Selection of Plant Species for Particulate Matter Removal in Urban Environments by Considering Multiple Ecosystem (Dis)Services and Environmental Suitability
Abstract
1. Introduction
2. Methodology
2.1. Determine the Effective Plant Species and the Effective Leaf Traits in PM Accumulation
2.2. Determine the Criteria for Building Leaf Traits, Leaf SIRM, and Services and Disservices Models
2.3. Consolidate Data from the Literature for the Criteria Included in the Respective Models
2.4. Assign Scores and Weights to the Criteria Included in the Respective Models
2.4.1. Leaf Traits Model
2.4.2. Leaf SIRM Model
2.4.3. Services and Disservices Model
2.5. Calculate the Weighted Average or Product (Π)-Value for Plant Species Included in the Respective Models
3. Results
3.1. Effective Plant Species for Reducing PM as Identified by the Leaf Traits and Leaf SIRM Models
3.2. Classification of Plant Species: Similarities and Differences between Leaf Traits and Leaf SIRM Models
3.3. Identifying Plant Species for Reducing PM in Urban Environments Considering the Environment Adaptability and Ecosystem (Dis)Services
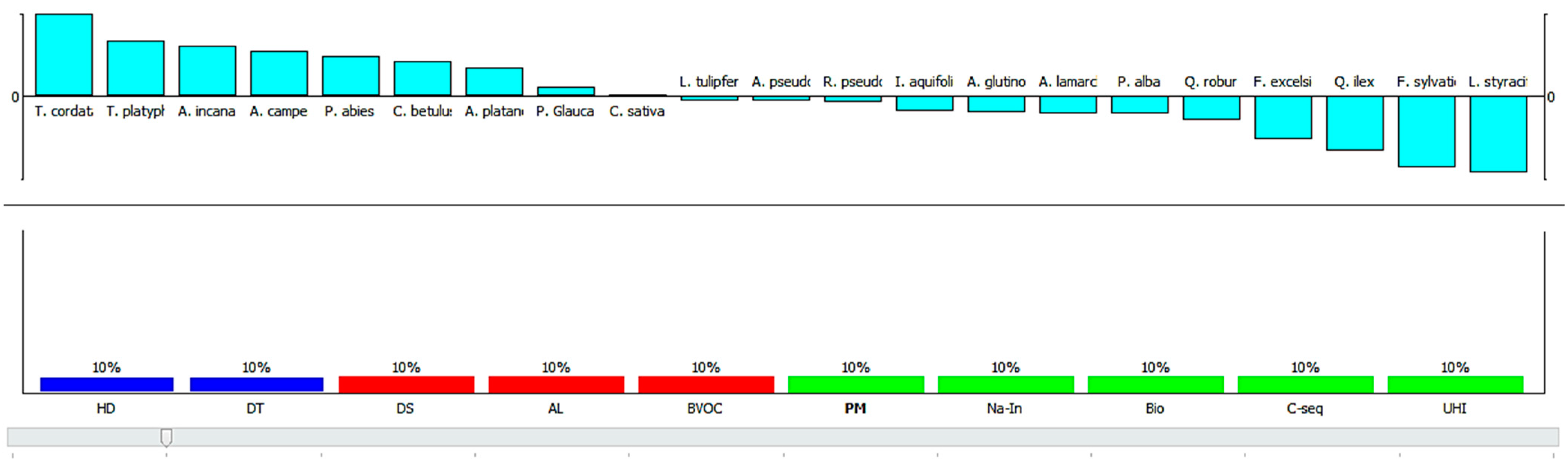
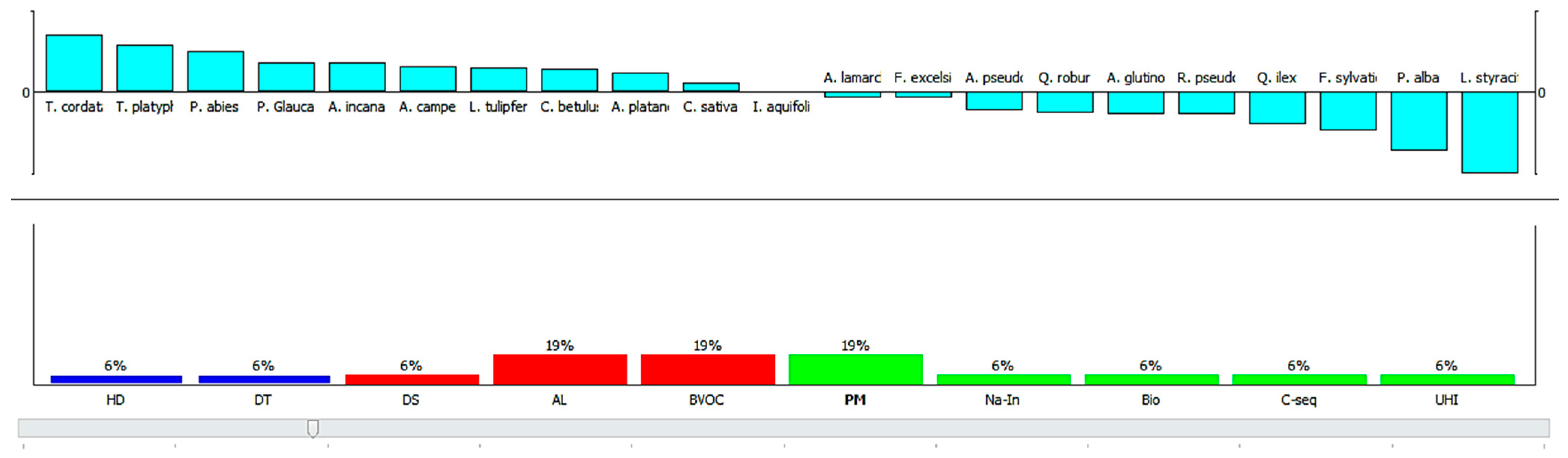
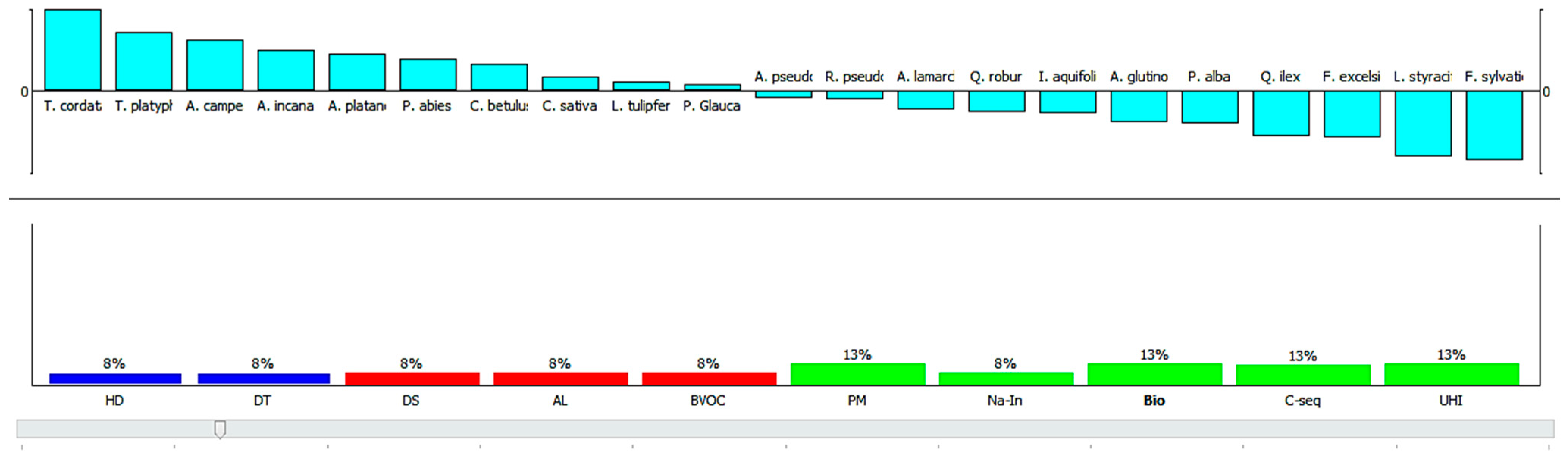
3.3.1. Scenario Analysis
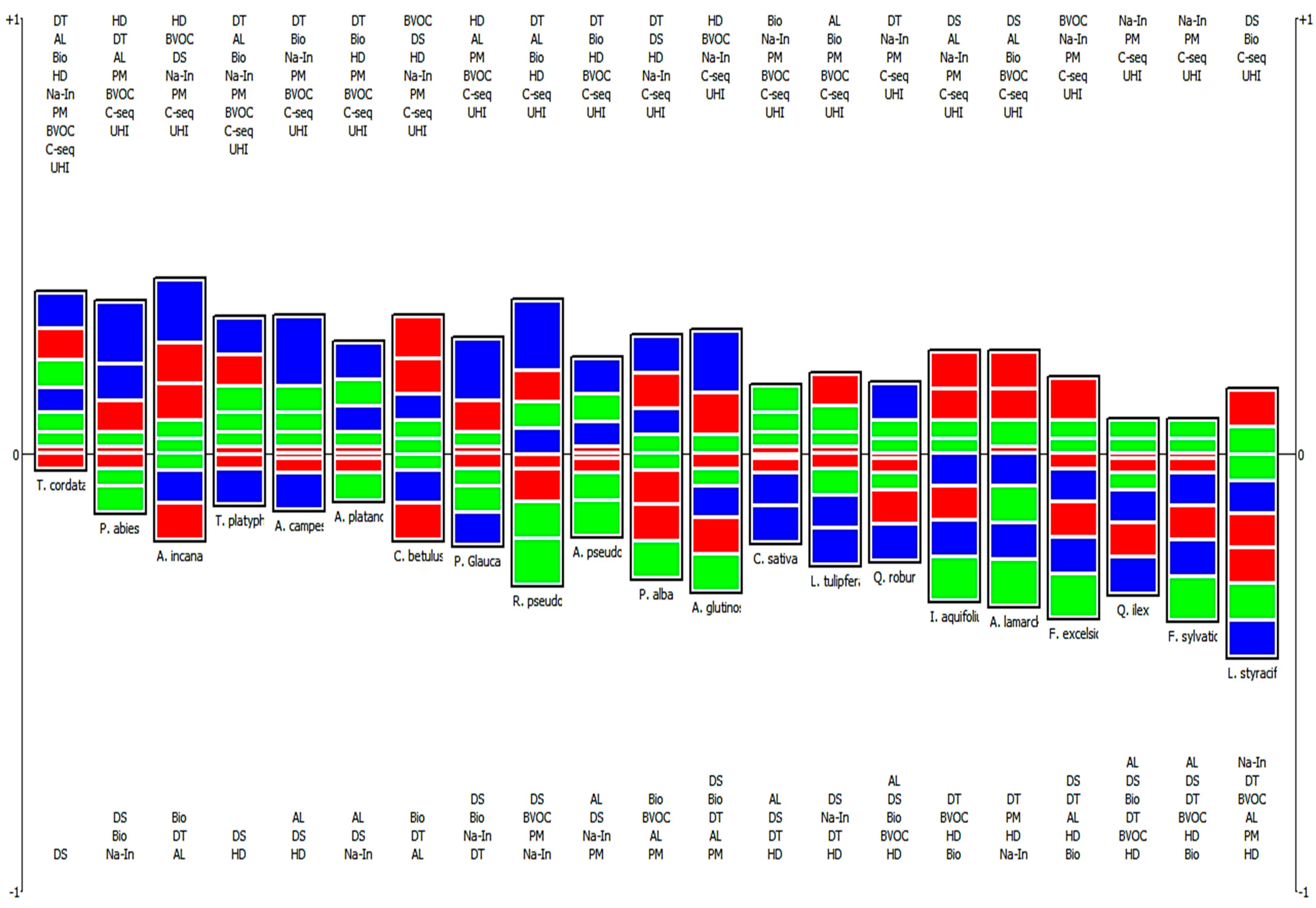
3.3.2. A Comprehensive Evaluation of Plant Species for Their Contribution in Ecosystem Services and Disservices
4. Discussion
4.1. Selection of Plant Species for PM Removal in Urban Environments: Similarities and Differences Using the Leaf Traits and Leaf SIRM Models
4.2. Enhancement of Plant Species Selection for Reducing PM in Urban Environments Using PROMETHEE
4.3. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzoulas, K.; Korpela, K.; Venn, S.; Yli-Pelkonen, V.; Kaźmierczak, A.; Niemela, J.; James, P. Promoting ecosystem and human health in urban areas using Green Infrastructure: A literature review. Landsc. Urban Plan. 2007, 81, 167–178. [Google Scholar] [CrossRef]
- Taylor, L.; Hochuli, D.F. Creating better cities: How biodiversity and ecosystem functioning enhance urban residents’ wellbeing. Urban Ecosyst. 2015, 18, 747–762. [Google Scholar] [CrossRef]
- Davies, P.; Corkery, L.; Nipperess, D.; Barnett, G.; Bishop, M.; Hochuli, D.; Hose, G.; Joei, C.; Lin, B.; Keane, A.; et al. Blueprint for Living Cities: Policy to Practice. National Green Infrastructure Network; National Green Infrastructure Network: Sydney, Australia, 2017. [Google Scholar]
- Millennium Ecosystem Assessment (MEA). Ecosystems and Human Well-Being: A Framework for Assessment; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- TEEB (The Economics of Ecosystems and Biodiversity). The Economics of Ecosystems and Biodiversity: Ecological and Economic Foundations; Earthscan: London, UK, 2010. [Google Scholar]
- Lyytimäki, J. Disservices of urban trees. In Routledge Handbook of Urban Forestry; Ferrini, F., van den Bosch, C.C.K., Fini, A., Eds.; Routledge: London, UK, 2017; pp. 164–176. [Google Scholar]
- Staas, L.; Beaumont, L.; Leishman, M. Which Plant Where: What We Heard: Documenting the Stakeholder Workshops; National Green Infrastructure Network: Sydney, Australia, 2017. [Google Scholar]
- Dwyer, J.F.; Nowak, D.J.; Noble, M.H. Sustaining urban forests. J. Arboric. 2003, 29, 49–55. [Google Scholar] [CrossRef]
- Berbés-Blázquez, M.; González, J.A.; Pascual, U. Towards an ecosystem services approach that addresses social power relations. Curr. Opin. Environ. Sustain. 2016, 19, 134–143. [Google Scholar] [CrossRef]
- Daw, T.; Brown, K.; Rosendo, S.; Pomeroy, R. Applying the ecosystem services concept to poverty alleviation: The need to disaggregate human well-being. Environ. Conserv. 2011, 34, 370–379. [Google Scholar] [CrossRef]
- Sikor, T.; Baggio, J.A. Can smallholders engage in tree plantations? An entitlements analysis from Vietnam. World Dev. 2014, 64, 101–112. [Google Scholar] [CrossRef]
- Brooks, E.G.E.; Smith, K.G.; Holland, R.A.; Poppy, G.M.; Eigenbrod, F. Effects of methodology and stakeholder disaggregation on ecosystem service valuation. Ecol. Soc. 2014, 19, 18. [Google Scholar] [CrossRef][Green Version]
- Hicks, C.C.; Cinner, J.E.; Stoeckl, N.; McClanahan, T.R. Linking ecosystem services and human-values theory. Conserv. Biol. 2015, 29, 1471–1480. [Google Scholar] [CrossRef]
- Lau, J.D.; Hicks, C.C.; Gurney, G.G.; Cinner, J.E. Disaggregating ecosystem service values and priorities by wealth, age, and education. Ecosyst. Serv. 2018, 29, 91–98. [Google Scholar] [CrossRef]
- Caceres, D.M.; Tapella, E.; Quetier, F.; Diaz, S. The social value of biodiversity and ecosystem services from the perspectives of different social actors. Ecol. Soc. 2015, 20, 62. [Google Scholar] [CrossRef]
- Milcu, A.I.; Leventon, J.; Hanspach, J.; Fischer, J. Disaggregated contributions of ecosystem services to human well-being in low-intensity farmland. Reg. Environ. Chang. 2015, 16, 117–163. [Google Scholar]
- Orenstein, D.E.; Groner, E. In the eye of the stakeholder: Changes in perceptions of ecosystem services across an international border. Ecosyst. Serv. 2014, 8, 185–196. [Google Scholar] [CrossRef]
- Sæbø, A.; Benedikz, T.; Randrup, T.B. Selection of trees for urban forestry in the Nordic countries. Urban For. Urban Green 2003, 2, 101–114. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Tripathi, B.D. Anticipated performance Index of some tree species considered for green belt development in and around an urban area: A case study of Varanasi city, India. J. Environ. Manag. 2008, 88, 1343–1349. [Google Scholar] [CrossRef]
- Nowak, D.J.; Heisler, G.M. Air Quality Effects of Urban Trees and Parks; National Recreation and Park Association: Ashburn, VA, USA, 2010; 46p. [Google Scholar]
- Pathak, V.; Tripathi, B.D.; Mishra, V.K. Evaluation of anticipated performance Index of some tree species for green belt development to mitigate traffic generated noise. Urban For. Urban Green. 2011, 10, 61–66. [Google Scholar] [CrossRef]
- Dobbs, C.; Escobedo, F.J.; Zipperer, W.C. A framework for developing urban forest ecosystem services and goods indicators. Landsc. Urban Plan. 2011, 99, 196–206. [Google Scholar] [CrossRef]
- Tiwary, A.; Williams, I.D.; Heidrich, O.; Namdeo, A.; Bandaru, V.; Calfapietra, C. Development of multi-functional streetscape green infrastructure using a performance index approach. Environ. Pollut. 2015, 208, 209–220. [Google Scholar] [CrossRef]
- O’Sullivan, O.S.; Holt, A.R.; Warren, P.H.; Evans, K.L. Optimising UK urban road verge contributions to biodiversity and ecosystem services with cost effective management. J. Environ. 2017, 191, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, Y.; Yan, P. Ranking the suitability of common urban tree species for controlling PM2.5 pollution. Atmos. Pollut. Res. 2015, 6, 267–277. [Google Scholar] [CrossRef]
- Muhammad, S.; Wuyts, K.; Samson, R. Atmospheric net particle accumulation on 96 plant species with contrasting morphological and anatomical leaf characteristics in a common garden experiment. Atmos. Environ. 2019, 202, 328–344. [Google Scholar] [CrossRef]
- Mareschal, B. Visual PROMETHEE 1.4 Manual. 2013. Available online: http://en.promethee-gaia.net/academic-edition.html (accessed on 2 February 2021).
- Breiman, L. Random forest. Mach. Learn. 2001, 45, 15–32. [Google Scholar]
- Bivand, R. classInt: Choose Univariate Class Intervals; R Package Version 0.3–3. 2019. Available online: https://github.com/r-spatial/classInt/ (accessed on 16 October 2022).
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Urban woodlands: Their role in reducing the effects of particulate pollution. Environ. Pollut. 1998, 99, 347–360. [Google Scholar] [CrossRef]
- Tiwary, A.; Sinnett, D.; Peachey, C.; Chalabi, Z.; Vardoulakis, S.; Fletcher, T.; Leonardi, G.; Grundy, C.; Azapagic, A.; Tony, R.; et al. An integrated tool to assess the role of new planting in PM10 capture and human health benefits: A case study in London. Environ. Pollut. 2009, 157, 2645–2653. [Google Scholar] [CrossRef]
- Tallis, M.; Taylor, G.; Sinnett, D.; Freer-Smith, P. Estimating the removal of atmospheric particulate pollution by the urban tree canopy of London, under current and future environments. Landsc. Urban Plan. 2011, 103, 129–138. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin, Germany, 2003; pp. 48–53. [Google Scholar]
- Vile, D.; Garnier, E.; Shipley, B.; Laurent, G.; Navas, M.L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Holder, C.D. The relationship between leaf hydrophobicity, water droplet retention, and leaf angle of common species in a semi-arid region of western United States. Agric. For. Meteorol. 2012, 152, 11–16. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Seasonal changes of leaf surface contamination in beech, oak, and ginkgo in relation to leaf micromorphology and wettability. New Phytol. 1998, 138, 91–98. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Li, Y.; Yu, Y.; Zhang, J. Seasoonal variations in leaf capturing of particulate matter, surface wettability and micromorphology in urban tree species. Front. Environ. Sci. Eng. 2013, 4, 579–588. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progress in superhydrophobic surface development. Soft Matter 2008, 84, 224–240. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to function application. J. Mater. Chem. 2008, 18, 621–663. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Adams, H., Adler, C., Aldunce, P., Ali, E., Begum, R.A., Betts, R., Kerr, R.B., Biesbroek, R., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Roloff, A.; Bärtels, A. Flora der Gehölzer; Eugen Ulmer Verlag: Stuttgart, Germany, 2014. [Google Scholar]
- Samson, R.; Ningal, T.F.; Tiwary, A.; Grote, R.; Fares, S.; Paoletti, E.; Calfapietra, C.; Saaroni, H.; Hiemstra, J.A.; Zhiyanski, U.V.; et al. Urban trees as environmental engineers. In The Urban Forest; Pearlmutter, D., Calfapietra, C., Samson, R., O’Brien, L., Ostoić, S.K., Sanesi, G., del Amo, R.A., Eds.; Future City 7; Springer International Publishing: New York, NY, USA, 2017; pp. 31–39. [Google Scholar]
- Samson, R.; Ningal, T.F.; Tiwary, A.; Grote, R.; Fares, S.; Saaroni, H.; Hiemstra, J.A.; Zhiyanski, M.; Vilhar, U.; Cariñanos, P.; et al. Species-specific information for enhancing ecosystem services. In The Urban Forest; Pearlmutter, D., Calfapietra, C., Samson, R., O’Brien, L., Ostoić, S.K., Sanesi, G., del Amo, R.A., Eds.; Future City 7; Springer International Publishing: New York, NY, USA, 2017; pp. 111–144. [Google Scholar]
- Cariñanos, P.; Adinolfi, C.; Díaz de la Guardia, C.; De Linares, C.; Casares-Porcel, M. Characterization of Allergen Emission Sources in Urban Areas. J. Environ. Qual. 2016, 45, 244–252. [Google Scholar] [CrossRef]
- Barwise, Y.; Kumar, P. Designing vegetation barriers for urban air pollution abatement: A practical review for appropriate plant species selection. Npj Clim. Atmos. Sci. 2020, 3, 12. [Google Scholar] [CrossRef]
- Singh, R.; Singh, M.P.; Singh, A.P. Ozone forming potential of tropical plant species of the Vidarbha region of Maharashtra state of India. Urban For. Urban Green. 2014, 13, 814–820. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Bacher, S.; Blackburn, T.M.; Dick, J.T.; Essl, F.; Evans, T.; Gaertner, M.; Hulme, P.E.; Kühn, I.; Mrugała, A.; et al. Defining the Impact of Non-Native Species. Conserv. Biol. 2014, 28, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.M.; Pyšek, P.; Rejmanek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Tsiaras, S.; Samara, T. Selection of the most suitable tree species in urban areas based on their capability of capturing heavy metals: A forest policy approach. Int. J. Sustain. Agric. Manag. Inform. 2019, 5, 15–24. [Google Scholar] [CrossRef]
- Kangas, A.; Kangas, J.; Pykäläinen, J. Outranking methods as tools in strategic natural resources planning. Silva Fenn. 2001, 35, 215–227. [Google Scholar] [CrossRef]
- Brans, J.P.; Mareschal, B. Promethee Methods. In Multiple Criteria Decision Analysis: State of the Art Surveys; Figueira, J., Greco, S., Ehrgott, M., Eds.; Springer: New York, NY, USA, 2005; pp. 163–186. [Google Scholar]
- Brans, J.P.; Vincke, P.; Mareschal, B. How to select and how to rank projects: The PROMETHEE method. Eur. J. Oper. Res. 1986, 24, 228–238. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.A.; Conway, T.M.; Eisenman, T.S.; Koeser, A.K.; Ordóñez Barona, C.; Locke, D.H.; Jenerette, G.D.; Östberg, J.; Vogt, J. Beyond ‘trees are good’: Disservices, management costs, and tradeoffs in urban forestry. Ambio 2020, 50, 615–630. [Google Scholar] [CrossRef]
- Mori, J.; Ferrini, F.; Sæbø, A. Air pollution mitigation by urban greening. Italus Hortus 2018, 25, 13–22. [Google Scholar] [CrossRef]
- Przybysz, A.; Popek, R.; Stankiewicz-Kosyl, M.; Zhu, C.Y.; Małecka-Przybysz, M.; Maulidyawati, T.; Mikowska, K.; Deluga, D.; Griżuk, K.; Sokalski-Wieczorek, J.; et al. Where trees cannot grow—Particulate matter accumulation by urban meadows. Sci. Total Environ. 2021, 785, 147310. [Google Scholar] [CrossRef]
- Popek, R.; Fornal-Pieniak, B.; Chyliński, F.; Pawełkowicz, M.; Bobrowicz, J.; Chrzanowska, D.; Piechota, N.; Przybysz, A. Not Only Trees Matter—Traffic-Related PM Accumulation by Vegetation of Urban Forests. Sustainability 2022, 14, 2973. [Google Scholar] [CrossRef]
- Gromke, C.; Ruck, B. Influence of trees on the dispersion of pollutants in an urban street canyon-Experimental investigation of the flow and concentration field. Atmos. Environ. 2007, 41, 3287–3302. [Google Scholar] [CrossRef]
- Gromke, C.; Ruck, B. Effects of trees on the dilution of vehicle exhaust emissions in urban street canyons. Int. J. Environ. Waste Manag. 2009, 4, 225–242. [Google Scholar] [CrossRef]
- Gromke, C.; Ruck, B. Pollutant concentration in street canyons of different aspect ratio with avenues of trees for various wind directions. Bound.-Layer Meteorol. 2012, 144, 41–64. [Google Scholar] [CrossRef]
- Wania, A.; Bruse, M.; Blond, N.; Weber, C. Analysing the influence of different street vegetation on traffic-induced particle dispersion using microscale simulations. J. Environ. Manag. 2011, 94, 91–101. [Google Scholar] [CrossRef]
- Vos, P.E.J.; Maiheu, B.; Vankerkom, J.; Janssen, S. Improving local air quality in cities; To tree or not to tree? Environ. Pollut. 2013, 183, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Khera, N.; Metha, V.; Sabata, B.C. Interrelationship of birds and habitat features in urban green spaces in Delhi, India. Urban For. Urban Green. 2009, 8, 187–196. [Google Scholar] [CrossRef]
- Farinha-Marques, P.; Lameiras, J.M.; Fernandes, C.; Silva, S.; Guiherme, F. Urban biodiversity: A review of current concepts and contributions to multidisciplinary approaches. Eur. J. Soc. Sci. Res. 2011, 24, 247–271. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in tree species ability to capture and retain airborne fine particulate matter (PM2.5). Nat.-Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Leonard, R.J.; McArthur, C.; Hochuli, D.F. Particulate matter deposition on roadside plants and the importance of leaf trait combinations. Urban For. Urban Green. 2016, 20, 249–253. [Google Scholar] [CrossRef]
- Singh, S.N.; Rao, D.N. Certain responses of wheat plants to cement dust pollution. Environ. Pollut. 1981, 24, 75–81. [Google Scholar] [CrossRef]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef]
- Czaja, M.; Kołton, A.; Muras, P. The complex issue of urban trees—Stress factor accumulation and ecological service possibilities. Forests 2020, 11, 932. [Google Scholar] [CrossRef]
- Vyshkvarkova, E.; Sukhonos, O. Compound extremes of air temperature and precipitation in Eastern Europe. Climate 2022, 10, 133. [Google Scholar] [CrossRef]

| Criterion | Data Source |
| Leaf Traits Leaf SIRM | Measured in September 2016, Muhammad et al. (2019) |
| Leaf Longevity | Missouri Botanical Garden http://www.missouribotanicalgarden.org/plantfinder/plantfindersearch.aspx [accessed on 20 December 2020] |
| Leaf Area Index | A Global Database of Field-observed Leaf Area Index in Woody Plant Species, 1932–2011 https://daac.ornl.gov/VEGETATION/guides/LAI_Woody_Plants.html [accessed on 21 November 2022] |
| Drought Tolerance, BVOC, Allergenicity, Food Source, Reduce Urban Heat Island Effect, Net Carbon Sequestration | The Urban Forest-Cultivating Green Infrastructure for People and the Environment, Samson, R., Ningal, T.F., Tiwary, A., Grote, R., Fares, S., Saaroni, H., Hiemstra, J.A., Zhiyanski, M., Vilhar. U., Cariñanos, P., Järvi, L., Przybysz, A., Moretti, M., Zürcher, N., 2017. The Urban Forest, Future City 7. Species–specific information for enhancing ecosystem services. Springer International Publishing. Pearlmutter, D., et al. (eds) pp. 111–144. |
| Pollination | Plants For A Future https://pfaf.org/user/default.aspx [accessed on 23 December 2020] |
| Hardiness | Urban Forest Ecosystems Institute https://selectree.calpoly.edu/ [accessed on 2 January 2021] |
| Invasive Potential | Invasive Species in Belgium https://ias.biodiversity.be/species/all [accessed on 5 January 2021] |
| Origin/Native Disease Susceptibility | Forest Ecology and Forest Management Group https://www.wur.nl/en/Research-Results/Chair-groups/Environmental-Sciences/Forest-Ecology-and-Forest-Management-Group/Education/Tree-database/Temperate-Species.htm [accessed on 10 January 2021] |
| Leaf Traits Model | Services and Disservices Model | |||||
|---|---|---|---|---|---|---|
| Criteria | Weight | Criteria | Equal | S1 | S2 | S3 |
| Single leaf area | 0.10 | PM mitigation | 0.10 | 0.19 | 0.16 | 0.13 |
| Leaf dissection index (LDI) | 0.09 | Supporting biodiversity | 0.10 | 0.06 | 0.06 | 0.13 |
| Leaf roundness | 0.11 | Urban heat island effect | 0.10 | 0.06 | 0.06 | 0.13 |
| Specific leaf area | 0.23 | Net carbon sequestration | 0.10 | 0.06 | 0.06 | 0.13 |
| Leaf wettability | 0.19 | Allergenicity | 0.10 | 0.19 | 0.06 | 0.08 |
| Trichome density | 0.28 | BVOC emissions | 0.10 | 0.19 | 0.06 | 0.08 |
| Native/invasive | 0.10 | 0.06 | 0.16 | 0.08 | ||
| Drought tolerance | 0.10 | 0.06 | 0.16 | 0.08 | ||
| Plant hardiness | 0.10 | 0.06 | 0.06 | 0.08 | ||
| Disease susceptibility | 0.10 | 0.06 | 0.16 | 0.08 | ||
| Plant Species | LA (0.10) | LDI (0.09) | TD (0.28) | Wettability (0.19) | SLA (0.23) | Roundness (0.11) | Longevity | LAI | Π | Performance |
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudotsuga menziesii (Mirb.) | 3 | 2 | 1 | 3 | 3 | 3 | 1.0 | 9.50 | 22.33 | +++ |
| Abies fraseri (Pursh.) | 3 | 1 | 1 | 3 | 3 | 3 | 1.0 | 9.01 | 20.35 | +++ |
| Picea abies (L.) | 3 | 2 | 1 | 1 | 3 | 3 | 1.0 | 7.80 | 15.37 | +++ |
| Pinus nigra (Arnold.) | 3 | 1 | 1 | 3 | 3 | 3 | 1.0 | 5.75 | 13.00 | +++ |
| Thuja plicata (Donn.) | 3 | 1 | 1 | 2 | 3 | 2 | 1.0 | 6.45 | 12.64 | +++ |
| Ilex aquifolium (L.) | 3 | 1 | 1 | 2 | 3 | 2 | 1.0 | 5.75 | 11.27 | +++ |
| Quercus ilex (L.) | 3 | 2 | 3 | 1 | 3 | 2 | 1.0 | 4.50 | 10.89 | +++ |
| Rhododendron (L.) | 2 | 2 | 1 | 3 | 3 | 3 | 1.0 | 4.50 | 10.13 | +++ |
| Carpinus betulus (L.) | 3 | 2 | 2 | 3 | 2 | 2 | 0.5 | 6.10 | 6.98 | +++ |
| Castanea sativa (Mill.) | 2 | 1 | 3 | 3 | 2 | 3 | 0.5 | 5.10 | 6.35 | +++ |
| Fagus sylvatica (L.) | 3 | 1 | 2 | 2 | 1 | 2 | 0.5 | 6.25 | 5.56 | +++ |
| Tilia platyphyllos (Scop.) | 2 | 1 | 3 | 2 | 1 | 1 | 0.5 | 5.95 | 5.50 | +++ |
| Quercus petraea (Matt.) | 3 | 1 | 3 | 1 | 2 | 2 | 0.5 | 5.15 | 5.41 | +++ |
| Alnus glutinosa (L.) | 2 | 2 | 2 | 3 | 1 | 1 | 0.5 | 5.20 | 4.81 | +++ |
| Tilia cordata (Mill.) | 2 | 2 | 1 | 2 | 1 | 1 | 0.5 | 6.85 | 4.73 | +++ |
| Acer campestre (L.) | 3 | 1 | 3 | 3 | 2 | 1 | 0.5 | 3.90 | 4.62 | +++ |
| Liriodendron tulipifera (L.) | 1 | 3 | 1 | 1 | 1 | 1 | 0.5 | 7.40 | 4.37 | +++ |
| Quercus rubra (L.) | 2 | 2 | 2 | 1 | 2 | 2 | 0.5 | 4.60 | 4.16 | +++ |
| Populus alba (L.) | 2 | 2 | 2 | 3 | 1 | 1 | 0.5 | 4.50 | 4.16 | +++ |
| Picea pungens glauca (Moench.) | 3 | 2 | 1 | 3 | 3 | 3 | 1.0 | 1.76 | 4.14 | +++ |
| Acer ginnala (Maxim.) | 3 | 1 | 1 | 3 | 2 | 1 | 0.5 | 4.55 | 4.12 | ++ |
| Acer platanoides (L.) | 2 | 1 | 2 | 2 | 2 | 1 | 0.5 | 4.55 | 4.10 | ++ |
| Quercus palustris (Münchh.) | 3 | 3 | 1 | 2 | 1 | 2 | 0.5 | 4.55 | 3.82 | ++ |
| Quercus robur (L.) | 3 | 1 | 1 | 1 | 2 | 2 | 0.5 | 4.55 | 3.50 | ++ |
| Liquidambar styraciflua (L.) | 2 | 2 | 1 | 2 | 1 | 1 | 0.5 | 4.80 | 3.31 | ++ |
| Betula pendula (Roth.) | 3 | 1 | 2 | 3 | 2 | 1 | 0.5 | 3.10 | 3.24 | ++ |
| Acer pseudoplatanus (L.) | 2 | 2 | 1 | 1 | 2 | 1 | 0.5 | 4.55 | 3.23 | ++ |
| Larix decidua (Mill.) | 3 | 1 | 1 | 1 | 3 | 3 | 0.5 | 3.05 | 2.87 | ++ |
| Robinia pseudoacacia (L.) | 3 | 2 | 3 | 1 | 1 | 2 | 0.5 | 2.90 | 2.84 | ++ |
| Fraxinus excelsior (L.) | 3 | 1 | 2 | 3 | 2 | 2 | 0.5 | 2.50 | 5.78 | ++ |
| Alnus incana (L.) | 2 | 1 | 2 | 2 | 2 | 1 | 0.5 | 3.00 | 2.70 | ++ |
| Corylus avellana (L.) | 2 | 1 | 2 | 3 | 1 | 1 | 0.5 | 2.54 | 2.24 | ++ |
| Salix viminalis (L.) | 3 | 1 | 3 | 1 | 1 | 3 | 0.5 | 1.54 | 1.52 | ++ |
| Salix repens (L.) | 3 | 1 | 3 | 2 | 2 | 3 | 0.5 | 1.23 | 1.48 | ++ |
| Salix rosmarinifolia (L.) | 3 | 3 | 3 | 1 | 2 | 3 | 0.5 | 1.23 | 1.47 | ++ |
| Viburnum lantana (L.) | 2 | 1 | 3 | 3 | 2 | 2 | 0.5 | 1.23 | 1.46 | ++ |
| Rosa rugosa (Thunb.) | 3 | 1 | 3 | 1 | 3 | 2 | 0.5 | 1.23 | 1.43 | ++ |
| Prunus spinosa (L.) | 3 | 1 | 3 | 2 | 2 | 2 | 0.5 | 1.23 | 1.41 | ++ |
| Cornus sanguinea (L.) | 2 | 1 | 3 | 3 | 2 | 1 | 0.5 | 1.23 | 1.40 | ++ |
| Viburnum opulus (L.) | 2 | 1 | 3 | 3 | 2 | 1 | 0.5 | 1.23 | 1.40 | ++ |
| Hibiscus syriacus (L.) | 3 | 1 | 3 | 3 | 1 | 2 | 0.5 | 1.23 | 1.38 | ++ |
| Salix aurita (L.) | 3 | 1 | 3 | 1 | 2 | 3 | 0.5 | 1.23 | 1.36 | + |
| Euonymus europaeus (L.) | 3 | 1 | 2 | 3 | 2 | 2 | 0.5 | 1.23 | 1.35 | + |
| Buddleja davidii (Franch.) | 3 | 2 | 3 | 1 | 2 | 2 | 0.5 | 1.23 | 1.35 | + |
| Lonicera xylosteum (L.) | 3 | 1 | 3 | 1 | 2 | 2 | 0.5 | 1.23 | 1.29 | + |
| Rosa rubiginosa (L.) | 3 | 1 | 2 | 3 | 2 | 1 | 0.5 | 1.23 | 1.29 | + |
| Syringa vulgaris (L.) | 2 | 1 | 1 | 3 | 3 | 2 | 0.5 | 1.23 | 1.26 | + |
| Larix kaempferi (Lamb.) | 3 | 1 | 1 | 1 | 2 | 3 | 0.5 | 1.45 | 1.20 | + |
| Ligustrum ovalifolium (Hasssk.) | 3 | 1 | 1 | 3 | 2 | 2 | 0.5 | 1.23 | 1.18 | + |
| Sambucus nigra (L.) | 2 | 1 | 2 | 3 | 1 | 2 | 0.5 | 1.23 | 1.15 | + |
| Salix cinerea (L.) | 3 | 1 | 3 | 1 | 1 | 2 | 0.5 | 1.23 | 1.15 | + |
| Ligustrum vulgare (L.) | 3 | 1 | 1 | 2 | 2 | 3 | 0.5 | 1.23 | 1.13 | + |
| Amelanchier lamarckii (Schroed.) | 3 | 2 | 1 | 2 | 2 | 2 | 0.5 | 1.23 | 1.12 | + |
| Rosa pimpinellifolia (L.) | 3 | 1 | 2 | 1 | 2 | 2 | 0.5 | 1.23 | 1.12 | + |
| Cornus alba (L.) | 2 | 1 | 3 | 1 | 1 | 2 | 0.5 | 1.23 | 1.09 | + |
| Prunus padus (L.) | 2 | 1 | 2 | 1 | 2 | 2 | 0.5 | 1.23 | 1.06 | + |
| Rhamnus frangula (L.) | 3 | 1 | 1 | 3 | 1 | 2 | 0.5 | 1.23 | 1.04 | + |
| Hippophae rhamnoides (L.) | 3 | 1 | 1 | 1 | 2 | 3 | 0.5 | 1.23 | 1.01 | + |
| Salix purpurea (L.) | 3 | 1 | 1 | 1 | 2 | 3 | 0.5 | 1.23 | 1.01 | + |
| Rosa glauca (Pourret.) | 3 | 2 | 1 | 1 | 2 | 2 | 0.5 | 1.23 | 1.00 | + |
| Lonicera tatarica (L.) | 3 | 2 | 1 | 1 | 2 | 1 | 0.5 | 1.23 | 0.93 | + |
| Plant Species | SIRM | Longevity | LAI | Π | Performance |
|---|---|---|---|---|---|
| Pseudotsuga menziesii (Mirb.) | 2 | 1.0 | 9.50 | 19.00 | +++ |
| Thuja plicata (Donn.) | 2 | 1.0 | 6.45 | 12.90 | +++ |
| Carpinus betulus (L.) | 3 | 0.5 | 6.10 | 9.15 | +++ |
| Abies fraseri (Pursh.) | 1 | 1.0 | 9.01 | 9.01 | +++ |
| Quercus ilex (L.) | 2 | 1.0 | 4.50 | 9.00 | +++ |
| Picea abies (L.) | 1 | 1.0 | 7.80 | 7.80 | +++ |
| Fagus sylvatica (L.) | 2 | 0.5 | 6.25 | 6.25 | +++ |
| Tilia platyphyllos (Scop.) | 2 | 0.5 | 5.95 | 5.95 | +++ |
| Acer campestre (L.) | 3 | 0.5 | 3.90 | 5.85 | +++ |
| Ilex aquifolium (L.) | 1 | 1.0 | 5.75 | 5.75 | +++ |
| Pinus nigra (Arnold.) | 1 | 1.0 | 5.75 | 5.75 | +++ |
| Quercus petraea (Matt.) | 2 | 0.5 | 5.15 | 5.15 | +++ |
| Castanea sativa (Mill.) | 2 | 0.5 | 5.10 | 5.10 | +++ |
| Acer platanoides (L.) | 2 | 0.5 | 4.55 | 4.55 | +++ |
| Quercus robur (L.) | 2 | 0.5 | 4.55 | 4.55 | +++ |
| Rhododendron (L.) | 1 | 1.0 | 4.50 | 4.50 | +++ |
| Liriodendron tulipifera (L.) | 1 | 0.5 | 7.40 | 3.70 | +++ |
| Picea pungens glauca (Moench.) | 2 | 1.0 | 1.76 | 3.52 | +++ |
| Tilia cordata (Mill.) | 1 | 0.5 | 6.85 | 3.43 | +++ |
| Alnus incana (L.) | 2 | 0.5 | 3.00 | 3.00 | +++ |
| Alnus glutinosa (L.) | 1 | 0.5 | 5.20 | 2.60 | ++ |
| Corylus avellana (L.) | 2 | 0.5 | 2.54 | 2.54 | ++ |
| Liquidambar styraciflua (L.) | 1 | 0.5 | 4.80 | 2.40 | ++ |
| Quercus rubra (L.) | 1 | 0.5 | 4.60 | 2.30 | ++ |
| Acer ginnala (Maxim.) | 1 | 0.5 | 4.55 | 2.28 | ++ |
| Acer pseudoplatanus (L.) | 1 | 0.5 | 4.55 | 2.28 | ++ |
| Quercus palustris (Münchh.) | 1 | 0.5 | 4.55 | 2.28 | ++ |
| Populus alba (L.) | 1 | 0.5 | 4.50 | 2.25 | ++ |
| Rosa rugosa (Thunb.) | 3 | 0.5 | 1.23 | 1.85 | ++ |
| Viburnum lantana (L.) | 3 | 0.5 | 1.23 | 1.85 | ++ |
| Viburnum opulus (L.) | 3 | 0.5 | 1.23 | 1.85 | ++ |
| Betula pendula (Roth.) | 1 | 0.5 | 3.10 | 1.55 | ++ |
| Salix viminalis (L.) | 2 | 0.5 | 1.54 | 1.54 | ++ |
| Larix decidua (Mill.) | 1 | 0.5 | 3.05 | 1.53 | ++ |
| Robinia pseudoacacia (L.) | 1 | 0.5 | 2.90 | 1.45 | ++ |
| Fraxinus excelsior (L.) | 1 | 0.5 | 2.50 | 1.25 | ++ |
| Amelanchier lamarckii (Schroed.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Euonymus europaeus (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Hippophae rhamnoides (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Lonicera tatarica (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Lonicera xylosteum (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Prunus spinosa (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Prunus padus (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Rhamnus frangula (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Rosa pimpinellifolia (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Rosa rubiginosa (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Salix aurita (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Salix cinerea (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Salix rosmarinifolia (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Salix repens (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Sambucus nigra (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Syringa vulgaris (L.) | 2 | 0.5 | 1.23 | 1.23 | ++ |
| Larix kaempferi (Lamb.) | 1 | 0.5 | 1.45 | 0.73 | + |
| Buddleja davidii (Franch.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Cornus alba (L.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Cornus sanguinea (L.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Hibiscus syriacus (L.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Ligustrum ovalifolium (Hassk.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Ligustrum vulgare (L.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Rosa glauca(Pourret.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Salix purpurea (L.) | 1 | 0.5 | 1.23 | 0.62 | + |
| Plant Species | Native/Invasive | Plant Hardiness | Drought Tolerance | Disease Susceptibility | Allergenicity | BVOC Emissions | PM Mitigation | Biodiversity | Net Carbon Sequestration | Urban Heat Island Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| Acer campestre (L.) | 3 | 1 | 3 | 1 | 2 | 2 | 3 | 3 | 1 | 2 |
| Acer platanoides (L.) | 2 | 2 | 2 | 1 | 2 | 2 | 3 | 3 | 2 | 3 |
| Acer pseudoplatanus (L.) | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 3 | 2 | 3 |
| Alnus glutinosa (L.) | 3 | 3 | 1 | 1 | 1 | 3 | 2 | 2 | 3 | 2 |
| Alnus incana (L.) | 3 | 3 | 1 | 3 | 1 | 3 | 3 | 2 | 2 | 2 |
| Amelanchier lamarckii (Schroed.) | 1 | 1 | 1 | 3 | 3 | 2 | 2 | 3 | 1 | 1 |
| Carpinus betulus (L.) | 3 | 2 | 1 | 3 | 1 | 3 | 3 | 2 | 2 | 2 |
| Castanea sativa (Mill.) | 3 | 1 | 1 | 1 | 2 | 2 | 3 | 3 | 3 | 3 |
| Fagus sylvatica (L.) | 3 | 1 | 1 | 1 | 2 | 1 | 3 | 1 | 3 | 3 |
| Fraxinus excelsior (L.) | 3 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 3 | 3 |
| Ilex aquifolium (L.) | 3 | 1 | 1 | 3 | 3 | 1 | 3 | 1 | 1 | 1 |
| Liquidambar styraciflua (L.) | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 3 | 2 | 2 |
| Liriodendron tulipifera (L.) | 2 | 1 | 1 | 1 | 3 | 2 | 3 | 3 | 2 | 3 |
| Picea abies (L.) | 2 | 3 | 2 | 1 | 3 | 2 | 3 | 2 | 2 | 2 |
| Picea pungens glauca (Moench.) | 2 | 3 | 1 | 1 | 3 | 2 | 3 | 2 | 2 | 2 |
| Populus alba (L.) | 3 | 2 | 2 | 3 | 1 | 1 | 2 | 2 | 3 | 3 |
| Quercus ilex (L.) | 3 | 1 | 1 | 1 | 2 | 1 | 3 | 2 | 2 | 2 |
| Quercus robur (L.) | 3 | 1 | 2 | 1 | 2 | 1 | 3 | 2 | 3 | 3 |
| Robinia pseudoacacia (L.) | 1 | 2 | 3 | 1 | 3 | 1 | 2 | 3 | 3 | 2 |
| Tilia cordata (Mill.) | 3 | 2 | 2 | 1 | 3 | 2 | 3 | 3 | 3 | 3 |
| Tilia platyphyllos (Scop.) | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 3 | 3 | 3 |
| Rank | Plant Species | Phi | Phi+ | Phi− |
|---|---|---|---|---|
| 1 | Tilia cordata (Mill.) | 0.34 | 0.44 | 0.11 |
| 2 | Picea abies (L.) | 0.21 | 0.41 | 0.20 |
| 3 | Alnus incana (L.) | 0.20 | 0.41 | 0.21 |
| 4 | Tilia platyphyllos (Scop.) | 0.19 | 0.35 | 0.16 |
| 5 | Acer campestre (L.) | 0.19 | 0.37 | 0.18 |
| 6 | Acer platanoides (L.) | 0.15 | 0.37 | 0.22 |
| 7 | Carpinus betulus (L.) | 0.12 | 0.36 | 0.25 |
| 8 | Picea pungens glauca (Moench.) | 0.06 | 0.31 | 0.26 |
| 9 | Robinia pseudoacacia (L.) | 0.05 | 0.38 | 0.33 |
| 10 | Acer pseudoplatanus (L.) | 0.03 | 0.34 | 0.31 |
| 11 | Populus alba (L.) | −0.01 | 0.34 | 0.35 |
| 12 | Alnus glutinosa (L.) | −0.03 | 0.30 | 0.33 |
| 13 | Castanea sativa (Mill.) | −0.05 | 0.21 | 0.26 |
| 14 | Liriodendron tulipfera (L.) | −0.07 | 0.22 | 0.29 |
| 15 | Quercus robur (L.) | −0.08 | 0.23 | 0.31 |
| 16 | Ilex aquifolium (L.) | −0.10 | 0.23 | 0.34 |
| 17 | Amelanchier lamarckii (Schroed.) | −0.11 | 0.26 | 0.37 |
| 18 | Fraxinus excelsior (L.) | −0.20 | 0.17 | 0.38 |
| 19 | Quercus ilex (L.) | −0.24 | 0.13 | 0.37 |
| 20 | Fagus sylvatica (L.) | −0.30 | 0.11 | 0.41 |
| 21 | Liquidambar styraciflua (L.) | −0.32 | 0.16 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, S.; Wuyts, K.; Samson, R. Selection of Plant Species for Particulate Matter Removal in Urban Environments by Considering Multiple Ecosystem (Dis)Services and Environmental Suitability. Atmosphere 2022, 13, 1960. https://doi.org/10.3390/atmos13121960
Muhammad S, Wuyts K, Samson R. Selection of Plant Species for Particulate Matter Removal in Urban Environments by Considering Multiple Ecosystem (Dis)Services and Environmental Suitability. Atmosphere. 2022; 13(12):1960. https://doi.org/10.3390/atmos13121960
Chicago/Turabian StyleMuhammad, Samira, Karen Wuyts, and Roeland Samson. 2022. "Selection of Plant Species for Particulate Matter Removal in Urban Environments by Considering Multiple Ecosystem (Dis)Services and Environmental Suitability" Atmosphere 13, no. 12: 1960. https://doi.org/10.3390/atmos13121960
APA StyleMuhammad, S., Wuyts, K., & Samson, R. (2022). Selection of Plant Species for Particulate Matter Removal in Urban Environments by Considering Multiple Ecosystem (Dis)Services and Environmental Suitability. Atmosphere, 13(12), 1960. https://doi.org/10.3390/atmos13121960







