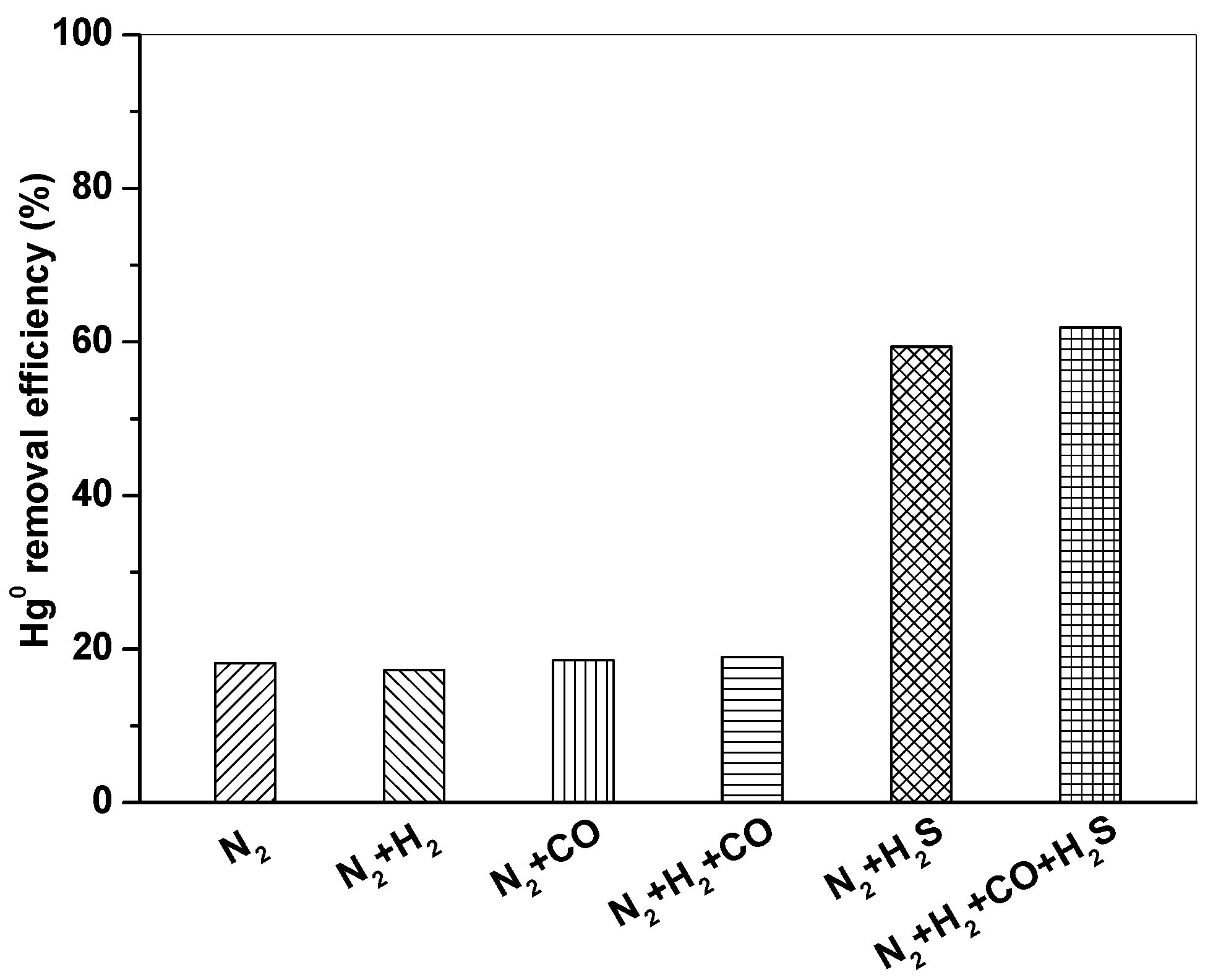Abstract
Simultaneous removal of Hg0 and H2S over a regenerable activated coke supported Fe2O3 catalyst (Fe2O3/AC) was studied in simulated coal-derived syngas. It was found that the Fe2O3/AC catalyst exhibited high capability for Hg0 and H2S removal, which was attributed to the catalytic oxidation activity of Fe2O3. Its capability for Hg0 and H2S removal increased with an increase of Fe2O3 loading amount, and the highest was at 150 °C for Hg0 removal. CO and H2 showed no obvious effect on Hg0 removal by Fe2O3/AC, while H2S had a promotion effect, which was due to S and FeSx produced by the H2S reaction on Fe2O3/AC. The results of SEM-EDX and the temperature programmed desorption experiment (TPD) revealed that Fe2O3 played a critical role in Hg0 oxidation, and HgS was generated upon the reaction of Hg0 with H2S on Fe2O3/AC. The used Fe2O3/AC catalyst after Hg0 and H2S removal could be effectively regenerated and still had high capability for Hg0 and H2S removal.
1. Introduction
Mercury in coal can release into the atmosphere during coal conversion processes and cause serious harm to human health and ecological environments due to its toxicity, bioaccumulation, and persistence [1,2]. It was reported that the annual anthropogenic mercury emission is about 1000 to 6000 tons, and coal conversion processes were the primary emission source [3,4,5]. Currently, more than 140 countries and regions have signed The Minamata Convention on Mercury treaty to limit mercury emission and use [6,7].
Coal gasification is considered as a promising clean coal technology and will be widely used in the near future. However, there are several pollutants in syngas, such as Hg and H2S, and they must be removed before further use of syngas. It was reported that the total Hg concentration in coal gasification syngas was higher than that in coal combustion flue gas, and Hg0 was the dominant form in syngas, accounting for 93–99% [8,9,10,11]. In addition, Hg0 is more difficult to remove than oxidized mercury (Hg2+) and particulate-bound mercury (Hgp) due to its high volatility and low solubility. Therefore, Hg control in syngas was more difficult and challenging than that in flue gas. Currently, a variety of studies have been carried out for Hg removal from coal combustion flue gas. However, little research has focused on, or been reported on, Hg removal from coal-derived syngas. Several types of sorbents have been studied for Hg0 removal from syngas, including activated carbon, noble metals, metal oxides, and metal sulfides [12,13,14,15,16,17,18,19,20,21]. Among these sorbents, activated carbon has been widely studied as a promising adsorbent. However, the disadvantages of a large consumption of activated carbon and the high cost limited its industrial application [22]. Noble metals, particularly Pd and Pt sorbents, exhibited high Hg0 removal ability. However, the high cost of the noble metal sorbents could be a concern and restricted their industrial application. Metal oxides with the advantages of high activity, convenient regeneration, and low cost have good development potential. Iron-based metal oxides adsorbents have attracted widespread attention due to their high adsorption activity, low cost, and easy preparation recovery. Therefore, it is considered as a promising cost-effective technology for mercury removal from syngas.
H2S is another pollutant existing in coal-derived syngas, which can cause severe corrosion to equipment and catalyst poisoning. Nowadays, a lot of research has been carried out for H2S removal in syngas. Iron-based sorbents, especially Fe2O3 or supported Fe2O3, are widely used for H2S removal due to their high adsorption capacity and low cost [23,24,25]. Recently, Fe2O3 was proposed and studied as sorbent to remove mercury in coal-derived syngas [26,27]. It was suggested that S and FeSx, formed during H2S removal by Fe2O3, were beneficial for Hg0 removal. In this regard, Fe2O3-based sorbents may be a candidate active for simultaneous removal Hg0 and H2S. Thus, the cost of Hg0 and H2S removal will be significantly lower.
In this work, an activated coke supported Fe2O3 catalyst (Fe2O3/AC) was prepared and used for simultaneous removal of Hg0 and H2S in simulated coal-derived syngas. Since Fe2O3 has high catalytic oxidation activity for Hg0 and Fe2O3-based sorbents are widely used for H2S removal, the Fe2O3/AC catalyst should have high capability for Hg0 and H2S simultaneous removal due to the combination of catalytic oxidation activity of Fe2O3 and adsorption ability of AC. Thus, the influences of Fe2O3 loading and temperature and syngas components were studied, as well as the regeneration of Fe2O3/AC after Hg0 and H2S removal.
2. Experimental
2.1. Fe2O3/AC Catalyst Preparation
A commercial granule activated coke (AC) was purchased from Shanxi Xinhua Chemical Technology Co., Ltd. (Taiyuan, China) with sizes of 0.25–0.60 mm and used as the support. Fe2O3/AC catalysts were prepared by pore volume impregnation of activated coke with an Fe(NO3)3 aqueous solution. After impregnating, it was stewed at room temperature overnight, dried at 50 °C for 5 h and at 110 °C for 5 h in air. Then, it was calcined at 300 °C for 3 h in N2 and pre-oxidized at 250 °C for 5 h in air. Several Fe2O3/AC catalysts with different Fe2O3 loading (wt.%) were prepared and named according to the Fe2O3 loading amount in Fe2O3/AC. Fe1.0/AC referred to Fe2O3/AC catalyst with 1.0% Fe2O3 loading, for example. The properties of AC and Fe2O3/AC catalysts are shown in Table 1.

Table 1.
Properties of AC and Fe2O3/AC catalysts.
2.2. Hg0 and H2S Removal Experiment
Hg0 and H2S removal experiments were carried out in a fixed-bed reactor in simulated coal-derived syngas as shown in Figure 1. An amount of 0.2 g Fe2O3/AC catalyst was loaded for each experiment in 80–200 °C in simulated coal-derived syngas containing 10% H2, 15% CO, 1500 ppm H2S, 80 μg/m3 Hg0, and balance N2. The total flow rate was 100 mL/min, corresponding to a space velocity of about 15,000 h−1. Gas-phase Hg0 was generated by a Hg0 permeation tube (VICI Metronics, Washington, DC, USA), which was immersed in a constant temperature water bath, and a certain flow of nitrogen was used as the carrier gas. All of the gas tubes for Hg0 delivery were heated by heating tape and kept at 120 °C to avoid the possible adsorption of Hg0. After the furnace was program heated up to the desired temperature, Hg0 and H2S removal experiments were carried out at constant temperature for 4 h. The Hg0 concentration before and after the reactor was continuously measured by a RA-915M mercury analyzer (Lumex Instruments, Saint Petersburg, Russia). The exhaust gas was evacuated after activated carbon treatment. The performance of the Fe2O3/AC catalyst for Hg0 and H2S removal was evaluated by removal efficiency (E), which was defined as follows:
where C0 and C1 are the Hg0 concentration and H2S concentration in the inlet and outlet gas, respectively.
E = [C0 − C1]/C0 × 100%
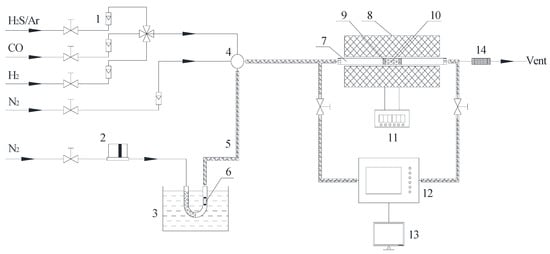
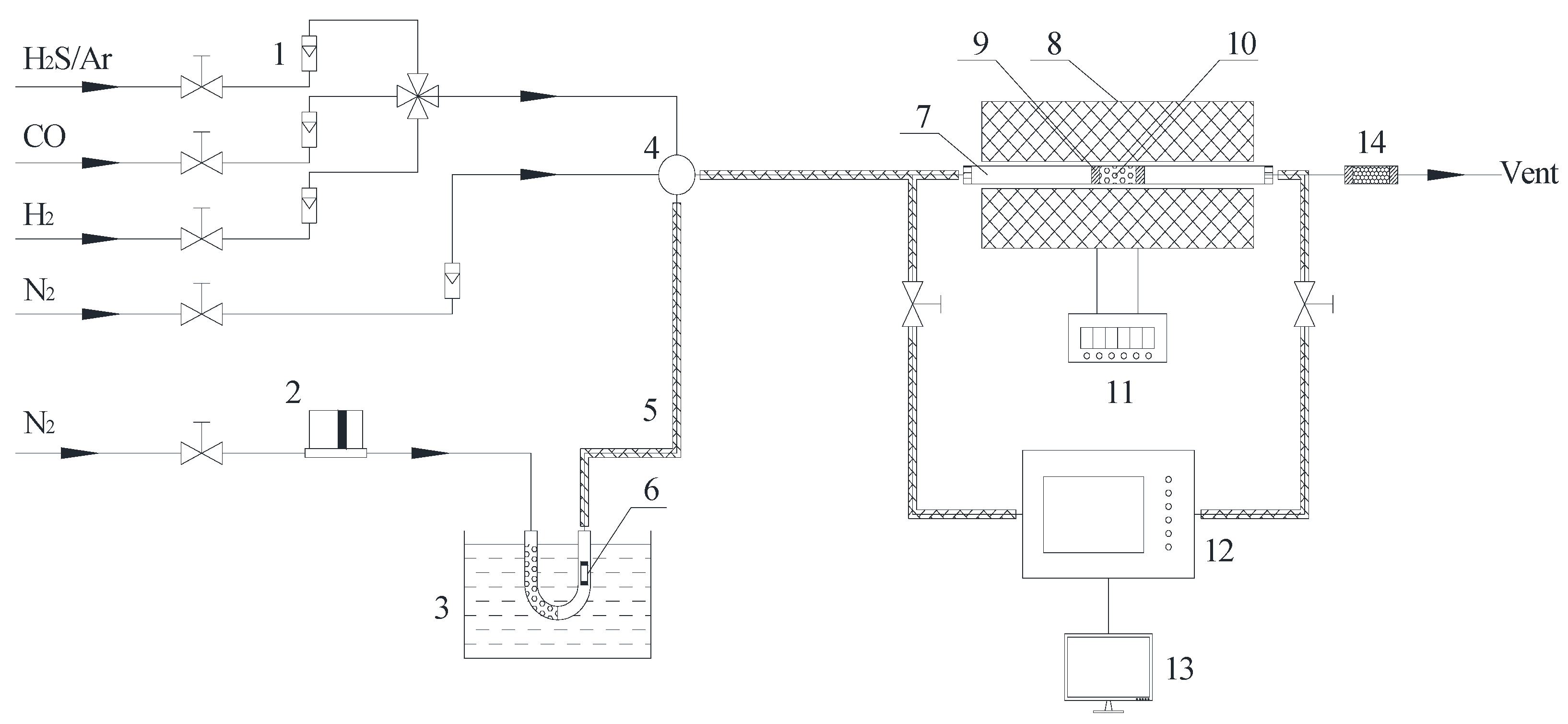
Figure 1.
Schematic diagram of Hg0 and H2S removal experiment: (1) rotameter; (2) mass flow controller; (3) water bath; (4) gas mixing chamber; (5) heating tape; (6) Hg0 permeation tube; (7) quartz reactor; (8) furnace; (9) quartz wool; (10) catalyst; (11) temperature controller; (12) mercury/H2S analyzer; (13) computer; (14) tail gas cleaner.
2.3. Temperature Programmed Desorption Experiment
A temperature programmed desorption experiment (TPD) was conducted to investigate Hg speciation on the Fe2O3/AC catalyst after Hg0 and H2S removal for 4 h upon heating to 600 °C in Ar. About 5 mg Hg0-adsorbed Fe2O3/AC catalyst was loaded in a quartz tube reactor, purged for 30 min in N2, and then heated up to 600 °C at a heating rate of 10 °C/min in Ar with a flow rate of 100 mL/min. The Hg0 in the effluent gas of the reactor was analyzed directly by RA-915M mercury analyzer. The total Hg was measured by bubbling the effluent gas through a KBH4 solution to reduce possible existing Hg2+ to Hg0 before the mercury analyzer.
2.4. Hg Content Analysis
The amounts of Hg adsorbed by Fe2O3/AC catalyst and AC were measured by following the Chinese national standard, GB/T 16659-1996, in which the Hg0-adsorbed Fe2O3/AC catalysts were digested by 2H2SO4 + 5HNO3 with V2O5, and the dissolved Hg2+ was analyzed by an atomic fluorescence spectrometer (Beijing Jitian Instruments Co., Ltd., Beijing, China) with KBH4 as a reducing agent. All the measurements were duplicated, and the relative deviation was less than 5%.
2.5. Characterization
BET surface area and pore structure of the AC and Fe2O3/AC were measured by BET analysis (TriStar 3000, Micromeritics Instrument Corporation, Shanghai, China) using N2 adsorption at −196 °C. The samples were initially purged at 150 °C for 3 h before adsorption.
X-ray photoelectron spectroscopy (XPS) analysis was carried out on an ESCALAB250 spectrometer (VG Scientific Ltd., London, UK) with Al Kα source at 10 KV. The binding energies of Hg 4f were scanned and calibrated by the C 1s peak at 284.6 eV.
Scanning electron microscope-energy dispersive X-ray (SEM-EDX) was used to characterize the microstructure of Fe2O3/AC catalysts and the elemental composition and distribution on the surface of Fe2O3/AC catalysts by a LEO435vp instrument (Leica, Cambridge, UK).
3. Results and Discussion
3.1. Role of Fe2O3 in Hg0 and H2S Removal
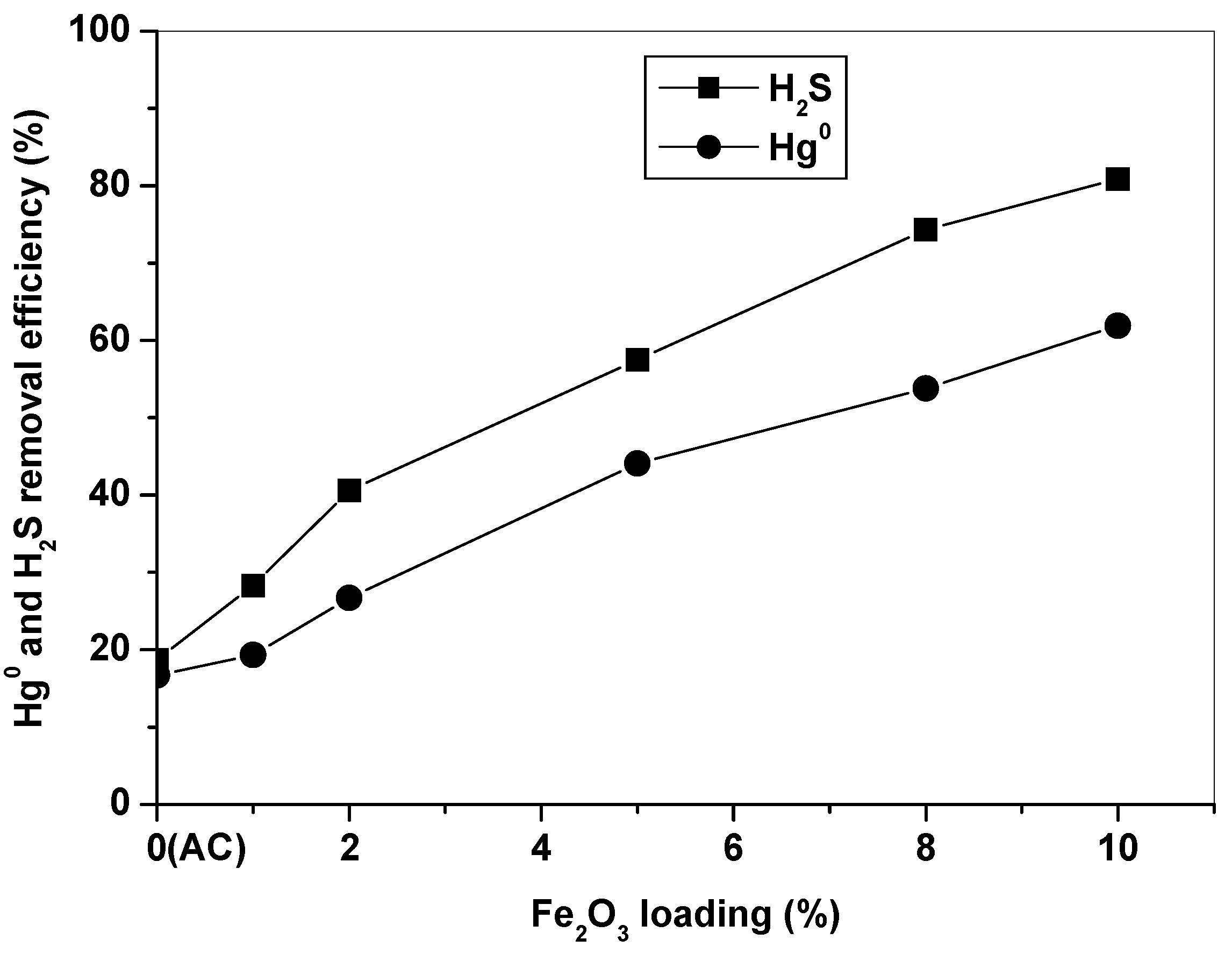
Figure 2 compares Hg0 and H2S removal efficiencies of AC and Fe2O3/AC catalysts with different Fe2O3 loading amounts at 150 °C for 4 h in simulated syngas. It can be seen that the AC carrier had low Hg0 and H2S removal efficiencies in 4 h, about 16% and 19%, respectively. Supporting Fe2O3 onto AC can significantly improve its Hg0 and H2S removal capabilities, and the capability of Fe2O3/AC increased with an increase of the Fe2O3 loading amount. The Hg0 and H2S removal efficiencies reached 62% and 81% for Fe10/AC. The Fe2O3/AC catalyst has high simultaneous Hg0 and H2S removal capability, which will significantly reduce and/or avoid use of noble metals for single Hg0 removal. Simultaneous removal of Hg0 and H2S will not only simplify the process, but also significantly reduce the operating costs. It is obvious that Fe2O3 was of great importance for Hg0 and H2S removal over Fe2O3/AC. Thus, the Fe10/AC catalyst was selected to evaluate Hg0 and H2S removal activity of the Fe2O3/AC catalyst in the following experiments.
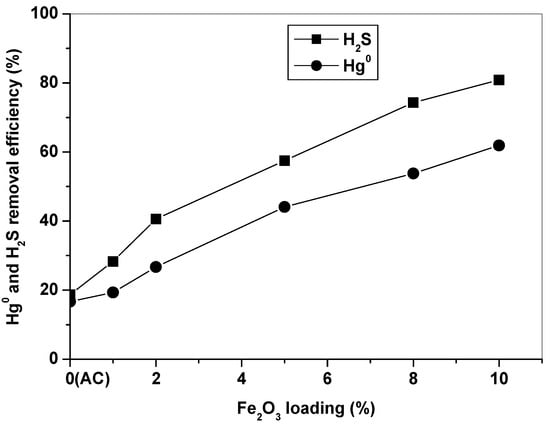
Figure 2.
Effect of Fe2O3 loading on Hg0 and H2S removal for 4 h.
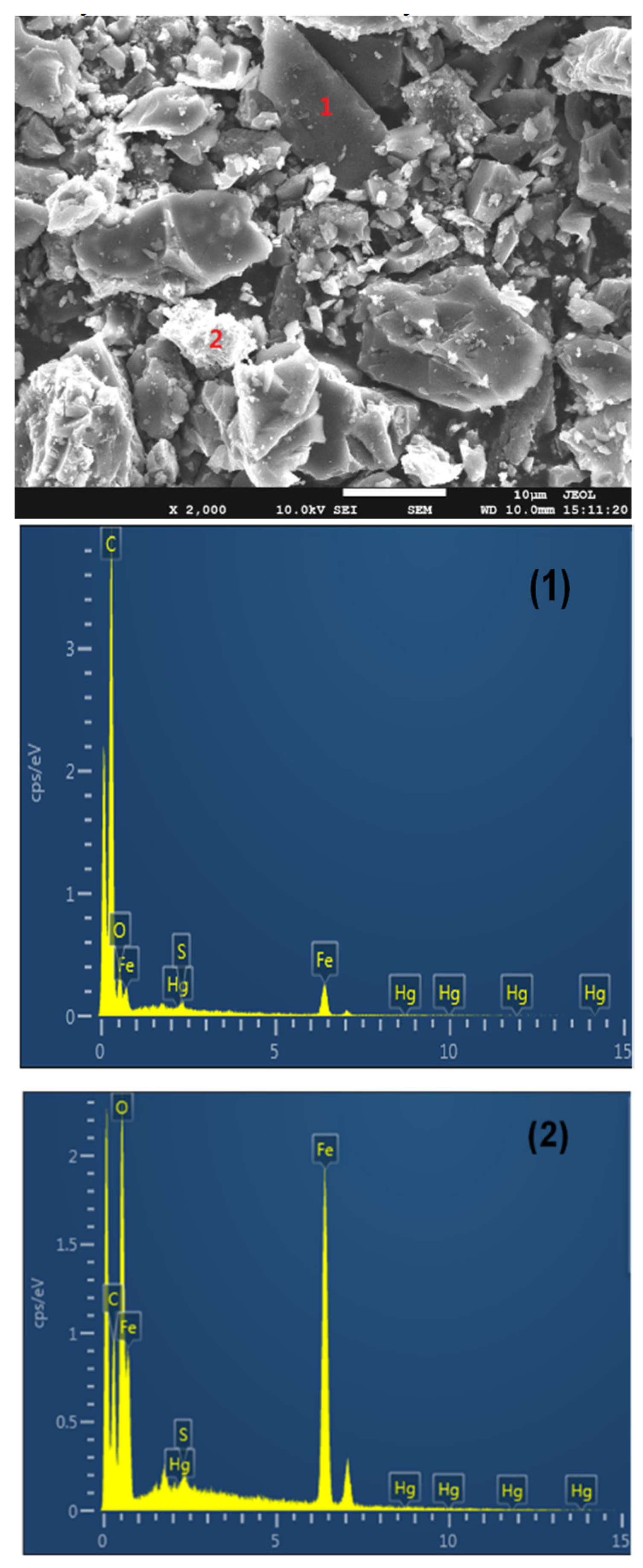
SEM-EDX was used to measure the distribution of Fe, Hg, and S on the surface of the Fe2O3/AC catalyst after Hg0 and H2S removal, and the results are shown in Figure 3. It can be seen that there are two kinds of distinguishing positions (named as 1 and 2) on Fe2O3/AC catalyst surface. EDX analysis results showed that position 1 contained mainly carbon with little Hg and S, while position 2 contained mainly Fe2O3 with more Hg and S. These indicated that Hg0 and H2S trended to be captured in the positions containing more Fe2O3. Thus, it can be concluded that the Fe2O3 played a critical role in Hg0 and H2S removal mainly due to its catalytic oxidation activity.
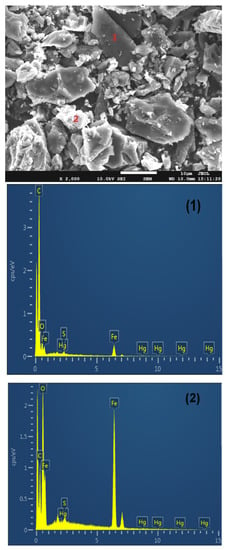
Figure 3.
SEM-EDX analysis of Fe2O3 after Hg0 and H2S removal ((1) position 1, (2) position 2).
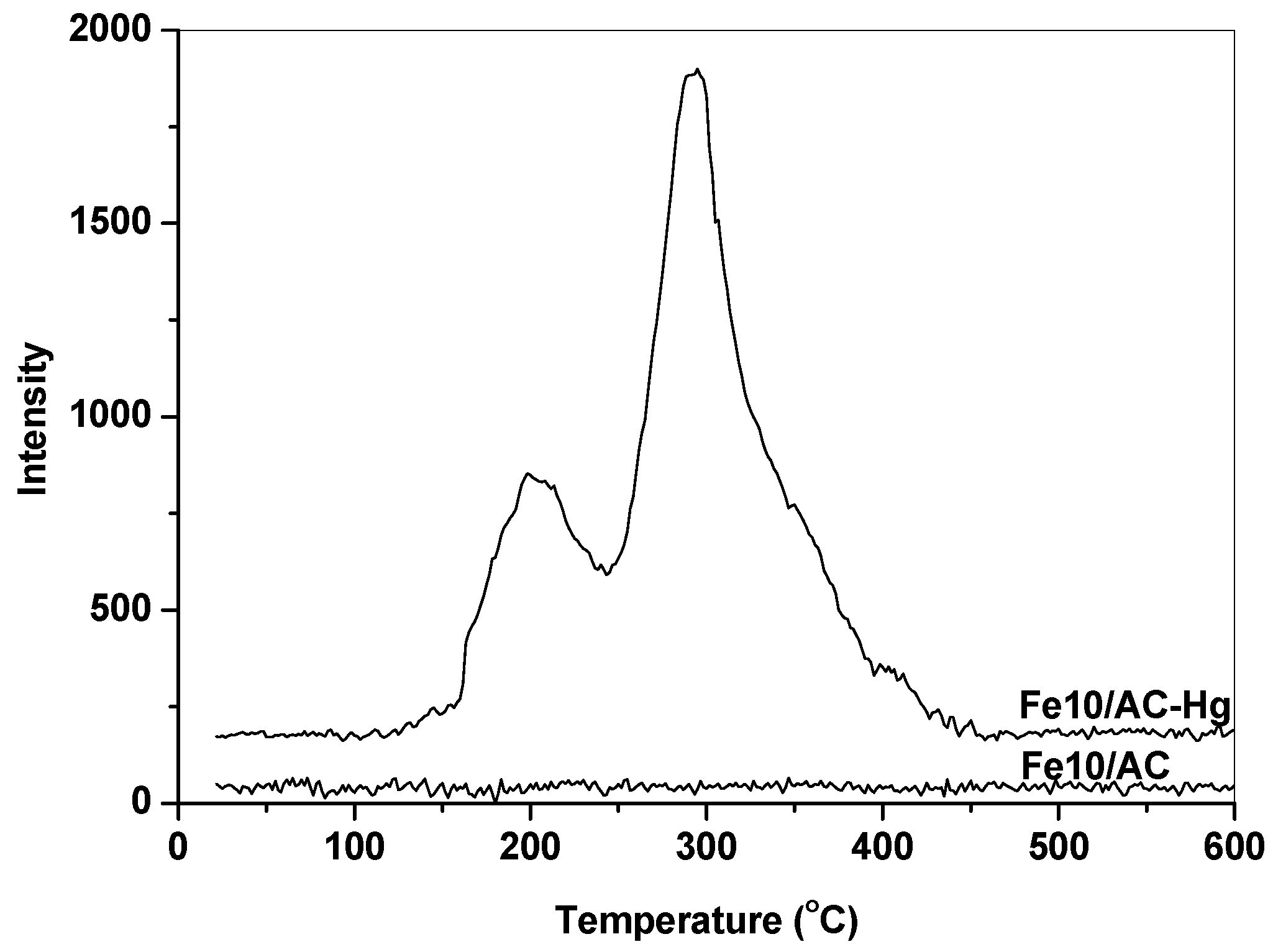
To confirm the catalytic oxidation activity of Fe2O3 in Hg0 removal, TPD experiments were conducted to identify the speciation of Hg adsorbed over Fe2O3/AC. Figure 4 shows the Hg release behaviors of the fresh (Fe10/AC) and spent Fe10/AC (Fe10/AC-Hg) catalysts upon heating to 600 °C in Ar. It can be seen that the fresh Fe10/AC sample showed no Hg release during the TPD process. As for the spent Fe10/AC sample, however, it showed two obvious Hg release peaks at about 198 °C and 296 °C, which could be attributed to HgS (black) and HgS (red) [28,29,30]. This indicated that the form of Hg captured over Fe2O3/AC were mainly Hg2+ compounds, confirming the oxidation of Hg0 to Hg2+ by Fe2O3 and the reaction of Hg2+ with H2S to form HgS. These were consistent with the results of Hg0 oxidation and the reaction on Fe2O3-based catalysts [12,31].
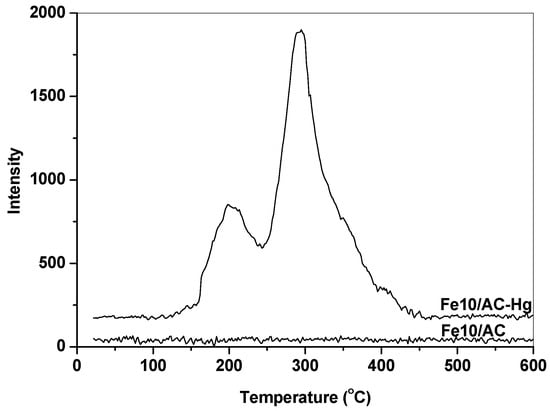
Figure 4.
Hg release of the fresh and spent Fe10/AC samples during the TPD process.
XPS analyses were also conducted to confirm the catalytic oxidation activity of Fe2O3 and identify the speciation of Hg captured over Fe2O3/AC; however, no valuable information was obtained due to the interferences of Si in SiO2 of the AC support (7.6%) [32].
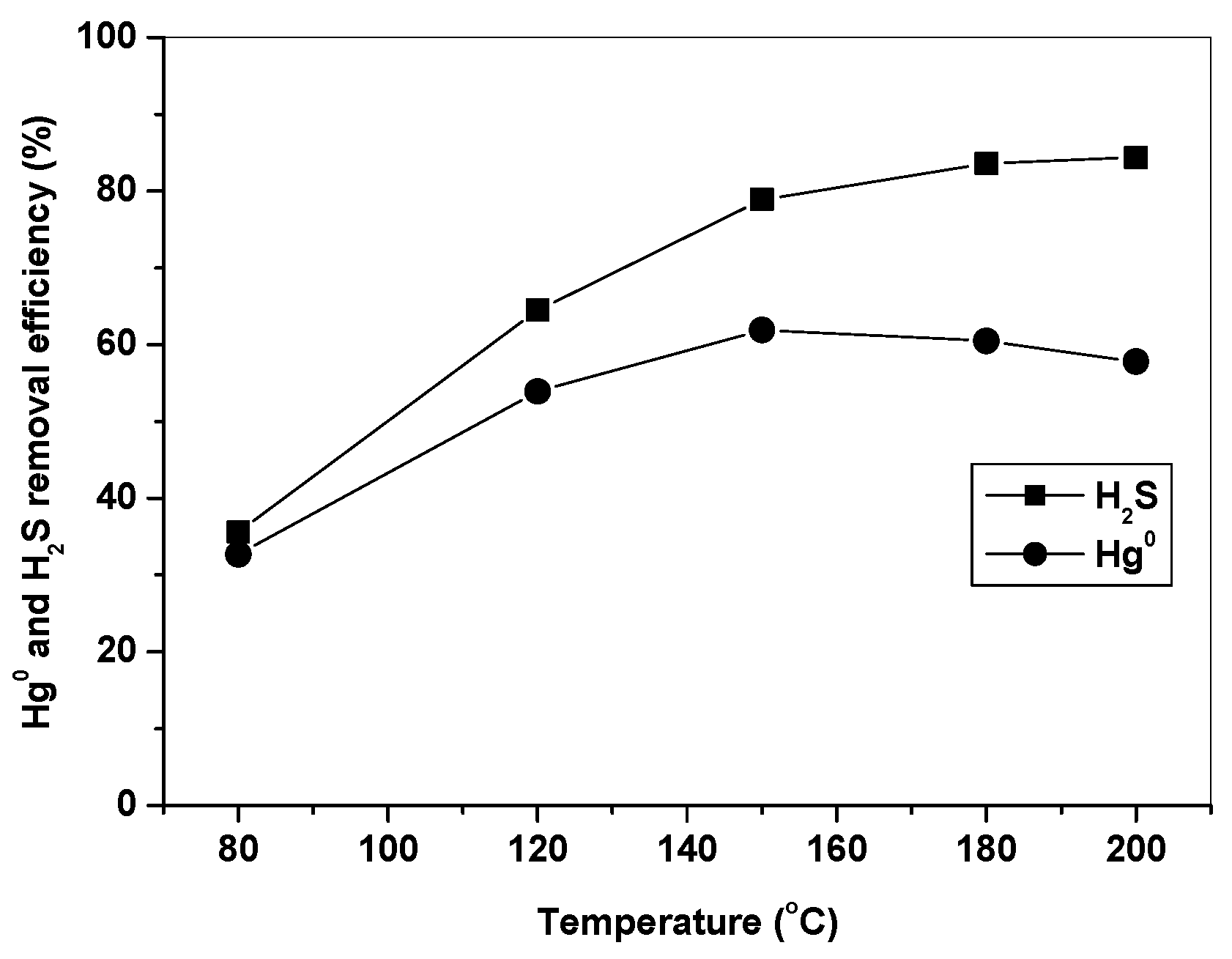
3.2. Effect of Temperature on Hg0 and H2S Removal
Figure 5 shows Hg0 and H2S removal efficiencies of Fe10/AC at different temperatures in simulated syngas. It can be seen that H2S removal efficiency increased as the temperature increased from 80 to 200 °C, while Hg0 removal efficiency firstly increased at 80–150 °C and then decreased at 150–200 °C. It is well understood that the catalytic activity of Fe2O3 increased with an increase of temperature, leading to a higher H2S removal efficiency at high temperature. However, the trend of Hg0 removal efficiency over Fe10/AC indicated that adsorption might be another important factor for Hg0 removal, which was favored at low temperature. The starting temperature of Hg0 release at around 150 °C in Figure 4 was very important, which indicated that Hg captured over Fe2O3/AC could release as the temperature increased above 150 °C. This explained the trend of Hg0 removal efficiency in Figure 5 and suggested that adsorption was an important effect factor in Hg0 removal [33,34]. Thus, it can be concluded that the effect of temperature on Hg0 removal over Fe2O3/AC depended on the combination effect of oxidation and adsorption at different temperature.
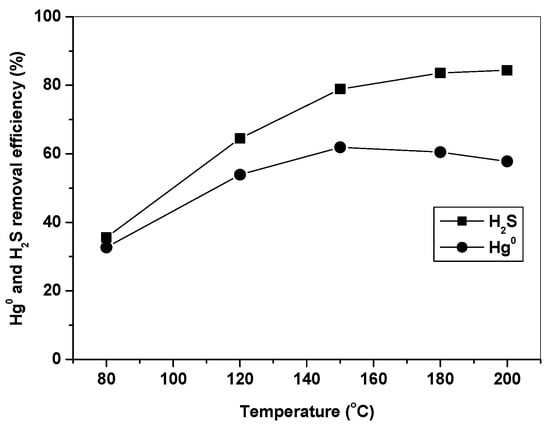
Figure 5.
Effect of temperature on Hg0 and H2S removal by Fe10/AC.
3.3. Effect of Syngas Components on Hg0 Removal
Figure 6 shows Hg0 removal by Fe2O3/AC at 150 °C in different atmospheres (N2, N2 + 10% H2, N2 + 15% CO, N2 + 10% H2 + 15% CO, N2 + 1500 ppm H2S, and N2 + 10% H2 + 15% CO+1500 ppm H2S). It can be seen that Hg0 removal efficiency of Fe2O3/AC was very low in N2 atmosphere, only about 18%. Hg0 removal efficiency had no obvious change in the presence of H2 and CO. When H2S was introduced, Hg0 removal efficiency significantly increased and reached to above 60%, indicating that H2S had a promotion effect and played an important role in Hg0 removal by Fe2O3/AC.
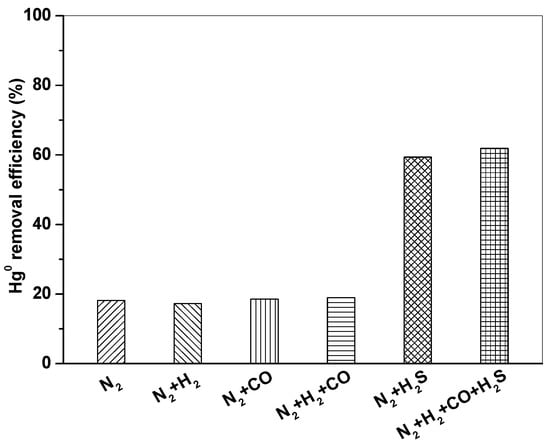
Figure 6.
Effect of syngas components on Hg0 removal by Fe2O3/AC. (Reaction condition: CHg0 = 80 μg/m3, GHSV = 15,000 h−1, T = 150 °C.)
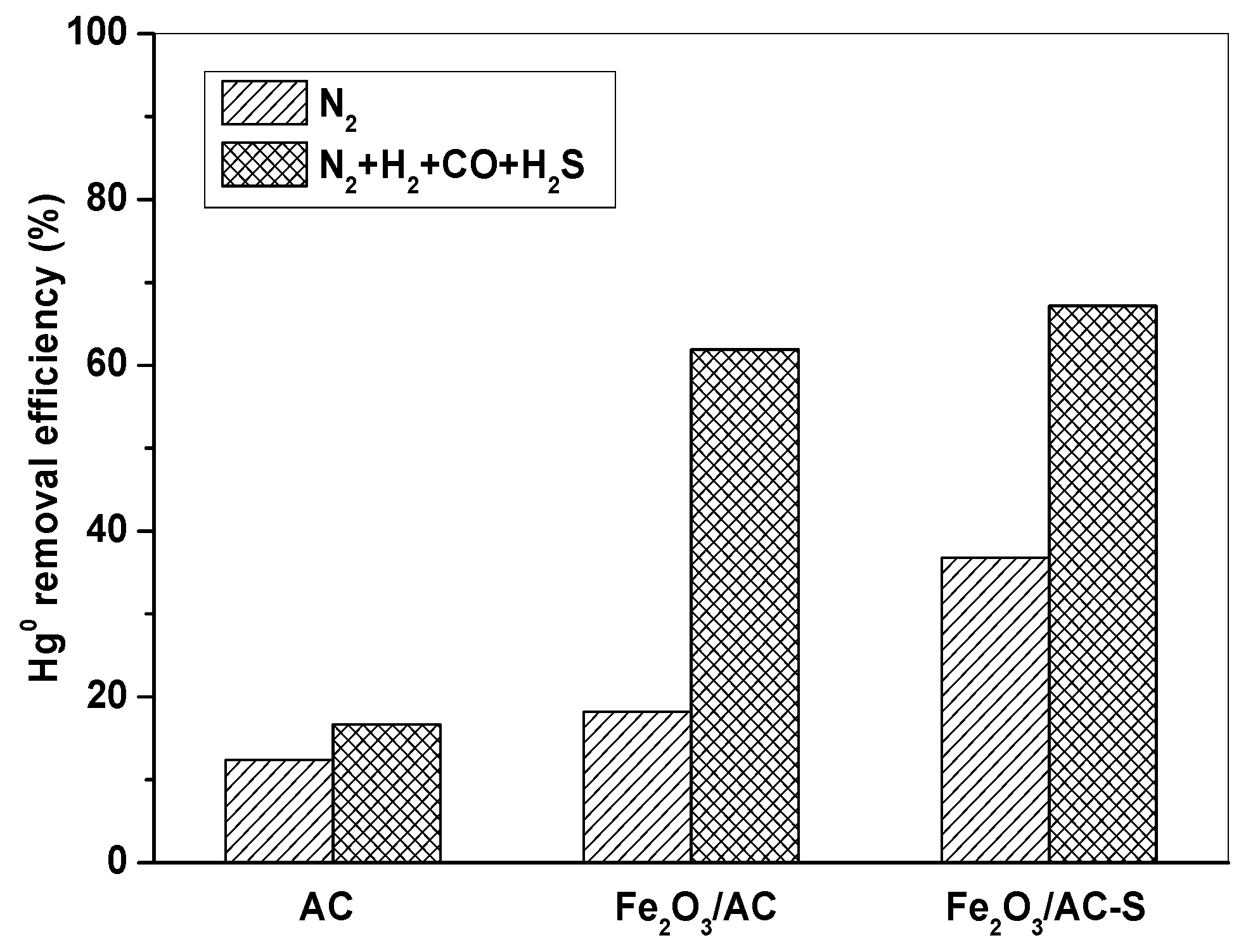
In order to further investigate the role of H2S, the Fe2O3/AC catalyst was firstly pre-adsorbed with H2S for 4 h to prepare the Fe2O3/AC-S catalyst, and then it was used to remove Hg0. Figure 7 shows Hg0 removal by AC, Fe2O3/AC, and Fe2O3/AC-S at 150 °C in N2 and simulated syngas (N2 + 10% H2 + 15% CO + 1500 ppm H2S). It can be seen that Fe2O3/AC-S showed much higher Hg0 removal capability than AC and Fe2O3/AC in both N2 and N2 + H2 + CO + H2S atmospheres. This was mainly because H2S may form Sx and FeSx on the Fe2O3/AC-S catalyst, which could react with Hg0 to form HgS and thus improve the capability of Fe2O3/AC-S for Hg0 removal [12,31].
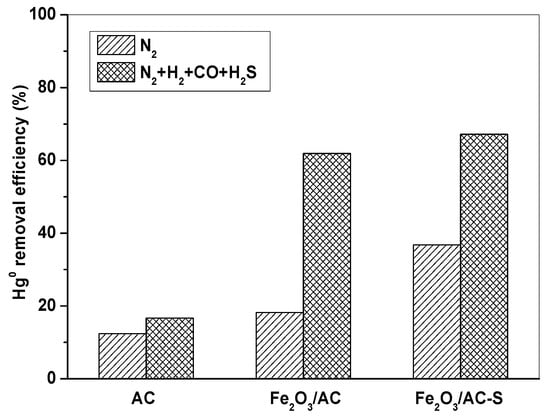
Figure 7.
Comparison of Hg0 removal by AC, Fe2O3/AC, and Fe2O3/AC-S. (Reaction condition: 10% H2, 15% CO, 1500 ppm H2S, balance N2, CHg0 = 80 μg/m3, GHSV = 15,000 h−1, T = 150 °C.)
3.4. Regeneration and Reuse of Fe2O3/AC after Hg0 and H2S Removal
The Fe2O3/AC catalyst after Hg0 and H2S removal (denoted as Fe2O3/AC-Hg) was thermally regenerated at 400 °C for 90 min in an Ar atmosphere and then was used again to remove Hg0 and H2S in simulated syngas at 150 °C. Table 2 shows the Hg and S content of the Fe2O3/AC catalyst before and after regeneration. It can be seen that the S content of the fresh Fe2O3/AC catalyst surface was only 3.7 mg/g, while the Fe2O3/AC-Hg catalyst contains a much higher S amount of 202.3 mg/g. The contents of Hg and S on the regenerated Fe2O3/AC catalyst (denoted as Fe2O3/AC-R) was much lower than those of Fe2O3/AC-Hg, and the contents of Hg and S were both close to the fresh Fe2O3/AC catalyst, indicating that Hg and S adsorbed on Fe2O3/AC almost completely released during the regeneration process.

Table 2.
Analysis of Hg and S contents before and after regeneration of Fe2O3/AC.
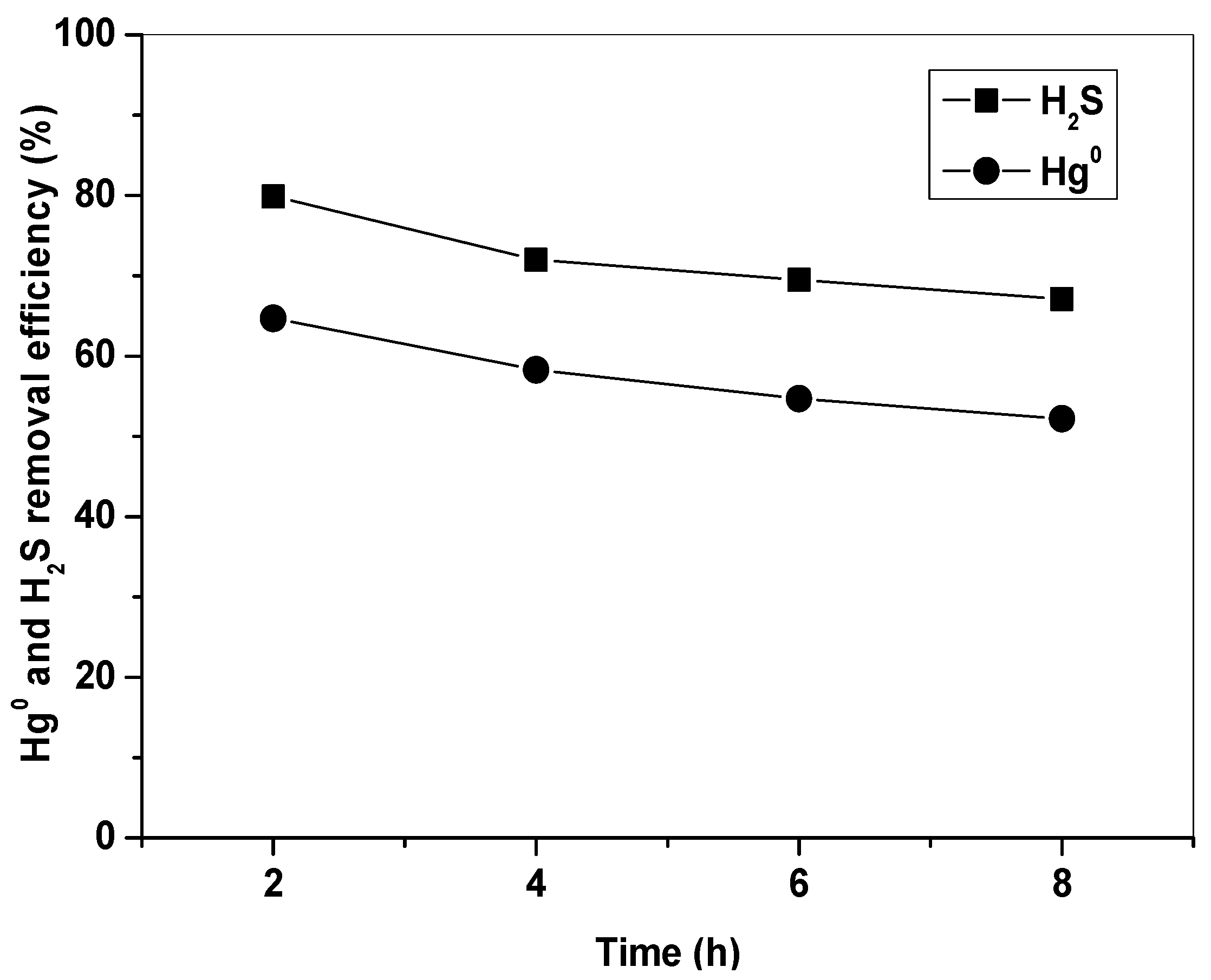
Figure 8 shows Hg0 and H2S removal by Fe2O3/AC-R at 150 °C in simulated syngas. It can be seen that the Fe2O3/AC-R catalyst still had a high capability to remove Hg0 and H2S. The initial removal efficiencies of Hg0 and H2S were about 65% and 80%, and they were still kept above 52% and 67% at 8 h. To further investigate the reuse performance of the Fe2O3/AC catalyst, five cycles of Hg0 and H2S removal–regeneration experiments were carried out. The results showed that the Fe2O3/AC catalyst still had a satisfactory Hg0 and H2S removal capability, and Hg0 and H2S removal efficiencies were above 46% and 50%. These indicated that the Fe2O3/AC catalyst had good regeneration and reuse performance. Compared with the poor regeneration performance, narrow temperature range, low capacity, and high operation cost of activated carbon for Hg0 removal, simultaneous removal of Hg0 and H2S by Fe2O3/AC catalyst will significantly simplify the process and reduce costs.
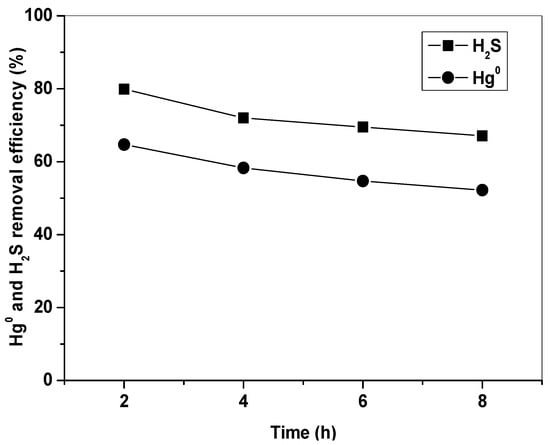
Figure 8.
Removal of Hg0 and H2S by Fe2O3/AC after regeneration. (Reaction condition: 10% H2, 15% CO, 1500 ppm H2S, balance N2, CHg0 = 80 μg/m3, GHSV = 15,000 h−1, T = 150 °C.)
4. Conclusions
The Fe2O3/AC catalyst had high capability to remove Hg0 and H2S at 150–200 °C, which was mainly due to the catalytic oxidation of Fe2O3, and Hg0 and H2S formed HgS on Fe2O3/AC. Hg0 and H2S removal capability of Fe2O3/AC increased with the Fe2O3 loading amount. Although H2S and Hg0 had competitive adsorption on Fe2O3 active sites, H2S still promoted Hg0 removal over Fe2O3/AC, mainly because of the formed S and FeSx by the reaction of H2S on Fe2O3/AC, which could react with Hg0 to form HgS. The Fe2O3/AC catalyst had good regeneration performance after Hg0 and H2S removal, and the regenerated Fe2O3/AC still had high capability to remove Hg0 and H2S.
The Fe2O3/AC catalyst not only had high oxidation activity, but also had high adsorption capacity, leading to its high simultaneous removal capacity of Hg0 and H2S. Compared to activated carbon and noble metals adsorbents, the Fe2O3/AC catalyst had the advantages of high capacity, good regeneration performance, low cost, and no negative effect of H2S. Simultaneous removal of Hg0 and H2S by the Fe2O3/AC catalyst will significantly simplify the process and reduce costs. Fe2O3/AC is a superior and promising catalyst for simultaneous removal of Hg0 and H2S in coal-derived syngas.
Author Contributions
Conceptualization, J.W.; methodology, J.W., W.Q. and J.Z.; investigation, Y.F. and H.W.; data curation, Y.F. and G.B.; writing—original draft preparation, Y.F. and H.W.; writing—review and editing, J.W., W.Q. and G.B.; visualization, W.Q.; supervision, J.Z.; project administration, J.W. and W.Q.; funding acquisition, J.W., W.Q. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21203003, Beijing, China), Foundation of State Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering (2020-KF-28, Yinchuan, China), and Anhui Provincial Discipline (Professional) Top Talent Academic Funding Project (gxbjZD2021062, Hefei, China).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The observation datasets are available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2019, 245, 125586. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; Lu, Z.; Levin, L.; Thackray, C.P.; Sunderland, E. Global and regional trends in mercury emissions and concentrations, 2010–2015. Atmospheric Environ. 2018, 201, 417–427. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- UN Environment. Global Mercury Assessment 2018. UN Environment Programme Chemicals and Health Branch: Geneva, Switzerland, 2019. Available online: https://www.unep.org/resources/publication/global-mercury-assessment-2018 (accessed on 20 December 2021).
- Charvát, P.; Klimeš, L.; Pospíšil, J.; Klemeš, J.J.; Varbanov, P.S. An overview of mercury emissions in the energy industry—A step to mercury footprint assessment. J. Clean. Prod. 2020, 267, 122087. [Google Scholar] [CrossRef]
- Lancet, T. Minamata Convention on mercury: A contemporary reminder. Lancet 2017, 390, 822. [Google Scholar] [CrossRef]
- Bank, M.S. The mercury science-policy interface: History, evolution and progress of the Minamata Convention. Sci. Total Environ. 2020, 722, 137832. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.; Ergüdenler, A.; Grace, J.; Watkinson, A.; Herod, A.; Dugwell, D.; Kandiyoti, R. Control of gasifier mercury emissions in a hot gas filter: The effect of temperature. Fuel 2001, 80, 623–634. [Google Scholar] [CrossRef]
- Pavlish, J.H.; Sondreal, E.A.; Mann, M.D.; Olson, E.S.; Galbreath, K.C.; Laudal, D.L.; Benson, S.A. State review of mercury control options for coal-fired power plants. Fuel Process. Technol. 2003, 82, 89–165. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Yang, Y.; Yu, Y.; Yan, X.; Zhang, Z. AMn2O4 (A=Cu, Ni and Zn) sorbents coupling high adsorption and regeneration performance for elemental mercury removal from syngas. J. Hazard. Mater. 2020, 388, 121738. [Google Scholar] [CrossRef] [PubMed]
- Dennis, Y.L.; David, L.G.; Donald, R. Study of mercury speciation from simulated coal gasification. Ind. Eng. Chem. Res. 2004, 43, 5400–5404. [Google Scholar] [CrossRef]
- Han, L.; Lv, X.; Wang, J.; Chang, L. Palladium–Iron Bimetal Sorbents for Simultaneous Capture of Hydrogen Sulfide and Mercury from Simulated Syngas. Energy Fuels 2012, 26, 1638–1644. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, T.; Liu, X.; Xu, J.; Deng, L.; Li, C.; Liu, J.; Xu, M. Mechanistic investigation of elemental mercury adsorption over silver-modified vanadium silicate: A DFT study. J. Hazard. Mater. 2021, 404, 124108. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, J.-S.; Zhou, Q.-X.; Xu, X.-Y.; Xie, C. Elemental mercury removal from coal gas by CeMnTi sorbents and their regeneration performance. J. Zhejiang Univ. A 2021, 22, 222–234. [Google Scholar] [CrossRef]
- Li, D.; Han, J.; Han, L.; Wang, J.; Chang, L. Pd/activated carbon sorbents for mid-temperature capture of mercury from coal-derived fuel gas. J. Environ. Sci. 2014, 26, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Yang, Y.; Yu, Y.; Yan, X.; Zhang, Z. Experimental and DFT studies of the role of H2S in Hg0 removal from syngas over CuMn2O4 sorbent. Chem. Eng. J. 2020, 391, 123616. [Google Scholar] [CrossRef]
- Han, L.; He, X.; Yue, C.; Hu, Y.; Li, L.; Chang, L.; Wang, H.; Wang, J. Fe doping Pd/AC sorbent efficiently improving the Hg0 removal from the coal-derived fuel gas. Fuel 2016, 182, 64–72. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Wu, J.; Liu, Y. Novel carbon-based sorbents for elemental mercury removal from gas streams: A review. Chem. Eng. J. 2020, 391, 123514. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Fang, Y.; Huang, J.; Wang, Y. Catalytic oxidation and stabilized adsorption of elemental mercury from coal-derived fuel gas. Energy Fuels 2012, 26, 1629–1637. [Google Scholar] [CrossRef]
- Jain, A.; Seyed-Reihani, S.-A.; Fischer, C.C.; Couling, D.; Ceder, G.; Green, W.H. Ab initio screening of metal sorbents for elemental mercury capture in syngas streams. Chem. Eng. Sci. 2010, 65, 3025–3033. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Ju, F.; Han, L.; Chang, L.; Bao, W. Supercritical hydrothermal synthesis of zeolites from coal fly ash for mercury removal from coal derived gas. Fuel Process. Technol. 2015, 136, 96–105. [Google Scholar] [CrossRef]
- Zhao, W.; Geng, X.; Lu, J.; Duan, Y.; Liu, S.; Hu, P.; Xu, Y.; Huang, Y.; Tao, J.; Gu, X. Mercury removal performance of brominated biomass activated carbon injection in simulated and coal-fired flue gas. Fuel 2020, 285, 119131. [Google Scholar] [CrossRef]
- Ren, X.; Chang, L.; Li, F.; Xie, K. Study of intrinsic sulfidation behavior of Fe2O3 for high temperature H2S removal. Fuel 2010, 89, 883–887. [Google Scholar] [CrossRef]
- Tian, H.; Wu, J.; Zhang, W.; Yang, S.; Li, F.; Qi, Y.; Zhou, R.; Qi, X.; Zhao, L.; Wang, X. High performance of Fe nanoparticles/carbon aerogel sorbents for H2S Removal. Chem. Eng. J. 2017, 313, 1051–1060. [Google Scholar] [CrossRef]
- Yang, C.; Florent, M.; de Falco, G.; Fan, H.; Bandosz, T.J. ZnFe2O4/activated carbon as a regenerable adsorbent for catalytic removal of H2S from air at room temperature. Chem. Eng. J. 2020, 394, 124906. [Google Scholar] [CrossRef]
- Liu, T.; Xue, L.; Guo, X. Study of Hg0 removal characteristics on Fe2O3 with H2S. Fuel 2015, 160, 189–195. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Mei, J.; Hong, Q.; Yang, S. Recovering gaseous Hg0 using sulfureted phosphotungstic acid modified γ-Fe2O3 from power plants burning Hg-rich coal for centralized control. J. Hazard. Mater. 2021, 407, 124381. [Google Scholar] [CrossRef] [PubMed]
- Rumayor, M.; Díaz-Somoano, M.; López-Antón, M.; Ochoa-González, R.; Martínez-Tarazona, M. Temperature programmed desorption as a tool for the identification of mercury fate in wet-desulphurization systems. Fuel 2015, 148, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Uddin, A.; Nagano, S.; Ozaki, M.; Sasaoka, E. Fundamental Study on Decomposition Characteristics of Mercury Compounds over Solid Powder by Temperature-Programmed Decomposition Desorption Mass Spectrometry. Energy Fuels 2010, 25, 144–153. [Google Scholar] [CrossRef]
- Uddin, A.; Ozaki, M.; Sasaoka, E.; Wu, S. Temperature-Programmed Decomposition Desorption of Mercury Species over Activated Carbon Sorbents for Mercury Removal from Coal-Derived Fuel Gas†. Energy Fuels 2009, 23, 4710–4716. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Han, L.; Chang, L.; Bao, W. Simultaneous removal of hydrogen sulfide and mercury from simulated syngas by iron-based sorbents. Fuel 2013, 103, 73–79. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Liu, Z. Gas-phase elemental mercury capture by a V2O5/AC catalyst. Fuel Process. Technol. 2010, 91, 676–680. [Google Scholar] [CrossRef]
- Hou, W.; Zhou, J.; Qi, P.; Gao, X.; Luo, Z. Effect of H2S/HCl on the removal of elemental mercury in syngas over CeO2–TiO2. Chem. Eng. J. 2014, 241, 131–137. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Dong, Y.; Qin, W.; Zhang, Q.; Lu, L.; Zhang, Y. Oxidation and adsorption of gas-phase Hg0 over a V2O5/AC catalyst. RSC Adv. 2016, 6, 77553–77557. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).