Assessment of Climate-Driven Variations in Malaria Transmission in Senegal Using the VECTRI Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
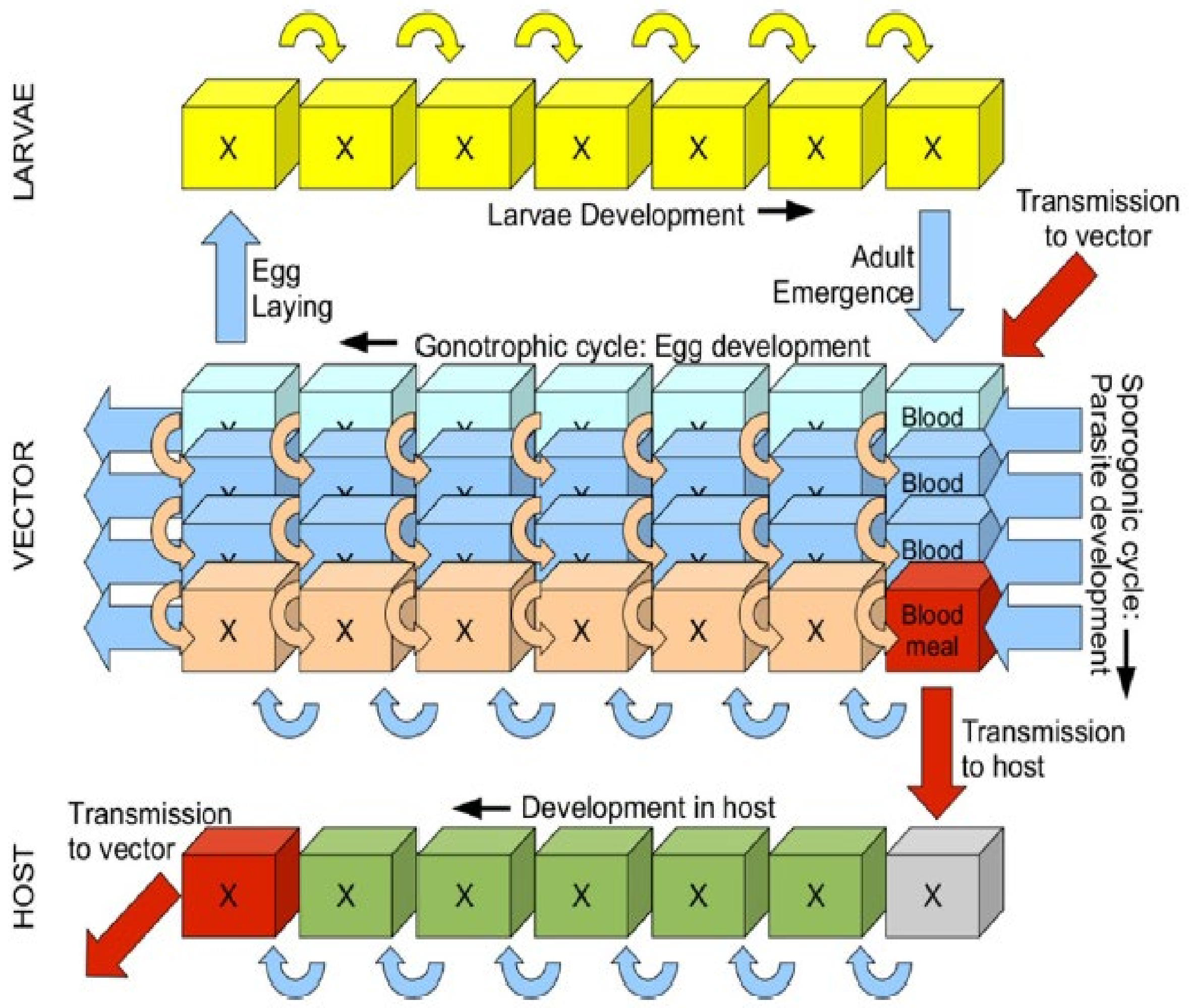
2.2. VECTRI Malaria Model
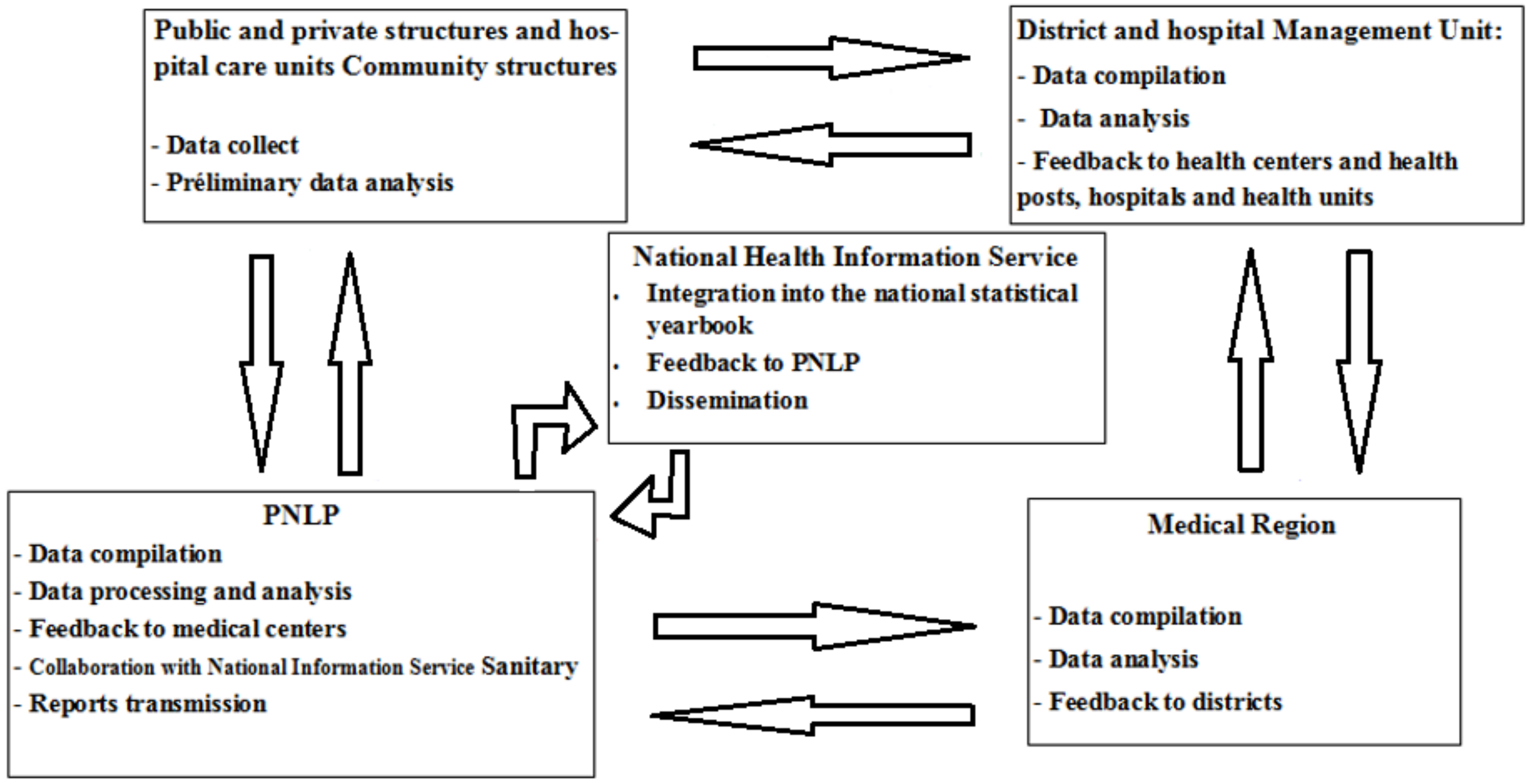
2.3. Malaria Data Surveillance
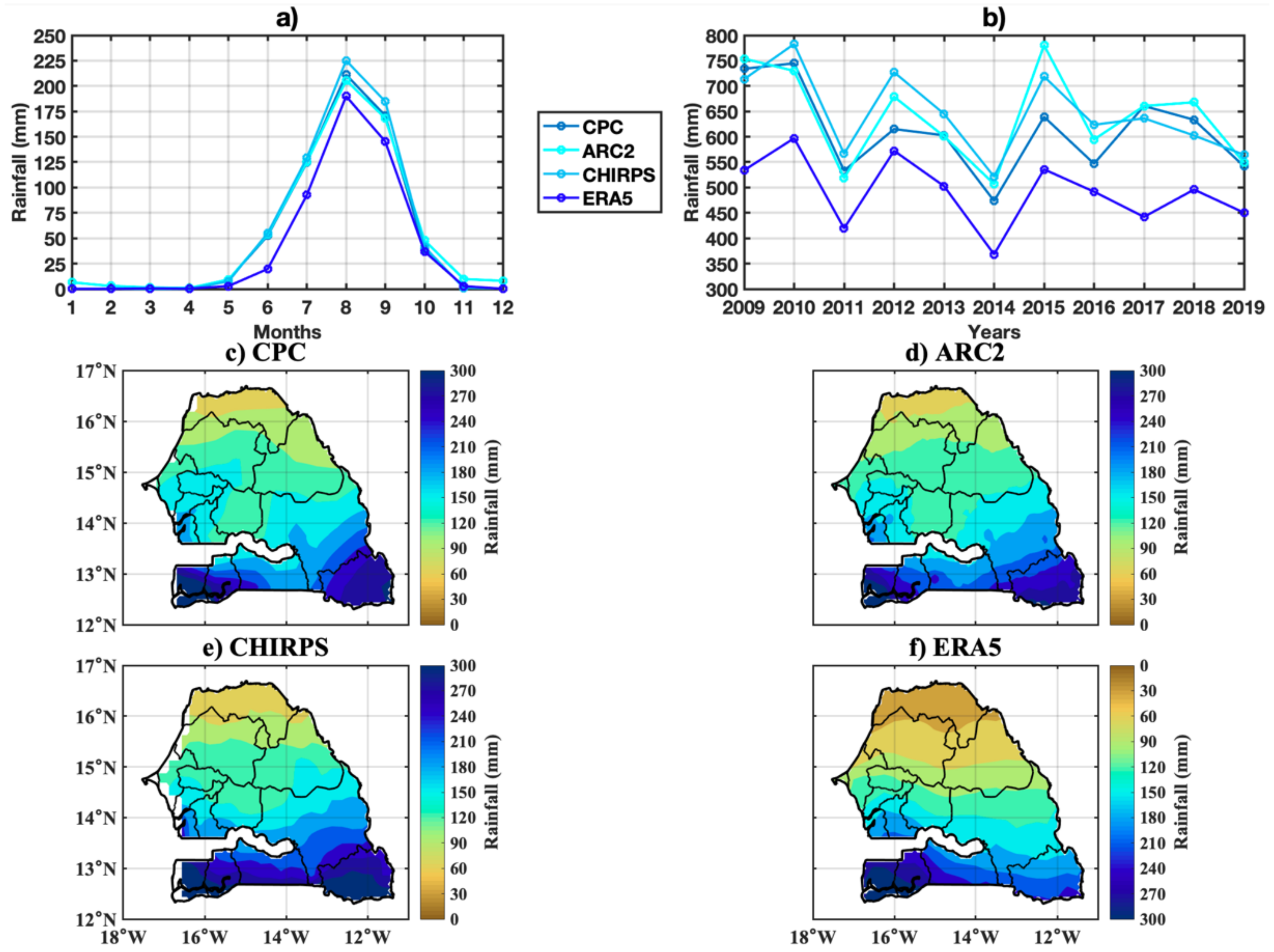
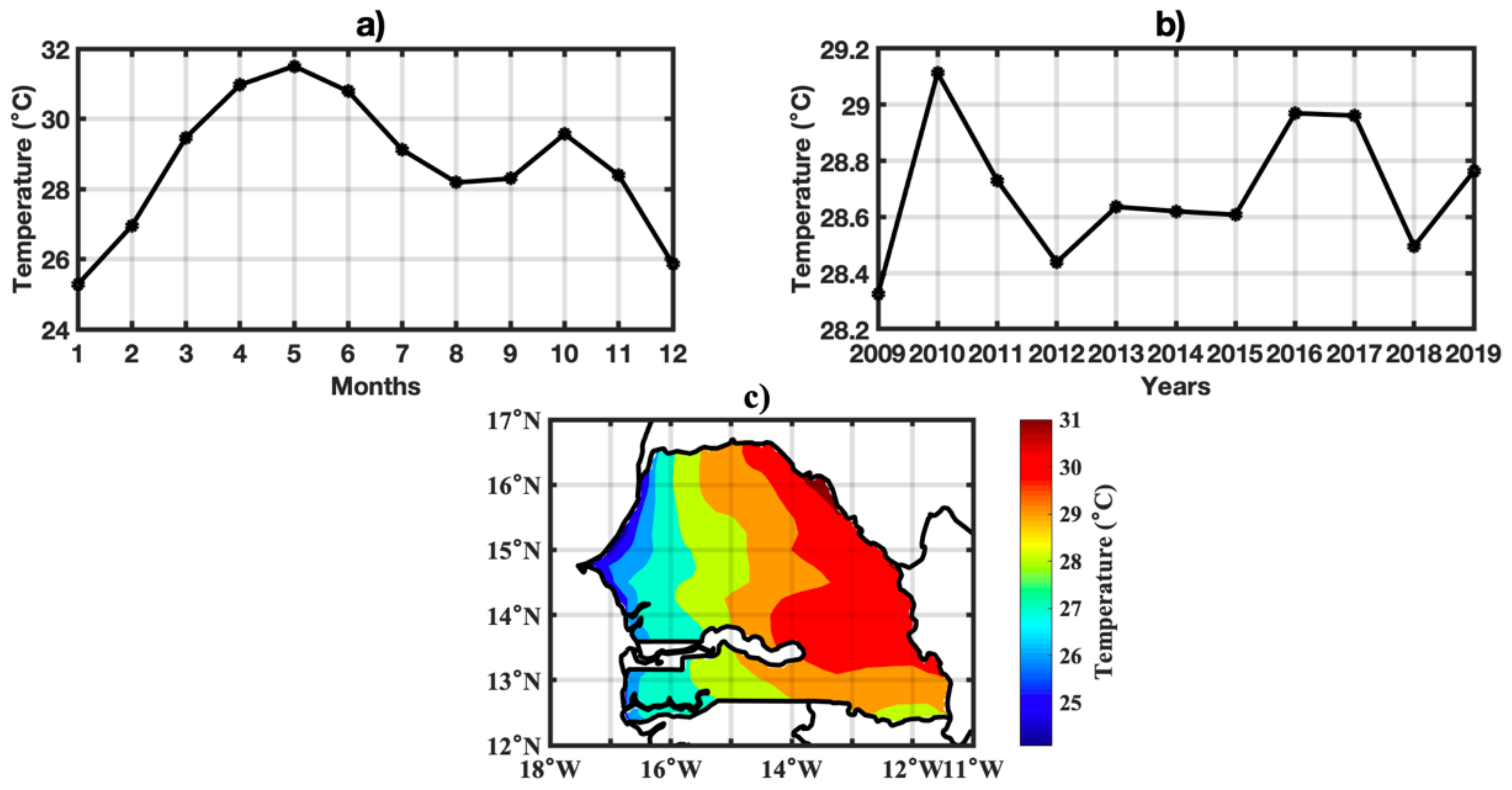
2.4. Climate Dataset
2.4.1. ERA5-Land
2.4.2. CHIRPS
2.4.3. ARC2
2.4.4. CPC
3. Results and Discussion
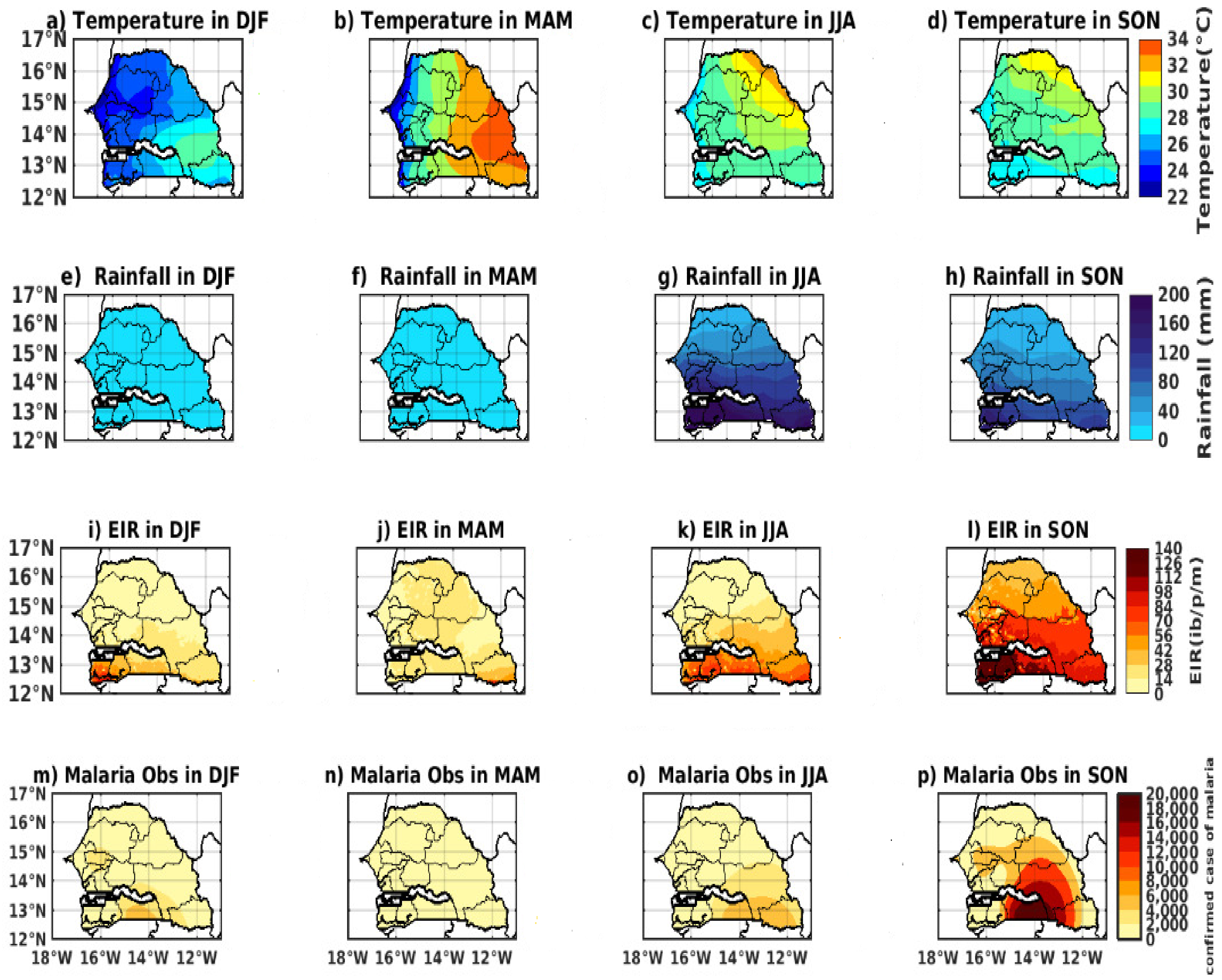
3.1. Spatio-Temporal Variability of Rainfall and Temperature over Senegal
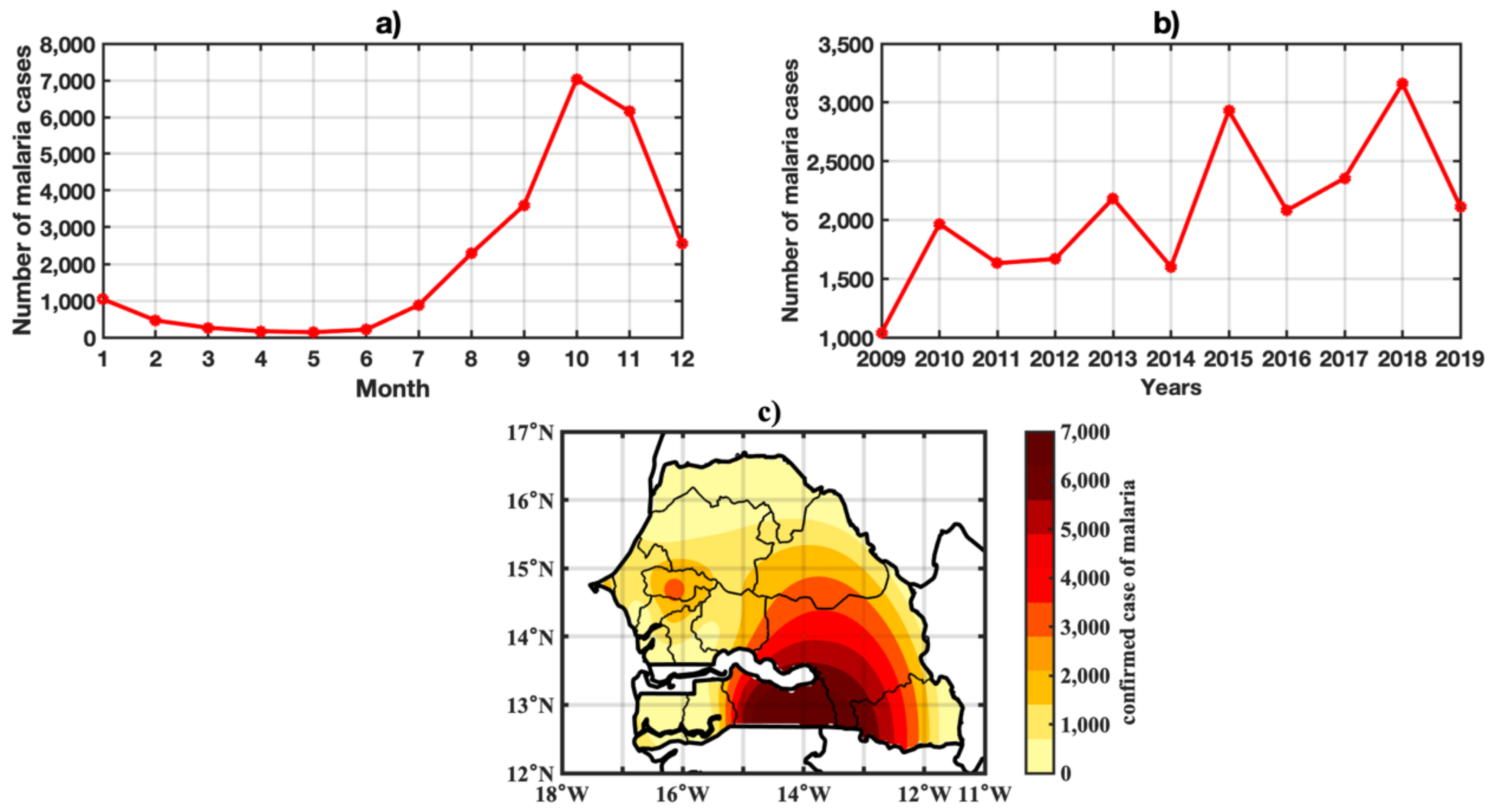
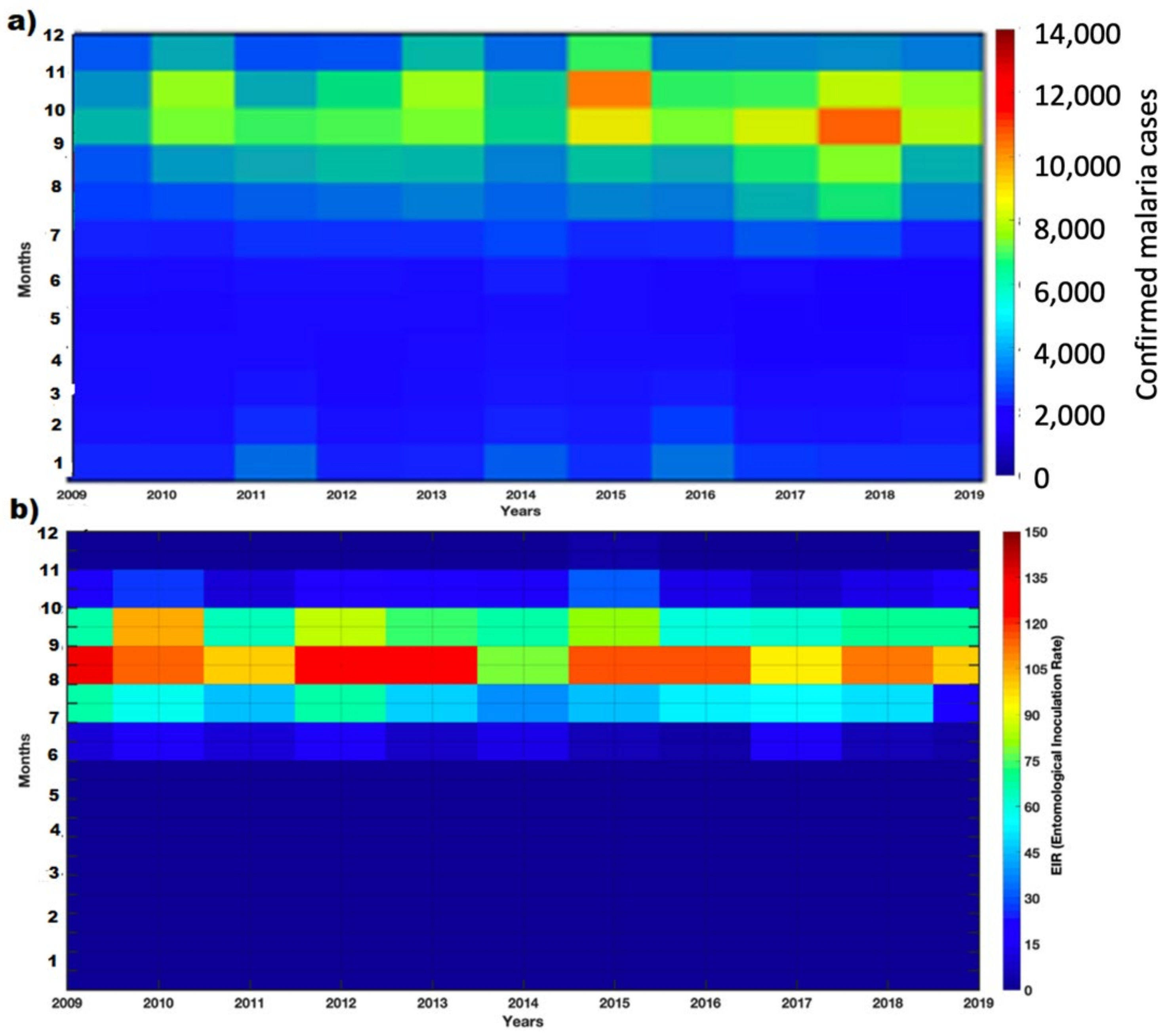
3.2. Spatio-Temporal Variability of Observed Malaria Cases
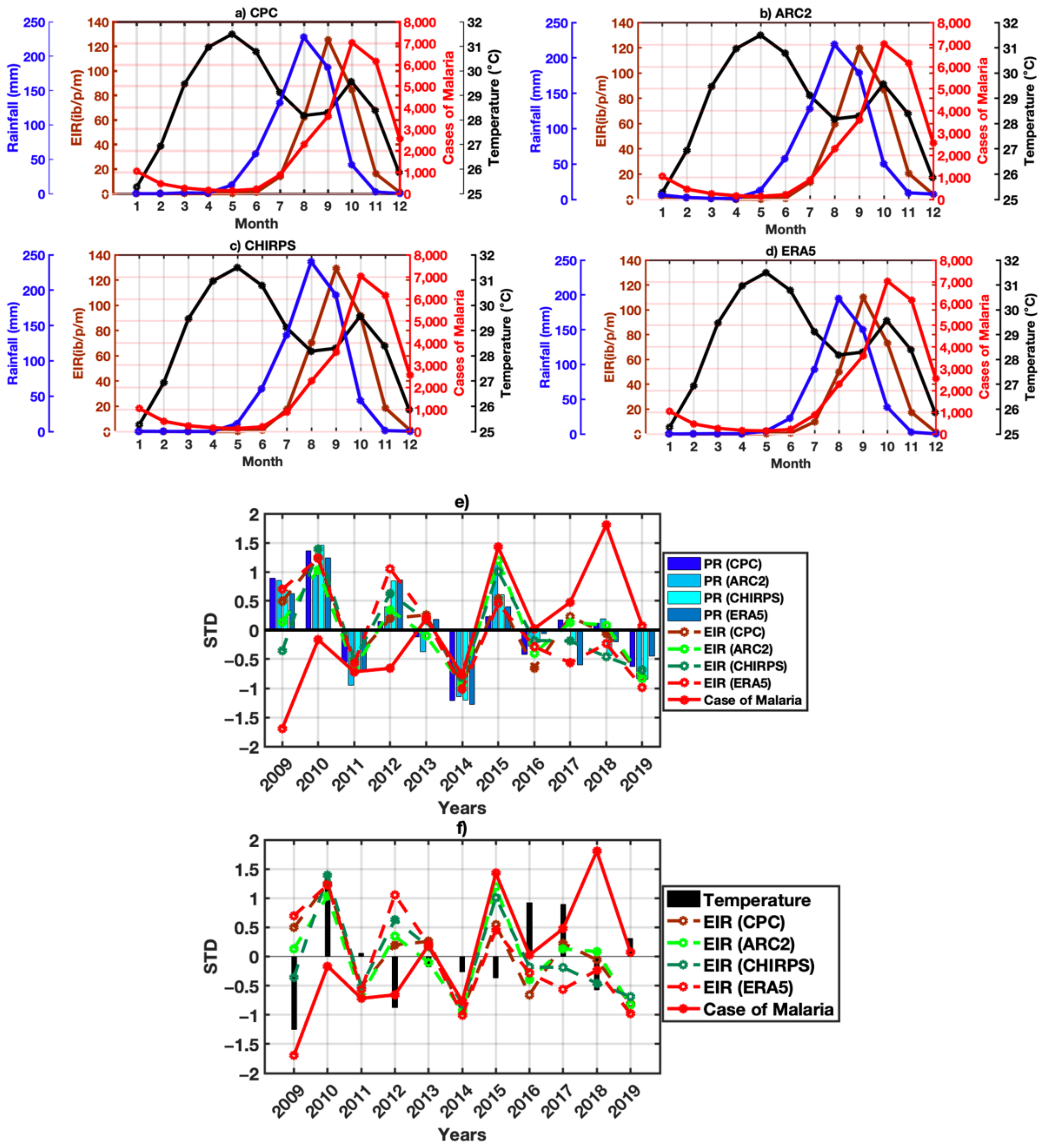
3.3. Relationship between Malaria and Climate Parameters
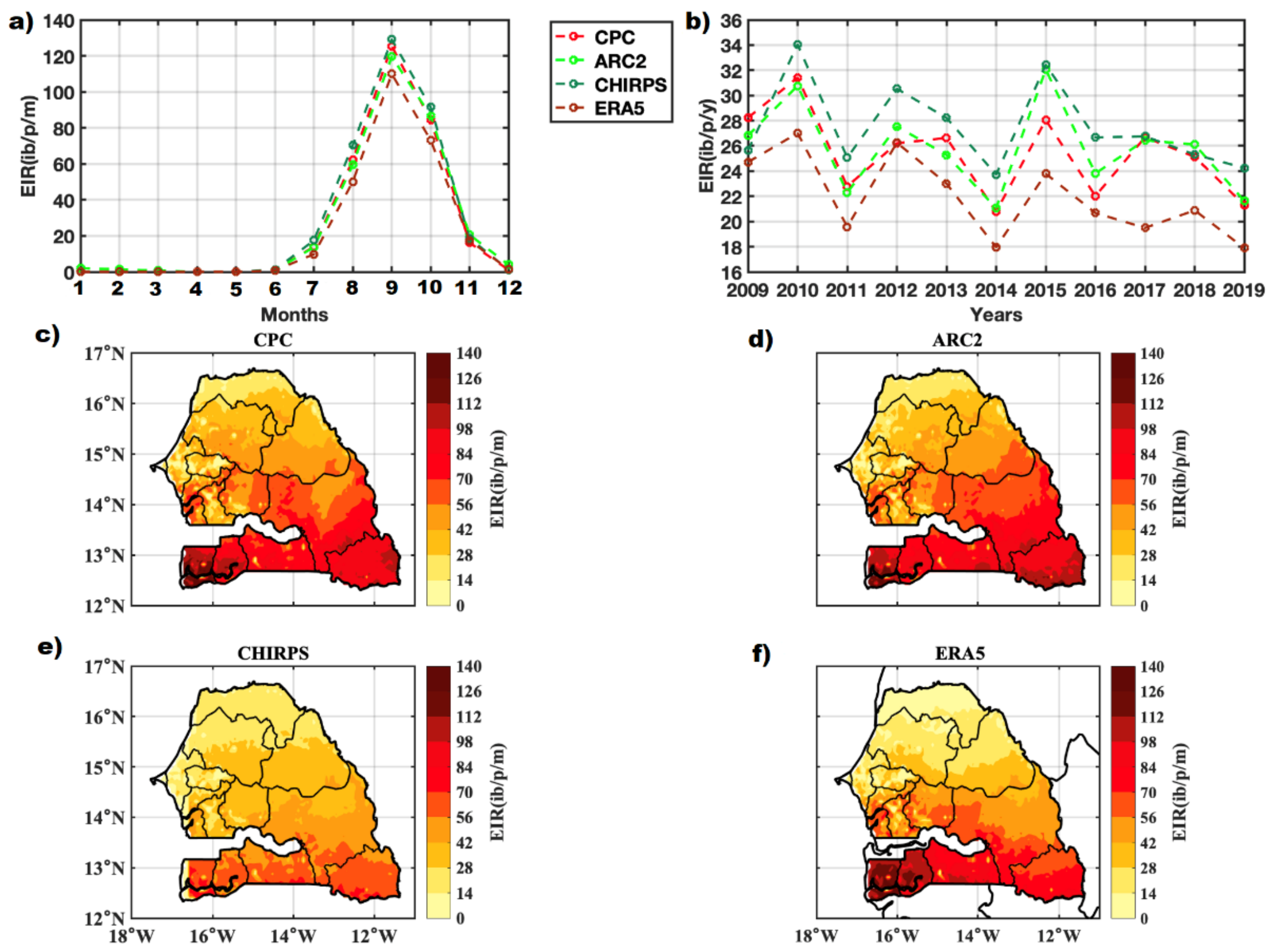
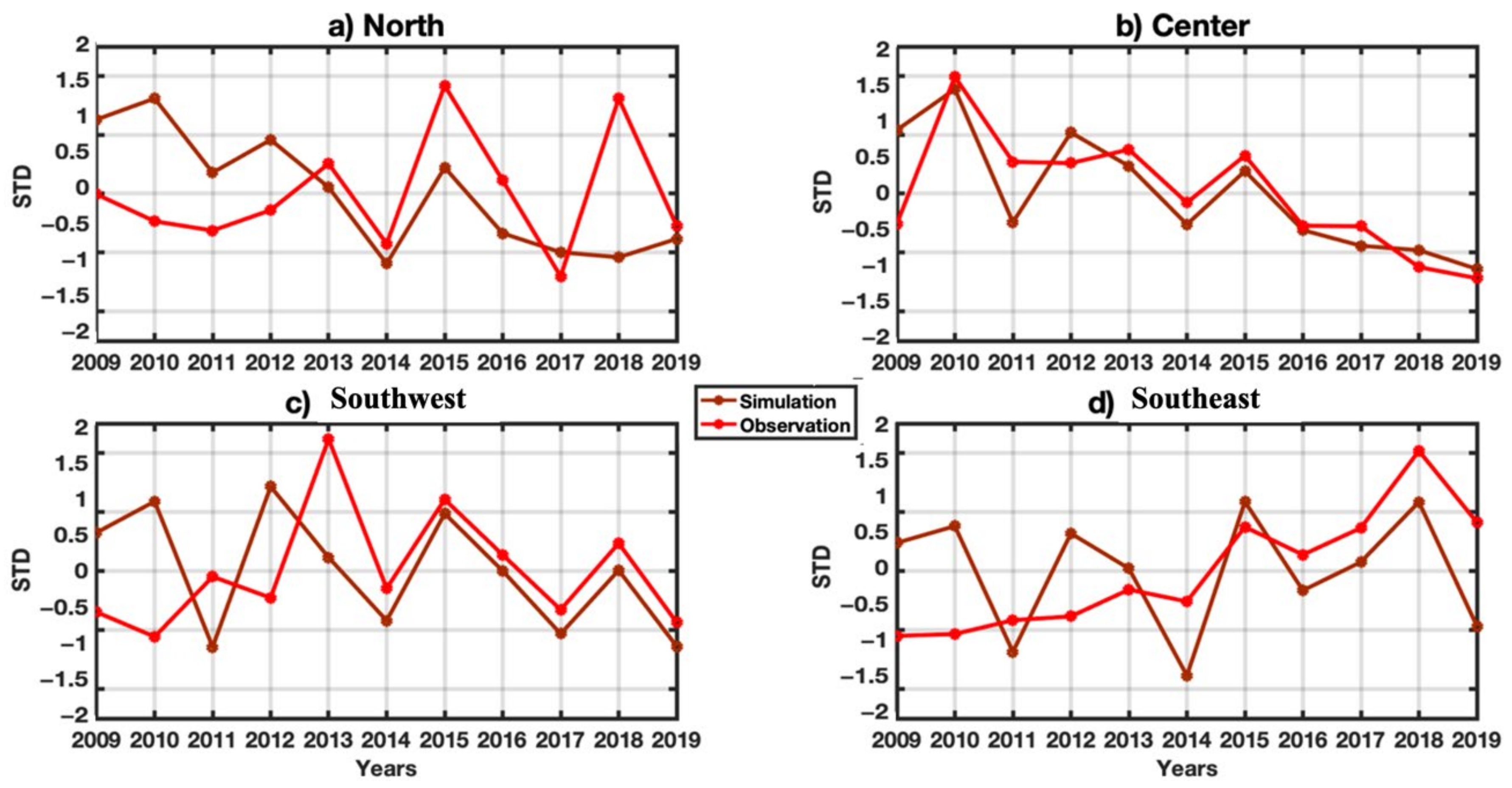
3.4. VECTRI Performance with Observed Malaria Cases
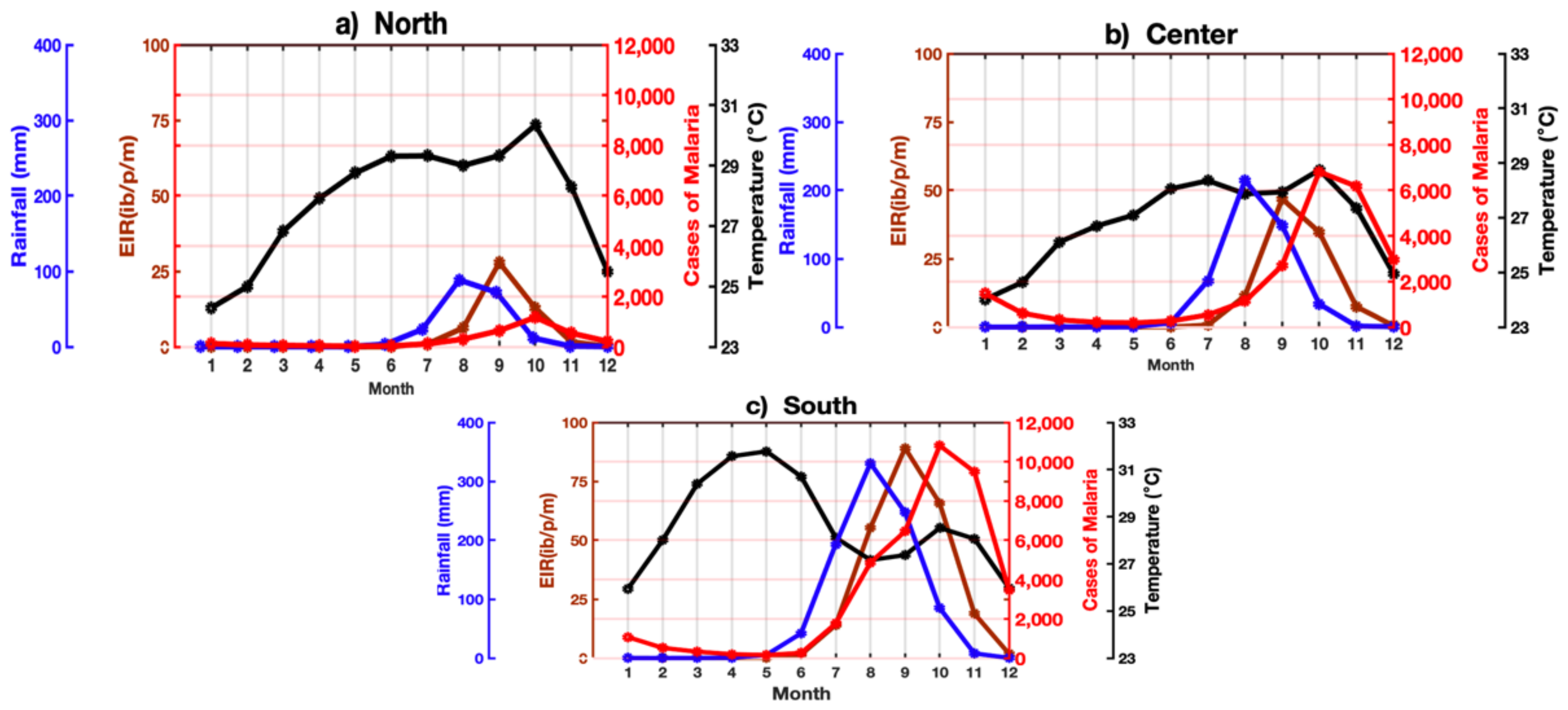
3.5. Malaria and Climate Parameters by Agro-Climatic Zones
3.5.1. Annual Cycle
3.5.2. Seasonal Study of Malaria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Elipe, A.; Otero, A.; van Herp, M.; Aguirre-Jaime, A. Forecasting Malaria Incidence Based on Monthly Case Reports and Environmental Factors in Karuzi, Burundi, 1997–2003. Malar. J. 2007, 6, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachs, J.; Malaney, P. The Economic and Social Burden of Malaria. Nature 2002, 415, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, P.; Robert, V. (Eds.) Les Anophèles: Biologie, Transmission du Plasmodium et Lutte Antivectorielle; Didactiques, IRD Éditions: Marseille, France, 2017; ISBN 978-2-7099-2283-8. [Google Scholar]
- Laporta, G.Z.; Linton, Y.-M.; Wilkerson, R.C.; Bergo, E.S.; Nagaki, S.S.; Sant’Ana, D.C.; Sallum, M.A.M. Malaria Vectors in South America: Current and Future Scenarios. Parasites Vectors 2015, 8, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diouf, I.; Rodriguez Fonseca, B.; Caminade, C.; Thiaw, W.M.; Deme, A.; Morse, A.P.; Ndione, J.-A.; Gaye, A.T.; Diaw, A.; Ndiaye, M.K.N. Climate Variability and Malaria over West Africa. Am. J. Trop. Med. Hyg. 2020, 102, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.J.E.; Walter, K.S.; Wesolowski, A.; Buckee, C.O.; Shevliakova, E.; Tatem, A.J.; Boos, W.R.; Weinberger, D.M.; Pitzer, V.E. Identifying Climate Drivers of Infectious Disease Dynamics: Recent Advances and Challenges Ahead. Proc. R. Soc. B. 2017, 284, 20170901. [Google Scholar] [CrossRef]
- Patz, J.A.; Campbell-Lendrum, D.; Holloway, T.; Foley, J.A. Impact of Regional Climate Change on Human Health. Nature 2005, 438, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E. Changement climatique et maladies à transmission vectorielle: Une analyse régionale. Bull. De L’Organ. Mond. De La Santé La Rev. Int. De Santé Publique Recl. D’Artic. 2001, 4, 62–72. [Google Scholar]
- Tantchou, J.C.; Ndoye, T. Ndoye Tidiane, 2009, La société sénégalaise face au paludisme. Politiques, savoirs et acteurs. Dakar-Paris, Crepos & Karthala. J. Afr. 2011, 245–247. [Google Scholar] [CrossRef]
- World Meteorological Organization; United Nations Environment Programme; Swiatowa Organizacja Zdrowia. Changement Climatique et Santé Humaine—Risques et Mesures à Prendre: Résumé; Organisation Mondiale de la Santé Genève: Geneva Switzerland, 2004; ISBN 978-92-4-259081-4. [Google Scholar]
- Pierrat, C. Risque palustre: Appréhender la vulnérabilité des individus à l’échelle locale (Sud du Bénin). VertigO-La Rev. Électronique En Sci. De L’environ. 2012, 11. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Paaijmans, K.P.; Johnson, L.R.; Balzer, C.; Ben-Horin, T.; de Moor, E.; McNally, A.; Pawar, S.; Ryan, S.J.; Smith, T.C.; et al. Optimal Temperature for Malaria Transmission Is Dramatically Lower than Previously Predicted. Ecol. Lett. 2013, 16, 22–30. [Google Scholar] [CrossRef]
- Smith, D.L.; Ellis McKenzie, F. Statics and Dynamics of Malaria Infection in Anopheles Mosquitoes. Malar. J. 2004, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Hoshen, M.B.; Morse, A.P. A Weather-Driven Model of Malaria Transmission. Malar. J. 2004, 3, 32. [Google Scholar] [CrossRef] [Green Version]
- Yamana, T.K.; Eltahir, E.A. Early Warnings of the Potential for Malaria Transmission in Rural Africa Using the Hydrology, Entomology and Malaria Transmission Simulator (HYDREMATS). Malar. J. 2010, 9, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndiaye, O.; Goddard, L.; Ward, M.N. Using Regional Wind Fields to Improve General Circulation Model Forecasts of July-September Sahel Rainfall. Int. J. Climatol. 2009, 29, 1262–1275. [Google Scholar] [CrossRef]
- Ngom, D.; Fall, T.; Sarr, O.; Diatta, S.; Akpo, L.E. Caractéristiques écologiques du peuplement ligneux de la réserve de biosphère du Ferlo (Nord Sénégal). J. App. Biosci. 2013, 65. [Google Scholar] [CrossRef] [Green Version]
- Besancenot, J.-P.; Handschumacher, P.; Ndione, J.-A.; Mbaye, I.; Laaidi, K. Climat, eau et santé au Sahel ouest-africain. Sci. Et Changements Planétaires/Sécheresse 2004, 15, 233–241. [Google Scholar]
- Mabaso, M.L.H.; Smith, T.; Ross, A.; Craig, M. Environmental predictors of the seasonality of malaria transmission in africa: The challenge. Am. J. Trop. Med. Hyg. 2007, 76, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, C.G. Seasonality of precipitation in the United States. Ann. Assoc. Am. Geogr. 1970, 60, 593–597. [Google Scholar] [CrossRef]
- Reiner, R.C., Jr.; Le Menach, A.; Kunene, S.; Ntshalintshali, N.; Hsiang, M.S.; Perkins, T.A.; Greenhouse, B.; Tatem, A.J.; Cohen, J.M.; Smith, D.L. Mapping Residual Transmission for Malaria Elimination. eLife 2015, 4, e09520. [Google Scholar] [CrossRef] [PubMed]
- Afrane, Y.A.; Githeko, A.K.; Yan, G. The Ecology of Anopheles Mosquitoes under Climate Change: Case Studies from the Effects of Environmental Changes in East Africa Highlands. Ann. N. Y. Acad. Sci. 2012, 1249, 204–210. [Google Scholar] [CrossRef]
- Appawu, M.; Owusu-Agyei, S.; Dadzie, S.; Asoala, V.; Anto, F.; Koram, K.; Rogers, W.; Nkrumah, F.; Hoffman, S.L.; Fryauff, D.J. Malaria Transmission Dynamics at a Site in Northern Ghana Proposed for Testing Malaria Vaccines. Trop. Med. Int. Health 2004, 9, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Fontenille, D.; Lochouarn, L.; Diatta, M.; Sokhna, C.; Dia, I.; Diagne, N.; Lemasson, J.-J.; Ba, K.; Tall, A.; Rogier, C.; et al. Four Years’ Entomological Study of the Transmission of Seasonal Malaria in Senegal and the Bionomics of Anopheles Gambiae and A. Arabiensis. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 647–652. [Google Scholar] [CrossRef]
- Shililu, J.; Ghebremeskel, T.; Mengistu, S.; Fekadu, H.; Zerom, M.; Mbogo, C.; Githure, J.; Novak, R.; Brantly, E.; Beier, J.C. High Seasonal Variation in Entomologic Inoculation Rates in Eritrea, a Semi-Arid Region of Unstable Malaria in Africa. Am. J. Trop. Med. Hyg. 2003, 69, 607–613. [Google Scholar] [CrossRef]
- Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Vittor, A.Y.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of anopheles darlingi, the primary vector of falciparum malaria in the peruvian amazon. Am. J. Trop. Med. Hyg. 2006, 74, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Kelly-Hope, L.A.; McKenzie, F.E. The Multiplicity of Malaria Transmission: A Review of Entomological Inoculation Rate Measurements and Methods across Sub-Saharan Africa. Malar. J. 2009, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tompkins, A.M.; Ermert, V. A Regional-Scale, High Resolution Dynamical Malaria Model That Accounts for Population Density, Climate and Surface Hydrology. Malar. J. 2013, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, M.H.; Snow, R.W.; le Sueur, D. A Climate-Based Distribution Model of Malaria Transmission in Sub-Saharan Africa. Parasitol. Today 1999, 15, 105–111. [Google Scholar] [CrossRef]
- Tompkins, A.M.; Thomson, M.C. Uncertainty in Malaria Simulations in the Highlands of Kenya: Relative Contributions of Model Parameter Setting, Driving Climate and Initial Condition Errors. PLoS ONE 2018, 13, e0200638. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, A.M.; Colón-González, F.J.; Di Giuseppe, F.; Namanya, D.B. Dynamical Malaria Forecasts Are Skillful at Regional and Local Scales in Uganda up to 4 Months Ahead. GeoHealth 2019, 3, 58–66. [Google Scholar] [CrossRef]
- Karypidou, M.C.; Almpanidou, V.; Tompkins, A.M.; Mazaris, A.D.; Gewehr, S.; Mourelatos, S.; Katragkou, E. Projected Shifts in the Distribution of Malaria Vectors Due to Climate Change. Clim. Chang. 2020, 163, 2117–2133. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dwivedi, S. Understanding the Effect of Climate Change in the Distribution and Intensity of Malaria Transmission over India Using a Dynamical Malaria Model. Int. J. Biometeorol. 2021, 65, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Diouf, I.; Rodriguez-Fonseca, B.; Deme, A.; Caminade, C.; Morse, A.; Cisse, M.; Sy, I.; Dia, I.; Ermert, V.; Ndione, J.-A.; et al. Comparison of Malaria Simulations Driven by Meteorological Observations and Reanalysis Products in Senegal. Ijerph 2017, 14, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 Global Reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Novella, N.S.; Thiaw, W.M. African Rainfall Climatology Version 2 for Famine Early Warning Systems. J. Appl. Meteorol. Climatol. 2013, 52, 588–606. [Google Scholar] [CrossRef]
- Chen, M.; Shi, W.; Xie, P.; Silva, V.B.S.; Kousky, V.E.; Wayne Higgins, R.; Janowiak, J.E. Assessing Objective Techniques for Gauge-Based Analyses of Global Daily Precipitation. J. Geophys. Res. 2008, 113, D04110. [Google Scholar] [CrossRef]
- Bodian, A.; Diop, L.; Panthou, G.; Dacosta, H.; Deme, A.; Dezetter, A.; Ndiaye, P.M.; Diouf, I.; Vischel, T. Recent Trend in Hydroclimatic Conditions in the Senegal River Basin. Water 2020, 12, 436. [Google Scholar] [CrossRef] [Green Version]
- Caminade, C.; Jones, A.E. Malaria in a Warmer West Africa. Nat. Clim. Chang. 2016, 6, 984–985. [Google Scholar] [CrossRef]
- Deme, A.; Gaye, A.T.; Hourdin, F. Chapter 3. Climate Projections in West Africa: The Obvious and the Uncertain. In Rural Societies in the Face of Climatic and Environmental Changes in West Africa; Sultan, B., Lalou, R., Sanni, M.A., Oumarou, A., Arame Soumaré, M., Eds.; IRD Éditions: Marseille, France, 2017; pp. 61–86. ISBN 978-2-7099-2424-5. Available online: https://books.openedition.org/irdeditions/12325 (accessed on 27 December 2021).
- Barbier, J.; Guichard, F.; Bouniol, D.; Couvreux, F.; Roehrig, R. Detection of Intraseasonal Large-Scale Heat Waves: Characteristics and Historical Trends during the Sahelian Spring. J. Clim. 2018, 31, 61–80. [Google Scholar] [CrossRef]
- Diouf, I.; Deme, A.; Ndione, J.-A.; Gaye, A.T.; Rodríguez-Fonseca, B.; Cissé, M. Climate and Health: Observation and Modeling of Malaria in the Ferlo (Senegal). Comptes Rendus Biol. 2013, 336, 253–260. [Google Scholar] [CrossRef]
- M’Bra, R.K.; Kone, B.; Soro, D.P.; N’krumah, R.T.A.S.; Soro, N.; Ndione, J.A.; Sy, I.; Ceccato, P.; Ebi, K.L.; Utzinger, J.; et al. Impact of Climate Variability on the Transmission Risk of Malaria in Northern Côte d’Ivoire. PLoS ONE 2018, 13, e0182304. [Google Scholar] [CrossRef]
- Laneri, K.; Bhadra, A.; Ionides, E.L.; Bouma, M.; Dhiman, R.C.; Yadav, R.S.; Pascual, M. Forcing Versus Feedback: Epidemic Malaria and Monsoon Rains in Northwest India. PLoS Comput. Biol. 2010, 6, e1000898. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hill, A.V.S. Dynamic Interactions in Malaria: Host Heterogeneity Meets Parasite Polymorphism. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1995, 261, 271–277. [Google Scholar] [CrossRef]
- Kapwata, T.; Wright, C.Y.; du Preez, D.J.; Kunene, Z.; Mathee, A.; Ikeda, T.; Landman, W.; Maharaj, R.; Sweijd, N.; Minakawa, N.; et al. Exploring Rural Hospital Admissions for Diarrhoeal Disease, Malaria, Pneumonia, and Asthma in Relation to Temperature, Rainfall and Air Pollution Using Wavelet Transform Analysis. Sci. Total Environ. 2021, 791, 148307. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal Biology of Mosquito-borne Disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef] [Green Version]
- Adewi, E. Variabilité Climatique et Paludisme a Kara, une Ville du Nord-Togo. 2012, p. 7. Available online: https://halshs.archives-ouvertes.fr/halshs-00730728 (accessed on 27 December 2021).
- Abiodun, G.J.; Maharaj, R.; Witbooi, P.; Okosun, K.O. Modelling the Influence of Temperature and Rainfall on the Population Dynamics of Anopheles Arabiensis. Malar. J. 2016, 15, 364. [Google Scholar] [CrossRef] [Green Version]
- Sultan, B.; Janicot, S. The West African Monsoon Dynamics. Part II: The “Preonset” and “Onset” of the Summer Monsoon. J. Clim. 2003, 16, 3407–3427. [Google Scholar] [CrossRef]
- Ryan, S.J.; McNally, A.; Johnson, L.R.; Mordecai, E.A.; Ben-Horin, T.; Paaijmans, K.; Lafferty, K.D. Mapping Physiological Suitability Limits for Malaria in Africa Under Climate Change. Vector Borne Zoonotic Dis. 2015, 15, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Altizer, S.; Dobson, A.; Hosseini, P.; Hudson, P.; Pascual, M.; Rohani, P. Seasonality and the Dynamics of Infectious Diseases. Ecol. Lett. 2006, 9, 467–484. [Google Scholar] [CrossRef] [Green Version]
- Asare, E.; Amekudzi, L. Assessing Climate Driven Malaria Variability in Ghana Using a Regional Scale Dynamical Model. Climate 2017, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- The Lancet A Commission on Climate Change. Lancet 2009, 373, 1659. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(09)60922-3/fulltext#articleInformation (accessed on 27 December 2021). [CrossRef]


| Clinical Datasets | Period | Region |
|---|---|---|
| Malaria Cases (observations) | 2009–2019 | 1. Dakar, 2. Diourbel, 3. Fatick, 4. Kaffrine, 5. Kaolack, 6. Kedougou, 7. Kolda, 8. Louga, 9. Matam, 10. Saint-Louis, 11. Sedhiou, 12. Tambacounda, 13. Thiès, 14. Ziguinchor |
| Climate Datasets | Period | Grid |
| ERA5 (rainfall and temperature) | 2009–2019 | 0.25° × 0.25° (25 km × 25 km) |
| CHIRPS (rainfall) | 2009–2019 | 0.05° × 0.05° (5 km × 5 km) |
| ARC2 (rainfall) | 2009–2019 | 0.1° × 0.1° (10 km × 10 km) |
| CPC (rainfall) | 2009–2019 | 0.25° × 0.25° (25 km × 25 km) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fall, P.; Diouf, I.; Deme, A.; Sene, D. Assessment of Climate-Driven Variations in Malaria Transmission in Senegal Using the VECTRI Model. Atmosphere 2022, 13, 418. https://doi.org/10.3390/atmos13030418
Fall P, Diouf I, Deme A, Sene D. Assessment of Climate-Driven Variations in Malaria Transmission in Senegal Using the VECTRI Model. Atmosphere. 2022; 13(3):418. https://doi.org/10.3390/atmos13030418
Chicago/Turabian StyleFall, Papa, Ibrahima Diouf, Abdoulaye Deme, and Doudou Sene. 2022. "Assessment of Climate-Driven Variations in Malaria Transmission in Senegal Using the VECTRI Model" Atmosphere 13, no. 3: 418. https://doi.org/10.3390/atmos13030418
APA StyleFall, P., Diouf, I., Deme, A., & Sene, D. (2022). Assessment of Climate-Driven Variations in Malaria Transmission in Senegal Using the VECTRI Model. Atmosphere, 13(3), 418. https://doi.org/10.3390/atmos13030418







