Quantifying Agricultural Drought Severity for Spring Wheat Based on Response of Leaf Photosynthetic Features to Progressive Soil Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Experimental Design
2.3. Field Data Collection
2.3.1. Available Soil Water Content
2.3.2. Leaf Water Potential
2.3.3. Photosynthetic Parameters (Gas Exchange Measurements)
2.4. Stomatal Conductance Models
2.4.1. Ball–Berry Model
2.4.2. Leuning Model
2.4.3. Medlyn Model
2.5. Statistical Analysis and Data Processing
3. Results
3.1. Response of Physiological Features to Soil Drying
3.2. Stomatal Conductance Models
4. Discussion
4.1. Feature Chosen to Quantify Agricultural Drought Severity
4.2. Classification of Agricultural Drought Severity Based on Response of Net Photosynthesis Rate to Available Soil Water Decrease
4.3. Parameters of Stomatal Conductance Models under Different Agricultural Drought Severities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ojima, D.S. Climate resilient management in response to flash droughts in the US Northern Great Plains. Curr. Opin. Environ. Sust. 2021, 48, 125–131. [Google Scholar] [CrossRef]
- Nguyen, H.; Wheeler, M.C.; Hendon, H.H.; Lim, E.P.; Otkin, J.A. The 2019 flash droughts in subtropical eastern Australia and their association with large-scale climate drivers. Weather Clim. Extrem. 2021, 32, 100321. [Google Scholar] [CrossRef]
- Osman, M.; Zaitchik, B.F.; Badr, H.S.; Christian, J.I.; Tadesse, T.; Otkin, J.A.; Anderson, M.C. Flash drought onset over the contiguous United States: Sensitivity of inventories and trends to quantitative definitions. Hydrol. Earth. Syst. Sci. 2021, 25, 565–581. [Google Scholar] [CrossRef]
- Chen, L.G.; Gottschalck, J.; Hartman, A.; Miskus, D.; Tinker, R.; Artusa, A. Flash drought characteristics based on US drought monitor. Atmosphere 2019, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Panu, U.S.; Sharma, T.C. Challenges in drought research: Some perspectives and future directions. Hydrolog. Sci. J. 2002, 47, S19–S30. [Google Scholar] [CrossRef] [Green Version]
- American Meteorological Society. Statement on meteorolgical drought. Bull. Am. Meteorol. Soc. 2004, 85, 771–773. [Google Scholar]
- Heim, R.R. A Review of Twentieth-Century Drought Indices Used in the United States. Bull. Am. Meteorol. Soc. 2002, 83, 1149–1165. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, B.; Srinivasan, R. Development and evaluation of Soil Moisture Deficit Index (SMDI) and Evapotranspiration Deficit Index (ETDI) for agricultural drought monitoring. Agric. For. Meteorol. 2005, 133, 69–88. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhou, G.S.; He, Q.J.; Zhou, L.; Ji, Y.H.; Lv, X.M. Capability of leaf water content and its threshold values in reflection of soil-plant water status in maize during prolonged drought. Ecol. Indic. 2021, 124, 107395. [Google Scholar] [CrossRef]
- Zhou, S.X.; Duursma, R.A.; Medlyn, B.E.; Kelly, J.W.; Prentice, I.C. How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agric. For. Meteorol. 2013, 182, 204–214. [Google Scholar] [CrossRef]
- Soltani, A.; Khooie, F.; Ghassemi, G.K.; Moghaddam, M. Thresholds for chickpea leaf expansion and transpiration response to soil water deficit. Field Crops Res. 2000, 68, 205–210. [Google Scholar] [CrossRef]
- Yang, X.L.; Wang, B.F.; Chen, L.; Li, P.; Cao, C.G. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 2019, 9, 3742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.W.; Zhang, Q.; Li, X.S.; Cao, K.F. Gas exchange and hydraulics in seedlings of Hevea brasiliensis during water stress and recovery. Tree Physiol. 2010, 30, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.N.; Zhuang, Q.L.; Tan, Z.L.; Dukes, J.S.; Zheng, B.Y.; Melillo, J.M. Do maize models capture the impacts of heat and drought stresses on yield? Using algorithm ensembles to identify successful approaches. Glob. Change Biol. 2016, 22, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress, Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Kendy, E.; Yu, Q.; Liu, C.M.; Shen, Y.J.; Sun, H.Y. Effect of soil water deficit on evapotranspiration, crop yield, and water use efficiency in the North China Plain. Agric. Water Manag. 2004, 64, 107–122. [Google Scholar] [CrossRef]
- Moene, A.F.; Dam, J.C. Transport in the Atmosphere-Vegetation-Soil Continuum; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Xu, Z.Z.; Zhou, G.S.; Shi, M.Z. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.; Rastetter, E.B.; Fernandes, D.N.; Goulden, M.L.; Wofsy, S.C.; Shaver, G.R.; Melillo, J.M.; Munger, J.W.; Fan, S.M.; Nadelhoffer, K.J. Modelling the soil-plant-atmosphere continuum in a Quercus-Acer stand at Harvard Forest, the regulation of stomatal conductance by light, nitrogen and soil/plant hydraulic properties. Plant Cell Environ. 1996, 19, 911–927. [Google Scholar] [CrossRef] [Green Version]
- Beeck, M.O.D.; Low, M.; Deckmyn, G.; Ceulemans, R. A comparison of photosynthesis-dependent stomatal models using twig cuvette field data for adult beech (Fagus sylvatica L.). Agric. For. Meteorol. 2010, 150, 531–540. [Google Scholar] [CrossRef]
- Bonan, G.B.; Williams, M.; Fisher, R.A.; Oleson, K.W. Modeling stomatal conductance in the Earth system, linking leaf water-use efficiency and water transport along the soil-plant-atmosphere continuum. Geosci. Model Dev. 2014, 7, 2193–2222. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.; Vico, G.; Katul, G.; Fay, P.A.; Polley, W.; Palmroth, S.; Porporato, A. Optimizing stomatal conductance for maximum carbon gain under water stress: A meta-analysis across plant functional types and climates. Funct. Ecol. 2011, 25, 456–467. [Google Scholar] [CrossRef]
- Xu, L.K.; Baldocchi, D.D. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol. 2003, 23, 865–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.N.; Lei, J.; Wang, R.Y.; Zhang, Q.; Qi, Y.; Zhang, K.; Guo, Q.; Wang, H.L. Environmental determination of spring wheat yield in a climatic transition zone under global warming. Int. J. Biometeorol. 2022, 66, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Ball, J.T.; Woodrow, I.E.; Berry, J.A. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In Progress in Photosynthesis Research, Proceedings of the VIIth International Congress on Photosynthesis, Providence, RI, USA, 10–15 August 1986; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1987; pp. 221–224. [Google Scholar]
- Ball, J.T. An Analysis of Stomatal Conductance; Stanford University: Stanford, CA, USA, 1988. [Google Scholar]
- Liu, F.L.; Andersen, M.N.; Jensen, C.R. Capability of the ‘Ball-Berry’ model for predicting stomatal conductance and water use efficiency of potato leaves under different irrigation regimes. Sci. Horticul. 2009, 122, 346–354. [Google Scholar] [CrossRef]
- Leuning, R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ. 1995, 18, 339–355. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.S.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; Angelis, P.D.; Freeman, M.; Wingate, L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Change Biol. 2011, 17, 2134–2144. [Google Scholar] [CrossRef] [Green Version]
- Casadebaig, P.; Debaeke, P.; Jérémie, L. Thresholds for leaf expansion and transpiration response to soil water deficit in a range of sunflower genotypes. Eur. J. Agron. 2008, 28, 646–654. [Google Scholar] [CrossRef]
- Sadras, V.O.; Milroy, S.P. Soil-water thresholds for the responses of leaf expansion and gas exchange: A review. Field Crops Res. 1996, 47, 253–266. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–906. [Google Scholar] [CrossRef]
- Baker, J.T.; Gitz, D.C.; Payton, P.; Wanjura, D.F.; Upchurch, D.R. Using leaf gas exchange to quantify drought in cotton irrigated based on canopy temperature measurements. Agron. J. 2007, 99, 637–644. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Escalona, J.M.; Galmes, J.; Gulias, J.; Lefi, E.K.; Martinez, S.F.; Moreno, M.T.; Ribas, M.; et al. Understanding down-regulation of photosynthesis under water stress, future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Misson, L.; Limousin, J.M.; Rodriguez, R.; Letts, M.G. Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant Cell Environ. 2010, 33, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, A.; Lebon, E.; Simonneau, T.; Wery, J. Towards a simple indicator of water stress in grapevine (Vitis vinifera L.) based on the differential sensitivities of vegetative growth components. Aust. J. Grape Wine R. 2005, 11, 306–315. [Google Scholar] [CrossRef]
- Brodribb, T. Dynamics of changing intercellular CO2 concentration (Ci) during drought and determination of minimum functional Ci. Plant Physiol. 1996, 111, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Xu, S.; Wang, J.; Lee, X.H. Influence of leaf water potential on diurnal changes in CO2 and water vapour fluxes. Bound. Lay. Meteorol. 2007, 124, 161–181. [Google Scholar] [CrossRef]
- Egea, G.; Verhoef, A.; González-Real, M.M.; Baille, A.; Nortes, P.A.; Domingo, R. Comparison of several approaches to modelling stomatal conductance in well-watered and drought-stressed almond trees. Acta Hortic. Sin. 2011, 922, 285–294. [Google Scholar] [CrossRef]
- Seidel, S.J.; Rachmilevitch, S.; Schütze, N.; Lazarovitch, N. Modelling the impact of drought and heat stress on common bean with two different photosynthesis model approaches. Environ. Modell. Softw. 2016, 81, 111–121. [Google Scholar] [CrossRef]
- Barnard, D.M.; Bauerle, W.L. The implications of minimum stomatal conductance on modeling water flux in forest canopies. J. Geophys. Res. Biogeosci. 2013, 118, 1322–1333. [Google Scholar] [CrossRef]
- Miner, G.L.; Bauerle, W.L.; Baldocchi, D.D. Estimating the sensitivity of stomatal conductance to photosynthesis: A review. Plant Cell Environ. 2016, 40, 1214–1238. [Google Scholar] [CrossRef] [PubMed]
- Egea, G.; Verhoef, A.; Vidale, P.L. Towards an improved and more flexible representation of water stress in coupled photosynthesis–stomatal conductance models. Agric. For. Meteorol. 2011, 151, 1370–1384. [Google Scholar] [CrossRef]
- Zhao, F.N.; Zhou, S.X.; Wang, R.Y.; Zhang, K.; Wang, H.L.; Yu, Q. Quantifying key model parameters for wheat leaf gas exchange under different environmental conditions. J. Integr. Agric. 2020, 19, 2188–2205. [Google Scholar] [CrossRef]
- Raab, N.; Meza, F.J.; Franck, N.; Bambach, N. Empirical stomatal conductance models reveal that the isohydric behavior of an Acacia caven Mediterranean Savannah scales from leaf to ecosystem. Agric. For. Meteorol. 2015, 213, 203–216. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T. Photosynthetic responses to stress in Mediterranean evergreens: Mechanisms and models. Environ. Exp. Bot. 2014, 103, 24–41. [Google Scholar] [CrossRef]
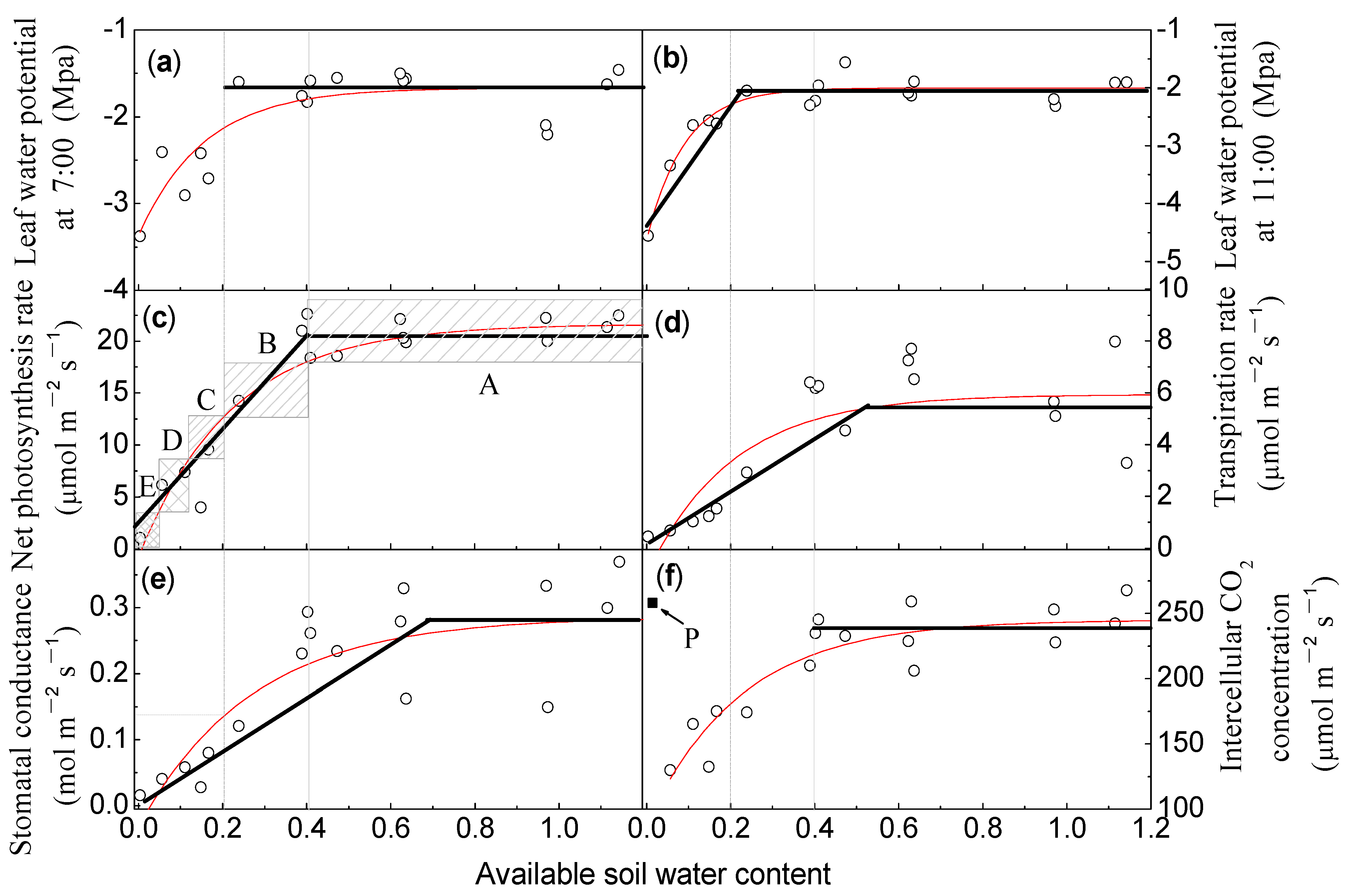
| Physiological Parameters | Equations | R2 | First Threshold/Second Threshold/Third Threshold | Intercept |
|---|---|---|---|---|
| Water potential at 7:00 | y = − 1.667 − 1.702 * exp(−6.415x) | 0.696 ** | 0.28/0.12/0.02 | / |
| Water potential at 11:00 | y = − 2.002 − 2.634 * exp(−11.072x) | 0.901 ** | 0.16/0.08/0.02 | / |
| Net photosynthesis rate | y = 21.642 − 22.444 * exp(−4.535x) | 0.866 ** | 0.2/0.12/0.04 | 0.008 |
| Transpiration rate | y = 5.936 − 6.957 * exp(−4.912x) | 0.577 ** | 0.28/0.12/0.08 | 0.03 |
| Stomatal conductance | y = 0.285 − 0.310 * exp(−3.700x) | 0.658 ** | 0.36/0.20/0.08 | 0.023 |
| Intercellular CO2 concentration | y = 245.090 − 157.000 * exp(−4.466x) | 0.785 ** | 0.2/0.12/0.04 | 0.1 |
| Physiological Parameters | Equations | R2 | Value Under Well-Water Conditions | Point of Intersection with the Value under Well-Water Conditions |
|---|---|---|---|---|
| Water potential at 7:00 | / | 0.643 | −1.699 | / |
| Water potential at 11:00 | y = 9.847 * x − 4.155 | 0.867 ** | −1.987 | 0.22 |
| Net photosynthesis rate | y = 46.998 * x + 1.376 | 0.741 * | 20.49 | 0.41 |
| Transpiration rate | y = 9.899 * x + 0.1092 | 0.727 ** | 5.76 | 0.57 |
| Stomatal conductance | y = 0.394 * x + 0.010 | 0.873 * | 0.286 | 0.69 |
| Intercellular CO2 concentration | / | 0.444 | 238 | / |
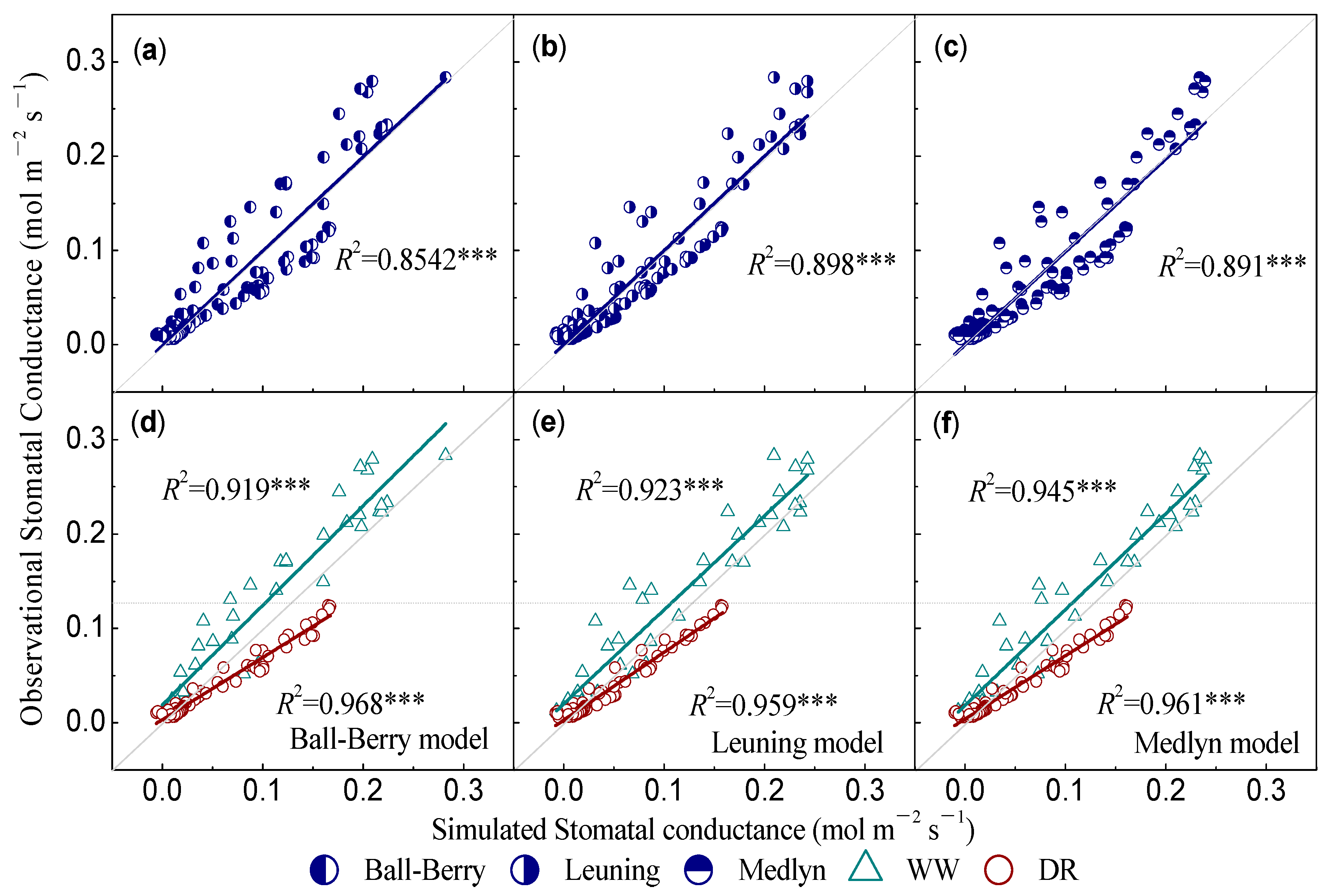
| Models | Class | a1 | b1 | a2 | b2 | c2 | a3 | b3 |
|---|---|---|---|---|---|---|---|---|
| Ball–Berry (1987) | N | 8.4619 | 0.0119 | / | / | / | / | / |
| WW | 8.933 | 0.031 | / | / | / | / | / | |
| DR | 5.5828 | 0.011 | / | / | / | / | / | |
| Leuning (1995) | N | / | / | 3.297 | 369.7 | 0.0085 | / | / |
| WW | / | / | 10.5968 | 0.99438 | 0.0256 | / | / | |
| DR | / | / | 2.9746 | 6.663 | 0.00935 | / | / | |
| Medlyn (2011) | N | / | / | / | / | / | 2.112 | 0.008 |
| WW | / | / | / | / | / | 2.284 | 0.025 | |
| DR | / | / | / | / | / | 1.019 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhang, B.; Zhao, F. Quantifying Agricultural Drought Severity for Spring Wheat Based on Response of Leaf Photosynthetic Features to Progressive Soil Drying. Atmosphere 2022, 13, 531. https://doi.org/10.3390/atmos13040531
Zhang K, Zhang B, Zhao F. Quantifying Agricultural Drought Severity for Spring Wheat Based on Response of Leaf Photosynthetic Features to Progressive Soil Drying. Atmosphere. 2022; 13(4):531. https://doi.org/10.3390/atmos13040531
Chicago/Turabian StyleZhang, Kai, Bo Zhang, and Funian Zhao. 2022. "Quantifying Agricultural Drought Severity for Spring Wheat Based on Response of Leaf Photosynthetic Features to Progressive Soil Drying" Atmosphere 13, no. 4: 531. https://doi.org/10.3390/atmos13040531
APA StyleZhang, K., Zhang, B., & Zhao, F. (2022). Quantifying Agricultural Drought Severity for Spring Wheat Based on Response of Leaf Photosynthetic Features to Progressive Soil Drying. Atmosphere, 13(4), 531. https://doi.org/10.3390/atmos13040531





