Abstract
The composition of airborne microflora in sawmills may vary to a great degree depending on the kind of timber being processed and the technology of production being used. Cases of allergy alveolitis and asthma have been reported in woodworkers who were exposed to wood dust largely infected with microorganisms. The aim of this review article is to identify studies where the microbial occupational exposure assessment was performed in sawmills and the characteristics of the contamination found, as well as to identify which sampling methods and assays were applied. This study reports the search of available data published regarding microbial occupational exposure assessment in environmental samples from sawmills, following the Preferred Reporting Items for Systematic Reviews (PRISMA) methodology. The most used sampling method was air sampling, impaction being the most common method. Regarding analytical procedures for microbial characterization, morphological identification of fungi and bacteria was the most frequent approach. Screening for fungal susceptibility to azoles was performed in two studies and four studies applied molecular tools. Regarding microbial contamination, high fungal levels were frequent, as well as high bacteria levels. Fungal identification evidenced Penicillium as the most frequent genera followed by Aspergillus sp. Mycotoxins were not assessed in any of the analyzed studies. Microbial occupational exposure assessment in sawmills is crucial to allow this risk characterization and management.
1. Introduction
Globally, the sawmill market is primarily driven by rising construction demand, which accounts for roughly 73.48 percent of total downstream consumption of sawmill in the world. Softwood and hardwood are the two types of sawmill raw materials. Its downstream use is diverse, and recently, building and furniture have gained prominence in a variety of sawmill areas [1].
Workers in sawmill industry may be exposed to allergic, carcinogenic, and immunotoxic agents, comprising wood derivatives (e.g., terpenes, resin acids) as well microorganisms that grow on timber (bacteria and fungi) and their products (endotoxins and mycotoxins) known as potential causative agents of health effects [2,3,4,5,6,7,8]. Exposure can result in decreased lung function, bronchial hyperresponsiveness, and a variety of disorders such as organic dust toxic syndrome (ODTS), allergic alveolitis, asthma, chronic bronchitis, rhinitis, mucous membrane irritation (MMI), contact dermatitis, and nasal cancer [9,10,11,12,13,14,15,16,17,18]. The majority of the negative effects generated by microorganisms linked with wood dust have an immunological basis. The most well-known are those produced by fungi, which may thrive in the right conditions on stored wood products (planks, chips) as a secondary wood infection [18].
Inhaling large amounts of spores and mycelial fragments of Aspergillus sp., Penicillium sp., Rhizopus sp., Paecilomyces sp., Mucor sp. and other fungi can result in a strong antibody response and respiratory disorders, most commonly allergic alveolitis (wood trimmer’s disease) or organic dust toxic syndrome in exposed workers [18,19,20,21,22,23,24,25,26,27]. Cases of allergy alveolitis and asthma have been reported in woodworkers who were exposed to wood dust largely infected with fungi during logging, debarking, and sawing tasks [18].
The composition of airborne microflora in sawmills may vary to a great degree depending on the kind of timber being processed and the technology of production [8,15,16,28]. In wood processing, preservation, and maintenance azole fungicides are used for the protection of spruce and pine fields [28,29]. To protect wood from wood-destroying basidiomycete fungus, sawmills, particularly those working with resinous timbers, typically use azole fungicides. This fungus can induce deterioration or blueing of wood, rendering it useless [28,30]. Propiconazole and tebuconazole are the most common azole compounds found in sawmills. In fact, these two compounds are among the five 14-demethylase inhibitors (DMIs) linked to clinical azoles and contributing to the rise in azole antifungal resistance [28,30,31,32]. Furthermore, Aspergillus section Fumigati azole antifungal resistance was already reported in this environment [28,29].
Portugal’s social and economic history is inextricably related to the products of the forest, where national economic organizations are world leaders in the production and trading of forest products [33]. Regarding the sawmill industry in Portugal, 2250 million euros were made with exportations in 2020, there were 8700 companies reported in the wood industry in 2019 and, consequently, about 56,000 workers account for this sector workforce [34].
Due to the lack of studies in Portuguese sawmills this study aimed to perform a systematic review to provide a broad overview of the state of art in the developed subject, describing the microbiological contamination reported in previous studies developed in sawmills and indicating which parameters and methods were applied to perform the microbial occupational exposure assessment in this setting. These study results will contribute to a sampling and analyses protocol proposal aiming to assess the occupational exposure to microbial contamination is this specific occupational environment.
2. Materials and Methods
2.1. Registration
The Preferred Reporting Items for Systematic Reviews (PRISMA) checklist [35] was completed (Supplementary Materials Table S1).
2.2. Search Strategy, Inclusion and Exclusion Criteria
This study reports the search of available data published between the period of 1 January 2000 and 30 September 2021. The search terms aimed to identify studies in microbial occupational exposure assessments, selecting studies on sawmills that included the terms “occupational exposure”, “sawmills”, with English as the chosen language. The databases chosen were PubMed, Scopus, Web of Science (WoS) and other sources, following the PRISMA methodology. This search strategy identified 441 papers in all databases. Articles that did not fulfil the inclusion criteria were not subjected to additional review (but some of them were used for introduction and discussion sections) (Table 1).

Table 1.
Inclusion and exclusion criteria in the articles selected.
2.3. Studies Selection and Data Extraction
The selection of the articles was performed through Rayyan, which is a free web-tool that greatly speeds up the process of screening and selecting papers for academics working on systematic reviews, in three rounds by three investigators (MD, BG, and RC). The first round consisted of a screening of all titles to exclude papers that were duplicated or unrelated to the subject, and then the included added to Rayyan for further analysis. The second round consisted of a screening of all abstracts. In the third round, the full texts of all potentially relevant studies were reviewed considering the inclusion and exclusion criteria. Potential divergences in the selection of the study were discussed and ultimately resolved by the remaining investigators (CV and SV). Data extraction was performed by two investigators (BG and RG) and reviewed by another (MD). The following information was manually extracted: (1) Database, (2) Title, (3) Country, (4) Occupational Environment, (5) Sampling Methods, (6) Analytical Methods, (7) Main Findings, and (8) References.
2.4. Quality Assessment
The assessment of the risk of bias was performed by two investigators (MD and CV). Within each study, we evaluated the risk of bias across three parameters divided as key criteria (Sampling Methods, Analytical Methods) and other criteria (data about metabolites). The risk of bias for each parameter was evaluated as “low”, “medium”, “high”, or “not applicable”. The studies for which all the key criteria and most of the other criteria are characterized as “high” were excluded.
3. Results
The flow diagram for selecting studies is shown in Figure 1. The initial database search yielded 441 studies, from which 133 abstracts were examined and 40 full texts were evaluated for eligibility. A total of 18 studies were rejected after examining the inclusion and exclusion criteria, primarily because they were related to biological samples collected from the sawmill workers. A total of 23 papers on microbial occupational exposure were chosen.
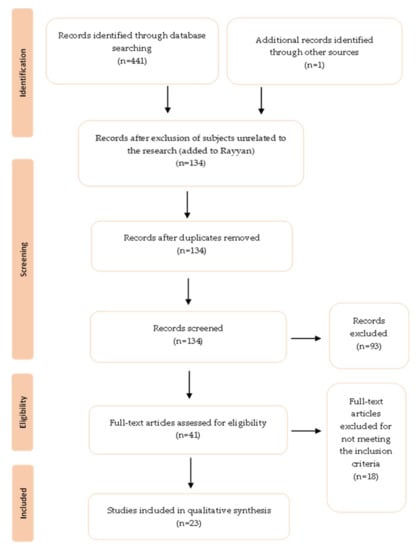
Figure 1.
PRISMA based selection of articles.
Characteristics and Data Obtained in the Selected Studies
Table 2 describes the main characteristics from the selected studies. From the selected studies (N = 23), 15 were conducted in the Europe, namely 5 in Norway [29,36,37,38,39], 4 in Poland [8,40,41,42], 2 in Switzerland [43,44], 2 in Croatia [45,46], 1 in Finland [47], 1 in Italy [48], and 1 in France [30]. Five studies from Canada [49,50,51,52,53], 1 from Korea [54], and 1 from Iran [55] were also analyzed. The majority of studies (15 out of 23–65.2%) analyzed environmental samples from small and medium size sawmills [18,28,36,37,38,39,40,41,42,43,44,45,46,47,48,49,51,52], 2 studies (8.7%) were performed in industrial sawmills [29,39], 2 studies (8.7%) in plywood hardwood processing companies [53], 1 (4.4%) in a manufacturing industry [51], 1 (4.4%) in carpentries [48], 1 (4.4%) in pellet production facilities [42], and 1 (4.4%) in a furniture factory [41].

Table 2.
Data selected from the chosen papers.
The most used sampling method was air sampling (19 out of 23–82.6%) [18,29,36,37,38,39,40,43,44,46,47,49,50,51,52,53,54,55]. Several studies used more than one active sampling method (8 out of 23–34.8%). Air collection through impaction was used in 16 studies (69.6%) [8,40,42,43,44,45,46,49,50,52,53,54,55], followed by filter air sampling in 11 studies (47.8%) [28,29,37,38,41,44,46,48,50,53,54], while 5 studies (21.7%) used the impingement method [29,47,49,50,52].
Passive methods were exclusively performed in 5 papers (21.7%) [28,40,41,47,53]. Dust samples collection was the most frequent methodology applied (N = 3) [28,41,53], one study collected wood samples [40] and the other performed surface samples [53].
Concerning analytical procedures for microbial characterization, 13 studies (56.5%) referred to fungi [28,29,37,38,39,41,45,46,47,48,50,51,53], 1 (4.4%) referred only to bacteria [40], while 9 (39.1%) encompassed fungi and bacteria [8,42,43,44,49,50,52,54,55]. Morphological identification was the most frequent approach. Fungal identification was accomplished through macroscopic and microscopic examination in 16 studies (69.6%) [8,28,29,41,42,43,44,45,46,49,50,51,52,53,54,55]. Regarding bacterial identification, 5 studies (21.7%) used biochemical tests [8,40,42,50,55].
Screening for fungal susceptibility to azoles was performed in 2 studies (8.7%). For the screening of A. fumigatus azole resistance, 1 study (4.4%) used the EUCAST methods [53] and the other used both EUCAST and E-test methods [28].
Molecular tools were applied in 4 studies (17.4%). All performed DNA sequencing [28,29,39,42,55]. High fungal levels were frequent in 6 studies (26.1%) [8,44,45,46,50,54], as well as high bacteria levels in 4 studies (17.4%) [8,43,50,54]. Fungal identification evidence Penicillium as the most frequent genera [41,43,46,47,49,50,52,53,55]. Aspergillus sp. was also recurrent in 4 studies (17.4%) [8,29,42,46]. From all the sampling sites, 3 studies (13%) reported the sorting and green department as having the highest levels of fungal fragments [36,37,38]. Other working sites were also associated with potential microbial exposure as follows: saw departments [36,39], dry timber departments [37], and debarking site [49]. In fact, 7 studies (30.4%) report airborne fungi as potential agents for occupational health effects [8,41,42,44,45,46,50], as well as bacteria in 2 studies (8.7%) [40,56]. In what concerns mycological diversity, 3 studies (13%) report fungal bioaerosols variation between different indoor locations [39,49,51] and 4 studies (17.4%) evidence a significant influence of seasons in fungal aerosol composition [36,38,39,49].
4. Discussion
It is well known that sawmill workers are exposed to wood dust and multiple wood-associated chemicals and microbiota, including fungi [1,2,3,4,30,31]. Fungi and Gram-negative bacteria are major contaminants of wood dust, especially in hot and humid areas. Occupational inhalation exposure to wood dust and its associated bioaerosols (composed by fungi, bacteria, endotoxins, mycotoxins, and much more) has been associated with adverse respiratory effects [5,6,7,8,9]. Health outcomes associated with the inhalation of wood dust have been reported in several studies [5,9,11,12,13,15,16,17,18,19,20] as well as a significant association between inhalation of wood dust and an increased prevalence of respiratory symptoms [13,21,22,23,28] and decreased lung functional capacity [55]. Considering the papers included in this review, most of them (21 out of 23) used air as an environmental matrix, impaction being the most frequent sampling method used (15 out of 23). This sampling approach relies solely on culture-based methods, which can have advantages and disadvantages. The inflammatory and/or cytotoxic potential can affect the microorganism viability [56,57] which makes this method beneficial since it allows us to rely on the microbial composition to draw conclusions regarding the inflammatory potential variation [57,58]. In impaction sampling devices, a specific flow rate (depending on the type of environment) is defined to collect particles [59] by using its inertia to drive deposition on a collection media by promoting particle separation through an air stream [60]. However, since it only allows to evaluate culturable microorganisms, the microbial load can be underestimated, due to the high velocity of the air flow that may result in microorganisms’ cell damage [61,62]. Moreover, it is important to highlight that indoor air is not homogeneous in space or time, it can always change depending on the type and intensity of the activity developed in that space [63]. Therefore, the sampling time must be adequate to the environment in study and work tasks being developed. For example when using high volume samplers in highly contaminated areas, it is crucial to employ short sampling intervals and lower flow rates for airborne fungal sampling [64]. Nevertheless, active sampling methods, namely impaction devices, have already proved to be very useful in the characterization of occupational exposure to fungi in several studies, by presenting the most diversified fungal contamination in comparison with all sampling methods applied [28,51,61,65,66].
Passive sampling methods were also used, even if in a smaller number (3 out of 23 papers, including studies with one or more sampling methods). There is evidence that ventilation, building design, environmental features [67], or water infiltrations and damage [68], geographical location [69], as well as the type of task developed in each working site [36,49] can alter fungi and bacteria found indoors. Different working sites were identified with potential for microbial exposure namely the ones that include sawing and drying, mainly because the cells in hardwood are firmly bonded, and kiln drying renders them less elastic, resulting in cell breakage and tiny airborne dust [70,71].
With so many factors impacting microbial contamination indoors, passive sampling approaches are anticipated to be more reliable than active sampling methods since they can collect contamination over a longer period of time, thus covering all expected fluctuations [72,73]. The passive sampling method used in all three studies was the collection of wood dust, which both acute and chronic exposures may serve as a sensitizer and irritant on the human body, mostly affecting the respiratory system and skin [56].
Several researchers [67,73,74,75,76,77,78] have begun to collect and analyze from indoor environments a similar matrix (settled dust) as part of their microbial contamination exposure assessments. Settled dust reservoirs have been described as having the ability to anticipate microbial levels in indoor air, as well as being more repeatable than active sampling approaches [67]. Furthermore, it has been documented as an environmental support for bacterial development, and is thus regarded as a bacterial contamination reservoir [79].
Considering all the described advantages and disadvantages of both active and passive sampling methods and in order to assess microbial exposure, sampling approaches in occupational environments should comprise more than one type of sampling method [28,29,62,67,73,76]. Furthermore, and as it was seen in one study, settled dust should be included in sampling protocols combined with impaction methods because when these two methods are combined, the sensitivity of the assessment increases, and the impaction samplers’ shortcomings are eliminated [58,80].
The majority of articles (15 out of 23) relied solely on culture-based methods to perform microorganisms’ identification; nevertheless, and as expected, this assay also has its drawbacks that may influence the studies accuracy, such as the specificities of each species (growth rates and requirements), that can affect the other species in a mixed culture. A very common example regarding growth rate, is the overgrowth of some species that limit the growth of other species due to chemical competition [74].
Molecular tools are well known for their features of precision, high analytical sensitivity of detection, speed, and the ability to detect and identify dead or dormant microorganisms, as well as toxigenic strains from microorganisms [58,74,80,81,82]. However, culture-based methods should be used every time that the exposure route is mainly happening by inhalation, due to the reasons addressed before [56,57]. Thus, culture-based methods and molecular tools should be used side by side as it was seen in a few studies (4 out of 23) of this review.
Regarding the contamination present in all studies, as previously mentioned, majority of studies reported airborne fungi as a potential agent for occupational health effects (10 out of 23) since the prevalent genera were Penicillium (9 out 23) and Aspergillus (4 out of 23). Aspergillus sp. can be found everywhere and are easily disseminated in the air. Because the conidia of the Aspergillus genus are so small, they can readily be inhaled and colonize the upper and lower respiratory tracts of those who have been exposed [83,84]. Therefore, and as a consequence of a high exposure to opportunistic Aspergillus sp. (both in clinical and environment) the number of infections in immunocompromised patients has increased, as well as the antifungal resistance. It is known that Aspergillus species with a pathogenic potential, such as A. flavus, A. niger, A. terreus, A. versicolor, A. calidoustus, and A. nidulans [29,85], can lead to several health outcomes such as allergic bronchopulmonary aspergillosis and chronic pulmonary aspergillosis [58,86]. Additionally, it is also crucial to evaluate those species resistance to azoles, as it was performed in two studies of this review, in which the authors made a screening for A. fumigatus susceptibility to azoles. Azole resistance is a growing issue in A. fumigatus, threatening clinical improvements made possible by the use of azole antifungals in the treatment of Aspergillus-related disorders [28]. While some fungal species have innate azole resistance, acquired azole resistance has been found in fungi from occupational environments, such as sawmills, where azole fungicides (14-alpha demethylase inhibitors, DMI) used for timber preservation may exert some selection pressure on fungal populations [29]. Therefore, the use of azole fungicides to protect the wood reinforces the idea of performing a screening of susceptibility to azoles, specifically in this occupational environment.
Despite the methods used for the microbial occupational exposure assessment in these studies, it is important to highlight other methods and analysis that allowed a more complete assessment of sawmills’ workers occupational exposure, such as the assessment to fungal allergens [87]. Sawmill workers are exposed to large levels of allergenic fungus on a regular basis, which can cause respiratory problems and asthma [8,87,88]. Microscopical spore counts and culture-based approaches have historically been used to measure fungus exposure [89]. There are, however, various immunoassays to measure environmental antigens [90] like the enzyme-linked immunosorbent test (ELISA) [87]. Another method commonly used in the studies of this review (9 out of 23) was the limulus amoebocyte lysate assay (LAL) to analyze and quantify endotoxins, and the field emission scanning electron microscopy (FESEM) to analyze fungal particles.
It is important to highlight that none of the studies included mycotoxins assessment. Mycotoxins are secondary metabolites created by fungi, and together with endotoxins and glucans, they make products of fungi and bacteria that are present in the organic dust produced by organic materials, including soil, plants, animals, food, and faeces, and inhaled by workers in a variety of industries [91]. Some mycotoxins can have serious human health effects when ingested, but their health effects following inhalation or dermal contact are insufficiently documented [91].
Specific fungal genera, primarily Aspergillus, Penicillium, Alternaria, Fusarium, and Claviceps, produce mycotoxins [91,92,93], such as aflatoxin B1 (produced mainly by Aspergillus flavus and Aspergillus parasiticus), ochratoxin (produced by both Aspergillus and Penicillium), trichothecenes, zearalenone, fumonisins B1 and B2, and some emerging mycotoxins like fusaproliferin, moniliformin, beauvericin and enniatins (produced mainly by Fusarium species), ergot alkaloids, (produced by Claviceps) and altenuene, alternariol, alternariol methyl ether, altertoxin, and tenuazonic acid (produced by Alternaria species) [91,93,94,95]. Two of them (Penicillium and Aspergillus) were found with the highest prevalence in this setting.
Mycotoxins can exist in the environment even when no visible fungi are present [91,96], since they can withstand adverse environmental factors such as high or low temperatures and can persist long after the death and disintegration of the fungal species responsible for their production. Even after being exposed to temperatures such as boiling or roasting operations, they are difficult to eradicate or inactivate from the source [91,97]. The majority of mycotoxins are non-volatile, nevertheless, they can be found in airborne dust [88,92,93], as well as in fungal spores and fragments [91,96,97]. As a result, dust, spores, and hyphae fragments in the air can carry mycotoxins to the lungs [91,96,97]. Moreover, in other cases, exposure in the workplace happens primarily by inhalation, notably through airborne dust [88,93,94,95,98,99,100,101,102]. Mucous membrane irritation, skin rash, nausea, immune system suppression, acute or chronic liver damage, acute or chronic central nervous system damage, endocrine changes, and cancer are all signs and effects of inhaling mycotoxins [91,97,103,104,105].
As previously reported by Viegas and colleagues [91], although the health effects of exposure to some mycotoxins through eating of contaminated food are well documented, few research has looked into the health implications of mycotoxins through inhalation or skin contact and absorption, which are probably the main routes of exposure in the sawmills industry. To understand the main determinants that may have an impact on exposure, it is particularly important to properly characterize occupational exposure through the identification of current mycotoxins, their levels, duration, and main routes of exposure associated with specific occupational environments. In addition, to allow comparisons between research standardized techniques (sampling and analysis) are required [91].
Finally, the geographical distribution of the studies included in this review is also something to consider since most of them (15 out of 23) were conducted in Europe. Thus, it is evident that there is a lack of investigation regarding microbial exposure in this occupational environment in the rest of the world. Moreover, looking more closely at the distribution of studies in Europe, the imbalance in the various areas is also perceptible since most studies are from Northern Europe (6 out of 15) and Central Europe (8 out of 15), leaving areas like Western Europe and Southern Europe with one study each, and Eastern Europe without studies regarding this subject.
Combining the findings of this review with the lack of information, it is possible to highlight the need to increase investigation regarding microbial occupational exposure in sawmills all over the world. This paper’s findings should be considered, when preparing sampling campaigns and laboratory resources, to achieve an accurate microbial occupational exposure assessment in Portuguese sawmills.
5. Conclusions
This review allowed to identify the sampling methods and assays already employed to assess occupational exposure to microbial contamination in sawmills and to identify the knowledge gaps in what concerns this risk characterization.
Sawmill workers are exposed to several microbial contaminants in their workplace. Exposure to bacteria and fungi has been already reported, as well as bacteria metabolites (namely endotoxins). However, mycotoxins’ assessment was not yet performed and, therefore, the risk from this exposure was not estimated.
No papers were found reporting the occupational microbiological exposure in sawmills located in Portugal. Therefore, microbial occupational exposure assessment in Portuguese sawmills is crucial to better characterize this risk, and to identify the measures to be taken into account in order to protect the workers.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/atmos13020266/s1, Table S1. PRISMA Checklist.
Author Contributions
Conceptualization, M.D., C.V. and S.V.; methodology, M.D., C.V.; formal analysis, B.G., R.C. and M.D.; investigation, M.D. and C.V.; resources, M.D., C.V. and S.V.; writing—original draft preparation, M.D., C.V., P.P. and S.V.; writing—review and editing, M.D., C.V. and S.V.; supervision, C.V.; project administration, M.D and C.V.; funding acquisition, M.D.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds through the FCT—Fundação para a Ciência e Tecnologia, I.P., within the scope of the PhD Grant UI/BD/151431/2021.
Acknowledgments
H&TRC authors gratefully acknowledge the FCT/MCTES national support through the UIDB/05608/2020 and UIDP/05608/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sawmill Market Share 2021: Global Industry Size, Growth, Trend, Demand, Top Players, Opportunities and Forecast to 2026 with Leading Regions and Countries Data. Available online: https://www.marketwatch.com/press-release/sawmill-market-share-2021-global-industry-size-growth-trend-demand-top-players-opportunities-and-forecast-to-2026-with-leading-regions-and-countries-data-2021-12-09 (accessed on 18 October 2021).
- Cox, C.S.; Wathes, C.M. Bioaerosols Handbook; CRC Press: Boca Raton, FL, USA; Available online: https://www.taylorfrancis.com/books/edit/10.1201/9781003070023/bioaerosols-handbook-christopher-cox-christopher-wathes (accessed on 15 December 2021).
- Demers, P.A.; Teschke, K.; Kennedy, S.M. What to do about softwood? A review of respiratory effects and recommendations regarding exposure limits. Am. J. Ind. Med. 1997, 31, 385–398. [Google Scholar] [CrossRef]
- Dennekamp, M.; Demers, P.; Bartlett, K.; Davies, H.; Teschke, K. Endotoxin exposure among softwood lumber mill workers in the canadian province of british columbia. Ann. Agric. Environ. Med. 1999, 6, 141–146. [Google Scholar]
- Enarson, D.A.; Chan-Yeung, M. Characterization of health effects of wood dust exposures. Am. J. Ind. Med 1990, 17, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.G.; Graneek, B.J.; Lacey, J.; Nieuwenhuijsen, M.J.; Williamson, P.A.M.; Venables, K.M.; Newman Taylor, A.J. Respiratory symptoms, immunological responses and aeroallergen concentrations at a sawmill. Occup. Environ. Med. 1994, 51, 165–172. [Google Scholar] [CrossRef]
- Whitehead, L.W. Health effects of wood dust-relevance for an occupational standard. Am. Ind. Hyg. Assoc. J. 1982, 43, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Krysińska-Traczyk, E.; Prazmo, Z.; Skoŕska, C.; Sitkowska, J. Exposure to airborne microorganisms in polish sawmills. Ann. Agric. Environ. Med. 2001, 8, 71–80. [Google Scholar]
- Burry, J.N. Contact dermatitis from radiata pine. Contact Dermat. 1976, 2, 262–263. [Google Scholar] [CrossRef]
- Demers, P.A.; Kennedy, S.M.; Teschke, K.; Davies, H.; Bartlett, K. In Proceedings of the 12th International Symposium on Epidemiology in Occupational Health (ISEOH), Harare, Zimbabwe, 16-19 September 1997; Abstract 38.
- De Zotti, R.; Gubian, F. Asthma and rhinitis in wooding workers. Allergy Asthma Proc. 1996, 17, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.F.; Shy, C.M. Respiratory health effects from occupational exposure to wood dusts. Scand. J. Work Environ. Health 1988, 14, 1–15. [Google Scholar] [CrossRef][Green Version]
- Hedenstierna, G.; Alexandersson, R.; Wimander, K.; Rosén, G. Exposure to terpenes: Effects on pulmonary function. Int. Arch. Occup. Environ. Health 1983, 51, 191–198. [Google Scholar] [CrossRef]
- Malmberg, P.O.; Rask-Andersen, A.; Larsson, K.A.; Stjernberg, N.; Sundblad, B.M.; Eriksson, K. Increased bronchial responsiveness in workers sawing scots pine. Am. J. Respir. Crit. Care Med. 1996, 153, 948–952. [Google Scholar] [CrossRef]
- Mandryk, J.; Alwis, K.U.; Hocking, A.D. Work-related symptoms and dose-response relationships for personal exposures and pulmonary function among woodworkers. Am. J. Ind. Med. 1999, 35, 481–490. [Google Scholar] [CrossRef]
- Mandryk, J.; Alwis, K.U.; Hocking, A.D. Effects of personal exposures on pulmonary function and work-related symptoms among sawmill workers. Ann. Occup. Hyg. 2000, 44, 281–289. [Google Scholar] [CrossRef]
- Health Effects of Exposure to Wood Dust & Wood Dust References|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/docs/wooddust/default.html (accessed on 10 December 2021).
- Dutkiewicz, J.; Skórska, C.; Dutkiewicz, E.; Matuszyk, A.; Sitkowska, J.; Krysińska-Traczyk, E. Response of sawmill workers to work-related airborne allergens. Ann. Agric. Environ. Med. 2001, 8, 81–90. [Google Scholar]
- Belin, L. Sawmill alveolitis in sweden. Int. Arch. Allergy Appl. Immunol. 1987, 82, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Eduard, W. Assessment of Mould Spore Exposure and Relations to Symptoms in Wood Trimmers|Wda. Available online: https://library.wur.nl/WebQuery/wda/abstract/577407 (accessed on 7 December 2021).
- Eduard, W.; Sandven, P.; Levy, F. Serum IgG antibodies to mold spores in two norwegian sawmill populations: Relationship to respiratory and other work-related symptoms. Am. J. Ind. Med. 1993, 24, 207–222. [Google Scholar] [CrossRef]
- Jäppinen, P.; Haahtela, T.; Liira, J. Chip pile workers and mould exposure. A preliminary clinical and hygienic survey. Allergy 1987, 42, 545–548. [Google Scholar] [CrossRef]
- Kolmodin-Hedman, B.; Blomquist, G.; Löfgren, F. Chipped wood as a source of mould exposure. Eur. J. Respir. Dis. Suppl. 1987, 154, 44–51. [Google Scholar]
- Minárik, L.; Mayer, M.; Votrubová, V.; Ürgeová, N.; Dutkiewicz, J. Allergic alveolitis. Wiad Lek 2020, 73, 1593–1599. (In Polish) [Google Scholar]
- Rask-Andersen, A.; Land, C.J.; Enlund, K.; Lundin, A. inhalation fever and respiratory symptoms in the trimming department of swedish sawmills. Am. J. Ind. Med. 1994, 25, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Van Assendelft, A.H.; Raitio, M.; Turkia, V. Fuel chip-induced hypersensitivity pneumonitis caused by penicillium species. Chest 1985, 87, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Wimander, K.; Belin, L. Recognition of allergic alveolitis in the trimming department of a swedish sawmill. Eur. J. Respir. Dis. Suppl. 1980, 107, 163–167. [Google Scholar]
- Jeanvoine, A.; Rocchi, S.; Reboux, G.; Crini, N.; Crini, G.; Millon, L. Azole-resistant Aspergillus fumigatus in sawmills of Eastern France. J. Appl. Microbiol. 2017, 123, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Almeida, B.; Aranha Caetano, L.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of norwegian sawmills. Int. J. Environ. Health Res. 2020, 12, 23. [Google Scholar] [CrossRef]
- Gisi, U. Assessment of selection and resistance risk for demethylation inhibitor fungicides in Aspergillus fumigatus in Agriculture and medicine: A critical review. Pest Manag. Sci. 2014, 70, 352–364. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.J.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of azole-resistant aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013, 9, e1003633. [Google Scholar] [CrossRef]
- Deslandes, L. Advisory committee on paper and wood products—Portugal general economic situation. Advisory Committee on Paper and Wood Products 2008, 1–5. [Google Scholar]
- Associação das Indústrias de Madeira e Mobiliário de Portugal (AIMMP). Available online: https://aimmp.pt/ (accessed on 8 November 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Afanou, K.A.; Eduard, W.; Laier Johnsen, H.B.; Straumfors, A. Fungal fragments and fungal aerosol composition in sawmills. Saudi J. Biol. Sci. 2018, 62, 559–570. [Google Scholar] [CrossRef]
- Straumfors, A.; Olsen, R.; Daae, H.L.; Afanou, A.; McLean, D.; Corbin, M.; Mannetje, A.; Ulvestad, B.; Bakke, B.; Johnsen, H.L.; et al. Exposure to wood dust, microbial components, and terpenes in the norwegian sawmill industry. Ann. Work. Expo. Health 2018, 62, 674–688. [Google Scholar] [CrossRef]
- Straumfors, A.; Corbin, M.; McLean, D.; Mannetje, A.; Olsen, R.; Afanou, A.; Daae, H.-L.; Skare, Ø.; Ulvestad, B.; Laier Johnsen, H.; et al. Exposure determinants of wood dust, microbial components, resin acids and terpenes in the saw- and planer mill industry. Ann. Work. Expo. Health 2020, 64, 282–296. [Google Scholar] [CrossRef]
- Straumfors, A.; Foss, O.A.H.; Fuss, J.; Mollerup, S.K.; Kauserud, H.; Mundra, S. The inhalable mycobiome of sawmill workers: Exposure characterization and diversity. Appl. Environ. Microbiol. 2019, 85, e01448-19. [Google Scholar] [CrossRef]
- Prażmo, Z.; Dutkiewicz, J.; Cholewa, G. Gram-negative bacteria associated with timber as a potential respiratory hazard for woodworkers. Aerobiologia 2000, 16, 275–279. [Google Scholar] [CrossRef]
- Rogoziński, T.; Szwajkowska-Michałek, L.; Dolny, S.; Andrzejak, R.; Perkowski, J. The evaluation of microfungal contamination of dust created during woodworking in furniture factories. Med. Pract. 2015, 65, 705–713. [Google Scholar] [CrossRef]
- Górny, R.L.; Gołofit-Szymczak, M.; Cyprowski, M.; Stobnicka-Kupiec, A. Nasal lavage as analytical tool in assessment of exposure to particulate and microbial aerosols in wood pellet production facilities. Sci. Total Environ. 2019, 697, 134018. [Google Scholar] [CrossRef] [PubMed]
- Oppliger, A.; Rusca, S.; Charrière, N.; Vu Duc, T.; Droz, P.-O. Assessment of bioaerosols and inhalable dust exposure in swiss sawmills. Ann. Occup. Hyg. 2005, 49, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Rusca, S.; Charrière, N.; Droz, P.O.; Oppliger, A. Effects of bioaerosol exposure on work-related symptoms among swiss sawmill workers. Int. Arch. Occup. Environ. Health 2008, 81, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ljubičić Ćalušić, A.; Varnai, V.M.; Cavlović, A.O.; Segvić Klarić, M.; Beljo, R.; Prester, L.; Macan, J. Respiratory health and breath condensate acidity in sawmill workers. Int. Arch. Occup. Environ. Health 2013, 86, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Klarić, M.Š.; Varnai, V.M.; Calušić, A.L.; Macan, J. Occupational exposure to airborne fungi in two croatian sawmills and atopy in exposed workers. Ann. Agric. Environ. Med. 2012, 19, 213–219. [Google Scholar] [PubMed]
- Roponen, M.; Seuri, M.; Nevalainen, A.; Hirvonen, M.-R. Fungal spores as such do not cause nasal inflammation in mold exposure. Inhal. Toxicol. 2002, 14, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Gioffrè, A.; Marramao, A.; Iannò, A. Airborne microorganisms, endotoxin, and dust concentration in wood factories in Italy. Ann. Occup. Hyg. 2012, 56, 161–169. [Google Scholar] [CrossRef][Green Version]
- Duchaine, C.; Mériaux, A.; Thorne, P.S.; Cormier, Y. Assessment of particulates and bioaerosols in eastern Canadian sawmills. AIHAJ 2000, 61, 727–732. [Google Scholar] [CrossRef]
- Duchaine, C.; Mériaux, A. Airborne microfungi from eastern canadian sawmills. Can. J. Microbiol. 2000, 46, 612–617. [Google Scholar] [CrossRef]
- Verma, D.K.; Demers, C.; Shaw, D.; Verma, P.; Kurtz, L.; Finkelstein, M.; des Tombe, K.; Welton, T. Occupational health and safety issues in ontario sawmills and veneer/plywood plants: A pilot study. J. Environ. Public Health 2010, 2010, 526487. [Google Scholar] [CrossRef] [PubMed]
- Cormier, Y.; Mérlaux, A.; Duchaine, C. Respiratory health impact of working in sawmills in Eastern Canada. Arch. Environ. Health 2000, 55, 424–430. [Google Scholar] [CrossRef]
- Veillette, M.; Cormier, Y.; Israël-Assayaq, E.; Meriaux, A.; Duchaine, C. Hypersensitivity pneumonitis in a hardwood processing plant related to heavy mold exposure. J. Occup. Environ. Hyg. 2006, 3, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, H.; Lee, I. Microbial exposure assessment in sawmill, livestock feed industry, and metal working fluids handling industry. Saf. Health Work. 2010, 1, 183–191. [Google Scholar] [CrossRef]
- Neghab, M.; Jabari, Z.; Kargar Shouroki, F. Functional disorders of the lung and symptoms of respiratory disease associated with occupational inhalation exposure to wood dust in iran. Epidemiol. Health 2018, 40, e2018031. [Google Scholar] [CrossRef] [PubMed]
- Croston, T.L.; Nayak, A.P.; Lemons, A.R.; Goldsmith, W.T.; Gu, J.K.; Germolec, D.R.; Beezhold, D.H.; Green, B.J. Influence of Aspergillus fumigatus conidia viability on murine pulmonary MicroRNA and MRNA expression following subchronic inhalation exposure. Clin. Exp. Allergy 2016, 46, 1315–1327. [Google Scholar] [CrossRef]
- Dias, M.; Viegas, C. Fungal prevalence on waste industry—Literature review. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Oxford, UK, 2021; pp. 99–106. [Google Scholar] [CrossRef]
- Timm, M.; Madsen, A.M.; Hansen, J.V.; Moesby, L.; Hansen, E.W. Assessment of the total inflammatory potential of bioaerosols by using a granulocyte assay. Appl. Environ. Microbiol. 2009, 75, 7655–7662. [Google Scholar] [CrossRef]
- Beard, J.T.; Iachetta, F.A.; Lilleleht, L.U. APTI (Air Pollution Training Institute) Course 427: Combustion Evaluation, Student Manual; PB-80-207798; Associated Environmental Consultants: Charlottesville, VA, USA, 1980. [Google Scholar]
- Santos, J.; Ramos, C.; Vaz-Velho, M.; Vasconcelos Pinto, M. Occupational exposure to biological agents. In Advances in Safety Management and Human Performance; Arezes, P.M., Boring, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–67. [Google Scholar]
- Dias, M.; Sousa, P.; Viegas, C. Occupational exposure to bioburden in portuguese ambulances. In Occupational and Environmental Safety and Health III; Arezes, P.M., Baptista, J.S., Carneiro, P., Castelo Branco, J., Costa, N., Duarte, J., Guedes, J.C., et al., Eds.; Studies in Systems, Decision and Control; Springer International Publishing: Cham, Switzerland, 2022; pp. 167–173. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Y.; Wang, Y.; Huang, J.; Dong, X.; Chen, Z.; Lai, Y. Particulate matter capturing via naturally dried ZIF-8/Graphene aerogels under harsh conditions. iScience 2019, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- International Labour Organization. Encyclopaedia of Occupational Health and Safety; International Labour Organization: Geneve, Switzerland, 1998. [Google Scholar]
- Černá, K.; Wittlingerová, Z.; Zimová, M.; Janovský, Z. Methods of sampling airborne fungi in working environments of waste treatment facilities. Int. J. Occup. Med. Environ. Health 2016, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.C.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; et al. Settled dust assessment in clinical environment: Useful for the evaluation of a wider bioburden spectrum. Int. J. Environ. Health Res. 2019, 31, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, H.K.; Täubel, M.; Jayaprakash, B.; Vepsäläinen, A.; Pasanen, P.; Hyvärinen, A. Quantitative assessment of microbes from samples of indoor air and dust. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 231–241. [Google Scholar] [CrossRef]
- Meadow, J.F.; Altrichter, A.E.; Kembel, S.W.; Kline, J.; Mhuireach, G.; Moriyama, M.; Northcutt, D.; O’Connor, T.K.; Womack, A.M.; Brown, G.Z.; et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air 2014, 24, 41–48. [Google Scholar] [CrossRef]
- Emerson, J.B.; Keady, P.B.; Brewer, T.E.; Clements, N.; Morgan, E.E.; Awerbuch, J.; Miller, S.L.; Fierer, N. Impacts of flood damage on airborne bacteria and fungi in homes after the 2013 colorado front range flood. Environ. Sci. Technol. 2015, 49, 2675–2684. [Google Scholar] [CrossRef]
- Barberán, A.; Dunn, R.R.; Reich, B.J.; Pacifici, K.; Laber, E.B.; Menninger, H.L.; Morton, J.M.; Henley, J.B.; Leff, J.W.; Miller, S.L.; et al. The ecology of microscopic life in household dust. Proc. Biol. Sci. 2015, 282, 1139. [Google Scholar] [CrossRef]
- Saejiw, N.; Chaiear, N.; Sadhra, S. Exposure to wood dust and its particle size distribution in a rubberwood sawmill in Thailand. J. Occup. Environ. Hyg. 2009, 6, 483–490. [Google Scholar] [CrossRef]
- Hinds, W.C. Basic for size-selective sampling for wood dust. Appl. Ind. Hyg. 1988, 3, 67–72. [Google Scholar] [CrossRef]
- Environmental Sciences—Editorial Contacts|Springer. Available online: https://www.springer.com/gp/environmental-sciences/contact-us?gclid=Cj0KCQiAweaNBhDEARIsAJ5hwbfngBdU3g9WbmW82SZiL1UWlTNh5X98xQXKlgPqR1IA2ymwa6znC6gaAo2FEALw_wcB (accessed on 5 November 2021).
- Viegas, C.; Almeida, B.; Dias, M.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Faria, T.; Martins, V.; Marta Almeida, S. Assessment of Children’s Potential Exposure to Bioburden in Indoor Environments. Atmosphere 2020, 11, 993. [Google Scholar] [CrossRef]
- Viegas, C. Sampling Methods for an Accurate Mycobiota Occupational Exposure Assessment: Overview of Several Ongoing Projects; Taylor & Francis: Abingdon, UK, 2018; pp. 7–11. [Google Scholar] [CrossRef]
- Park, J.-H.; Sulyok, M.; Lemons, A.R.; Green, B.J.; Cox-Ganser, J.M. Characterization of fungi in office dust: Comparing results of microbial secondary metabolites, fungal internal transcribed spacer region sequencing, viable culture and other microbial indices. Indoor Air 2018, 28, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Viegas, S. Characterization of occupational exposure to fungal burden in portuguese bakeries. Microorganisms 2019, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Gomes, B.; Dias, M.; Carolino, E.; Aranha Caetano, L. Aspergillus section Fumigati in firefighter headquarters. Microorganisms 2021, 9, 2112. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.; Viegas, S. EXPOsE: Establishing protocols to assess occupational exposure to bioburden in clinical environments. In Proceedings of the OH2019—The Premier Conference for Occupational Hygiene in the UK, Brighton, UK, 1–4 April 2019. [Google Scholar]
- Bouillard, L.; Michel, O.; Dramaix, M.; Devleeschouwer, M. Bacterial contamination of indoor air, surfaces, and settled dust, and related dust endotoxin concentrations in healthy office buildings. Ann. Agric. Environ. Med. 2005, 12, 187–192. [Google Scholar] [PubMed]
- Viegas, C.; Faria, T.; Meneses, M.; Carolino, E.; Viegas, S.; Gomes, A.Q.; Sabino, R. Analysis of surfaces for characterization of fungal burden—Does it matter? Int. J. Occup. Med. Environ. Health 2016, 29, 623–632. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- MacNeil, L.; Kauri, T.; Robertson, W. Molecular techniques and their potential application in monitoring the microbiological quality of indoor air. Can. J. Microbiol. 1995, 41, 657–665. [Google Scholar] [CrossRef]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Infectious diseases society of america. treatment of aspergillosis: Clinical practice guidelines of the infectious diseases society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef]
- Viegas, C.; Caetano, L.A.; Viegas, S. Occupational exposure to Aspergillus Section Fumigati: Tackling the knowledge gap in Portugal. Environ. Res. 2021, 194, 110674. [Google Scholar] [CrossRef]
- Varga, V.; Kocsubé, S.; Szigeti, G.; Baranyi, N.; Téth, B. Aspergillus mycotoxins. In Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 165–186. [Google Scholar]
- Lamoth, F. Aspergillus fumigatus-related species in clinical practice. Front. Microbiol. 2016, 7, 683. [Google Scholar] [CrossRef] [PubMed]
- Prester, L.; Macan, J. Determination of Alt a 1 (Alternaria alternata) in poultry farms and a sawmill using ELISA. Med. Mycol. 2010, 48, 298–302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hessel, P.A.; Herbert, F.A.; Melenka, L.S.; Yoshida, K.; Michaelchuk, D.; Nakaza, M. Lung health in sawmill workers exposed to pine and spruce. Chest 1995, 108, 642–646. [Google Scholar] [CrossRef]
- Niemeier, R.T.; Sivasubramani, S.K.; Reponen, T.; Grinshpun, S.A. Assessment of fungal contamination in moldy homes: Comparison of different methods. J. Occup. Environ. Hyg. 2006, 3, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.; Portnoy, J.; Sever, M.; Arbes, S.; Vaughn, B.; Zeldin, D.C. Comparison of enzyme immunoassay-based assays for environmental Alternaria alternata. Ann. Allergy Asthma Immunol. 2006, 97, 350–356. [Google Scholar] [CrossRef]
- Iversen, M.; Kirychuk, S.; Drost, H.; Jacobson, L. Human health effects of dust exposure in animal confinement buildings. J. Agric. Saf. Health 2000, 6, 283–288. [Google Scholar] [CrossRef]
- Viegas, S.; Viegas, C.; Oppliger, A. Occupational exposure to mycotoxins: Current knowledge and prospects. Ann. Work. Expo. Health 2018, 62, 923–941. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Barkai-Golan, R.; Paster, N. Mouldy fruits and vegetables as a source of mycotoxins: Part 1. World Mycotoxin J. 2008, 1, 147–159. [Google Scholar] [CrossRef]
- Huttunen, K.; Korkalainen, M. Microbial secondary metabolites and knowledge on inhalation effects. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Viegas, C., Viegas, S., Quintal Gomes, A., Taubel, M., Sabino, R., Eds.; Springer Nature: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Lavicoli, I.; Brera, C.; Carelli, G.; Caputi, R.; Marinaccio, A.; Miraglia, M. External and internal dose in subjects occupationally exposed to ochratoxin A. Int. Arch. Occup. Environ. Health 2002, 75, 381–386. [Google Scholar] [CrossRef]
- Halstensen, A.S. Species-specific fungal DNA in airborne dust as surrogate for occupational mycotoxin exposure? Int. J. Mol. Sci. 2008, 9, 2543–2558. [Google Scholar] [CrossRef] [PubMed]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Flannigan, B. Mycotoxins in the air. Int. Biodeterior. 1987, 23, 73–78. [Google Scholar] [CrossRef]
- Brera, C.; Caputi, R.; Miraglia, M.; Iavicoli, I.; Salerno, A.; Carelli, G. Exposure assessment to mycotoxins in workplaces: Aflatoxins and ochratoxin A occurrence in airborne dusts and human sera. Microchem. J. 2002, 73, 167–173. [Google Scholar] [CrossRef]
- Brasel, T.L.; Martin, J.M.; Carriker, C.G.; Wilson, S.C.; Straus, D.C. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Appl. Environ. Microbiol. 2005, 71, 7376–7388. [Google Scholar] [CrossRef]
- Mayer, S.; Curtui, V.; Usleber, E.; Gareis, M. Airborne mycotoxins in dust from grain elevators. Mycotoxin Res. 2007, 23, 94–100. [Google Scholar] [CrossRef]
- Mayer, S. Occupational exposure to mycotoxins and preventive measures. In Environmental Mycology in Public Health: Fungi and Mycotoxins Risk Assessment and Mana-Gement; Viegas, C., Pinheiro, A.C., Sabino, R., Viegas, S., Brandão, J., Verissimo, C., Eds.; Academic Press: Waltham, MA, USA, 2007; ISBN 978-0-12-411471-5. [Google Scholar]
- Olsen, J.H.; Dragsted, L.; Autrup, H. Cancer risk and occupational exposure to aflatoxins in denmark. Br. J. Cancer 1988, 58, 392–396. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).