discussion
4.1. Effect of Feces-Cleaning Frequency on NH3 and GHG Concentrations
4.2. Effect of Temperature on NH3 and GHGs Concentration
4.3. Deodorization Effect of Microbial Preparations
4.4. Deodorization Effect of VenaZn Deodorant
5. Conclusions
- (1)
- The overall trends in the average concentrations of NH3, CO2, N2O, and CH4 in the experimental rabbit house first increased and then decreased from 12:00 to 12:00 the next day during the winter. The manure removal frequency had a significant impact on the average concentrations of NH3, CO2, and CH4 in the rabbit house. Cleaning feces from animal houses two and three times a day significantly decreased the NH3 concentration, and, in contrast, cleaning four times a day increased the NH3 concentration in the rabbit house; increasing the manure removal frequency significantly reduced the CO2 and CH4 concentration in the rabbit house. Considering the average concentrations of NH3, CO2, N2O, and CH4 in the rabbit house and the economic cost, it is better to clean feces from animal houses twice a day.
- (2)
- The average NH3 and CO2 concentrations declined significantly within 3 days in the summer and winter, and N2O concentration declined within 3 days in the summer but not in the winter. There was no effect on the CH4 concentration in the summer or in the winter after the microbial deodorant was sprayed. Therefore, it was better to spray microbial deodorant twice a week on Monday and Thursday to reduce the concentrations of NH3, CO2, N2O, and CH4 in the rabbit house.
- (3)
- The average concentrations of NH3, CO2, N2O, and CH4 first showed a decreasing trend and then an increasing within 5 days to 7 days in the summer and winter after the VenaZn deodorant had been sprayed in the rabbit house, and the concentrations of NH3, CO2, N2O, and CH4 were significantly lower on the third and fourth days than they were on the other days.
- (4)
- The average NH3, CO2, and N2O concentrations in the winter were higher than they were in the summer, and the average CH4 concentration was higher in the summer than it was in the winter in the experimental rabbit house.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivier, J.G.J.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2020. [Google Scholar]
- Olivier, J.G.; Schure, K.; Peters, J. Trends in Global CO2 and Total Greenhouse Gas Emissions; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017; Volume 5, pp. 1–11. [Google Scholar]
- Havlík, P.; Valin, H.; Herrero, M.; Obersteiner, M.; Schmid, E.; Rufino, M.C.; Mosnier, A.; Thornton, P.K.; Böttcher, H.; Conant, R.T.; et al. Climate change mitigation through livestock system transitions. Proc. Natl. Acad. Sci. USA 2014, 111, 3709–3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mara, F.P. The significance of livestock as a contributor to global greenhouse gas emissions today and in the near future. Anim. Feed Sci. Technol. 2011, 166–167, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, M.; Lu, X.; Caro, D.; Gao, J.; Zhang, J.; Cullen, B.; Li, Q. Emissions of non-CO2 greenhouse gases from livestock in China during 2000–2015: Magnitude, trends and spatiotemporal patterns. J. Environ. Manag. 2019, 242, 40–45. [Google Scholar] [CrossRef]
- Wei, S.; Bai, Z.H.; Chadwick, D.; Hou, Y.; Qin, W.; Zhao, Z.Q.; Jiang, R.F.; Ma, L. Greenhouse gas and ammonia emissions and mitigation options from livestock production in peri-urban agriculture: Beijing—A case study. J. Clean. Prod. 2018, 178, 515–525. [Google Scholar] [CrossRef]
- Huang, X.; Song, Y.; Li, M.; Li, J.; Huo, Q.; Cai, X.; Zhu, T.; Hu, M.; Zhang, H. A high-resolution ammonia emission inventory in China. Glob. Biogeochem. Cycles 2012, 26, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Zhao, Y.; Henze, D.K.; Zhu, L.; Song, Y.; Paulot, F.; Liu, X.; Pan, Y.; Lin, Y.; et al. Agricultural ammonia emissions in China: Reconciling bottom-up and top-down estimates. Atmos. Chem. Phys. 2018, 18, 339–355. [Google Scholar] [CrossRef] [Green Version]
- Ma, S. High-resolution assessment of ammonia emissions in China: Inventories, driving forces and mitigation. Atmos. Environ. 2020, 229, 117458. [Google Scholar] [CrossRef]
- Webb, J.; Sommer, S.G.; Kupper, T.; Groenestein, K.; Hutchings, N.J.; Eurich-Menden, B.; Rodhe, L.; Misselbrook, T.H.; Amon, B. Emissions of Ammonia, Nitrous Oxide and Methane during the Management of Solid Manures. In Agroecology and Strategies for Climate Change; Springer: Dordrecht, The Netherlands, 2012; pp. 67–107. [Google Scholar]
- Wu, S.-Y.; Hu, J.-L.; Zhang, Y.; Aneja, V.P. Modeling atmospheric transport and fate of ammonia in North Carolina—Part II: Effect of ammonia emissions on fine particulate matter formation. Atmos. Environ. 2008, 42, 3437–3451. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; Van Cleemput, O. Closing the global N2O budget: Nitrous oxide emissions through the agricultural nitrogen cycle. Nutr. Cycl. Agroecosyst. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- Pinder, R.W.; Davidson, E.A.; Goodale, C.L.; Greaver, T.L.; Herrick, J.D.; Liu, L. Climate change impacts of US reactive nitrogen. Proc. Natl. Acad. Sci. USA 2012, 109, 7671–7675. [Google Scholar] [CrossRef] [Green Version]
- Banhazi, T.; Seedorf, J.; Rutley, D.; Pitchford, W. Identification of risk factors for sub-optimal housing conditions in Australian piggeries: Part 2. Airborne pollutants. J. Agric. Saf. Health 2008, 14, 21–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, D.A.; Donham, K.J.; Olenchock, S.A.; Popendorf, W.J.; Van Fossen, D.S.; Burmeister, L.F.; Merchant, J.A. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. Am. J. Respir. Crit. Care Med. 1995, 151, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, B. Study on exposure of pig farm workers to bioaerosols, immunologic reactivity and health effects. Ann. Agric. Environ. Med. 1998, 5, 169. [Google Scholar] [PubMed]
- Donham, K.J.; Cumro, D.; Reynolds, S. Synergistic Effects of Dust and Ammonia on the Occupational Health Effects of Poultry Production Workers. J. Agromed. 2002, 8, 57–76. [Google Scholar] [CrossRef]
- Nicolai, R.; Pohl, S. Understanding livestock odors. Fact Sheets 2005, 105. [Google Scholar]
- Thorne, P.S. Environmental Health Impacts of Concentrated Animal Feeding Operations: Anticipating Hazards—Searching for Solutions. Environ. Health Perspect. 2007, 115, 296–297. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Hristov, A.N.; Arogo, J.; Sheffield, R. A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst. Eng. 2008, 100, 453–469. [Google Scholar] [CrossRef]
- Liu, S.; Ni, J.-Q.; Radcliffe, J.S.; Vonderohe, C.E. Mitigation of ammonia emissions from pig production using reduced dietary crude protein with amino acid supplementation. Bioresour. Technol. 2017, 233, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Le, P.D.; Aarnink, A.J.A.; Jongbloed, A.W. Odour and ammonia emission from pig manure as affected by dietary crude protein level. Livest. Sci. 2009, 121, 267–274. [Google Scholar] [CrossRef]
- Guarino, M.; Fabbri, C.; Navarotto, P.; Valli, L.; Moscatelli, G.; Rossetti, M.; Mazzotta, V. Ammonia, Methane and Nitrous Oxide Emissions and Particulate Matter Concentrations in Two Different Buildings for Fattening Pigs. In Proceedings of the International Symposium on Gaseous and Odor Emissions from Animal Production Facilities 2003, Jutland, Denmark, 1–4 June 2003; pp. 140–149. [Google Scholar]
- Philippe, F.X.; Nicks, B. Review on greenhouse gas emissions from pig houses: Production of carbon dioxide, methane and nitrous oxide by animals and manure. Agric. Ecosyst. Environ. 2015, 199, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, F.A.; Chambers, B.J.; Walker, A.W. Ammonia Emissions from Broiler Litter and Laying Hen Manure Management Systems. Biosyst. Eng. 2004, 89, 175–185. [Google Scholar] [CrossRef]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Monteny, G.-J.; Bannink, A.; Chadwick, D. Greenhouse gas abatement strategies for animal husbandry. Agric. Ecosyst. Environ. 2006, 112, 163–170. [Google Scholar] [CrossRef]
- Lim, T.-T.; Heber, A.J.; Ni, J.-Q.; Kendall, D.C.; Richert, B.R. Effects of Manure Removal Strategies on Odor and Gas Emission from Swine Finishing. In Proceedings of the 2002 ASAE Annual Meeting, Chicago, IL, USA, 28–31 July 2002; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2002; p. 1. [Google Scholar]
- Cai, L.; Yu, J.; Zhang, J.; Qi, D. The effects of slatted floors and manure scraper systems on the concentrations and emission rates of ammonia, methane and carbon dioxide in goat buildings. Small Rumin. Res. 2015, 132, 103–110. [Google Scholar] [CrossRef]
- Niu, H. Effect of Barn Type and Feeding Technology on Barn Environment and Behavior of Beef Cattle in the Winter; Nanjing Agriculture University: Nanjing, China, 2015. [Google Scholar]
- Wood, J.D.; Gordon, R.J.; Wagner-Riddle, C.; Dunfield, K.E.; Madani, A. Relationships between Dairy Slurry Total Solids, Gas Emissions, and Surface Crusts. J. Environ. Qual. 2012, 41, 694–704. [Google Scholar] [CrossRef]
- Smith, K.; Cumby, T.; Lapworth, J.; Misselbrook, T.; Williams, A. Natural crusting of slurry storage as an abatement measure for ammonia emissions on dairy farms. Biosyst. Eng. 2007, 97, 464–471. [Google Scholar] [CrossRef]
- Zhu, J. A review of microbiology in swine manure odor control. Agric. Ecosyst. Environ. 2000, 78, 93–106. [Google Scholar] [CrossRef]
- Jongebreur, A.A.; Monteny, G.J. Prevention and Control of Losses of Gaseous Nitrogen Compounds in Livestock Operations: A Review. Sci. World J. 2001, 1, 844–851. [Google Scholar] [CrossRef]
- Philippe, F.-X.; Cabaraux, J.-F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Pu, S.; Rong, X.; Zhu, J.; Zeng, Y.; Yue, J.; Lim, T.; Long, D. Short-Term Aerial Pollutant Concentrations in a Southwestern China Pig-Fattening House. Atmosphere 2021, 12, 103. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, B.; Ni, J.-Q.; Xue, W.; Zhu, Z.; Li, X.; Zou, G. New insights into concentrations, sources and transformations of NH3, NOx, SO2 and PM at a commercial manure-belt layer house. Environ. Pollut. 2020, 262, 114355. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.H.; Long, D.B.; Liu, H.Z. The Emission of air pollutants in fattening pig houses. In Proceedings of the 10th International Livestock Environment Symposium (ILES X), Omaha, NE, USA, 25–27 September 2018. [Google Scholar]
- Amon, B.; Kryvoruchko, V.; Fröhlich, M.; Amon, T.; Pöllinger, A.; Mösenbacher, I.; Hausleitner, A. Ammonia and greenhouse gas emissions from a straw flow system for fattening pigs: Housing and manure storage. Livest. Sci. 2007, 112, 199–207. [Google Scholar] [CrossRef]
- Pereira, J.; Fangueiro, D.; Misselbrook, T.H.; Chadwick, D.R.; Coutinho, J.; Trindade, H. Ammonia and greenhouse gas emissions from slatted and solid floors in dairy cattle houses: A scale model study. Biosyst. Eng. 2011, 109, 148–157. [Google Scholar] [CrossRef]
- Lu, Y.; Fu, L.; Lu, Y.; Hugenholtz, F.; Ma, K. Effect of temperature on the structure and activity of a methanogenic archaeal community during rice straw decomposition. Soil Biol. Biochem. 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Husted, S. Seasonal Variation in Methane Emission from Stored Slurry and Solid Manures; 0047-2425; Wiley Online Library: Hoboken, NJ, USA, 1994. [Google Scholar]
- Li, X. Establishment and Application of Real-Time Monitoring System for the Swine Harmful Gases Emissions; Graduate School of Chinese Academy of Agricultural Sciences: Beijing, China, 2012. [Google Scholar]
- Haeussermann, A.; Hartung, E.; Gallmann, E.; Jungbluth, T. Influence of season, ventilation strategy, and slurry removal on methane emissions from pig houses. Agric. Ecosyst. Environ. 2006, 112, 115–121. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Liao, X.; Wang, W.; Mao, C.; Mi, J.; Wu, Y.; Wang, Y. A low-cost deodorizing spray net device for the removal of ammonia emissions in livestock houses. J. Clean. Prod. 2021, 318, 128516. [Google Scholar] [CrossRef]
- Chen, G.; Zhan, K.; Chen, L.; Li, J.; Liu, W. Preparation of solid-state deodorant and its ammonia-reducing effect in layers’ house. Trans. Chin. Soc. Agric. Eng. 2012, 28, 163–170. [Google Scholar]
- Ma, H.; Li, F.; Niyitanga, E.; Chai, X.; Wang, S.; Liu, Y. The Odor Release Regularity of Livestock and Poultry Manure and the Screening of Deodorizing Strains. Microorganisms 2021, 9, 2488. [Google Scholar] [CrossRef]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological methods for odor treatment—A review. J. Clean. Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Kennes, C.; Rene, E.R.; Veiga, M.C. Bioprocesses for air pollution control. J. Chem. Technol. Biotechnol. 2009, 84, 1419–1436. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, Y.; Yong, X.; Wu, X.; Jia, H.; Wong, J.W.C.; Wu, H.; Zhou, J. Odor emission and microbial community succession during biogas residue composting covered with a molecular membrane. Bioresour. Technol. 2020, 297, 122518. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S.; Matusiak, K.; Powałowski, S.; Pielech-Przybylska, K.; Makowski, K.; Nowak, A.; Rosowski, M.; Komorowski, P.; Gutarowska, B. A novel microbial-mineral preparation for the removal of offensive odors from poultry manure. Int. Biodeterior. Biodegrad. 2017, 119, 299–308. [Google Scholar] [CrossRef]
- Kuroda, K.; Waki, M.; Yasuda, T.; Fukumoto, Y.; Tanaka, A.; Nakasaki, K. Utilization of Bacillus sp. strain TAT105 as a biological additive to reduce ammonia emissions during composting of swine feces. Biosci. Biotechnol. Biochem. 2015, 79, 1702–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matulaitis, R.; Juškienė, V.; Juška, R. Gaseous emissions from manure as affected by microbial-based additive and temperature. Vet. Med. Zoot 2013, 64, 86. [Google Scholar]
- Fenxia, Y.; Ruifen, Z.; Yangfang, Y. Preparation of complex microbial adsorbent of deodorization and its application to deodorization. Trans. CSAE 2008, 24, 254–257. [Google Scholar]
- Choi, I.-H.; Lee, H.-J.; Kim, D.-H.; Lee, Y.-B.; Kim, S.-C. Evaluation of Probiotics on Animal Husbandry and Environmental Management as Manure Additives to Reduce Pathogen and Gas Emissions in Pig Slurry. J. Environ. Sci. Int. 2015, 24, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Salem, W.; Leitner, D.R.; Zingl, F.G.; Schratter, G.; Prassl, R.; Goessler, W.; Reidl, J.; Schild, S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 2015, 305, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; Karan, R.; Sinha, A.; Khare, S.K. Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 2011, 102, 1516–1520. [Google Scholar] [CrossRef]
- Esa, Y.A.M.; Sapawe, N. A short review on zinc metal nanoparticles synthesize by green chemistry via natural plant extracts. Mater. Today Proc. 2020, 31, 386–393. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. -Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Conte, A.; Speranza, B.; Sinigaglia, M.; Del Nobile, M.A. Effect of Lemon Extract on Foodborne Microorganisms. J. Food Prot. 2007, 70, 1896–1900. [Google Scholar] [CrossRef]
- Hindi, N.K.K.; Chabuck, Z.A.G. Antimicrobial Activity of Different Aqueous Lemon Extracts-Nada Khazal Kadhim Hindi1 2013. J. Appl. Pharm. Sci. 2013, 3, 074–078. [Google Scholar]
- Yang, C.S.; Chung, J.Y.; Yang, G.-y.; Chhabra, S.K.; Lee, M.-J. Tea and tea polyphenols in cancer prevention. J. Nutr. 2000, 130, 472S–478S. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wan, X.; Zhan, J.; Ho, C.-T. Focusing on the recent progress of tea polyphenol chemistry and perspectives. Food Sci. Hum. Wellness 2022, 11, 437–444. [Google Scholar] [CrossRef]
- Turkmen, N.; Velioglu, Y.S.; Sari, F.; Polat, G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 2007, 12, 484–496. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, T. Antimicrobial activities of tea polyphenol on phytopathogens: A review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef] [Green Version]
- Mbata, T.; Debiao, L.; Saikia, A. Antibacterial activity of the crude extract of Chinese green tea (Camellia sinensis) on Listeria monocytogenes. Afr. J. Biotechnol. 2008, 7, 10. [Google Scholar]
- Laireiter, C.M.; Schnabel, T.; Köck, A.; Stalzer, P.; Petutschnigg, A.; Oostingh, G.J.; Hell, M. Active anti-microbial effects of larch and pine wood on four bacterial strains. BioResources 2014, 9, 273–281. [Google Scholar] [CrossRef]
- Kim, K.Y.; Davidson, P.; Chung, H.J. Antimicrobial Effectiveness of Pine Needle Extract on Foodborne Illness Bacteria. J. Microbiol. Biotechnol. 2000, 10, 227–232. [Google Scholar]
| Season | Temperature (°C) | Relative Humidity (%) | |
|---|---|---|---|
| Experiment 1 | Winter | 14.93 | 73.77 |
| Experiment 2 | Summer | 26.04 | 81.04 |
| Winter | 12.39 | 77.98 |
| Treatment | NH3 (mg/m3) | CO2 (mg/m3) | N2O (mg/m3) | CH4 (mg/m3) |
|---|---|---|---|---|
| A | 16.17 ± 4.87a | 1803 ± 341a | 0.796 ± 0.037b | 4.13 ± 0.79a |
| B | 14.31 ± 3.82b | 1681 ± 183b | 0.817 ± 0.047a | 2.80 ± 0.82b |
| C | 14.71 ± 4.88b | 1590 ± 225b | 0.803 ± 0.026ab | 2.29 ± 0.78c |
| D | 16.95 ± 6.13a | 1623 ± 191b | 0.799 ± 0.029b | 2.32 ± 0.71c |
| NH3 (mg/m3) | CO2 (mg/m3) | N2O (mg/m3) | CH4 (mg/m3) | |
|---|---|---|---|---|
| Summer | ||||
| 1d | 3.50 ± 0.47c | 944 ± 33c | 0.89 ± 0.04c | 7.20 ± 0.40b |
| 2d | 3.44 ± 0.55c | 929 ± 29c | 0.91 ± 0.04bc | 8.07 ± 0.74a |
| 3d | 3.64 ± 0.86c | 1014 ± 124b | 0.91 ± 0.03bc | 7.04 ± 0.75b |
| 4d | 4.25 ± 1.25b | 1056 ± 100a | 0.93 ± 0.05b | 5.42 ± 1.74d |
| 5d | 4.67 ± 1.03a | 1090 ± 86a | 0.99 ± 0.06a | 6.13 ± 1.78c |
| 6d | 3.91 ± 1.12b | 1006 ± 60b | 0.97 ± 0.06a | 5.99 ± 1.85cd |
| Mean value | 3.94 ± 0.49 | 1007 ± 63 | 0.93 ± 0.04 | 6.61 ± 1.00 |
| Winter | ||||
| 1d | 5.86 ± 0.49c | 1350 ± 84c | 1.02 ± 0.04a | 0.46 ± 0.24a |
| 2d | 6.20 ± 0.59c | 1348 ± 140c | 1.02 ± 0.03a | 0.40 ± 0.34ab |
| 3d | 5.89 ± 1.42c | 1326 ± 176c | 1.00 ± 0.05b | 0.34 ± 0.25bc |
| 4d | 6.68 ± 0.50b | 1368 ± 77bc | 1.00 ± 0.04b | 0.41 ± 0.24ab |
| 5d | 7.92 ± 0.78a | 1406 ± 126ab | 0.99 ± 0.02c | 0.27 ± 0.28c |
| 6d | 7.59 ± 0.65a | 1399 ± 102ab | 1.01 ± 0.04ab | 0.40 ± 0.32ab |
| 7d | 7.61 ± 0.80a | 1430 ± 137a | 1.02 ± 0.03a | 0.47 ± 0.32a |
| Mean value | 6.82 ± 0.88 | 1375 ± 37 | 1.01 ± 0.01 | 0.39 ± 0.07 |
| NH3 (mg/m3) | CO2 (mg/m3) | N2O (mg/m3) | CH4 (mg/m3) | |
|---|---|---|---|---|
| Summer | ||||
| 1d | 4.73 ± 1.06a | 1038 ± 153a | 0.89 ± 0.04a | 6.21 ± 2.54b |
| 2d | 3.91 ± 1.29b | 994 ± 107b | 0.87 ± 0.04b | 5.77 ± 2.51bc |
| 3d | 2.37 ± 0.46c | 898 ± 43c | 0.82 ± 0.02c | 4.98 ± 2.42cd |
| 4d | 2.67 ± 0.70c | 913 ± 57c | 0.82 ± 0.03c | 4.88 ± 1.67d |
| 5d | 4.23 ± 1.11b | 1010 ± 96ab | 0.91 ± 0.03a | 7.24 ± 2.01a |
| Mean value | 3.58 ± 1.02 | 809 ± 62 | 0.86 ± 0.04 | 5.81 ± 0.97 |
| Winter | ||||
| 1d | 7.93 ± 1.05a | 1403 ± 77a | 1.00 ± 0.03b | 0.23 ± 0.47a |
| 2d | 6.62 ± 1.23bc | 1261 ± 141b | 0.98 ± 0.02c | 0.02 ± 0.05b |
| 3d | 5.53 ± 0.39d | 1202 ± 66c | 0.97 ± 0.03c | 0.01 ± 0.03b |
| 4d | 5.81 ± 0.60d | 1173 ± 61c | 0.95 ± 0.01d | 0.00 ± 0.00b |
| 5d | 6.36 ± 0.72c | 1184 ± 102c | 0.95 ± 0.01d | 0.00 ± 0.00b |
| 6d | 6.99 ± 0.55b | 1278 ± 69b | 1.03 ± 0.02a | 0.04 ± 0.07b |
| 7d | 7.70 ± 0.70a | 1384 ± 73a | 1.00 ± 0.01b | 0.01 ± 0.01b |
| Mean value | 6.70 ± 0.90 | 1269 ± 93 | 0.98 ± 0.03 | 0.04 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, J.; Shao, L.; Huan, H.; Qin, F.; Zhai, P.; Yang, J.; Pan, X. Effects of Manure Removal Frequencies and Deodorants on Ammonia and GHG Concentrations in Livestock House. Atmosphere 2022, 13, 1033. https://doi.org/10.3390/atmos13071033
Zhang X, Li J, Shao L, Huan H, Qin F, Zhai P, Yang J, Pan X. Effects of Manure Removal Frequencies and Deodorants on Ammonia and GHG Concentrations in Livestock House. Atmosphere. 2022; 13(7):1033. https://doi.org/10.3390/atmos13071033
Chicago/Turabian StyleZhang, Xia, Jian Li, Le Shao, Hailin Huan, Feng Qin, Pin Zhai, Jie Yang, and Xiaoqing Pan. 2022. "Effects of Manure Removal Frequencies and Deodorants on Ammonia and GHG Concentrations in Livestock House" Atmosphere 13, no. 7: 1033. https://doi.org/10.3390/atmos13071033
APA StyleZhang, X., Li, J., Shao, L., Huan, H., Qin, F., Zhai, P., Yang, J., & Pan, X. (2022). Effects of Manure Removal Frequencies and Deodorants on Ammonia and GHG Concentrations in Livestock House. Atmosphere, 13(7), 1033. https://doi.org/10.3390/atmos13071033





