Abstract
Background: Variations in atmospheric pressure (AP) are known to affect blood pressure (BP). We assessed the effect of AP on BP, and the major fatal and nonfatal complications thereof (i.e., stroke, myocardial infarction, and pulmonary emboli). Methods: In this observational cohort study, 250 hypertensive patients (aged 65–92 years old) were followed for 3.5–5.4 years in a primary care clinic. Cox proportional hazard regression was performed to define the associations between AP, clinical, demographic and environmental factors, and major complications such as stroke, myocardial infarction, etc. Results: AP fluctuated between 1007 and 1024 millibars (MB). A total of 132 patients (53%) developed various complications, of which 13 (9.8%) were fatal. Among all fatalities, 93 of 119 nonfatal cases and 7 of 13 fatal cases occurred at AP < 1013 MB. A Cox regression analysis showed that low AP (AP < 1013 MB) had a higher hazard ratio (HR) on hypertension (HTN) complications among all demographic, clinical and environmental parameters. Conclusions: Most major complications were associated with very low APs. Low AP was the best predictive risk-factor for major complications of HTN.
1. Introduction
The effects of atmospheric pressure (AP) have long been a subject of interest [1,2,3,4,5,6,7,8,9]. AP is an environmental element that affects human health. In the setting of hypertension (HTN), variations in AP during the four seasons are known to affect blood pressure (BP) [1,2,3,4,5,6]. In temperate and continental climates, AP is high in summer, when the air temperature is also high. In winter, the situation is opposite: high AP is related to very low temperatures. In the Eastern Mediterranean region, climate conditions are stable during winter and AP is mostly high. [1,2,3,4,5,6,7,8]. Although factors such as cyclones and anticyclones may contribute remarkably to atmospheric pressure, the effects of these climate components on weather phenomena in Israel were shown to be relatively minor and accounted for barometric pressure and temperature excursions of approximately 1.4% during the entire year [9,10]. In bio-environmental studies, AP seemed to be the most objective meteorological factor, because it has the same effect on humans, whether indoors and outdoors [4]. Information on whether AP affects BP is inconsistent. Several reports found no correlation between atmospheric pressure and elevated BP [4,5,6]. However, several articles have related BP complications, such as myocardial infarction, strokes, or aortic dissection, to the effects of cold [5,6,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. AP exerts an effect on the autonomic nervous system. Associations between an increase in coronary heart disease and atmospheric pressure were shown to exhibit a V-shape, with a minimum of daily event rates at 1016 MB. Catecholamines are elevated in cold weather, activating the sympathetic nervous system [6,8,17]. Previous studies on the relationship between AP and BP with temperature changes in the same location showed minimal, if any, change in BP [4,18,19,20,21]. However, it has been shown that BP decreases in countries with warmer, tropical climates [1,2,3,4,5,6,13,14]. A significant, inverse relationship between AP and BP during spring days was observed, as it was for systolic blood pressure systolic blod pressure [SBP] during winter nights [4]. Relatively higher AP during winter and lower AP during summer is the most common prevailing pattern in Israel (Israel Meteorological Service).
HTN is a recognized risk factor for cardiovascular and cerebrovascular events. These complications are increased during winter in comparison to summer [6,13,14,15,16,17,18]. Previously, we have reported greater seasonal BP variations in geriatric patients with HTN, whose mean SBP and diastolic blood pressure diastolic blood pressure [DBP] were higher during winter, as compared with summer [6]. The winter–summer difference was above 30 mmHg for SBP and above 14 mmHg for DBP. It has also been observed that more fatal events occur in winter than in summer seasons, mostly due to strokes and cardiovascular events among the elderly [6,13,14,15]. The aim of the current study was to present the effects of AP on the major complications of HTN (stroke, myocardial infarction, and pulmonary emboli) during a period of 5 years.
2. Methods
2.1. Patients
All patients in this observational cohort study were diagnosed with HTN, and treated and followed-up in a Maccabi Health System primary care outpatient clinic from January 2012 to December 2016.
Patients with elevated BP (SBP 141–200 mmHg and DBP 90–115 mmHg) and age ≥65 years were recruited for the study. Exclusion criteria were previous cerebrovascular accident, recent ST elevation myocardial infarction, acute coronary syndrome, congestive heart failure, advanced oncologic disease, severe pulmonary disease, kidney disease (creatinine > 1.4 mg/dL), anemia (hemoglobin < 12 g%), secondary HTN, hyper- or hypothyroid function, and cognitive disorders.
All patients received antihypertensive treatment, which included ACE inhibitors, angiotensin receptor blockers, calcium channel blockers, diuretics, β-blockers, or α-blockers. The patients continued their regular daily activities and ingested their usual amounts of caffeine, tobacco, and alcohol.
2.2. Study Protocol
The AP in the examination room was measured at every patient’s visit. Room temperature was maintained at a constant 23 °C with air conditioning.
Patients’ current and past medical histories were recorded, and they underwent a physical examination, including weight measurements, electrocardiograms, chest X-rays, examinations of the retina, and echocardiographs. Blood tests included analyses of kidney function, electrolyte levels, and complete blood counts. Blood pressure was measured after a 20-minute rest, which is suitable for adaptation to indoor conditions of temperature and AP, as appropriate to the Eastern Mediterranean region, where very low temperatures (<0 °C) and high temperatures (>35 °C) are rare (Israel Meteorological Service); thus, adaptation is relatively rapid. BP was measured using a mercury sphygmomanometer with a standard-sized cuff (12 cm × 35 cm). For patients with BMI > 27 kg/m2 and/or with large upper arm, a 16 × 36 cm cuff was used. For patients with an upper arm circumference of 45–52 cm, a 16 × 42 cm (extra-large) cuff was used. Mean BP values were calculated. SBP ≤ 140 mmHg and DBP ≤ 90 mmHg were considered normal. All BP measurements were performed in the same examination room, by the same physician (G.C.).
The patients were followed at intervals as indicated clinically, which did not exceed 3 months, during the 5-year period, at the same room temperature. AP and BP were recorded at each visit. Ambient AP, i.e., mean sea level pressure (MSLP) measurements in millibars (MB), were obtained from the Israel Meteorological Service, (Bet Dagan, Israel Meteorological Service). Mean year-round AP values are presented in the figure. However, to associate blood pressure with AP, readings on the visit days were specifically recorded for data analysis.
Body mass index (BMI) and age were determined on the first visit and BMI was reassessed every 6 months.
Antihypertensive treatment included diuretics for 65% (disothiazide—56%, furosemide—24%, and spironolactone—16%), beta-blockers—58%; calcium channel blockers—48%; angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers—72%; and alpha blockers—11%. Supplementary antihypertension treatment was required during high APs of 1019–1024 MB in 24% of the patients.
2.3. Statistical Analysis
The main outcome variables were major HTN complications (fatal outcomes and stroke, myocardial infarction, and pulmonary emboli). We also investigated the relationship of AP with SBP, DBP, and mean BP.
The analysis investigated the relationship of the outcome variable with age, gender, body mass index (BMI), outdoor temperature, severe HTN (SBP > 160), and AP. Mean values and frequencies of the outcome variables in subgroups defined by gender, age, and BMI were compared. The risk of outcomes was analyzed using a Cox regression on each factor of interest. Bonferroni corrections for multiple comparisons were used when evaluating the p-values of different regressions. All p-values were two-sided and p-values < 0.05 were considered statistically significant. Calculations were performed using SAS 9.4 (mainly Proc MIXED and PHREG).
3. Results
Based on the inclusion and exclusion criteria, 272 outpatients with HTN were eligible to participate in this longitudinal, prospective study. A total of 22 patients were excluded due to noncompliance or lack of follow-up information. The remaining 250 patients (mean age, 74.8 ± 14.2 years; range, 65–92) were followed for at least 3.5 years and up to 5.4 years. Most patients were not obese (218/250). During follow-up, BMI did not change significantly and did not affect BP.
AP fluctuated from 1007 to 1024 MB during the 5-year study. Table 1 shows the results of the SBP and DBP under the same weather conditions for both men and women and for both sexes combined. The overall mean difference (Δ) in SBP (between AP 1024 and 1007) was 18 ± 0.3 mmHg (19 ± 5.9 mmHg for men, as compared with 18.01 ± −6.3 mmHg for women, p (t-test) = 0.23). The mean ΔDBP under the same AP fluctuations was 6.0 ± 0.4 mmHg (6 ± 1.4 mmHg for men and 6 ± 0.7 mmHg for women). After analyzing BP differences during consecutive, gradual increases in AP from 1007 to 1024 MB (i.e., 1007 to 1009, 1009 to 1013, 1013 to 1016, etc.), we did not find differences in changes in BP between men and women. Supplementary antihypertension treatment was required during high AP (1019–1024 MB) in 24% of selected patients. Figure 1 displays the mean monthly atmospheric pressure and temperature changes through the 5-year observation period.

Table 1.
Systolic and diastolic blood pressure by atmospheric pressure and sex.
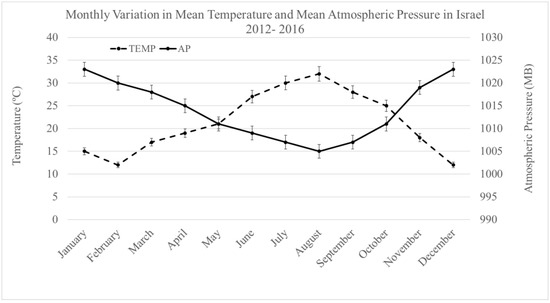
Figure 1.
Monthly variation in mean atmospheric pressure (MB) and temperature during the 5–year observation period.
Table 2 lists the Pearson correlations (R) of the mean differences in SBP and DBP measurements at AP 1024 MB minus AP 1007 MB, according to age group. Strong correlations with SBP were found for age ranges of 65–69 years (14 ± 11.3 mmHg, R for SBP = 0.42, p < 0.002) and 70–75 years (16 ± 13.5 mmHg, R for SBP = 0.41, p < 0.003), and in DBP for age ranges of 65–69 years (9 ± 8.5, R for DBP = 0.38, p < 0.004) and 70–75 years 7 ± 0.9 (R for DBP = 0.41, p < 0.02).

Table 2.
Pearson correlations between changes in systolic and diastolic blood pressure measurements at atmospheric pressure differences of 1024 and 1007 MB, according to age.
For Pearson correlations of mean SBP and DBP variations at APs ranging from 1024 to 1007 MB according to BMI, significant differences in SBP were observed at BMI values of 21–25 and 26–30. They were 17 ± 0.4 mmHg (R = 0.41) and 19 ± 14.5 mmHg (R = 0.42), respectively (p < 0.01). For DBP, at the same BMI, the values were 16 ± 4.8 mmHg (R = 0.37) and 16 ± 9.3 mmHg (R = −0.40), respectively.
Table 3 demonstrates the association between nonfatal complications and mortality related to AP changes. A total of 132 patients (53%) developed complications, of which 13 (9.8%) were fatal: 7 (5.3%) of the total complications were a myocardial infarction, and 9 of the 132 patients (6.8%) developed stroke during conditions of decreased AP. Nonfatal complications, including weakness and headaches, were significantly more common at APs of 1007 and 1009 MB.

Table 3.
Distribution of related complications and mortality according to atmospheric pressure (n = 132).
To define which AP and other factors had the strongest effect on HTN complications, Cox univariate regression was performed for each parameter of interest. Table 4 displays the hazard ratios (HRs) of the main clinical, seasonal, and demographic characteristics related to HTN on major complications. HTN is a risk factor for major complications of stroke, myocardial infarction, and pulmonary embolism. An HR ratio of 2.831 (95% CI 1.752–3.232; p = 0.002) was seen for AP. However, HTN (SBP > 160) and gender were also significant factors that predicted major complications.

Table 4.
Hazard ratios (HRs) of the major complications (hospitalizations due to stroke, myocardial infarction, pulmonary embolism, or fatality) for the main parameters of interest.
4. Discussion
The results of this study demonstrate a direct relationship between AP and SBP and DBP among hypertensive patients. This was remarkable at APs above 1016 MB.
Our findings do not support previous studies that reported an inverse relationship between AP and arterial BP [19,20,21]. In previous reports, low AP was associated with an increased incidence of cardiovascular disease [22,23,24,25,26,27,28,29,30,31,32,33], probably related to dysregulation of the autonomic and endocrine systems, resulting in higher BP [13,19,22]. We would expect more cardiovascular complications related to higher blood pressure; however, this study had contrasting results. Low BP concurrent with low AP was associated with a higher rate of complications.
BP fluctuations among our study participants were related to age and BMI. Relatively younger participants (65–75 years of age) experienced significant BP variations compared with patients older than 75 years (p < 0.05), at APs ranging from 1007 to 1024 MB. A possible explanation for this effect may be inadequate autonomic nervous system function among elderly individuals, impacting the control of BP. Moreover, hypertensive patients with BMI 21–30 kg/m2 showed a higher correlation of BP changes at these atmospheric conditions, as compared with patients with BMI 18–20 or 30–35 kg/m2 (p < 0.05).
The present investigation found that major nonfatal complications—myocardial infarction, stroke, and pulmonary emboli—were inversely related to AP levels, which highlights the effect of decreased AP on lowering both SBP and DBP. The same effect was found for fatal complications. From among gender, age, BMI, outdoor temperature, HTN, and AP, the highest HR was found for AP. This does not agree with the common appreciation that high BP is a major risk factor for cerebro- and cardiovascular events. A strongly negative association between AP and HTN-associated complications was also observed for nonfatal manifestations, such as weakness and headaches, which were markedly more common at lower APs (p < 0.001). As mentioned, Cox regression demonstrated that, in the current study, AP had the strongest effect on major complications of HTN. However, severe HTN and gender were significant predictors as well. Our previous studies showed the relationship between ambient temperature and health complications [6.22]. Our objective in this work was to consider the contribution of AP alone. This does not mean that a common effect of both environmental components does not exist.
5. Limitations
A limitation of this study was the relatively small study group. Some risk-factors for cerebrovascular events, e.g., smoking and family history, were not included in the analysis. Additionally, parameters such as humidity, water vapor pressure, and wind speed were not assessed in this study, and UTCI was not calculated. Further investigations are warranted with larger cohorts of patients, as well as taking into account extensive series of environmental parameters, to further support our observations.
The results of the current study, including the relationship between higher APs and lower temperatures, are true for the Eastern Mediterranean region and might differ from those of other regions.
6. Conclusions
This study found AP to be the strongest risk factor for major complications of HTN. Most major complications were found at low APs.
BP levels in hypertensive patients were directly correlated with high AP (>1016 MB). Significant changes in BP associated with varying AP were observed at relatively younger age ranges (65–75 years) and in non-obese patients (BMI 18–20 kg/m2).
Stricter controls of BP by carefully adjusting antihypertensive medication and doses should be maintained during periods of low AP.
Author Contributions
G.C. and I.G. responsible for conception, design and analysis, interpretation of data, major revision and final approval of the manuscript submitted. E.K. and L.C. performed the experiments and treated the patients. G.C. drafted and revised the manuscript. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The study was funded by internal departmental resources.
Institutional Review Board Statement
The study was approved by the Maccabi Health Services Ethics Committee. It is registered at Clinical Trials.gov, registration number 0097-17-BBL.
Informed Consent Statement
All participants provided written informed consent prior to data collection. Patients agreed to publication. Identifying information is not included.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
The authors would like to acknowledge Faye Schreiber for editing the manuscript. She is an employee of Meir Medical Center.
Conflicts of Interest
The authors declare that they have no competing financial or non-financial interests, whatsoever.
Abbreviations
| AP | atmospheric pressure |
| BMI | body mass index |
| BP | blood pressure |
| DBP | diastolic blood pressure |
| HR | hazard ratio |
| MB | millibars |
| HTN | hypertension |
| SBP | systolic blood pressure |
References
- Brennan, P.J.; Greenberg, G.; Miall, W.E.; Thompson, S.G. Seasonal variation in arterial blood pressure. Br. Med. J. 1982, 285, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Hozawa, A.; Kuriyama, S.; Shimazu, T.; Ohmori-Matsuda, K.; Tsuji, I. Seasonal variation in home blood pressure measurements and relation to outside temperature in Japan. Clin. Exp. Hypertens. 2011, 33, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Obayashi, K.; Iwamoto, J.; Tone, N.; Okamoto, N.; Tomioka, K.; Kurumatani, N. Stronger association of indoor temperature than outdoor temperature with blood pressure in colder months. J. Hypertens. 2014, 32, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, M.; Cieślik-Guerra, U.I.; Kotas, R.; Mazur, P.; Marańda, W. Evaluation of the impact of atmospheric pressure in different seasons on blood pressure in patients with arterial hypertension. Int. J. Occup. Med. Environ. Health 2016, 29, 783–792. [Google Scholar] [CrossRef]
- Verberkmoes, N.J.; Hamad, M.A.S.; Ter Woorst, J.F.; Tan, M.E.; Peels, C.H.; van Straten, A. Impact of temperature and atmospheric pressure on the incidence of major acute cardiovascular events. Nethrlands Heart J. 2012, 20, 193–196. [Google Scholar] [CrossRef]
- Charach, G.; Rabinovich, P.; Weintraub, M. Seasonal changes in blood pressure and frequency of related complications in elderly Israeli patients with essential hypertension. Gerontology 2004, 50, 315–321. [Google Scholar] [CrossRef]
- Cornelius, H. On airs, waters, and places. Apud Johannem Elsevirium 1658, 11, 152. (In Latin) [Google Scholar]
- Yackerson, N.S.; Bromberg, L.; Adler, B.; Aizenberg, A. Possible effects of changes in the meteorological state over semi-arid areas on the general well-being of weather-sensitive patients. Environ. Health 2012, 11, 26. [Google Scholar] [CrossRef]
- Danet, S.; Richard, S.; Montaye, M.; Beaushant, S.; Graux, C.; Cottel, D.; Marecaux, N.; Amouyel, P. Unhealthy effect of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary death. Circulation 1999, 100, e1–e7. [Google Scholar] [CrossRef]
- Saarini, H.; Ziv, B.; Bitan, A.; Alpert, P. Eastern Wind Storms over Israel. Theor. Appl. Climatol. 1998, 59, 61–77. [Google Scholar] [CrossRef]
- Morabito, M.; Crisci, A.; Orlandini, S.; Maracchi, G.; Gensini, G.F.; Modesti, P.A. A synoptic approach to weather conditions discloses a relationship with ambulatory blood pressure in hypertensives. Am. J. Hypertens. 2008, 21, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Niwa, M.; Tanaka, T. Difference of intensity and disparity in impact of climate on several vascular diseases. Heart Vessel. 2012, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Curwen, M. Excess winter mortality, temperature: A British phenomenon? Health Trends 1990, 22, 169–175. [Google Scholar]
- Feigin, V.L.; Anderson, C.S.; Anderson, N.E.; Broad, J.B.; Pledger, M.J.; Bonita, R. Is there a temporal pattern in occurrence of subarachnoid hemorrhage in the southern hemisphere? Pooled data from 3 large, population-based incidence studies in Australia, 1981–1997. Stroke 2001, 32, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Giaconi, S.; Ghione, S.; Palombo, C.; Genovesi-Ebert, A.; Marabotti, C.; Fommei, E.; Donato, L. Seasonal influences on blood pressure in high normal to mild hypertensive range. Hypertension 1989, 14, 22–27. [Google Scholar] [CrossRef]
- Stout, R.W.; Crawford, V. Seasonal variations in fibrinogen concentrations among elderly people. Lancet 1991, 338, 9–13. [Google Scholar] [CrossRef]
- Brueren, M.M.; Schouten, B.J.; Schouten, H.J.; van Weel, C.; de Leeuw, P.W.; Ree, J.W. No relevant seasonal influences on office and ambulatory blood pressure. Data from a study in borderline hypertensive primary care patients. Am. J. Hypertens. 1998, 11, 602–605. [Google Scholar] [CrossRef]
- Abdulla, K.; Taka, M. Climatic effects on blood pressure in normotensive and hypertensive subjects. Postgrad. Med. J. 1988, 64, 23–26. [Google Scholar] [CrossRef]
- Woodhouse, P.R.; Khaw, K.; Plummer, M. Seasonal variations of blood pressure and its ambient temperature in an elderly population. J. Hypertens. 1993, 11, 1267–1274. [Google Scholar] [CrossRef]
- Skrobowski, A. The Impact of Selected Atmospheric Conditions on Blood Pressure; Wojskowa Akademia Medyczna: Warszawa, Poland, 1998. [Google Scholar]
- Weinbacher, M.; Martina, B.; Bart, T.; Drewe, J.; Gasser, P.; Gyr, K. Blood pressure and atmospheric pressure. Ann. N. Y. Acad. Sci. 1996, 783, 335–336. [Google Scholar] [CrossRef]
- Charach, G.; Shochat, M.; Argov, O.; Weintraub, M.; Charach, L.; Rabinovich, A.; Ayzenberg, O.; George, J. Seasonal changes in blood pressure: Cardiac and cerebrovascular morbidity and mortality. World J. Hypertens. 2013, 3, 18. [Google Scholar] [CrossRef]
- Dawson, J.; Weir, C.; Wright, F.; Bryden, C.; Aslanyan, S.; Lees, K.; Bird, W.; Walters, M. Associations between meteorological variables and acute stroke hospital admissions in the west of Scotland. Acta Neurol. Scand. 2008, 117, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Law, H.Y.; Wong, G.K.; Chan, D.T.; Wong, L.; Poon, W.S. Meteorological factors and aneurysmal subarachnoid haemorrhage in Hong Kong. Hong Kong Med. J. 2009, 15, 85–89. [Google Scholar] [PubMed]
- Turin, T.C.; Kita, Y.; Murakami, Y.; Rumana, N.; Sugihara, H.; Morita, Y.; Tomioka, N.; Okayama, A.; Nakamura, Y.; Abbott, R.D.; et al. Higher stroke incidence in the spring season regardless of conventional risk factors: Takashima Stroke Registry, Japan, 1988–2001. Stroke 2008, 39, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Hessman-Kosaris, A. The Impact of Weather on Mood; Diogenes: Warszawa, Poland, 1998. [Google Scholar]
- Kriszbacher, I.; Czopf, L.; Bódis, J. The effects of seasonal variations and weather conditions on the occurrence of heart attacks in Hungary between 2000–2004. Orv. Hetil. 2007, 148, 731–736. [Google Scholar] [CrossRef]
- Rumana, N.; Kita, Y.; Turin, T.C.; Murakami, Y.; Sugihara, H.; Morita, Y.; Tomioka, N.; Okayama, A.; Nakamura, Y.; Abbott, R.D.; et al. Seasonal pattern of incidence and case fatality of acute myocardial infarction in a Japanese population (from the Takashima AMI Registry, 1988 to 2003). Am. J. Cardiol. 2008, 102, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Kristal-Boneh, E.; Harari, G.; Green, M.S. Seasonal change in 24-hour blood pressure and heart rate is greater among smokers than nonsmokers. Hypertension 1997, 30, 436–441. [Google Scholar] [CrossRef]
- Shinkawa, A.; Ueda, K.; Hasuo, Y.; Kiyohara, Y.; Fujishima, M. Seasonal variation in stroke incidence in Hisayama, Japan. Stroke 1990, 1, 1262–1267. [Google Scholar] [CrossRef]
- Rusticucci, M.; Bettolli, M.L.; de los Angeles, M. Association between weather conditions and the number of patients at the emergency room in an Argentine hospital. Int. J. Biomet. 2002, 46, 42–51. [Google Scholar] [CrossRef]
- Seto, T.B.; Mittleman, M.A.; Davis, R.B.; Taira, D.A.; Kawachi, I. Seasonal variation in coronary artery disease mortality in Hawaii: Observational study. BMJ 1998, 316, 1946–1947. [Google Scholar] [CrossRef]
- Lanska, D.J.; Hoffmann, R.G. Seasonal variation in stroke mortality rates. Neurology 1999, 52, 984–990. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).