Simultaneous Removal of Hg0 and H2S over a Regenerable Fe2O3/AC Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Fe2O3/AC Catalyst Preparation
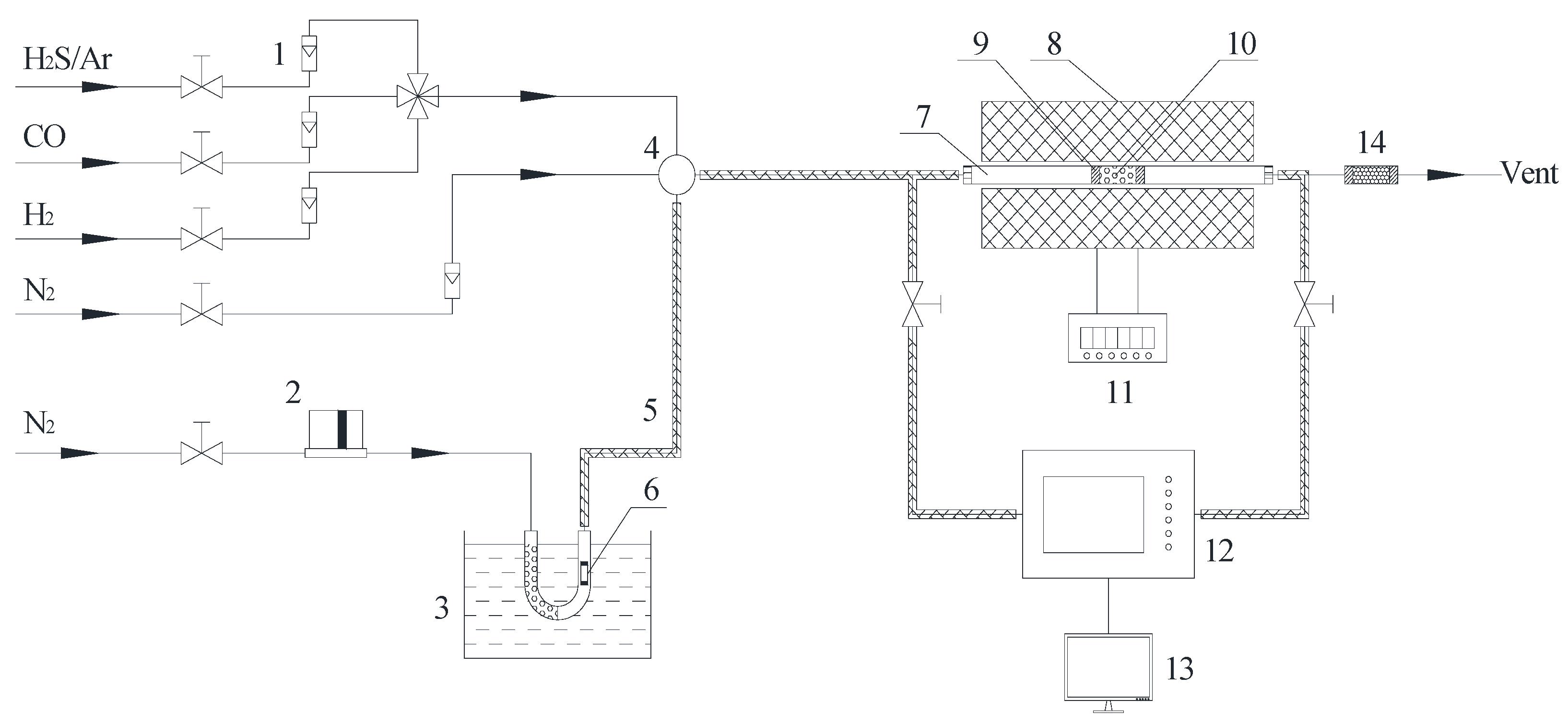
2.2. Hg0 and H2S Removal Experiment
2.3. Temperature Programmed Desorption Experiment
2.4. Hg Content Analysis
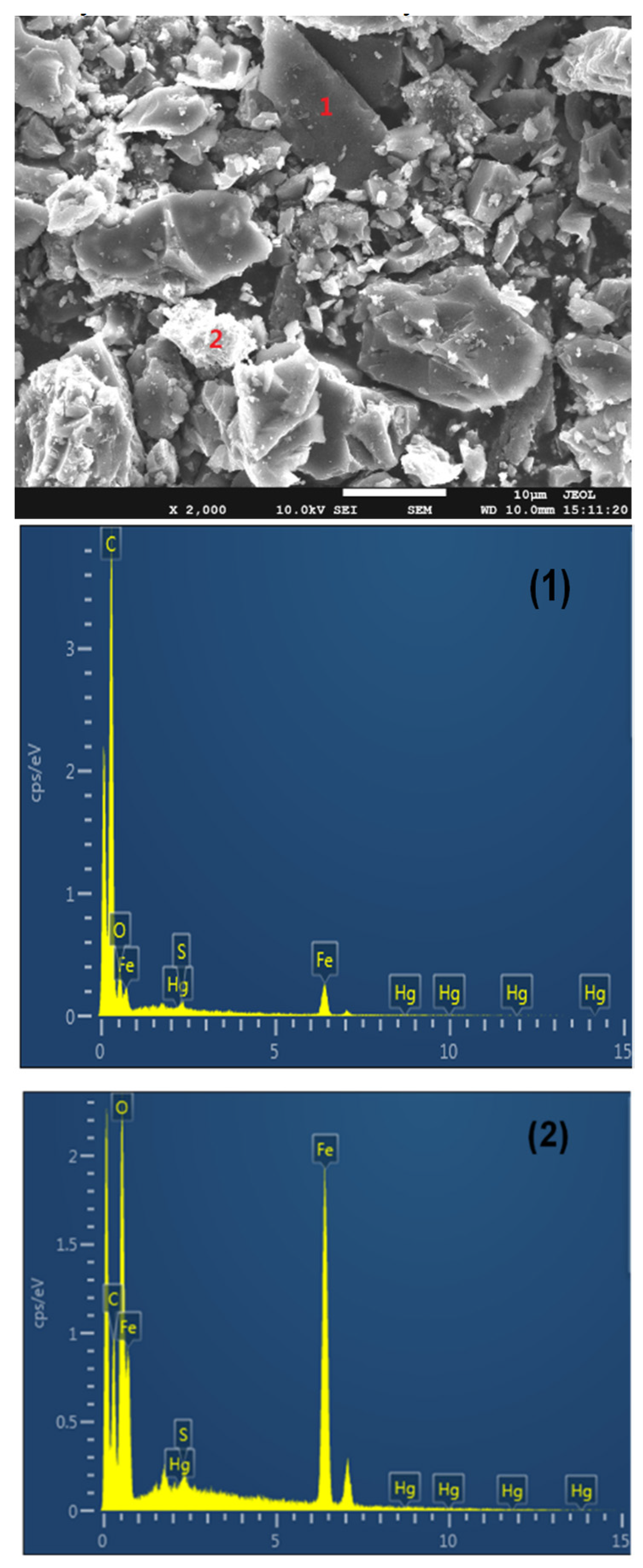
2.5. Characterization
3. Results and Discussion
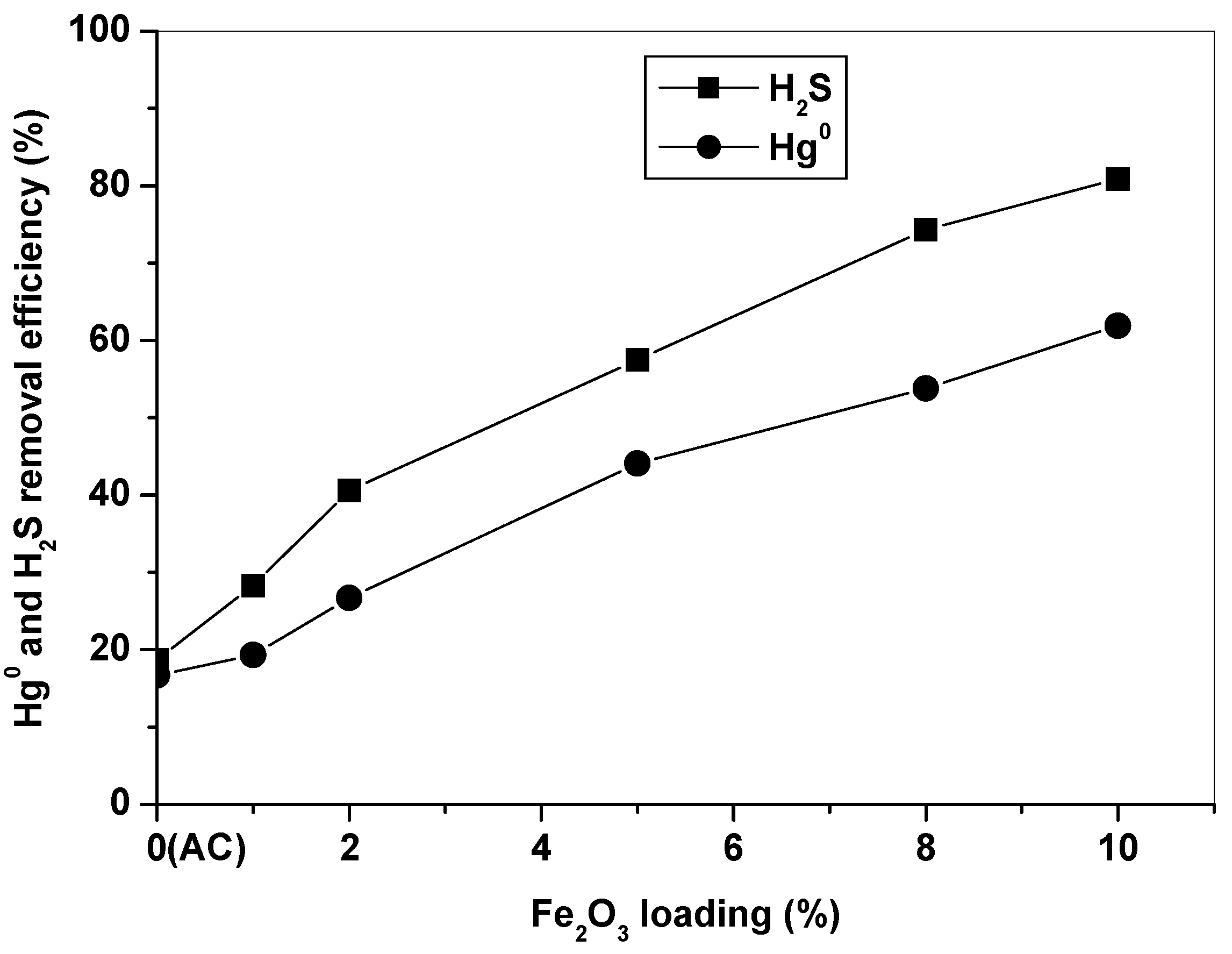
3.1. Role of Fe2O3 in Hg0 and H2S Removal
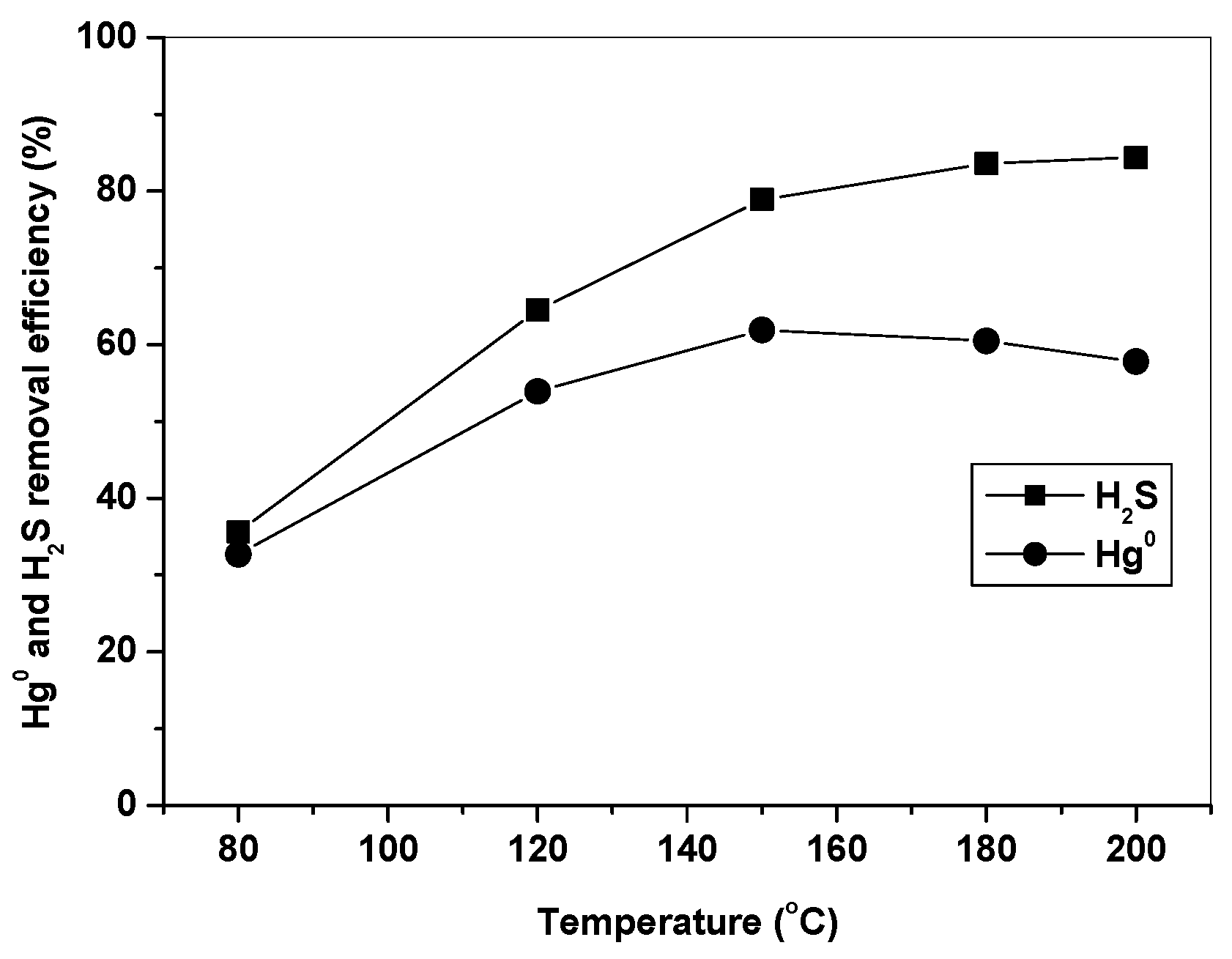
3.2. Effect of Temperature on Hg0 and H2S Removal
3.3. Effect of Syngas Components on Hg0 Removal
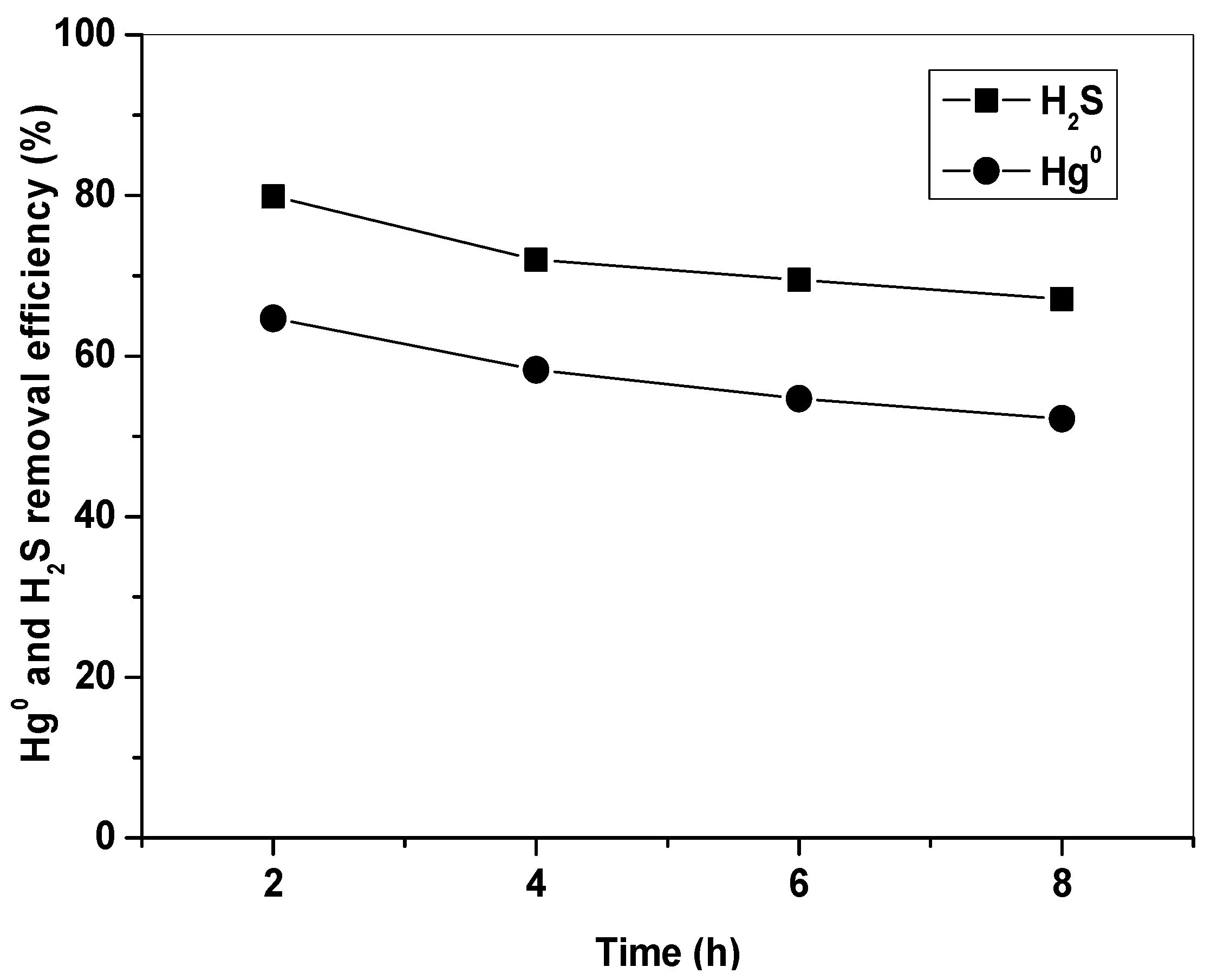
3.4. Regeneration and Reuse of Fe2O3/AC after Hg0 and H2S Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2019, 245, 125586. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; Lu, Z.; Levin, L.; Thackray, C.P.; Sunderland, E. Global and regional trends in mercury emissions and concentrations, 2010–2015. Atmospheric Environ. 2018, 201, 417–427. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- UN Environment. Global Mercury Assessment 2018. UN Environment Programme Chemicals and Health Branch: Geneva, Switzerland, 2019. Available online: https://www.unep.org/resources/publication/global-mercury-assessment-2018 (accessed on 20 December 2021).
- Charvát, P.; Klimeš, L.; Pospíšil, J.; Klemeš, J.J.; Varbanov, P.S. An overview of mercury emissions in the energy industry—A step to mercury footprint assessment. J. Clean. Prod. 2020, 267, 122087. [Google Scholar] [CrossRef]
- Lancet, T. Minamata Convention on mercury: A contemporary reminder. Lancet 2017, 390, 822. [Google Scholar] [CrossRef]
- Bank, M.S. The mercury science-policy interface: History, evolution and progress of the Minamata Convention. Sci. Total Environ. 2020, 722, 137832. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.; Ergüdenler, A.; Grace, J.; Watkinson, A.; Herod, A.; Dugwell, D.; Kandiyoti, R. Control of gasifier mercury emissions in a hot gas filter: The effect of temperature. Fuel 2001, 80, 623–634. [Google Scholar] [CrossRef]
- Pavlish, J.H.; Sondreal, E.A.; Mann, M.D.; Olson, E.S.; Galbreath, K.C.; Laudal, D.L.; Benson, S.A. State review of mercury control options for coal-fired power plants. Fuel Process. Technol. 2003, 82, 89–165. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Yang, Y.; Yu, Y.; Yan, X.; Zhang, Z. AMn2O4 (A=Cu, Ni and Zn) sorbents coupling high adsorption and regeneration performance for elemental mercury removal from syngas. J. Hazard. Mater. 2020, 388, 121738. [Google Scholar] [CrossRef] [PubMed]
- Dennis, Y.L.; David, L.G.; Donald, R. Study of mercury speciation from simulated coal gasification. Ind. Eng. Chem. Res. 2004, 43, 5400–5404. [Google Scholar] [CrossRef]
- Han, L.; Lv, X.; Wang, J.; Chang, L. Palladium–Iron Bimetal Sorbents for Simultaneous Capture of Hydrogen Sulfide and Mercury from Simulated Syngas. Energy Fuels 2012, 26, 1638–1644. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, T.; Liu, X.; Xu, J.; Deng, L.; Li, C.; Liu, J.; Xu, M. Mechanistic investigation of elemental mercury adsorption over silver-modified vanadium silicate: A DFT study. J. Hazard. Mater. 2021, 404, 124108. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, J.-S.; Zhou, Q.-X.; Xu, X.-Y.; Xie, C. Elemental mercury removal from coal gas by CeMnTi sorbents and their regeneration performance. J. Zhejiang Univ. A 2021, 22, 222–234. [Google Scholar] [CrossRef]
- Li, D.; Han, J.; Han, L.; Wang, J.; Chang, L. Pd/activated carbon sorbents for mid-temperature capture of mercury from coal-derived fuel gas. J. Environ. Sci. 2014, 26, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Yang, Y.; Yu, Y.; Yan, X.; Zhang, Z. Experimental and DFT studies of the role of H2S in Hg0 removal from syngas over CuMn2O4 sorbent. Chem. Eng. J. 2020, 391, 123616. [Google Scholar] [CrossRef]
- Han, L.; He, X.; Yue, C.; Hu, Y.; Li, L.; Chang, L.; Wang, H.; Wang, J. Fe doping Pd/AC sorbent efficiently improving the Hg0 removal from the coal-derived fuel gas. Fuel 2016, 182, 64–72. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Wu, J.; Liu, Y. Novel carbon-based sorbents for elemental mercury removal from gas streams: A review. Chem. Eng. J. 2020, 391, 123514. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Fang, Y.; Huang, J.; Wang, Y. Catalytic oxidation and stabilized adsorption of elemental mercury from coal-derived fuel gas. Energy Fuels 2012, 26, 1629–1637. [Google Scholar] [CrossRef]
- Jain, A.; Seyed-Reihani, S.-A.; Fischer, C.C.; Couling, D.; Ceder, G.; Green, W.H. Ab initio screening of metal sorbents for elemental mercury capture in syngas streams. Chem. Eng. Sci. 2010, 65, 3025–3033. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Ju, F.; Han, L.; Chang, L.; Bao, W. Supercritical hydrothermal synthesis of zeolites from coal fly ash for mercury removal from coal derived gas. Fuel Process. Technol. 2015, 136, 96–105. [Google Scholar] [CrossRef]
- Zhao, W.; Geng, X.; Lu, J.; Duan, Y.; Liu, S.; Hu, P.; Xu, Y.; Huang, Y.; Tao, J.; Gu, X. Mercury removal performance of brominated biomass activated carbon injection in simulated and coal-fired flue gas. Fuel 2020, 285, 119131. [Google Scholar] [CrossRef]
- Ren, X.; Chang, L.; Li, F.; Xie, K. Study of intrinsic sulfidation behavior of Fe2O3 for high temperature H2S removal. Fuel 2010, 89, 883–887. [Google Scholar] [CrossRef]
- Tian, H.; Wu, J.; Zhang, W.; Yang, S.; Li, F.; Qi, Y.; Zhou, R.; Qi, X.; Zhao, L.; Wang, X. High performance of Fe nanoparticles/carbon aerogel sorbents for H2S Removal. Chem. Eng. J. 2017, 313, 1051–1060. [Google Scholar] [CrossRef]
- Yang, C.; Florent, M.; de Falco, G.; Fan, H.; Bandosz, T.J. ZnFe2O4/activated carbon as a regenerable adsorbent for catalytic removal of H2S from air at room temperature. Chem. Eng. J. 2020, 394, 124906. [Google Scholar] [CrossRef]
- Liu, T.; Xue, L.; Guo, X. Study of Hg0 removal characteristics on Fe2O3 with H2S. Fuel 2015, 160, 189–195. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Mei, J.; Hong, Q.; Yang, S. Recovering gaseous Hg0 using sulfureted phosphotungstic acid modified γ-Fe2O3 from power plants burning Hg-rich coal for centralized control. J. Hazard. Mater. 2021, 407, 124381. [Google Scholar] [CrossRef] [PubMed]
- Rumayor, M.; Díaz-Somoano, M.; López-Antón, M.; Ochoa-González, R.; Martínez-Tarazona, M. Temperature programmed desorption as a tool for the identification of mercury fate in wet-desulphurization systems. Fuel 2015, 148, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Uddin, A.; Nagano, S.; Ozaki, M.; Sasaoka, E. Fundamental Study on Decomposition Characteristics of Mercury Compounds over Solid Powder by Temperature-Programmed Decomposition Desorption Mass Spectrometry. Energy Fuels 2010, 25, 144–153. [Google Scholar] [CrossRef]
- Uddin, A.; Ozaki, M.; Sasaoka, E.; Wu, S. Temperature-Programmed Decomposition Desorption of Mercury Species over Activated Carbon Sorbents for Mercury Removal from Coal-Derived Fuel Gas†. Energy Fuels 2009, 23, 4710–4716. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Han, L.; Chang, L.; Bao, W. Simultaneous removal of hydrogen sulfide and mercury from simulated syngas by iron-based sorbents. Fuel 2013, 103, 73–79. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Liu, Z. Gas-phase elemental mercury capture by a V2O5/AC catalyst. Fuel Process. Technol. 2010, 91, 676–680. [Google Scholar] [CrossRef]
- Hou, W.; Zhou, J.; Qi, P.; Gao, X.; Luo, Z. Effect of H2S/HCl on the removal of elemental mercury in syngas over CeO2–TiO2. Chem. Eng. J. 2014, 241, 131–137. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Dong, Y.; Qin, W.; Zhang, Q.; Lu, L.; Zhang, Y. Oxidation and adsorption of gas-phase Hg0 over a V2O5/AC catalyst. RSC Adv. 2016, 6, 77553–77557. [Google Scholar] [CrossRef]

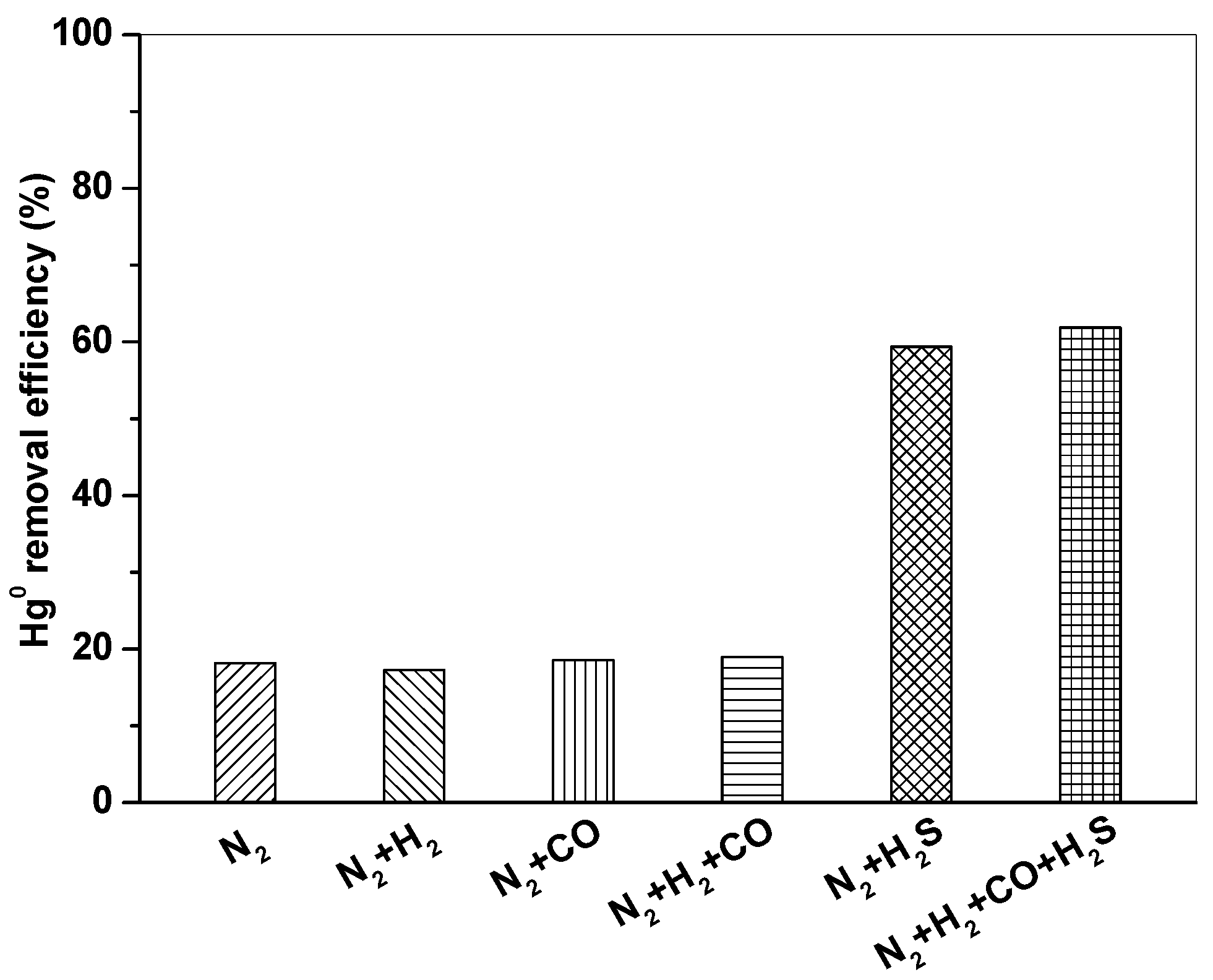

| Name | BET Area (m2/g) | Average Pore Diameter (nm) | Micropore Volume (cm3/g) |
|---|---|---|---|
| AC | 960 | 2.013 | 0.244 |
| Fe1.0/AC | 883 | 2.039 | 0.305 |
| Fe2.0/AC | 943 | 2.171 | 0.337 |
| Fe5.0/AC | 918 | 2.189 | 0.321 |
| Fe8.0/AC | 873 | 2.393 | 0.265 |
| Fe10/AC | 852 | 2.332 | 0.280 |
| Sample | Hg Content (μg/g) | S Content (mg/g) |
|---|---|---|
| Fresh Fe2O3/AC | — | 3.7 |
| Fe2O3/AC-Hg | 5.95 | 202.3 |
| Fe2O3/AC-R | 0.06 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Fang, Y.; Wang, H.; Bai, G.; Qin, W.; Zhang, J. Simultaneous Removal of Hg0 and H2S over a Regenerable Fe2O3/AC Catalyst. Atmosphere 2022, 13, 425. https://doi.org/10.3390/atmos13030425
Wang J, Fang Y, Wang H, Bai G, Qin W, Zhang J. Simultaneous Removal of Hg0 and H2S over a Regenerable Fe2O3/AC Catalyst. Atmosphere. 2022; 13(3):425. https://doi.org/10.3390/atmos13030425
Chicago/Turabian StyleWang, Junwei, Yao Fang, Huan Wang, Guoliang Bai, Wei Qin, and Jianli Zhang. 2022. "Simultaneous Removal of Hg0 and H2S over a Regenerable Fe2O3/AC Catalyst" Atmosphere 13, no. 3: 425. https://doi.org/10.3390/atmos13030425
APA StyleWang, J., Fang, Y., Wang, H., Bai, G., Qin, W., & Zhang, J. (2022). Simultaneous Removal of Hg0 and H2S over a Regenerable Fe2O3/AC Catalyst. Atmosphere, 13(3), 425. https://doi.org/10.3390/atmos13030425





