Passive Sampling as a Tool to Assess Atmospheric Pesticide Contamination Related to Vineyard Land Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sampling Procedure
2.3. Sample Extraction and Analysis
2.4. Quality Assurance and Quality Control (QA/QC)
2.5. Derivation of Sampling Rates and Estimation of Air Concentrations from PUF-PAS
3. Results and Discussion
3.1. Pesticide Air Concentrations Determined by Active Sampling
3.2. Pesticide Air Concentrations Determined by PAS
3.3. PAS-Derived Air Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Pesticides Use. Available online: http://www.fao.org/faostat/en/#data/RP/visualize (accessed on 4 October 2021).
- Kookana, R.S.; Baskaran, S.; Naidu, R. Pesticide fate and behaviour in Australian soils in relation to contamination and management of soil and water: A review. Soil Res. 1998, 36, 715. [Google Scholar] [CrossRef]
- Petrovic, M.; Gonzalez, S.; Barcelo, D. Analysis and removal of emerging contaminants in wastewater and drinking water. TrAC Trends Anal. Chem. 2003, 22, 685–696. [Google Scholar] [CrossRef]
- Alvarez, D.A.; Petty, J.D.; Huckins, J.N.; Jones-Lepp, T.L.; Getting, D.T.; Goddard, J.P.; Manahan, S.E. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ. Toxicol. Chem. 2014, 23, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Werhli, B. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.-C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar]
- Kosikowska, M.; Biziuk, M. Review of the determination of pesticide residues in ambient air. TrAC Trends Anal. Chem. 2010, 29, 1064–1072. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar]
- Córdoba Gamboa, L.; Solano Diaz, K.; Ruepert, C.; van Wendel de Joode, B. Passive monitoring techniques to evaluate environmental pesticide exposure: Results from the Infant’s Environmental Health study (ISA). Environ. Res. 2020, 184, 109243. [Google Scholar]
- Ohlander, J.; Fuhrimann, S.; Basinas, I.; Cherrie, J.W.; Galea, K.S.; Povey, A.C.; van Tongeren, M.; Harding, A.-H.; Jones, K.; Vermeulen, R.; et al. Systematic review of methods used to assess exposure to pesticides in occupational epidemiology studies, 1993–2017. Occup. Environ. Med. 2020, 77, 357–367. [Google Scholar]
- Shen, L.; Wania, F.; Lei, Y.D.; Teixeira, C.; Muir, D.C.G.; Bidleman, T.F. Atmospheric Distribution and Long-Range Transport Behavior of Organochlorine Pesticides in North America. Environ. Sci. Technol. 2005, 39, 409–420. [Google Scholar]
- Schummer, C.; Mothiron, E.; Appenzeller, B.M.R.; Wennig, R.; Millet, M. Gas/particle partitioning of currently used pesticides in the atmosphere of Strasbourg (France). Air Qual. Atmos. Health 2010, 3, 171–181. [Google Scholar]
- Schummer, C.; Tuduri, L.; Briand, O.; Appenzeller, B.M.; Millet, M. Application of XAD-2 resin-based passive samplers and SPME–GC–MS/MS analysis for the monitoring of spatial and temporal variations of atmospheric pesticides in Luxembourg. Environ. Pollut. 2012, 170, 88–94. [Google Scholar]
- Fuhrimann, S.; Klánová, J.; Přibylová, P.; Kohoutek, J.; Dalvie, M.A.; Röösli, M.; Degrendele, C. Qualitative assessment of 27 current-use pesticides in air at 20 sampling sites across Africa. Chemosphere 2020, 258, 127333. [Google Scholar]
- Isogai, N.; Hogarh, J.N.; Seike, N.; Kobara, Y.; Oyediran, F.; Wirmvem, M.J.; Ayonghe, S.N.; Fobil, J.; Masunaga, S. Atmospheric monitoring of organochlorine pesticides across some West African countries. Environ. Sci. Pollut. Res. 2018, 25, 31828–31835. [Google Scholar] [CrossRef]
- Shunthirasingham, C.; Oyiliagu, C.E.; Cao, X.; Gouin, T.; Wania, F.; Lee, S.-C.; Pozo, K.; Harner, T.; Muir, D.C.G. Spatial and temporal pattern of pesticides in the global atmosphere. J. Environ. Monit. 2010, 12, 1650. [Google Scholar] [CrossRef]
- Daly, G.L.; Lei, Y.D.; Teixeira, C.; Muir, D.C.G.; Wania, F. Pesticides in Western Canadian Mountain Air and Soil. Environ. Sci. Technol. 2007, 41, 6020–6025. [Google Scholar] [CrossRef]
- Bengtson Nash, S.M.; Wild, S.J.; Hawker, D.W.; Cropp, R.A.; Hung, H.; Wania, F.; Xiao, H.; Bohlin-Nizzetto, P.; Bignert, A.; Broomhall, S. Persistent Organic Pollutants in the East Antarctic Atmosphere: Inter-Annual Observations from 2010 to 2015 Using High-Flow-Through Passive Sampling. Environ. Sci. Technol. 2017, 51, 13929–13937. [Google Scholar] [CrossRef]
- Hung, H.; Kallenborn, R.; Breivik, K.; Su, Y.; Brorström-Lundén, E.; Olafsdottir, K.; Thorlacius, J.M.; Leppänen, S.; Bossi, R.; Skov, H.; et al. Atmospheric monitoring of organic pollutants in the Arctic under the Arctic Monitoring and Assessment Programme (AMAP): 1993–2006. Sci. Total Environ. 2010, 408, 2854–2873. [Google Scholar] [CrossRef]
- Octaviani, M.; Stemmler, I.; Lammel, G.; Graf, H.F. Atmospheric Transport of Persistent Organic Pollutants to and from the Arctic under Present-Day and Future Climate. Environ. Sci. Technol. 2015, 49, 3593–3602. [Google Scholar] [CrossRef]
- Gil, Y.; Sinfort, C. Emission of pesticides to the air during sprayer application: A bibliographic review. Atmos. Environ. 2005, 39, 5183–5193. [Google Scholar]
- van der Werf, H.M.G. Assessing the impact of pesticides on the environment. Agric. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar]
- Van den Berg, F.; Kubiak, R.; Benjey, W.G.; Majewski, M.S.; Yates, S.R.; Reeves, G.L.; Smelt, J.H.; van der Linden, A.M.A. Emission of Pesticides into the Air. In Fate of Pesticides in the Atmosphere: Implications for Environmental Risk Assessment; van Dijk, H.F.G., van Pul, W.A.J., de Voogt, P., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 195–218. [Google Scholar]
- Hayward, S.J.; Gouin, T.; Wania, F. Comparison of Four Active and Passive Sampling Techniques for Pesticides in Air. Environ. Sci. Technol. 2010, 44, 3410–3416. [Google Scholar]
- White, L.M.; Ernst, W.R.; Julien, G.; Garron, C.; Leger, M. Ambient air concentrations of pesticides used in potato cultivation in Prince Edward Island, Canada. Pest Manag. Sci. 2006, 62, 126–136. [Google Scholar] [PubMed]
- Herkert, N.J.; Spak, S.N.; Smith, A.; Schuster, J.K.; Harner, T.; Martinez, A.; Hornbuckle, K.C. Calibration and evaluation of PUF-PAS sampling rates across the Global Atmospheric Passive Sampling (GAPS) network. Environ. Sci. Process. Impacts 2018, 20, 210–219. [Google Scholar] [PubMed]
- Petrich, N.T.; Spak, S.N.; Carmichael, G.R.; Hu, D.; Martinez, A.; Hornbuckle, K.C. Simulating and Explaining Passive Air Sampling Rates for Semivolatile Compounds on Polyurethane Foam Passive Samplers. Environ. Sci. Technol. 2013, 47, 8591–8598. [Google Scholar] [CrossRef][Green Version]
- Moeckel, C.; Harner, T.; Nizzetto, L.; Strandberg, B.; Lindroth, A.; Jones, K.C. Use of Depuration Compounds in Passive Air Samplers: Results from Active Sampling-Supported Field Deployment, Potential Uses, and Recommendations. Environ. Sci. Technol. 2009, 43, 3227–3232. [Google Scholar] [PubMed]
- Persoon, C.; Hornbuckle, K.C. Calculation of Passive Sampling Rates from Both Native PCBs and Depuration Compounds in Indoor and Outdoor Environments. Chemosphere 2009, 74, 917–923. [Google Scholar] [CrossRef]
- Pozo, K.; Harner, T.; Wania, F.; Muir, D.C.G.; Jones, K.C.; Barrie, L.A. Toward a Global Network for Persistent Organic Pollutants in Air: Results from the GAPS Study. Environ. Sci. Technol. 2016, 40, 4867–4873. [Google Scholar] [CrossRef]
- Gouin, T.; Wania, F.; Ruepert, C.; Castillo, L.E. Field Testing Passive Air Samplers for Current Use Pesticides in a Tropical Environment. Environ. Sci. Technol. 2008, 42, 6625–6630. [Google Scholar] [CrossRef]
- Hazrati, S.; Harrad, S. Calibration of polyurethane foam (PUF) disk passive air samplers for quantitative measurement of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs): Factors influencing sampling rates. Chemosphere 2017, 67, 448–455. [Google Scholar]
- Wania, F.; Shen, L.; Lei, Y.D.; Teixeira, C.; Muir, D.C.G. Development and Calibration of a Resin-Based Passive Sampling System for Monitoring Persistent Organic Pollutants in the Atmosphere. Environ. Sci. Technol. 2003, 37, 1352–1359. [Google Scholar] [CrossRef]
- Shoeib, M.; Harner, T. Characterization and Comparison of Three Passive Air Samplers for Persistent Organic Pollutants. Environ. Sci. Technol. 2002, 36, 4142–4151. [Google Scholar]
- Jaward, F.M.; Farrar, N.J.; Harner, T.; Sweetman, A.J.; Jones, K.C. Passive Air Sampling of PCBs, PBDEs, and Organochlorine Pesticides Across Europe. Environ. Sci. Technol. 2004, 38, 34–41. [Google Scholar]
- Bidleman, T.F.; Laudon, H.; Nygren, O.; Svanberg, S.; Tysklind, M. Chlorinated pesticides and natural brominated anisoles in air at three northern Baltic stations. Environ. Pollut. 2017, 225, 381–389. [Google Scholar]
- Harner, T.; Su, K.; Genualdi, S.; Karpowicz, J.; Ahrens, L.; Mihele, C.; Schuster, J.; Charland, J.-P.; Narayan, J. Calibration and application of PUF disk passive air samplers for tracking polycyclic aromatic compounds (PACs). Atmos. Environ. 2013, 75, 123–128. [Google Scholar]
- Vasiljevic, T.; Su, K.; Harner, T. A first look at atmospheric concentrations and temporal trends of phthalates in distinct urban sectors of the Greater Toronto Area. Atmos. Pollut. Res. 2021, 12, 173–182. [Google Scholar]
- Niu, S.; Harner, T.; Chen, R.; Parnis, J.M.; Saini, A.; Hageman, K. Guidance on the Application of Polyurethane Foam Disk Passive Air Samplers for Measuring Nonane and Short-Chain Chlorinated Paraffins in Air: Results from a Screening Study in Urban Air. Environ. Sci. Technol. 2021, 55, 11693–11702. [Google Scholar] [CrossRef]
- Meire, R.O.; Lee, S.C.; Yao, Y.; Targino, A.C.; Torres, J.P.M.; Harner, T. Seasonal and altitudinal variations of legacy and current-use pesticides in the Brazilian tropical and subtropical mountains. Atmos. Environ. 2012, 59, 108–116. [Google Scholar]
- Koblizkova, M.; Lee, S.C.; Harner, T. Sorbent impregnated polyurethane foam disk passive air samplers for investigating current-use pesticides at the global scale. Atmos. Pollut. Res. 2012, 3, 456–462. [Google Scholar] [CrossRef]
- Lévy, M.; Ba, H.; Pallares, C.; Pham-Huu, C.; Millet, M. Comparison and calibration of diverse passive samplers used for the air sampling of pesticides during a regional sampling monitoring campaign. Atmos. Pollut. Res. 2020, 11, 1217–1225. [Google Scholar]
- Coscollà, C.; Colin, P.; Yahyaoui, A.; Petrique, O.; Yusà, V.; Mellouki, A.; Pastor, A. Occurrence of currently used pesticides in ambient air of Centre Region (France). Atmos. Environ. 2010, 44, 3915–3925. [Google Scholar] [CrossRef]
- Coscollà, C.; Hart, E.; Pastor, A.; Yusà, V. LC-MS characterization of contemporary pesticides in PM10 of Valencia Region, Spain. Atmos. Environ. 2013, 77, 394–403. [Google Scholar] [CrossRef]
- Yusà, V.; Coscollà, C.; Millet, M. New screening approach for risk assessment of pesticides in ambient air. Atmos. Environ. 2014, 96, 322–330. [Google Scholar] [CrossRef]
- Villiot, A.; Chrétien, E.; Drab-Sommesous, E.; Rivière, E.; Chakir, A.; Roth, E. Temporal and seasonal variation of atmospheric concentrations of currently used pesticides in Champagne in the centre of Reims from 2012 to 2015. Atmos. Environ. 2018, 174, 82–91. [Google Scholar] [CrossRef]
- Tuduri, L.; Harner, T.; Hung, H. Polyurethane foam (PUF) disks passive air samplers: Wind effect on sampling rates. Environ. Pollut. 2006, 144, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Coscollà, C.; Yusà, V.; Beser, M.I.; Pastor, A. Multi-residue analysis of 30 currently used pesticides in fine airborne particulate matter (PM 2.5) by microwave-assisted extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8817–8827. [Google Scholar] [CrossRef]
- AIRAQ. Projet PHYTO’RIV: Evaluation des Niveaux en Produits Phytosanitaires Dans L’air Ambiant—Communes de Rauzan et de Saint-Symphorien (Campagne du 15/06/10 au 10/08/10); Rapport n ET/PP/12/01; AIRAQ: Mérignac, France, 2012; 27p. [Google Scholar]
- AIRAQ. Projet AIRES: Prévalence des Maladies Respiratoires et Allergiques Chez L’enfant en Milieu Rural Viticole et Exposition aux Polluants de L’air—Volet Métrologique 2011; Rapport n ET/MM/13/01; AIRAQ: Mérignac, France, 2013; 27p. [Google Scholar]
- Schummer, C.; Mothiron, E.; Appenzeller, B.M.R.; Rizet, A.-L.; Wennig, R.; Millet, M. Temporal variations of concentrations of currently used pesticides in the atmosphere of Strasbourg, France. Environ. Pollut. 2010, 158, 576–584. [Google Scholar] [CrossRef]
- Scheyer, A.; Morville, S.; Mirabel, P.; Millet, M. Gas/particle partitioning of lindane and current-used pesticides and their relationship with temperature in urban and rural air in Alsace region (east of France). Atmos. Environ. 2008, 42, 7695–7705. [Google Scholar] [CrossRef]
- Hart, E.; Coscollà, C.; Pastor, A.; Yusà, V. GC–MS characterization of contemporary pesticides in PM10 of Valencia Region, Spain. Atmos. Environ. 2012, 62, 118–129. [Google Scholar] [CrossRef]
- Degrendele, C.; Okonski, K.; Melymuk, L.; Landlová, L.; Kukučka, P.; Audy, O.; Kohoutek, J.; Čupr, P.; Klánová, J. Pesticides in the atmosphere: A comparison of gas-particle partitioning and particle size distribution of legacy and current-use pesticides. Atmos. Chem. Phys. 2016, 16, 1531–1544. [Google Scholar] [CrossRef]
- Degrendele, C.; Klánová, J.; Prokeš, R.; Příbylová, P.; Šenk, P.; Šudoma, M.; Röösli, M.; Dalvie, M.A.; Fuhrimann, S. Current use pesticides in soil and air from two agricultural sites in South Africa: Implications for environmental fate and human exposure. Sci. Total Environ. 2022, 807, 150455. [Google Scholar] [CrossRef]
- Houbraken, M.; Senaeve, D.; Fevery, D.; Spanoghe, P. Influence of adjuvants on the dissipation of fenpropimorph, pyrimethanil, chlorpyrifos and lindane on the solid/gas interface. Chemosphere 2015, 138, 357–363. [Google Scholar] [CrossRef]
- Das, S.; Hageman, K.J. Influence of Adjuvants on Pesticide Soil–Air Partition Coefficients: Laboratory Measurements and Predicted Effects on Volatilization. Environ. Sci. Technol. 2020, 54, 7302–7308. [Google Scholar] [CrossRef]
- Perine, J.; Anderson, J.C.; Kruger, G.R.; Abi-Akar, F.; Overmyer, J. Effect of nozzle selection on deposition of thiamethoxam in Actara® spray drift and implications for off-field risk assessment. Sci. Total Environ. 2021, 772, 144808. [Google Scholar] [CrossRef]
- Pflieger, M. Etude de la Dégradation Photochimique des Pesticides Adsorbés à la Surface de Particules Atmosphériques. Ph.D. Thesis, University of Bordeaux, Bordeaux, France, 2009; 293p. [Google Scholar]
- Socorro, J.; Durand, A.; Temime-Roussel, B.; Gligorovski, S.; Wortham, H.; Quivet, E. The persistence of pesticides in atmospheric particulate phase: An emerging air quality issue. Sci. Rep. 2016, 6, 33456. [Google Scholar] [CrossRef]
- Chaemfa, C.; Wild, E.; Davison, B.; Barber, J.L.; Jones, K.C. A study of aerosol entrapment and the influence of wind speed, chamber design and foam density on polyurethane foam passive air samplers used for persistent organic pollutants. J. Environ. Monit. 2009, 11, 1135. [Google Scholar] [CrossRef]
- Melymuk, L.; Robson, M.; Helm, P.A.; Diamond, M.L. Evaluation of passive air sampler calibrations: Selection of sampling rates and implications for the measurement of persistent organic pollutants in air. Atmos. Environ. 2011, 45, 1867–1875. [Google Scholar] [CrossRef]
- Dalvie, M.A.; Sosan, M.B.; Africa, A.; Cairncross, E.; London, L. Environmental monitoring of pesticide residues from farms at a neighbouring primary and pre-school in the Western Cape in South Africa. Sci. Total Environ. 2014, 466–467, 1078–1084. [Google Scholar] [CrossRef]
- Figueiredo, D.M.; Duyzer, J.; Huss, A.; Krop, E.J.M.; Gerritsen-Ebben, M.G.; Gooijer, Y.; Vermeulen, R.C.H. Spatio-temporal variation of outdoor and indoor pesticide air concentrations in homes near agricultural fields. Atmos. Environ. 2021, 262, 118612. [Google Scholar] [CrossRef]
- Climent, M.J.; Coscollà, C.; Lopez, A.; Barra, R.; Urrutia, R. Legacy and current-use pesticides (CUPs) in the atmosphere of a rural area in central Chile, using passive air samplers. Sci. Total Environ. 2019, 662, 646–654. [Google Scholar] [CrossRef]
- Scheyer, A.; Morville, S.; Mirabel, P.; Millet, M. Variability of atmospheric pesticide concentrations between urban and rural areas during intensive pesticide application. Atmos. Environ. 2007, 41, 3604–3618. [Google Scholar] [CrossRef]
- Holt, E.; Bohlin-Nizzetto, P.; Borůvková, J.; Harner, T.; Kalina, J.; Melymuk, L.; Klánová, J. Using long-term air monitoring of semi-volatile organic compounds to evaluate the uncertainty in polyurethane-disk passive sampler-derived air concentrations. Environ. Pollut. 2017, 220, 1100–1111. [Google Scholar]
- Estellano, V.H.; Pozo, K.; Efstathiou, C.; Pozo, K.; Corsolini, S.; Focardi, S. Assessing levels and seasonal variations of current-use pesticides (CUPs) in the Tuscan atmosphere, Italy, using polyurethane foam disks (PUF) passive air samplers. Environ. Pollut. 2015, 205, 52–59. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Ruiz, P.; Yusà, V.; Coscollà, C. Methodological Aspects for the Implementation of the Air Pesticide Control and Surveillance Network (PESTNet) of the Valencian Region (Spain). Atmosphere 2021, 12, 542. [Google Scholar]
- Cortes, S.; Pozo, K.; Llanos, Y.; Martinez, N.; Foerster, C.; Leiva, C.; Ustáriz, J.; Přibylová, P.; Klánová, J.; Jorquera, H. First measurement of human exposure to current use pesticides (CUPs) in the atmosphere of central Chile: The case study of Mauco cohort. Atmos. Pollut. Res. 2020, 11, 776–784. [Google Scholar]
- Fuentes, E.; López, A.; Ibáñez, M.; Yusà, V.; Muñoz, A.; Vera, T.; Borrás, E.; Calvete-Sogo, H.; Coscollà, C. Pesticide Inhalation Exposure of Applicators and Bystanders Using Conventional and Innovative Cropping Systems in the Valencian Region, Spain. Atmosphere 2021, 12, 631. [Google Scholar]
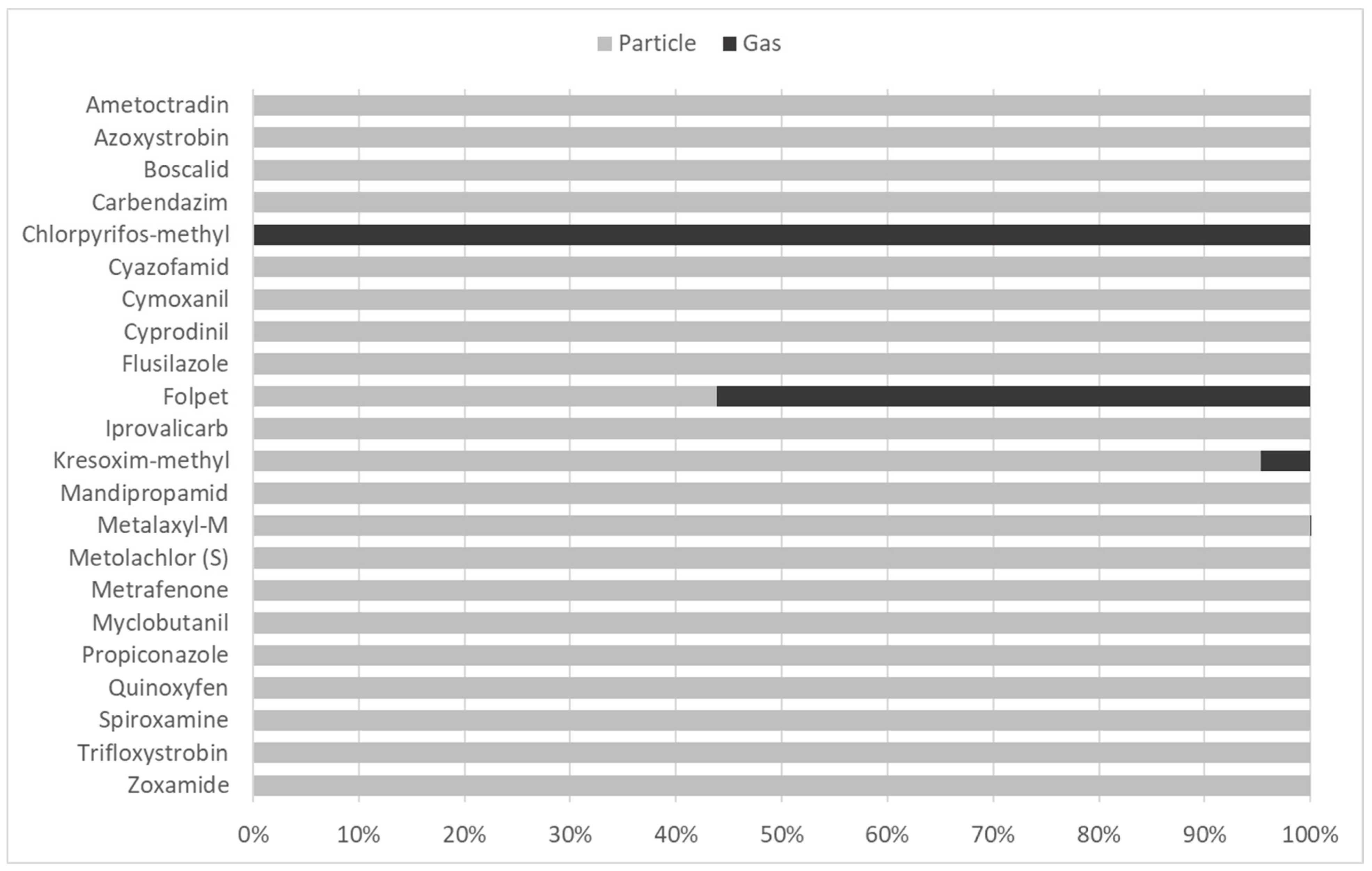

| Name | Type | Analysis | Vapor Pressure a at 20 °C (mPa) | Henry’s Law Constant a at 25 °C (Pa m3 moL−1) | log Koa a | Approved in France (in 2013) | Applied on the Vineyard Plot in 2013 |
|---|---|---|---|---|---|---|---|
| Ametoctradin | F | LC-MS/MS | 2.1 × 10−7 | 4.13 × 10−7 | 14.1 | Y | Y (17 May) |
| Atrazine | H | LC-MS/MS | 3.9 × 10−2 | 1.5 × 10−4 | 9.9 | N | - |
| Azoxystrobin | F | LC-MS/MS | 1.1 × 10−7 | 7.4 × 10−9 | 14.0 | Y | - |
| Bifenthrin | I | GC-MS/MS | 1.78 × 10−2 | 7.74 × 10−5 | 10.8 | N | - |
| Boscalid | F | LC-MS/MS | 7.2 × 10−2 | 5.18 × 10−5 | 10.6 | Y | Y (8 July) |
| Carbendazim | F | LC-MS/MS | 9.0 × 10−2 | 3.60 × 10−3 | 5.9 | N | - |
| Carbetamide | H | LC-MS/MS | 3.0 × 10−4 | 1.93 × 10−8 | 12.9 | Y | - |
| Chlorpyrifos-methyl | I | GC-MS/MS | 3.0 | 0.235 | 8.0 | Y | Y (1 July + 1 August) |
| Cyazofamid | F | LC-MS/MS | 1.33 × 10−2 | 4.03 × 10−2 | 7.9 | Y | Y (2 August) |
| Cymoxanil | F | LC-MS/MS | 1.5 × 10−1 | 3.3 × 10−5 | 8.5 | Y | - |
| Cyprodinil | F | LC-MS/MS | 5.10 × 10−1 | 6.6 × 10−3 | 9.5 | Y | - |
| Desipropylatrazine (DIA) | TP | LC-MS/MS | - | 9.8 × 10+2 | 1.5 | - | - |
| Diuron | H | LC-MS/MS | 1.15 × 10−3 | 2.0 × 10−6 | 11.9 | N | - |
| Fipronil | I | GC-MS/MS | 2 × 10−3 | 2.31 × 10−4 | 10.7 | N | - |
| Fipronil sulfide | TP | GC-MS/MS | - | - | - | - | - |
| Fipronil sulfone | TP | GC-MS/MS | - | - | - | - | - |
| Flazasulfuron | H | LC-MS/MS | 1.33 × 10−2 | 2.58 × 10−6 | 8.9 | Y | - |
| Fludioxonil | F | GC-MS/MS | 3.9 × 10−4 | 5.4 × 10−5 | 11.7 | Y | - |
| Flusilazole | F | LC-MS/MS | 3.9 × 10−2 | 2.7 × 10−4 | 10.8 | N | - |
| Folpet | F | GC-MS/MS | 2.1 × 10−2 | 8.0 × 10−3 | 8.5 | Y | Y (7 May + 17 June + 17 July) |
| Imidacloprid | I | LC-MS/MS | 4.0 × 10−7 | 1.7 × 10−10 | 13.7 | Y | - |
| Iprovalicarb | F | LC-MS/MS | 7.9 × 10−5 | 1.4 × 10−6 | 12.4 | Y | Y (17 June) |
| Kresoxim-methyl | F | LC-MS/MS | 2.3 × 10−3 | 3.6 × 10−4 | 10.2 | Y | Y (16 July) |
| Mandipropamid | F | LC-MS/MS | 9.4 × 10−4 | 9.2 × 10−5 | 10.6 | Y | Y (27 May) |
| Metalaxyl-M | F | LC-MS/MS | 3.3 | 3.5 × 10−5 | 9.5 | Y | Y (7 May) |
| Metolachlor (S) | H | LC-MS/MS | 3.7 | 2.2 × 10−3 | 9.1 | Y | - |
| Metrafenone | F | LC-MS/MS | 0.15 | 1.32 × 10−1 | 8.6 | Y | Y (27 May) |
| Myclobutanil | F | LC-MS/MS | 0.198 | 4.33 × 10−4 | 9.6 | Y | Y (7 June) |
| Propiconazole | F | LC-MS/MS | 5.6 × 10−2 | 9.2 × 10−5 | 11.1 | Y | - |
| Quinoxyfen | F | GC-MS/MS | 1.2 × 10−2 | 3.08 × 10−2 | 10.0 | Y | Y (17 May) |
| Simazine | H | LC-MS/MS | 8.1 × 10−4 | 5.6 × 10−5 | 9.9 | N | - |
| Spiroxamine | F | LC-MS/MS | 3.5 | 3.8 × 10−3 | 8.7 | Y | Y (17 June) |
| Tebufenpyrad | I | LC-MS/MS | 1.6 × 10−3 | 1.1 × 10−3 | 11.2 | Y | - |
| Terbuthylazine | H | LC-MS/MS | 0.152 | 2.3 × 10−3 | 9.4 | Y | - |
| Tetraconazole | F | GC-MS/MS | 0.18 | 3.6 × 10−4 | 10.4 | Y | Y (1 July) |
| Thiamethoxam | I | LC-MS/MS | 6.6 × 10−6 | 4.7 × 10−10 | 12.6 | Y | - |
| Trifloxystrobin | F | LC-MS/MS | 3.4 × 10−3 | 2.3 × 10−3 | 10.5 | Y | - |
| Zoxamide | F | LC-MS/MS | 1.3 × 10−2 | 6.59 × 10−3 | 9.3 | Y | Y (1 July) |
| Total Concentrations 1 pg·m−3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Detection Number (/5) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 (Rainy) | Median | Mean | Day 1 to Day 4 CV (%) | Minimum | Maximum |
| Ametoctradin | 5 | 234.1 | 880.5 | 1237.8 | 197.8 | 6.5 | 234.1 | 511.3 | 80% | 6.5 | 1237.8 |
| Azoxystrobin | 4 | 6.8 | 3.1 | 2.7 | 13.0 | <LQ | 3.1 | 5.1 | 75% | <LQ | 13 |
| Boscalid | 4 | 54.8 | 65.4 | 53.2 | 38.3 | <LQ | 53.2 | 42.3 | 21% | <LQ | 65.4 |
| Carbendazim | 4 | 15.8 | 5.8 | 4.4 | 3.8 | <LQ | 4.4 | 6.0 | 76% | <LQ | 15.8 |
| Chlorpyrifos-methyl | 5 | 2338.2 | 771.1 | 1494.9 | 691.5 | 285.6 | 771.1 | 1116.3 | 58% | 285.6 | 2338.2 |
| Cyazofamid | 4 | 51.0 | 121.5 | 62.6 | 59.6 | <LQ | 59.6 | 58.9 | 44% | <LQ | 121.5 |
| Cymoxanil | 5 | 102.2 | 156.8 | 122.2 | 77.0 | 17.2 | 102.2 | 95.1 | 29% | 17.2 | 156.8 |
| Cyprodinil | 5 | 10.1 | 32 | 17.8 | 8.2 | 3.7 | 10.1 | 14.4 | 64% | 3.7 | 32 |
| Flusilazole | 2 | 3.9 | <LQ | <LQ | 2.9 | <LQ | <LQ | 1.4 | 21% | <LQ | 3.9 |
| Folpet | 4/4 | 13,302.5 | 24,814.9 | 23,685.0 | 18,027.3 | na * | 20,856.2 | 19,957.4 | 27% | 13,302.5 | 24,814.9 |
| Iprovalicarb | 4 | 1.5 | 163.8 | 131.3 | 49.9 | <LQ | 49.9 | 69.3 | 86% | <LQ | 163.8 |
| Kresoxim-methyl | 5 | 89.5 | 230.5 | 125.6 | 143.5 | 53.6 | 125.6 | 128.5 | 41% | 53.6 | 230.5 |
| Mandipropamid | 5 | 162 | 383.6 | 182.3 | 115.2 | 2.4 | 162.0 | 169.1 | 56% | 2.4 | 383.6 |
| Metalaxyl-M | 5 | 195.3 | 411.2 | 291.4 | 177.1 | 108.7 | 195.3 | 236.7 | 40% | 108.7 | 411.2 |
| Metolachlor (S) | 5 | 210.3 | 165.3 | 142.3 | 99.6 | 68.6 | 142.3 | 137.2 | 30% | 68.6 | 210.3 |
| Metrafenone | 5 | 193.8 | 520.3 | 158.7 | 124.7 | 15.8 | 158.7 | 202.7 | 73% | 15.8 | 520.3 |
| Myclobutanil 2 | 5 | 35.6 | 1237.7 | 1779.1 | 235.2 | 87.4 | 235.2 | 675.0 | 101% | 35.6 | 1779.1 |
| Propiconazole | 4 | 10.4 | 16.1 | 5.7 | 3.5 | <LQ | 5.7 | 7.1 | 63% | <LQ | 16.1 |
| Quinoxyfen | 5 | 28.2 | 59.2 | 169.7 | 54.0 | 16.8 | 54.0 | 65.6 | 81% | 16.8 | 169.7 |
| Spiroxamine | 5 | 726.2 | 980.6 | 665.4 | 437.5 | 23.5 | 665.4 | 566.6 | 32% | 23.5 | 980.6 |
| Trifloxystrobin | 5 | 51.8 | 253.9 | 181.8 | 136.8 | 20.9 | 136.8 | 129.0 | 54% | 20.9 | 253.9 |
| Zoxamide | 5 | 24.5 | 73.1 | 111.8 | 63.1 | 1.4 | 63.1 | 54.8 | 53% | 1.4 | 111.8 |
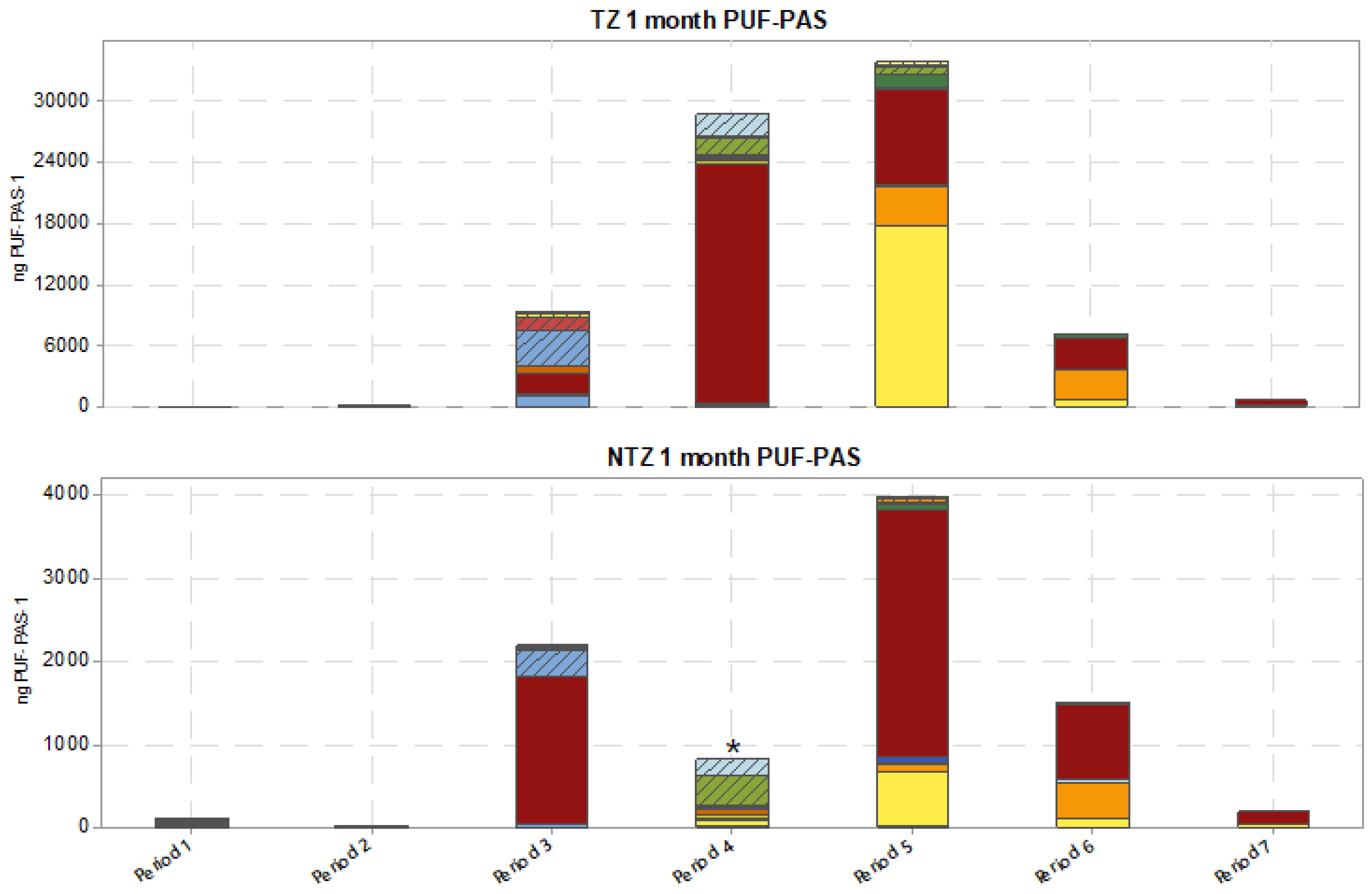
| Compound | Quantities Found in PUF-PAS (ng) on TZ in June (Period 4) | Quantities Found in PUF-PAS (ng) on NTZ in June (Period 4) | Quantities Found in PUF-PAS (ng) Next to HV-AAS Area in June | Average Concentration (Gas and Particulate Phases) Obtained during 5 Days (June) by DA80 HV-AAS (pg·m−3) | Estimated Rs for TZ (m3.d−1) | Estimated Rs for NTZ (m3.d−1) | Estimated Rs Next to HV-AAS Area (m3.d−1) | Mean Rs for TZ, NTZ, and HV-AAS Area (m3.d−1) |
|---|---|---|---|---|---|---|---|---|
| Ametoctradin | 58 | 34 | 46 | 511 | 4.1 | 2.4 | 3.3 | 3.2 |
| Azoxystrobin | <LQ | <LQ | <LQ | 5 | - | - | - | - |
| Boscalid | 79 | 56 | 93 | 42 | 67.2 | 47.6 | 79.1 | 64.6 |
| Carbendazim | 2 | 6 | 4 | 6 | 11.9 | 35.7 | 23.8 | 23.8 |
| Chlorpyrifos-methyl | 137 | <LQ | 61 | 1116 | 4.4 | - | 2.0 | 3.2 |
| Cyazofamid | 18 | 11 | 12 | 59 | 10.9 | 6.7 | 7.3 | 8.3 |
| Cymoxanil | <LQ | <LQ | <LQ | 95 | - | - | - | - |
| Cyprodinil | <LQ | <LQ | <LQ | 14 | - | - | - | - |
| Fludioxonil | 31 | <LQ | <LQ | <LQ | - | - | - | - |
| Flusilazole | <LQ | <LQ | <LQ | 1 | - | - | - | - |
| Folpet | 23,481 | * na | 5016 | 19,957 | 42.0 | - | 9.0 | 25.5 |
| Iprovalicarb | 414 | 54 | 299 | 69 | 214.3 | 28.0 | 154.8 | 132.3 |
| Kresoxim-methyl | 21 | 10 | 14 | 129 | 5.8 | 2.8 | 3.9 | 4.2 |
| Mandipropamid | 176 | 59 | 94 | 169 | 37.2 | 12.5 | 19.9 | 23.8 |
| Metalaxyl-M | 41 | 20 | 25 | 237 | 6.2 | 3.0 | 3.8 | 4.3 |
| Metolachlor (S) | 150 | <LQ | <LQ | 137 | 39.1 | - | - | 39.1 |
| Metrafenone | 42 | 17 | 23 | 203 | 7.4 | 3.0 | 4.0 | 4.8 |
| Myclobutanil | 1774 | 371 | 117 | 675 | 93.9 | 19.7 | 6.2 | 39.9 |
| Propiconazole | <LQ | <LQ | <LQ | 7 | - | - | - | - |
| Quinoxyfen | 51 | <LQ | 13 | 66 | 27.6 | - | 7.3 | 17.3 |
| Spiroxamine | 2193 | 189 | 1463 | 567 | 138.3 | 11.9 | 92.2 | 80.7 |
| Trifloxystrobin | 10 | 6 | 9 | 129 | 2.8 | 1.7 | 2.5 | 2.3 |
| Zoxamide | 6 | 3 | 5 | 55 | 3.9 | 1.9 | 3.2 | 3.0 |
| Atmospheric Concentration (pg·m−3) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 3 | Period 4 | Period 5 | Period 6 | Period 7 | ||||||||||
| TZ | NTZ | TZ | NTZ | TZ | NTZ | TZ | NTZ | TZ | NTZ | TZ | NTZ | TZ | NTZ | Max. | Min. | |
| Ametoctradin | - | - | - | - | 10,459 | 372 | 628 | 368 | 195 | 97 | 37 | 12 | - | - | 10,459 | 12 |
| Azoxystrobin | - | - | - | - | 7 | - | - | - | 188 | 152 | - | - | 7 | - | 188 | 7 |
| Boscalid | - | 233 | 61 | 53 | 79 | - | 705 | 500 | 157,277 | 5714 | 3908 | 602 | 1431 | 278 | 157,277 | 53 |
| Carbendazim | - | - | - | - | 43 | 21 | 18 | 54 | - | - | - | 5 | - | - | 54 | 5 |
| Chlorpyrifos-methyl | - | - | - | - | 714 | - | 2446 | - | 70,196 | 1714 | 28,602 | 4347 | 167 | 97 | 70,196 | 97 |
| Cyazofamid | - | 38 | - | - | 20 | 8 | 88 | 54 | 24 | - | 196 | 75 | 42 | 8 | 196 | 8 |
| Cyprodinil | - | - | 121 | - | - | - | - | - | 429 | 268 | - | - | - | - | 429 | 121 |
| Fipronil | - | - | 23 | - | - | - | - | - | 27 | - | 10 | - | - | - | 27 | 10 |
| Fipronil sulfide | - | - | 45 | - | - | - | - | - | 18 | - | 10 | - | - | - | 45 | 10 |
| Fipronil sulfone | - | - | 45 | - | - | - | - | - | - | - | 15 | - | - | - | 45 | 15 |
| Fludioxonil | - | - | - | - | 93 | - | 277 | - | 1107 | 607 | 56 | 41 | - | - | 1107 | 41 |
| Folpet | 234 | - | 481 | 81 | 6222 | 5597 | 93,179 | na * | 37,714 | 11,754 | 7465 | 2059 | 1426 | 475 | 93,179 | 81 |
| Iprovalicarb | - | 28 | - | - | 11 | - | 2957 | 386 | 507 | 50 | 33 | - | 11 | - | 2957 | 11 |
| Kresoxim-methyl | - | 27 | - | - | 44 | 15 | 192 | 92 | 10,888 | 476 | 858 | 21 | 57 | - | 10,888 | 15 |
| Mandipropamid | - | 78 | - | - | 6086 | 21 | 1571 | 527 | 304 | 54 | 77 | 10 | - | - | 6086 | 10 |
| Metalaxyl-M | - | 118 | - | - | 24,992 | 2489 | 385 | 188 | 179 | 28 | 21 | 16 | - | - | 24,992 | 16 |
| Metolachlor (S) | - | - | - | - | 10,579 | - | 1339 | - | - | 161 | - | - | - | - | 10,579 | 161 |
| Metrafenone | - | 52 | - | - | 1814 | 29 | 375 | 152 | 63 | 18 | - | - | - | - | 1814 | 18 |
| Myclobutanil | - | 83 | - | - | 32 | 5 | 10,219 | 2137 | 4303 | 52 | 46 | - | 18 | - | 10,219 | 5 |
| Quinoxyfen | - | - | - | - | 51 | 106 | 250 | - | 284 | 201 | 20 | 17 | - | - | 284 | 17 |
| Spiroxamine | - | 293 | - | - | 521 | 71 | 19,580 | 1688 | 509 | 54 | 26 | 15 | - | - | 19,580 | 15 |
| Trifloxystrobin | - | 14 | - | - | 34 | 11 | 143 | 86 | 43 | 29 | - | - | - | - | 143 | 11 |
| Zoxamide | - | 119 | - | - | 330 | 45 | 67 | 33 | 4777 | 145 | 140 | 19 | 61 | - | 4777 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, S.; Dévier, M.-H.; Cruz, J.; Duporté, G.; Barron, E.; Gaillard, J.; Le Menach, K.; Pardon, P.; Augagneur, S.; Flaud, P.-M.; et al. Passive Sampling as a Tool to Assess Atmospheric Pesticide Contamination Related to Vineyard Land Use. Atmosphere 2022, 13, 504. https://doi.org/10.3390/atmos13040504
Martin S, Dévier M-H, Cruz J, Duporté G, Barron E, Gaillard J, Le Menach K, Pardon P, Augagneur S, Flaud P-M, et al. Passive Sampling as a Tool to Assess Atmospheric Pesticide Contamination Related to Vineyard Land Use. Atmosphere. 2022; 13(4):504. https://doi.org/10.3390/atmos13040504
Chicago/Turabian StyleMartin, Stéphan, Marie-Hélène Dévier, Justine Cruz, Geoffroy Duporté, Emmanuelle Barron, Juliette Gaillard, Karyn Le Menach, Patrick Pardon, Sylvie Augagneur, Pierre-Marie Flaud, and et al. 2022. "Passive Sampling as a Tool to Assess Atmospheric Pesticide Contamination Related to Vineyard Land Use" Atmosphere 13, no. 4: 504. https://doi.org/10.3390/atmos13040504
APA StyleMartin, S., Dévier, M.-H., Cruz, J., Duporté, G., Barron, E., Gaillard, J., Le Menach, K., Pardon, P., Augagneur, S., Flaud, P.-M., Villenave, É., & Budzinski, H. (2022). Passive Sampling as a Tool to Assess Atmospheric Pesticide Contamination Related to Vineyard Land Use. Atmosphere, 13(4), 504. https://doi.org/10.3390/atmos13040504






