Climatic Signals on Growth Ring Variation in Salix herbacea: Comparing Two Contrasting Sites in Iceland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling
2.3. Microscopic Preparation
2.4. Growth Ring Measurements
2.5. Cross-Dating and Chronology Development
2.6. Data Standardization
2.7. Climate Data and Analysis of Climate–Growth Responses
3. Results
3.1. Chronology Development
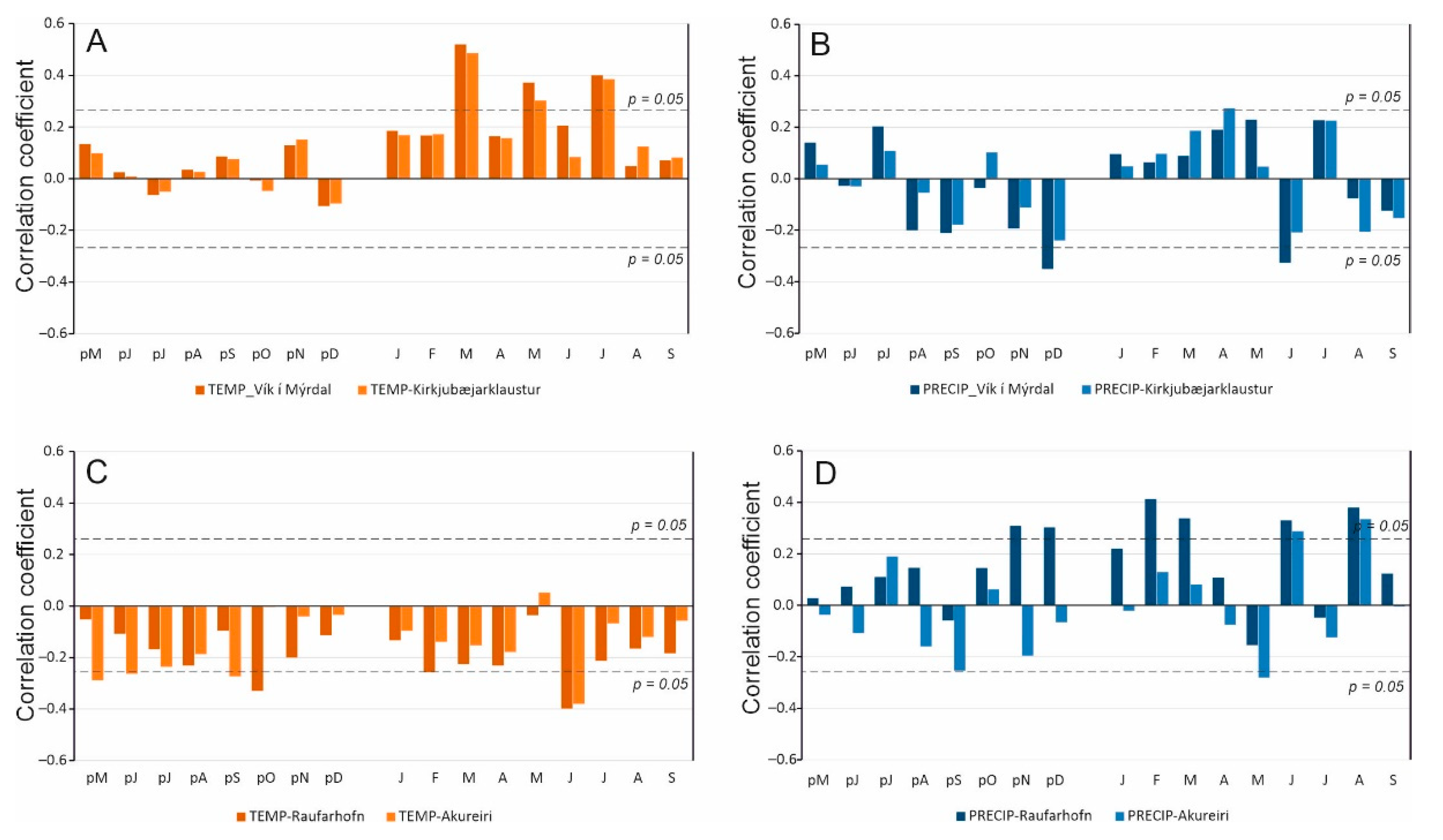
3.2. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AMAP, Snow, Water, Ice and Permafrost in the Arctic (SWIPA). (Oslo: Arctic Monitoring and Assessment Programme (AMAP)); The Arctic Monitoring and Assessment Programme: Tromsø, Norway, 2017. [Google Scholar]
- Raynolds, M.K.; Comiso, J.C.; Walker, D.A.; Verbyla, D. Relationship between satellite-derived land surface temperatures, arctic vegetation types, and NDVI. Remote Sens. Environ. 2008, 112, 1884–1894. [Google Scholar] [CrossRef]
- Wolf, A.; Blyth, E.; Harding, R.; Jacob, D.; Keup-Thiel, E.; Goettel, H.; Callaghan, T. Sensitivity of an ecosystem model to hydrology and temperature. Clim. Chang. 2008, 87, 75–89. [Google Scholar] [CrossRef][Green Version]
- Przybylak, R. The Climate of the Arctic Introduction. Atmos. Ocean. Sci. Lib. 2016, 52, 1–14. [Google Scholar]
- Reisch, C.; Schurm, S.; Poschlod, P. Spatial genetic structure and clonal diversity in an alpine population of Salix herbacea (salicaceae). Ann. Bot.-Lond. 2007, 99, 647–651. [Google Scholar] [CrossRef]
- Beerling, D.J. Salix herbacea L. J. Ecol. 1998, 86, 872–895. [Google Scholar] [CrossRef]
- Wijk, S. Performance of Salix-Herbacea in an Alpine Snow-Bed Gradient. J. Ecol. 1986, 74, 675–684. [Google Scholar] [CrossRef]
- Stocker, T.F.Q.D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC: The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Climate Change; Cambridge University: Cambridge, UK; New York, NY, USA, 2013; Volume 1535. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Academic Press: London, UK, 1976; p. 567. [Google Scholar]
- Myers-Smith, I.H.; Forbes, B.C.; Wilmking, M.; Hallinger, M.; Lantz, T.; Blok, D.; Tape, K.D.; Macias-Fauria, M.; Sass-Klaassen, U.; Levesque, E.; et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 2011, 6, 045509. [Google Scholar] [CrossRef]
- Elmendorf, S.C.; Henry, G.H.R.; Hollister, R.D.; Bjork, R.G.; Boulanger-Lapointe, N.; Cooper, E.J.; Cornelissen, J.H.C.; Day, T.A.; Dorrepaal, E.; Elumeeva, T.G.; et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Chang. 2012, 2, 453–457. [Google Scholar] [CrossRef]
- Forbes, B.C.; Fauria, M.M.; Zetterberg, P. Russian Arctic warming and ′greening′ are closely tracked by tundra shrub willows. Glob. Chang. Biol. 2010, 16, 1542–1554. [Google Scholar] [CrossRef]
- Buntgen, U.; Hellmann, L.; Tegel, W.; Normand, S.; Myers-Smith, I.; Kirdyanov, A.V.; Nievergelt, D.; Schweingruber, F.H. Temperature-induced recruitment pulses of Arctic dwarf shrub communities. J. Ecol. 2015, 103, 489–501. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Elmendorf, S.C.; Beck, P.S.A.; Wilmking, M.; Hallinger, M.; Blok, D.; Tape, K.D.; Rayback, S.A.; Macias-Fauria, M.; Forbes, B.C.; et al. Climate sensitivity of shrub growth across the tundra biome. Nat. Clim. Chang. 2015, 5, 887. [Google Scholar] [CrossRef]
- Opała-Owczarek, M.; Owczarek, P.; Łupikasza, E.; Boudreau, S.; Migała, K. Influence of climatic conditions on growth rings of Salix uva-ursi Pursh from the southeastern shore of Hudson Bay, Subarctic Canada. Arct. Antarct. Alp. Res. 2020, 52, 87–102. [Google Scholar] [CrossRef]
- Owczarek, P.; Opała, M. Dendrochronology and Extreme Pointer Years in the Tree-Ring Record (Ad 1951-2011) of Polar Willow from Southwestern Spitsbergen (Svalbard, Norway). Geochronometria 2016, 43, 84–95. [Google Scholar] [CrossRef]
- Owczarek, P.; Opała-Owczarek, M.; Migała, K. Post-1980s shift in the sensitivity of tundra vegetation to climate revealed by the first dendrochronological record from Bear Island (Bjornoya), western Barents Sea. Environ. Res. Lett. 2021, 16, 014031. [Google Scholar] [CrossRef]
- Weijers, S.; Buchwal, A.; Blok, D.; Loffler, J.; Elberling, B. High Arctic summer warming tracked by increased Cassiope tetragona growth in the world’s northernmost polar desert. Glob. Chang. Biol. 2017, 23, 5006–5020. [Google Scholar] [CrossRef] [PubMed]
- Le Moullec, M.; Buchwal, A.; van der Wal, R.; Sandal, L.; Hansen, B.B. Annual ring growth of a widespread high arctic shrub reflects past fluctuations in community-level plant biomass. J. Ecol. 2019, 107, 436–451. [Google Scholar] [CrossRef]
- Buchwal, A.; Rachlewicz, G.; Fonti, P.; Cherubini, P.; Gartner, H. Temperature modulates intra-plant growth of Salix polaris from a high Arctic site (Svalbard). Polar Biol. 2013, 36, 1305–1318. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Kerby, J.T.; Phoenix, G.K.; Bjerke, J.W.; Epstein, H.E.; Assmann, J.J.; John, C.; Andreu-Hayles, L.; Angers-Blondin, S.; Beck, P.S.A.; et al. Complexity revealed in the greening of the Arctic. Nat. Clim. Chang. 2020, 10, 106–117. [Google Scholar] [CrossRef]
- Opała-Owczarek, M.; Piroznikow, E.; Owczarek, P.; Szymanski, W.; Luks, B.; Kepski, D.; Szymanowski, M.; Wojtun, B.; Migała, K. The influence of abiotic factors on the growth of two vascular plant species (Saxifraga oppositifolia and Salix polaris) in the High Arctic. Catena 2018, 163, 219–232. [Google Scholar] [CrossRef]
- Weijers, S.; Pape, R.; Loffler, J.; Myers-Smith, I.H. Contrasting shrub species respond to early summer temperatures leading to correspondence of shrub growth patterns. Environ. Res. Lett. 2018, 13, 034005. [Google Scholar] [CrossRef]
- Martin, A.C.; Jeffers, E.S.; Petrokofsky, G.; Myers-Smith, I.; Macias-Fauria, M. Shrub growth and expansion in the Arctic tundra: An assessment of controlling factors using an evidence-based approach. Environ. Res. Lett. 2017, 12, 085007. [Google Scholar] [CrossRef]
- Forchhammer, M. Sea-ice induced growth decline in Arctic shrubs. Biol. Lett. 2017, 13, 20170122. [Google Scholar] [CrossRef] [PubMed]
- Weijers, S.; Broekman, R.; Rozema, J. Dendrochronology in the High Arctic: July air temperatures reconstructed from annual shoot length growth of the circumarctic dwarf shrub Cassiope tetragona. Quat. Sci. Rev. 2010, 29, 3831–3842. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Hallinger, M.; Blok, D.; Sass-Klaassen, U.; Rayback, S.A.; Weijers, S.; Trant, A.J.; Tape, K.D.; Naito, A.T.; Wipf, S.; et al. Methods for measuring arctic and alpine shrub growth: A review. Earth-Sci. Rev. 2015, 140, 1–13. [Google Scholar] [CrossRef]
- Georgsdottir, G.I.E.Ó. Effects of climate on the growth of Sitka spruce and Lodgepole pine in Heidmork (in Icelandic). Fraedathing Landbun. 2005, 1, 364–368. [Google Scholar]
- Eggertsson, O.; Gudmundsson, H.J. Age of birch (Betula pubescens Ehrh.) in Baejarstadarskogur and the effects of climate on growth and maturity (in Icelandic). Skograektarritid 2002, 2, 85–89. [Google Scholar]
- Owczarek, P. Dendrochronological dating of geomorphic processes in the High Arctic. Landf. Anal. 2010, 14, 45–56. [Google Scholar]
- Rossi, G.; Parolo, G.; Zonta, L.A.; Crawford, J.A.; Leonardi, A. Salix herbacea L. fragmented small population in the N-Apennines (Italy): Response to human trampling disturbance. Biodivers. Conserv. 2006, 15, 3881–3893. [Google Scholar] [CrossRef]
- Alsos, I.G.; Alm, T.; Normand, S.; Brochmann, C. Past and future range shifts and loss of diversity in dwarf willow (Salix herbacea L.) inferred from genetics, fossils and modelling. Glob. Ecol. Biogeogr. 2009, 18, 223–239. [Google Scholar] [CrossRef]
- Wheeler, J.A.; Cortes, A.J.; Sedlacek, J.; Karrenberg, S.; van Kleunen, M.; Wipf, S.; Hoch, G.; Bossdorf, O.; Rixen, C. The snow and the willows: Earlier spring snowmelt reduces performance in the low-lying alpine shrub Salix herbacea. J. Ecol. 2016, 104, 1041–1050. [Google Scholar] [CrossRef]
- Abeli, T.; Vamosi, J.C.; Orsenigo, S. The importance of marginal population hotspots of cold-adapted species for research on climate change and conservation. J. Biogeogr. 2018, 45, 977–985. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Poschlod, P.; Forêt, L. Growth Rings in Herbs and Shrubs: Life Span, Age Determination and stem Anatomy; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 2005; Volume 79. [Google Scholar]
- De Witte, L.C.; Armbruster, G.F.; Gielly, L.; Taberlet, P.; Stoecklin, J. AFLP markers reveal high clonal diversity and extreme longevity in four key arctic-alpine species. Mol. Ecol. 2012, 21, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, S.P.; Gudmundsson, M.T. Subglacial and intraglacial volcanic formations in Iceland. Jokull 2008, 58, 179–196. [Google Scholar]
- Jóhannesson, H.; Sæmundsson, K. Geological map of Iceland. Bedrock geology, scale 1:500.000. In Náttúrufræðistofnun Is-Lands, Reykjavík, 2nd ed.; Icelandic Institute of Natural History: Reykjavik, Iceland, 1998. [Google Scholar]
- Arnalds, O. Soils of Iceland. In World Soil Book Series; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–183. [Google Scholar]
- Arnalds, O. The Icelandic ‘rofabard’ soil erosion features. Earth Surf. Processes Landf. J. Br. Geomorphol. Res. Group 2000, 25, 17–28. [Google Scholar] [CrossRef]
- Arnalds, O.; Gisladottir, F.O.; Sigurjonsson, H. Sandy deserts of Iceland: An overview. J. Arid Environ. 2001, 47, 359–371. [Google Scholar] [CrossRef]
- Walker, D.A.; Raynolds, M.K.; Daniels, F.J.A.; Einarsson, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.S.; Markon, C.J.; Melnikov, E.S.; et al. The Circumpolar Arctic vegetation map. J. Veg. Sci. 2005, 16, 267–282. [Google Scholar] [CrossRef]
- Yurtsev, B.A. Floristic Division of the Arctic. J. Veg. Sci. 1994, 5, 765–776. [Google Scholar] [CrossRef]
- Wasowicz, P.; Pasierbinski, A.; Przedpelska-Wasowicz, E.M.; Kristinsson, H. Distribution Patterns in the Native Vascular Flora of Iceland. PLoS ONE 2014, 9, e102916. [Google Scholar] [CrossRef]
- Cunningham, L.K.; Austin, W.E.N.; Knudsen, K.L.; Eiriksson, J.; Scourse, J.D.; Wanamaker, A.D.; Butler, P.G.; Cage, A.G.; Richter, T.; Husum, K.; et al. Reconstructions of surface ocean conditions from the northeast Atlantic and Nordic seas during the last millennium. Holocene 2013, 23, 921–935. [Google Scholar] [CrossRef]
- Wanamaker, A.D.; Kreutz, K.J.; Schoene, B.R.; Pettigrew, N.; Borns, H.W.; Introne, D.S.; Belknap, D.; Maasch, K.A.; Feindel, S. Coupled North Atlantic slope water forcing on Gulf of Maine temperatures over the past millennium. Clim. Dyn. 2008, 31, 183–194. [Google Scholar] [CrossRef]
- Olafsdottir, R.; Guomundsson, H.J. Holocene land degradation and climatic change in northeastern Iceland. Holocene 2002, 12, 159–167. [Google Scholar] [CrossRef]
- Gartner, H.; Schweingruber, H.F. Microscopic Preparation Techniques for Plant. Stem Analysis; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 2013; 78p, ISBN 378-3-941300-76-7. [Google Scholar]
- Owczarek, P.; Latocha, A.; Wistuba, M.; Malik, I. Reconstruction of modern debris flow activity in the arctic environment with the use of dwarf shrubs (south-western Spitsbergen)—A new dendrochronological approach. Z. Geomorphol. 2013, 57, 75–95. [Google Scholar] [CrossRef]
- Bär, A.; Bräuning, A.; Löffler, J. Dendroecology of dwarf shrubs in the high mountains of Norway—A methodological approach. Dendrochronologia 2006, 24, 17–27. [Google Scholar] [CrossRef]
- Kolishchuk, V. Dendroclimatological study of prostrate woody plants. Methods Dendrochronol. 1990, 353, 51–55. [Google Scholar]
- Gärtner, H.; Lucchinetti, S.; Schweingruber, F.H. New perspectives for wood anatomical analysis in dendrosciences: The GSL1-microtome. Dendrochronologia 2014, 32, 47–51. [Google Scholar] [CrossRef]
- Instruments, R. WinDendro. An. Image Analysis System for Tree-Rings Analysis; Regent Instruments Inc.: Quebec City, QC, Canada, 2012. [Google Scholar]
- Stokes, M.A. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Holmes, R.L. Dendrochronology Program Library User’s Manual; Laboratory of tree-ring research, University of Arizona: Tucson, AZ, USA, 1994. [Google Scholar]
- Young, A.B.; Watts, D.A.; Taylor, A.H.; Post, E. Species and site differences influence climate-shrub growth responses in West Greenland. Dendrochronologia 2016, 37, 69–78. [Google Scholar] [CrossRef]
- Wilson, J.W. Annual Growth of Salix Arctica in High-Arctic. Ann. Bot.-Lond. 1964, 28, 71. [Google Scholar] [CrossRef]
- Woodcock, H.; Bradley, R.S. Salix arctica (Pall.): Its potential for dendroclimatological studies in the High Arctic. Dendrochronologia 1994, 12, 11–22. [Google Scholar]
- Schmidt, N.M.; Baittinger, C.; Forchhammer, M.C. Reconstructing century-long snow regimes using estimates of high arctic Salix arctica radial growth. Arct. Antarct. Alp. Res. 2006, 38, 257–262. [Google Scholar] [CrossRef]
- Zalatan, R.; Gajewski, K. Dendrochronological potential of Salix alaxensis from the Kuujjua River area, western Canadian Arctic. Tree-Ring Res. 2006, 62, 75–82. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Hellmann, L.; Tegel, W.; Braun, S.; Nievergelt, D.; Büntgen, U. Evaluating the wood anatomical and dendroecological potential of arctic dwarf shrub communities. IAWA J. 2013, 34, 485–497. [Google Scholar] [CrossRef]
- Traustason, B.; Snorrason, A. Spatial distribution of forests and woodlands in Iceland in accordance with the CORINE land cover classification. Icel. Agr. Sci. 2008, 21, 39–47. [Google Scholar]
- Hannak, N.; Eggertsson, O. The long-term effects of climatic factors on radial growth of downy birch (Betula pubescens) and rowan (Sorbus aucuparia) in East Iceland. Icel. Agr. Sci. 2020, 33, 73–87. [Google Scholar] [CrossRef]
- Levanic, T.; Eggertsson, O. Climatic effects on birch (Betula pubescens Ehrh.) growth in Fnjoskadalur valley, northern Iceland. Dendrochronologia 2008, 25, 135–143. [Google Scholar] [CrossRef]
- Piermattei, A.; Urbinati, C.; Tonelli, E.; Eggertsson, O.; Levanic, T.; Kaczka, R.J.; Andrew, C.; Schone, B.R.; Buntgen, U. Potential and limitation of combining terrestrial and marine growth records from Iceland. Glob. Planet. Chang. 2017, 155, 213–224. [Google Scholar] [CrossRef]
- Decaulne, A.; Eggertsson, O.; Saemundsson, P. A first dendrogeomorphologic approach of snow avalanche magnitude-frequency in Northern Iceland. Geomorphology 2012, 167, 35–44. [Google Scholar] [CrossRef]
- Eggertsson, Ó. Origin of the driftwood on the coasts of Iceland: A dendrochronological study. Jokull 1993, 43, 15–32. [Google Scholar]
- Buntgen, U.; Eggertsson, O.; Wacker, L.; Sigl, M.; Ljungqvist, F.C.; Di Cosmo, N.; Plunkett, G.; Krusic, P.J.; Newfield, T.P.; Esper, J.; et al. Multi-proxy dating of Iceland’s major pre-settlement Katla eruption to 822-823 CE. Geology 2017, 45, 783–786. [Google Scholar] [CrossRef]
- Bär, A.; Pape, R.; Bräuning, A.; Löffler, J. Growth-ring variations of dwarf shrubs reflect regional climate signals in alpine environments rather than topoclimatic differences. J. Biogeogr. 2008, 35, 625–636. [Google Scholar] [CrossRef]
- Opala, M.; Migala, K.; Owczarek, P. Two centuries-long dendroclimatic reconstruction based on Low Arctic Betula pubescens from Tromso Region, Northern Norway. Pol. Polar Res. 2016, 37, 457–476. [Google Scholar] [CrossRef]
- Duthorn, E.; Schneider, L.; Konter, O.; Schon, P.; Timonen, M.; Esper, J. On the hidden significance of differing micro-sites on tree-ring based climate reconstructions. Silva. Fenn. 2015, 49. [Google Scholar] [CrossRef]
- Esper, J.; Holzkamper, S.; Buntgen, U.; Schone, B.; Keppler, F.; Hartl, C.; St George, S.; Riechelmann, D.F.C.; Treydte, K. Site-specific climatic signals in stable isotope records from Swedish pine forests. Trees 2018, 32, 855–869. [Google Scholar] [CrossRef]
- Beil, I.; Buras, A.; Hallinger, M.; Smiljanic, M.; Wilmking, M. Shrubs tracing sea surface temperature—Calluna vulgaris on the Faroe Islands. Int. J. Biometeorol. 2015, 59, 1567–1575. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Arnalds, O.; Olafsson, H. Long-term variability of dust events in Iceland (1949-2011). Atmos. Chem. Phys. 2014, 14, 13411–13422. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Arnalds, O.; Olafsson, H. Long-term frequency and characteristics of dust storm events in Northeast Iceland (1949–2011). Atmos. Environ. 2013, 79, 883. [Google Scholar] [CrossRef]
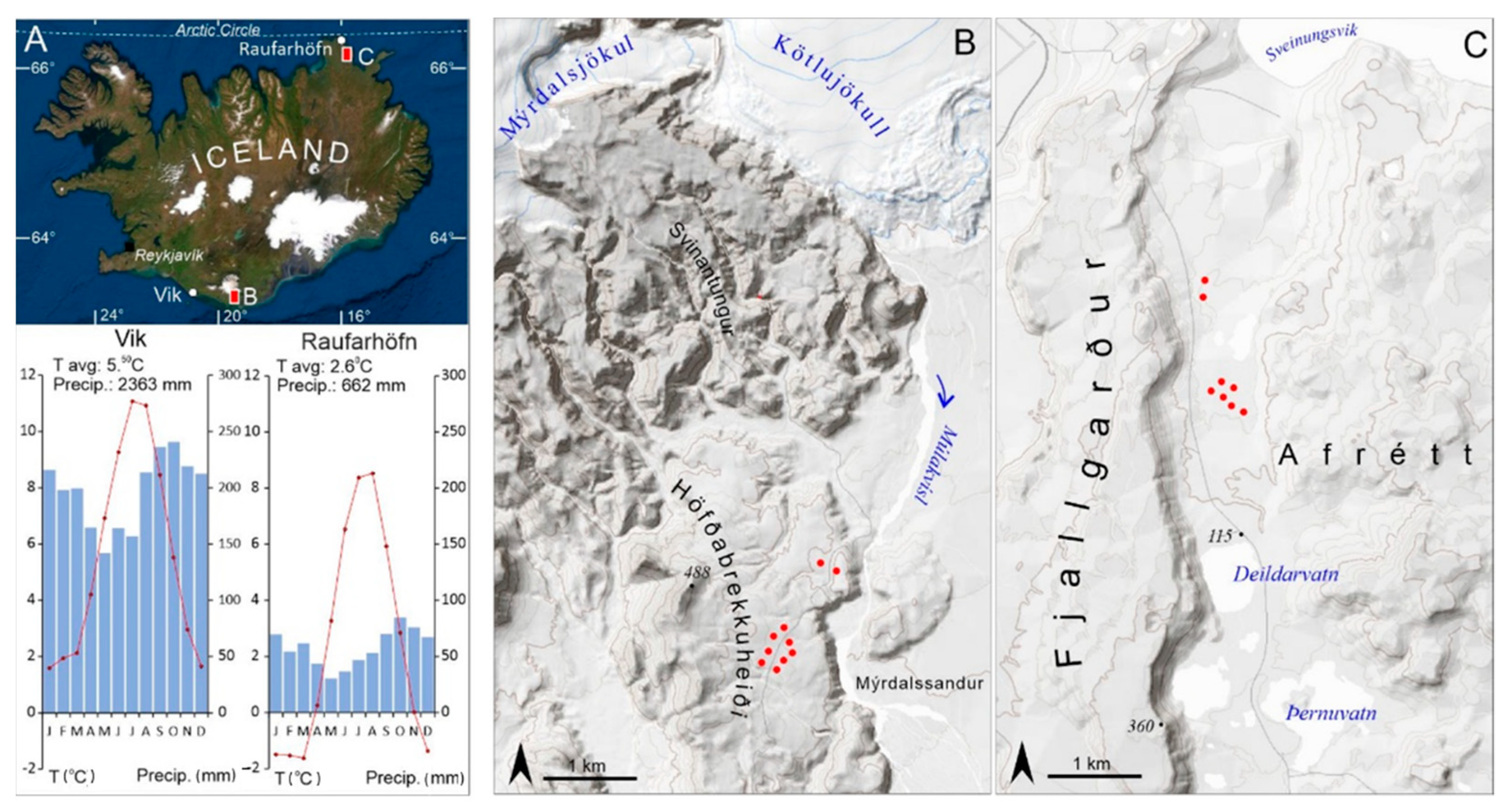
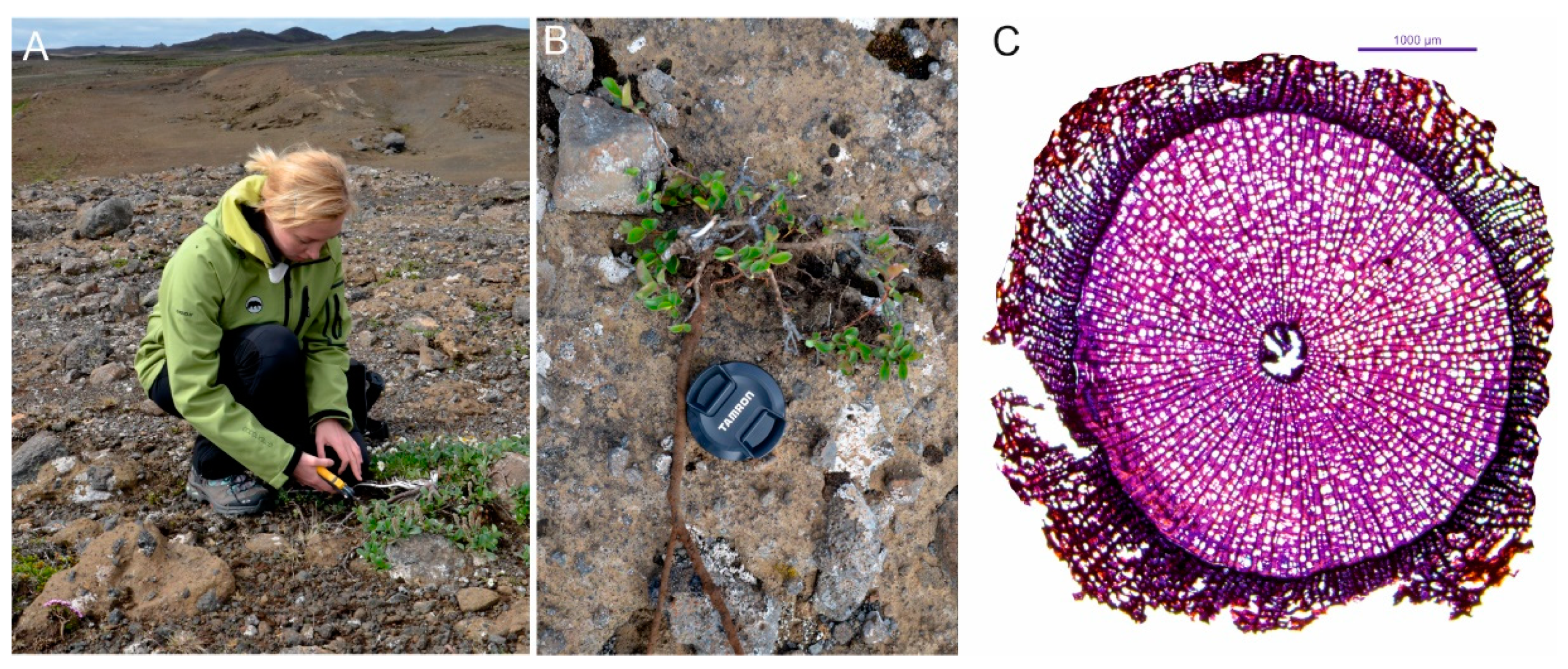
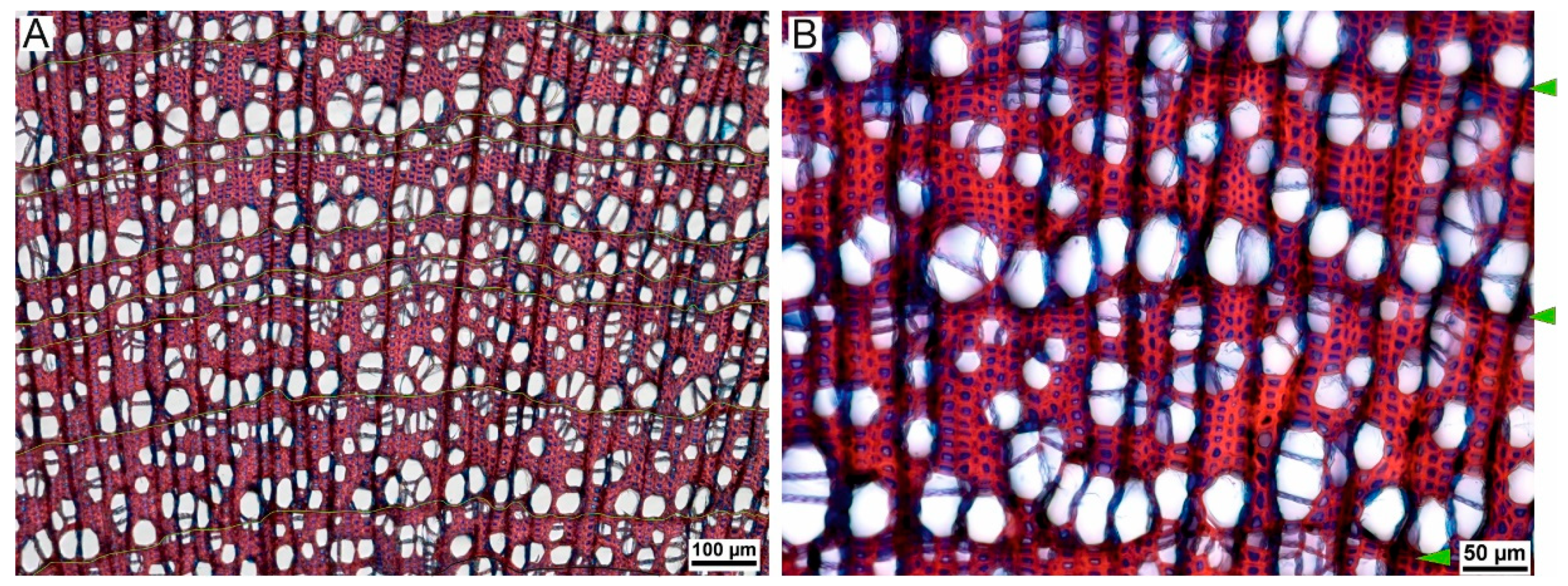
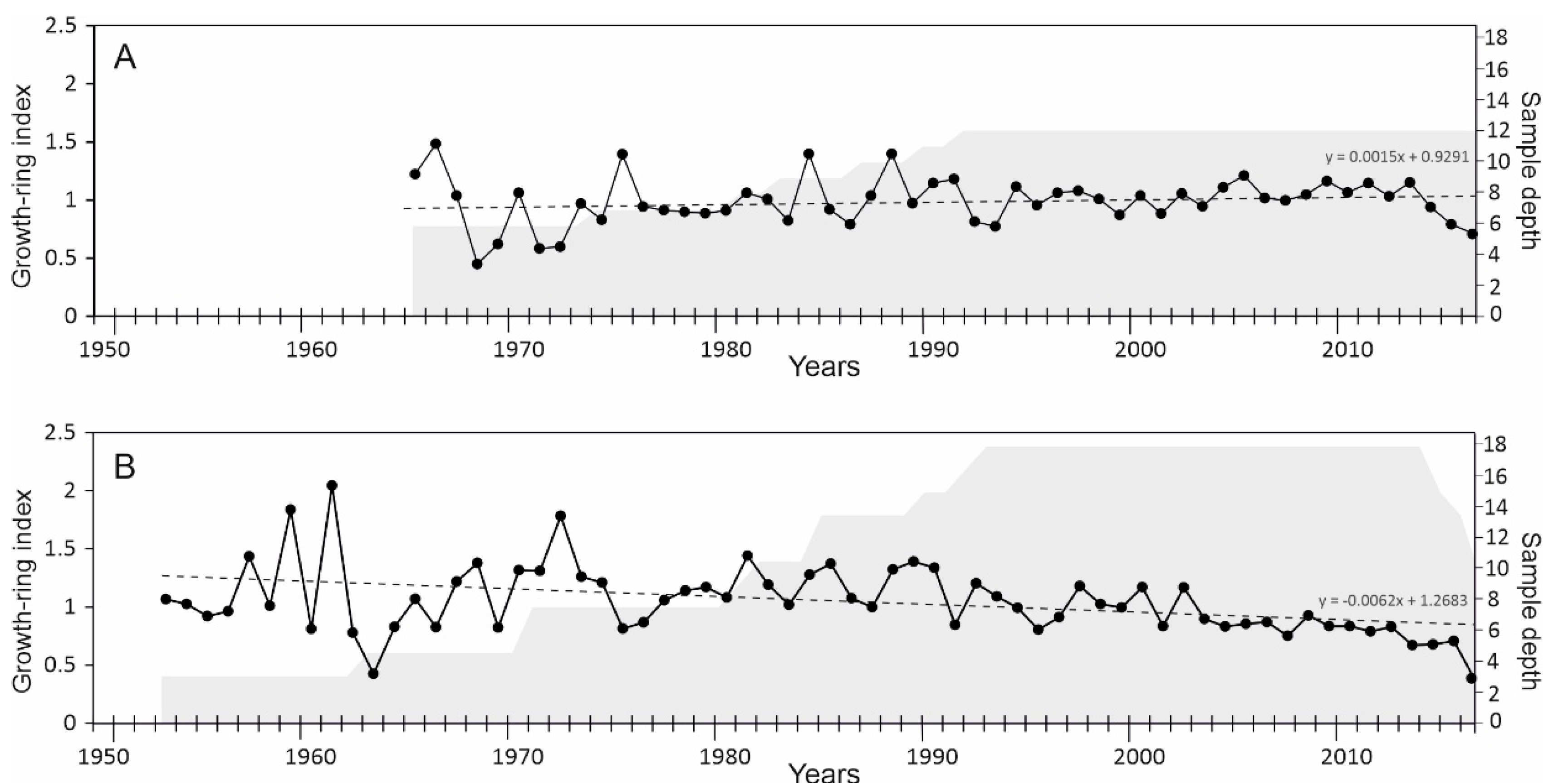
| No. | Station Name | Station Code | Location | Coordinates | Elevation | Distance to the Sampling Site | Timespan |
|---|---|---|---|---|---|---|---|
| 1 | Kirkjubæjarklaustur | 722 | S Iceland | 63.79° N, 18.05° W | 42 m a.s.l. | 51.0 km | 1930–2013 |
| 2 | Vík í Mýrdal | 798 | S Iceland | 63.42° N, 19.01° W | 31 m a.s.l. | 11.7 km | 1961–2013 |
| 3 | Raufarhöfn | 505 | NE Iceland | 66.45° N, 15.95° W | 4 m a.s.l. | 12.5 km | 1931–2021 |
| 4 | Akureyri | 422 | NE Iceland | 65.68° N, 18.09° W | 23 m a.s.l. | 122.5 km | 1949–2021 |
| Parameter/Site | Mýrdal | Afrétt |
|---|---|---|
| Number of samples (sampled/cross-dated) | 20/12 | 20/18 |
| Correlation between samples | 0.47 | 0.48 |
| Mean measurement (mm) | 32.59 | 32.72 |
| Mean sensitivity | 0.36 | 0.34 |
| First year | 1965 | 1953 |
| Last year | 2016 | 2016 |
| Coefficient of variation: before/after 1990 | 26.77/13.90 | 24.02/19.36 |
| Standard deviation: before/after 1990 | 0.26/0.13 | 0.27/0.17 |
| Trend per decade: entire chronology/after 1990 | −0.02/−0.02 * | −0.06/−0.18 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phulara, M.; Opała-Owczarek, M.; Owczarek, P. Climatic Signals on Growth Ring Variation in Salix herbacea: Comparing Two Contrasting Sites in Iceland. Atmosphere 2022, 13, 718. https://doi.org/10.3390/atmos13050718
Phulara M, Opała-Owczarek M, Owczarek P. Climatic Signals on Growth Ring Variation in Salix herbacea: Comparing Two Contrasting Sites in Iceland. Atmosphere. 2022; 13(5):718. https://doi.org/10.3390/atmos13050718
Chicago/Turabian StylePhulara, Mohit, Magdalena Opała-Owczarek, and Piotr Owczarek. 2022. "Climatic Signals on Growth Ring Variation in Salix herbacea: Comparing Two Contrasting Sites in Iceland" Atmosphere 13, no. 5: 718. https://doi.org/10.3390/atmos13050718
APA StylePhulara, M., Opała-Owczarek, M., & Owczarek, P. (2022). Climatic Signals on Growth Ring Variation in Salix herbacea: Comparing Two Contrasting Sites in Iceland. Atmosphere, 13(5), 718. https://doi.org/10.3390/atmos13050718








