Association between PM2.5 Exposure and Cardiovascular and Respiratory Hospital Admissions Using Spatial GIS Analysis
Abstract
1. Introduction
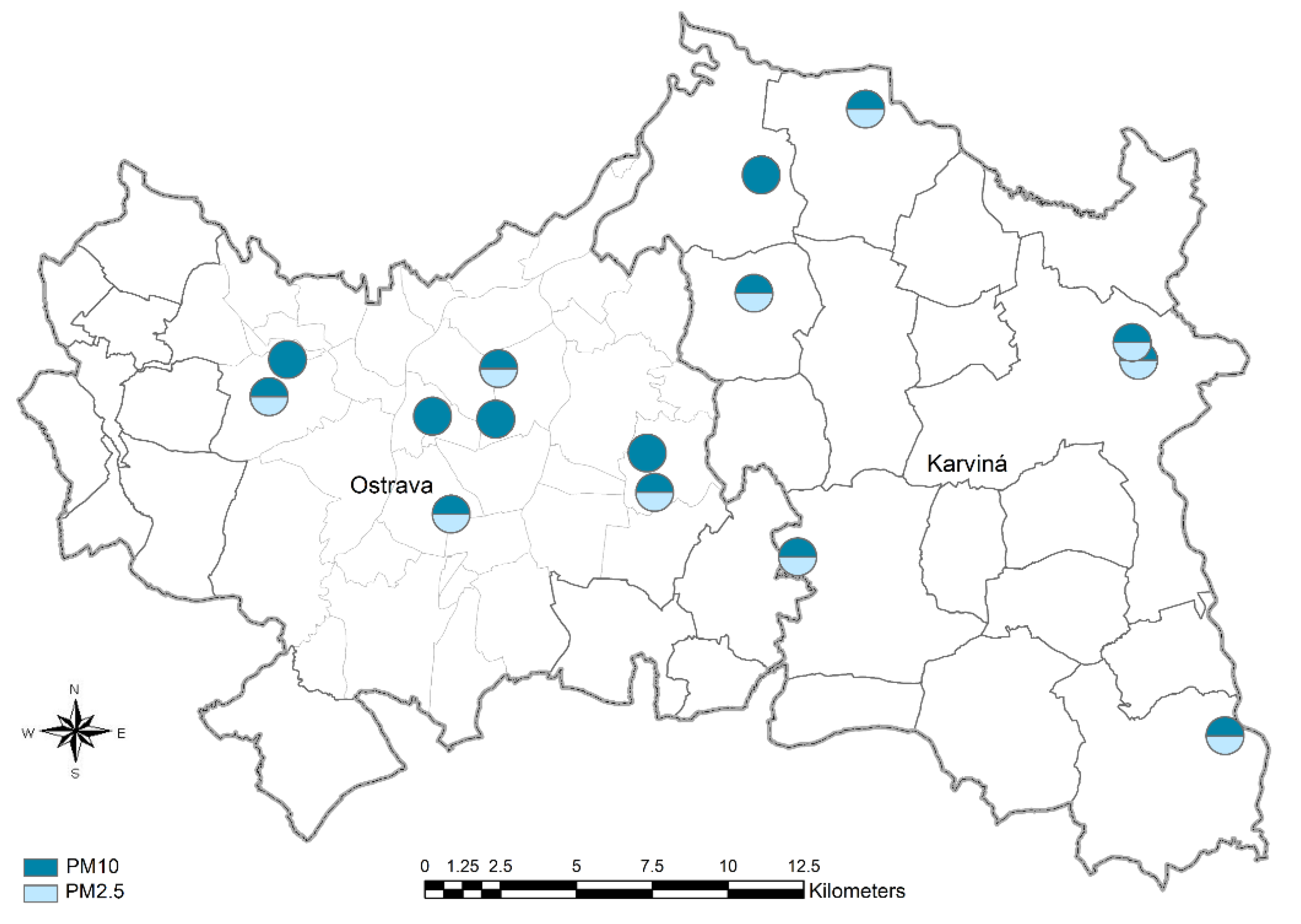
2. Materials and Methods
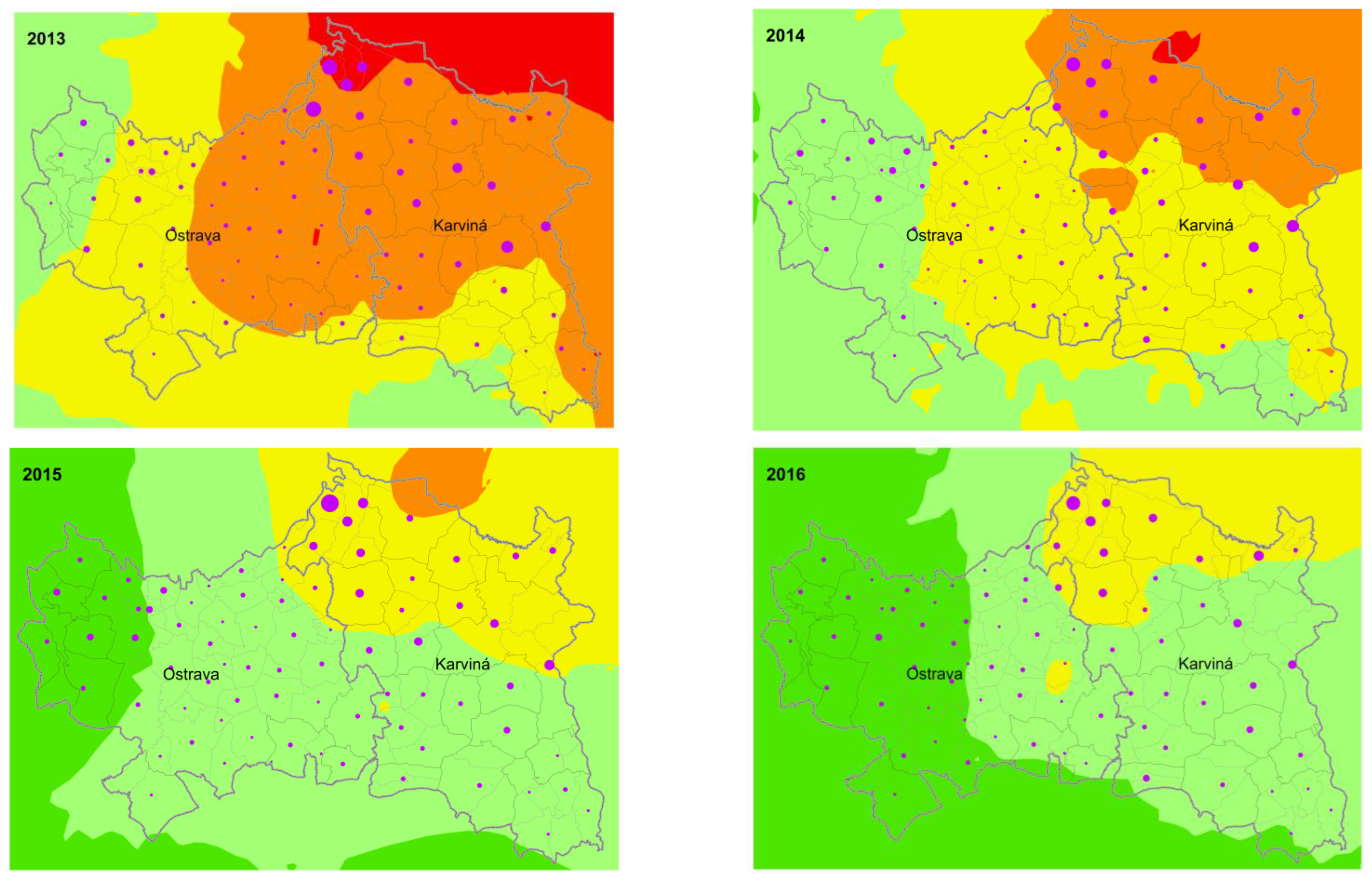
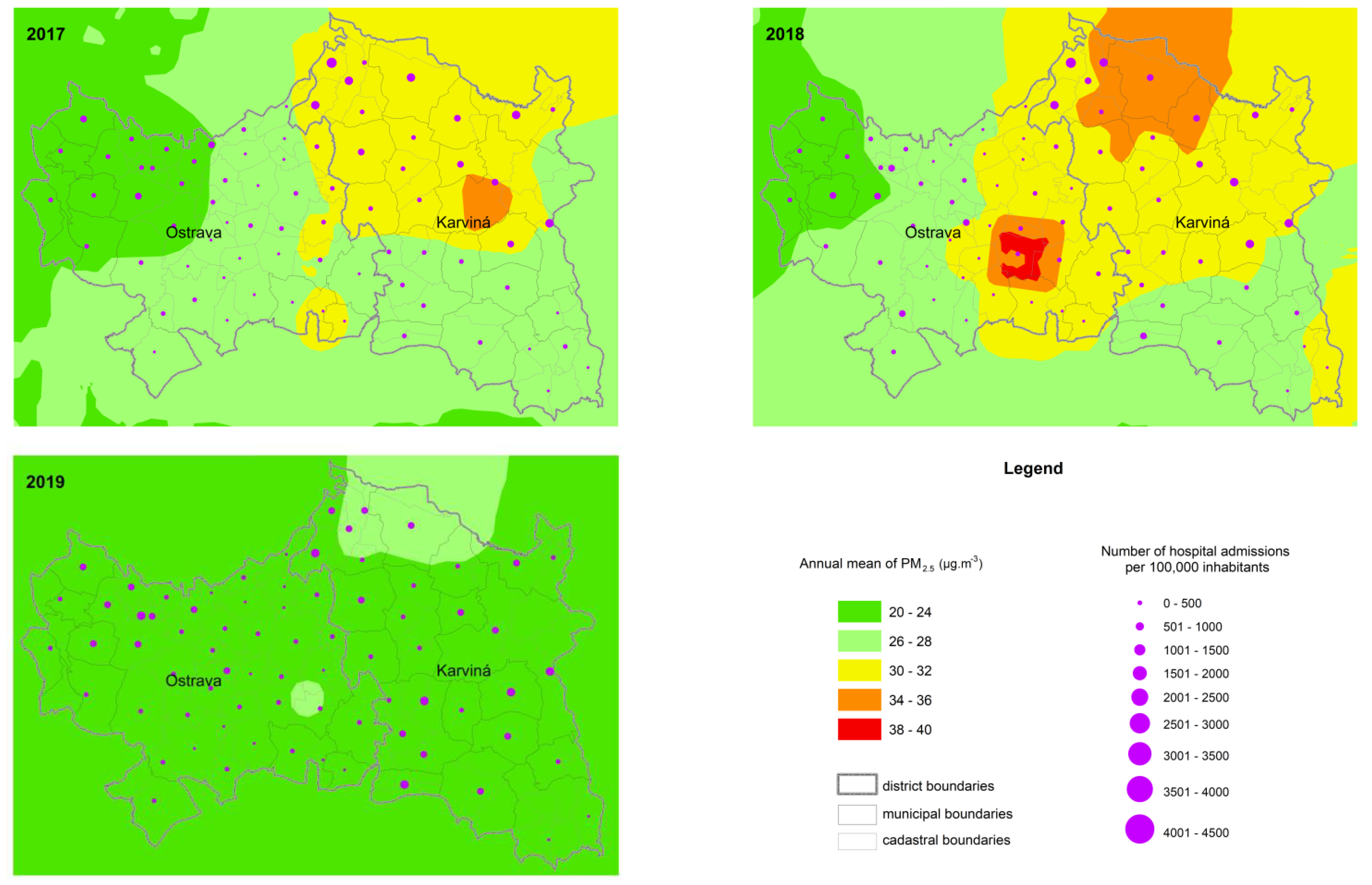
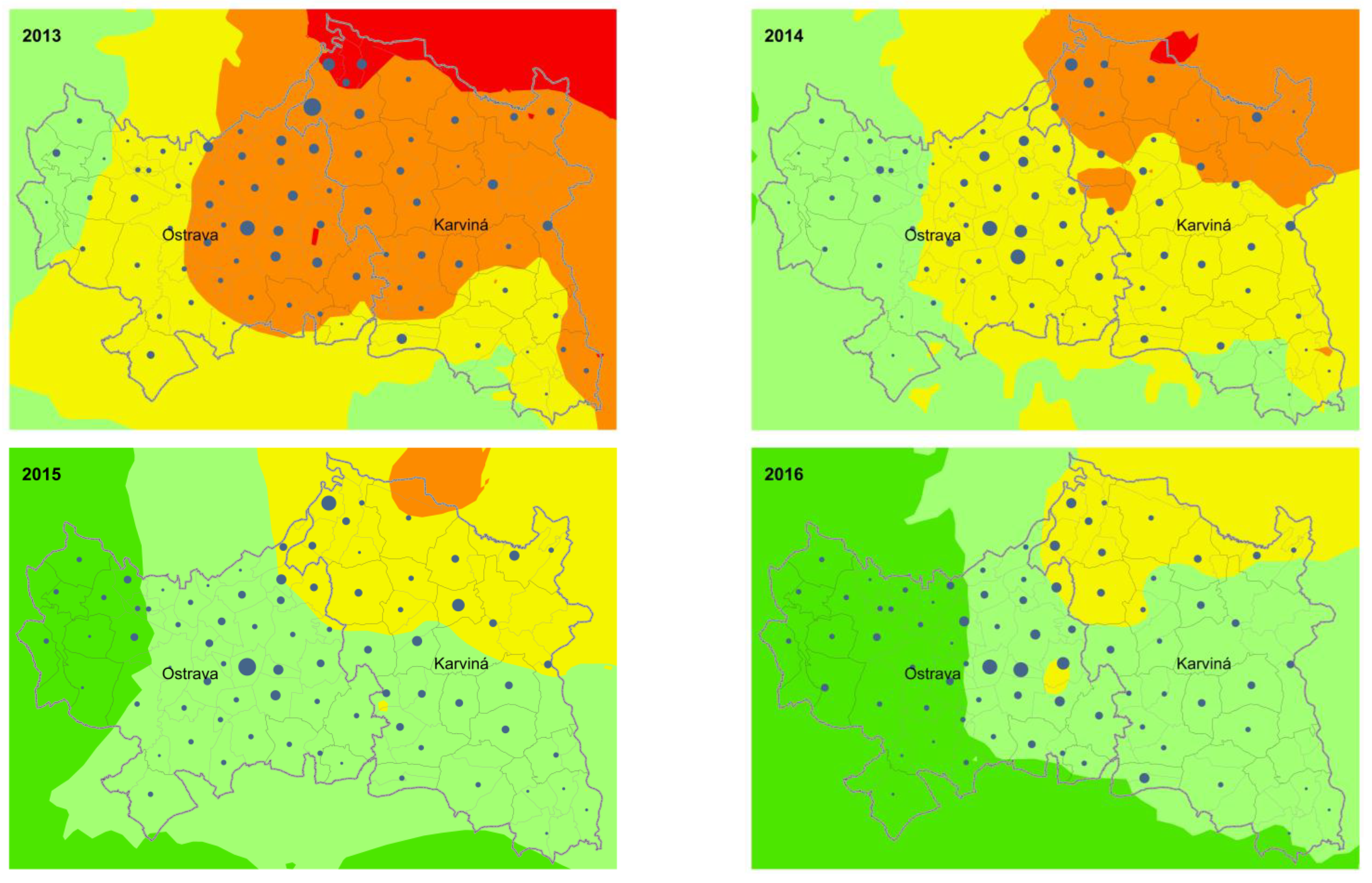
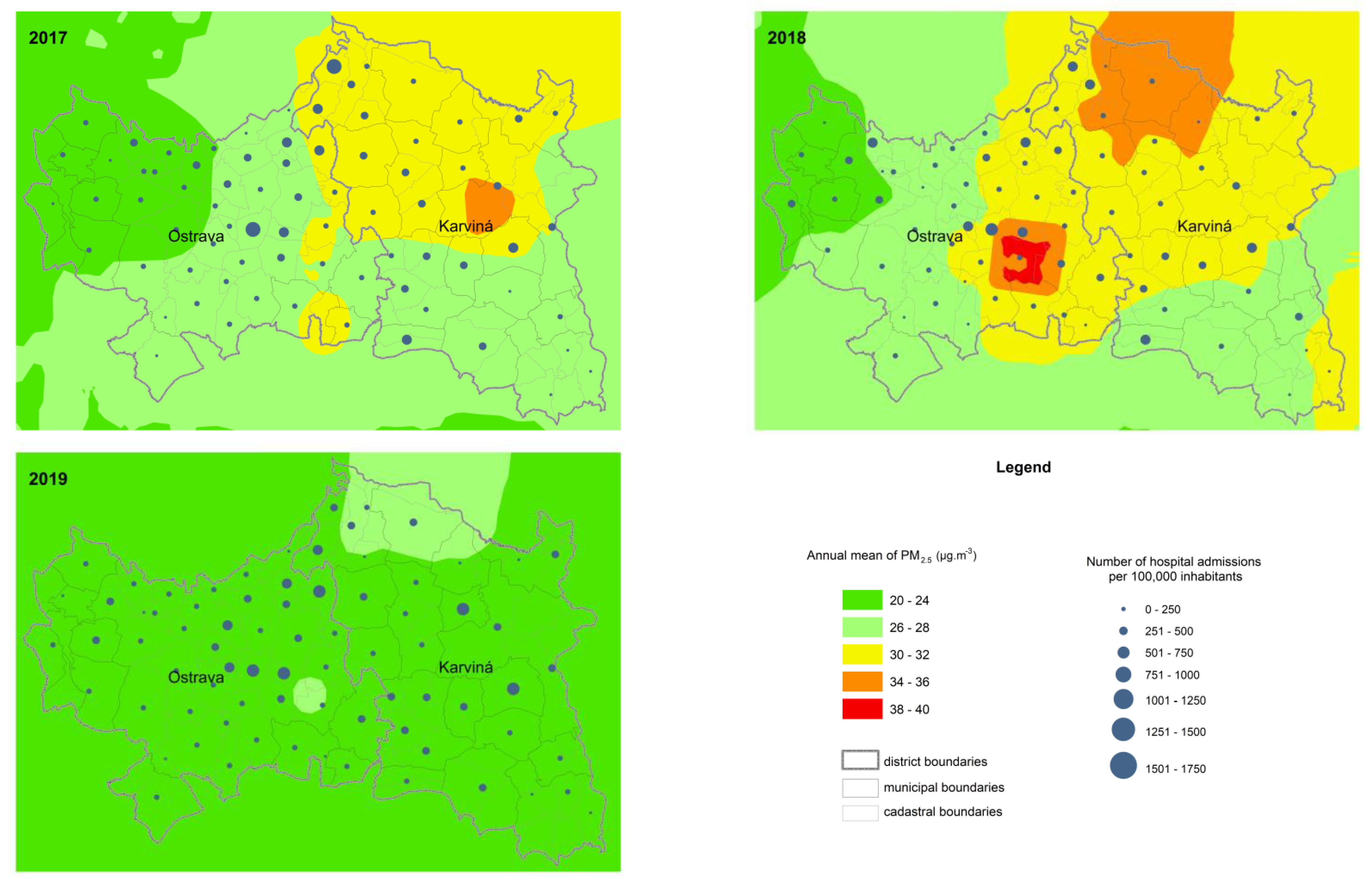
3. Results
4. Discussion
5. Conclusions
Key Messages
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Noncommunicable Diseases and Air Pollution. In Proceedings of the WHO European High-Level Conference on Noncommunicable Diseases: Time to Deliver—Meeting NCD Targets to Achieve Sustainable Development Goals in Europe, Ashgabat, Turkmenistan, 9–10 April 2019; p. 12. [Google Scholar]
- World Health Organization. Preventing Noncommunicable Diseases (NCDs) by Reducing Environmental Risk Factors; World Health Organization: Geneva, Switzerland, 2017; pp. 1–16. [Google Scholar]
- EEA. Air Quality in Europe—2020 Report; European Economic Area: Copenhagen, Denmark, 2020. [Google Scholar]
- Neira, M.; Prüss-Ustün, A.; Mudu, P. Reduce air pollution to beat NCDs: From recognition to action. Lancet 2018, 392, 1178–1179. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Lee, K.K.; McAllister, D.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Peel, J.L. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Lanzinger, S.; Schneider, A.; Breitner, S.; Stafoggia, M.; Erzen, I.; Dostal, M.; Pastorkova, A.; Bastian, S.; Cyrys, J.; Zscheppang, A.; et al. Ultrafine and fine particles and hospital admissions in central Europe results from the ufireg study. Am. J. Respir. Crit. Care Med. 2016, 194, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; Williams, G.; Schwartz, J.; Best, T.L.; Neller, A.H.; Petroeschevsky, A.L.; Simpson, R.W. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ. Health Perspect. 2006, 114, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Yee, J.; Cho, Y.A.; Yoo, H.J.; Yun, H.; Gwak, H.S. Short-term exposure to air pollution and hospital admission for pneumonia: A systematic review and meta-analysis. Environ. Health 2021, 20, 6. [Google Scholar] [CrossRef]
- Song, Q.; Christiani, D.C.; Wang, X.; Ren, J. The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2014, 11, 11822–11832. [Google Scholar] [CrossRef]
- Volná, V.; Hladký, D.; Seibert, R.; Krejčí, B. Transboundary Air Pollution Transport of PM10 and Benzo[a]pyrene in the Czech–Polish Border Region. Atmos 2022, 13, 341. [Google Scholar] [CrossRef]
- Černikovský, L.; Krejčí, B.; Blažek, Z.; Volná, V. Transboundary Air-Pollution Transport in the Czech-Polish Border Region between the Cities of Ostrava and Katowice. Cent. Eur. J. Public Health 2016, 24 (Suppl.), S45–S50. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Geneva PP; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tomášková, H.; Tomášek, I.; Šlachtová, H.; Polaufová, P.; Šplíchalová, A.; Michalík, J.; Feltl, D.; Lux, J.; Marsová, M. PM10 Air Pollution and Acute Hospital Admissions for Cardiovascular and Respiratory Causes in Ostrava. Cent. Eur. J. Public Health 2016, 24, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Tomášková, H.; Šlachtová, H.; Tomášek, I. Analýza Vztahu Koncentrací PM10, PM2.5 a PM1 a Zdravotních Ukazatelů v Silně Zatížené Oblasti v ČR. [Analysis of the Relationships between PM10, PM2.5 and PM1 Concentrations and Health Indicators in the Heavily Polluted Region of the Czech Republic]; Zdravotní Ústav se Sídlem v Ostravě: Ostrava, Czech Republic, 2021; ISBN 978-80-906887-1-1. (In Czech) [Google Scholar]
- Czech Hydrometeorological Institute: Graphic Yearbook. 2013. Available online: https://www.chmi.cz/files/portal/docs/uoco/isko/grafroc/13groc/gr13e/XII_mapovani_GB.html (accessed on 6 August 2022).
- De Marco, A.; Amoatey, P.; Khaniabadi, Y.O.; Sicard, P.; Hopke, P.K. Mortality and morbidity for cardiopulmonary diseases attributed to PM(2.5) exposure in the metropolis of Rome, Italy. Eur. J. Intern. Med. 2018, 57, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liu, X.; Liu, T.; Chen, D.; Jiao, K.; Wang, X.; Suo, J.; Yang, H.; Liao, J.; Ma, L. Effect of ambient fine particulates (PM2.5) on hospital admissions for respiratory and cardiovascular diseases in Wuhan, China. Respir. Res. 2021, 22, 128. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Yousefian, F.; Azimi, F.; Pasalari, H.; Momeniha, F.; Mokammel, A.; Gholampour, A.; Hassanvand, M.S.; Naddafi, K. Spatial homogeneity and heterogeneity of ambient air pollutants in Tehran. Sci. Total. Environ. 2019, 697, 134123. [Google Scholar] [CrossRef]
- Yousefian, F.; Faridi, S.; Azimi, F.; Aghaei, M.; Shamsipour, M.; Yaghmaeian, K.; Hassanvand, M.S. Temporal variations of ambient air pollutants and meteorological influences on their concentrations in Tehran during 2012–2017. Sci. Rep. 2020, 10, 292. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Mccreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Jarup, L.; Harrington, R.; Svartengren, M.; Han, I.K.; Oman-Strickland, P.; et al. Respiratory Effects of Exposure to Diesel Traffic in Persons with Asthma from the National Heart and Lung Institute, Imperial College, and Royal Brompton Hospital. N. Engl. J. Med. 2007, 357, 2348–2358. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Adar, S.D.; Barr, R.G.; Budoff, M.; Burke, G.L.; Curl, C.L.; Daviglus, M.L.; Roux, A.V.D.; Gassett, A.J.; Jacobs, D.R.; et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 2016, 388, 696–704. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, A.; Jin, X.; Natanzon, A.; Duquaine, D.; Brook, R.D.; Aguinaldo, J.G.; Fayad, Z.A.; Fuster, V.; Lippmann, M.; et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005, 294, 3003–3010. [Google Scholar] [CrossRef]
- Louwies, T.; Panis, L.I.; Kicinski, M.; De Boever, P.; Nawrot, T. Retinal Microvascular Responses to Short-Term Changes in Particulate Air Pollution in Healthy Adults. Environ. Health Perspect. 2013, 121, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Aztatzi-Aguilar, O.G.; Uribe-Ramírez, M.; Arias-Montaño, J.A.; Barbier, O.; De Vizcaya-Ruiz, A. Acute and subchronic exposure to air particulate matter induces expression of angiotensin and bradykinin-related genes in the lungs and heart: Angiotensin-II type-I receptor as a molecular target of particulate matter exposure. Part. Fibre Toxicol. 2012, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, P.; Lan, L.; Zhou, L.; Liu, R.; Sun, Q.; Ban, J.; Wang, W.; Xu, D.; Li, T. Short-term exposures to PM(2.5) and cause-specific mortality of cardiovascular health in China. Environ. Res. 2018, 161, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Bind, M.-A.C.; Schwartz, J. Particulate air pollution and survival in a COPD cohort. Environ. Health 2008, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Son, J.-Y.; Peng, R.D.; Wang, Y.; Dominici, F. Ambient PM2.5 and Risk of Hospital Admissions: Do Risks Differ for Men and Women? Epidemiology 2015, 26, 575–579. [Google Scholar] [CrossRef]
- Jin, J.-Q.; Han, D.; Tian, Q.; Chen, Z.-Y.; Ye, Y.-S.; Lin, Q.-X.; Ou, C.-Q.; Li, L. Individual exposure to ambient PM2.5 and hospital admissions for COPD in 110 hospitals: A case-crossover study in Guangzhou, China. Environ. Sci. Pollut. Res. 2022, 29, 11699–11706. [Google Scholar] [CrossRef]
- Sloan, C.D.; Philipp, T.J.; Bradshaw, R.K.; Chronister, S.; Barber, W.B.; Johnston, J.D. Applications of GPS-tracked personal and fixed-location PM(2.5) continuous exposure monitoring. J. Air Waste Manag. Assoc. 2016, 66, 53–65. [Google Scholar] [CrossRef]
| CU | Residents | Mean Concentrations of PM2.5 (µg·m−3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PM2.5+ (µg·m−3) | n | n | % | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
| ≤29 | 4 | 7762 | 1.3% | 26.5 | 23.0 | 22.0 | 22.5 | 24.0 | 20.0 |
| 30–31 | 8 | 56,099 | 9.3% | 28.0 | 25.0 | 24.5 | 25.3 | 26.5 | 20.0 |
| 32–33 | 15 | 74,391 | 12.4% | 29.3 | 26.3 | 24.5 | 26.0 | 27.5 | 20.0 |
| 34–35 | 34 | 335,447 | 55.8% | 31.5 | 28.2 | 26.9 | 28.4 | 31.1 | 21.6 |
| 36–37 | 11 | 101,485 | 16.9% | 33.5 | 30.0 | 29.1 | 30.0 | 31.6 | 22.9 |
| ≥38 | 5 | 26,115 | 4.3% | 32.4 | 29.2 | 29.2 | 30.0 | 32.0 | 23.6 |
| Celkem | 77 | 601,299 | 100.0% | 30.8 | 27.5 | 26.4 | 27.6 | 29.7 | 21.4 |
| Year | PM2.5+ (µg·m−3) | <30 | 30–31 | 32–33 | 34–35 | 36–37 | ≥38 | |
|---|---|---|---|---|---|---|---|---|
| Risk of hospitalization from cardiovascular causes (I00–I99) | 2013 | cases/100,000 | 751.3 | 752.0 | 695.4 | 823.5 | 1321.5 | 2046.6 |
| IRR | ref. | 1.01 | 0.93 | 1.10 | 1.77 * | 2.74 * | ||
| 95% CI | (0.76–1.35) | (0.71–1.24) | (0.85–1.46) | (1.36–2.34) | (2.08–3.65) | |||
| 2014 | cases/100,000 | 871.5 | 663.8 | 728.2 | 785.1 | 1280.6 | 1881.0 | |
| IRR | ref. | 0.76 | 0.83 | 0.90 | 1.46* | 2.15 * | ||
| 95% CI | (0.58–1.00) | (0.65–1.09) | (0.70–1.16) | (1.15–1.89) | (1.66–2.81) | |||
| 2015 | cases/100,000 | 890.3 | 676.1 | 614.0 | 773.7 | 1124.9 | 2003.0 | |
| IRR | ref. | 0.76 | 0.69 | 0.87 | 1.27 | 2.25 * | ||
| 95% CI | (0.59–1.00) | (0.54–0.90) | (0.69–1.12) | (0.99–1.64) | (1.75–2.94) | |||
| 2016 | cases/100,000 | 659.3 | 588.6 | 687.9 | 722.1 | 1146.6 | 1683.8 | |
| IRR | ref. | 0.90 | 1.05 | 1.10 | 1.75 * | 2.56 * | ||
| 95% CI | (0.67–1.23) | (0.78–1.43) | (0.83–1.48) | (1.32–2.36) | (1.92–3.50) | |||
| 2017 | cases/100,000 | 845.3 | 662.5 | 685.6 | 700.4 | 973.5 | 1336.2 | |
| IRR | ref. | 0.78 | 0.81 | 0.82 | 1.14 | 1.57 * | ||
| 95% CI | (0.60–1.03) | (0.62–1.06) | (0.65–1.07) | (0.89–1.49) | (1.21–2.08) | |||
| 2018 | cases/100,000 | 813.5 | 567.1 | 664.5 | 669.4 | 815.1 | 1441.2 | |
| IRR | ref. | 0.70 | 0.78 | 0.79 | 0.96 | 1.69 * | ||
| 95% CI | (0.53–0.93) | (0.60–1.03) | (0.62–1.02) | (0.75–1.25) | (1.30–2.23) | |||
| 2019 | cases/100,000 | 863.5 | 938.9 | 805.4 | 753.8 | 905.8 | 1132.0 | |
| IRR | ref. | 1.09 | 0.93 | 0.87 | 1.05 | 1.31 | ||
| 95% CI | (0.84–1.43) | (0.72–1.22) | (0.69–1.13) | (0.82–1.37) | (1.00–1.74) | |||
| Risk of hospitalization from respiratory causes (J00–J99) | 2013 | cases/100,000 | 325.0 | 322.0 | 384.7 | 594.4 | 587.9 | 694.4 |
| IRR | ref. | 1.00 | 1.19 | 1.85 * | 1.83 * | 2.15 * | ||
| 95% CI | (0.66–1.59) | (0.79–1.88) | (1.25–2.86) | (1.23–2.85) | (1.41–3.41) | |||
| 2014 | cases/100,000 | 271.5 | 333.4 | 312.7 | 599.6 | 495.8 | 659.8 | |
| IRR | ref. | 1.23 | 1.16 | 2.22 * | 1.83 * | 2.43 * | ||
| 95% CI | (0.78–2.04) | (0.74–1.91) | (1.45–3.59) | (1.19–2.99) | (1.54–4.03) | |||
| 2015 | cases/100,000 | 327.8 | 321.6 | 310.3 | 567.4 | 481.5 | 623.0 | |
| IRR | ref. | 1.00 | 0.96 | 1.76 * | 1.50 * | 1.94 * | ||
| 95% IS CI | (0.65–1.58) | (0.64–1.52) | (1.19–2.73) | (1.00–2.33) | (1.27–3.08) | |||
| 2016 | cases/100,000 | 362.0 | 301.6 | 337.8 | 578.6 | 509.8 | 583.0 | |
| IRR | ref. | 0.84 | 0.94 | 1.60 * | 1.41 | 1.61 * | ||
| 95% CI | (0.56–1.29) | (0.63–1.44) | (1.11–2.42) | (0.97–2.15) | (1.07–2.51) | |||
| 2017 | cases/100,000 | 263.8 | 372.3 | 403.2 | 512.3 | 437.2 | 647.6 | |
| IRR | ref. | 1.45 | 1.57 | 1.99 * | 1.70 * | 2.51 * | ||
| 95% CI | (0.91–2.42) | (1.00–2.60) | (1.28–3.26) | (1.09–2.81) | (1.58–4.22) | |||
| 2018 | cases/100,000 | 365.5 | 375.1 | 321.5 | 477.6 | 394.0 | 489.2 | |
| IRR | ref. | 1.04 | 0.89 | 1.32 | 1.09 | 1.36 | ||
| 95% CI | (0.70–1.60) | (0.60–1.37) | (0.91–2.00) | (0.74–1.67) | (0.90–2.13) | |||
| 2019 | cases/100,000 | 307.5 | 298.0 | 362.0 | 623.2 | 432.1 | 515.4 | |
| IRR | ref. | 0.96 | 1.17 | 2.02 | 1.40 | 1.67 * | ||
| 95% CI | (0.63–1.54) | (0.77–1.86) | (1.35–3.15) | (0.93–2.21) | (1.08–2.70) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomášková, H.; Šlachtová, H.; Dalecká, A.; Polaufová, P.; Michalík, J.; Tomášek, I.; Šplíchalová, A. Association between PM2.5 Exposure and Cardiovascular and Respiratory Hospital Admissions Using Spatial GIS Analysis. Atmosphere 2022, 13, 1797. https://doi.org/10.3390/atmos13111797
Tomášková H, Šlachtová H, Dalecká A, Polaufová P, Michalík J, Tomášek I, Šplíchalová A. Association between PM2.5 Exposure and Cardiovascular and Respiratory Hospital Admissions Using Spatial GIS Analysis. Atmosphere. 2022; 13(11):1797. https://doi.org/10.3390/atmos13111797
Chicago/Turabian StyleTomášková, Hana, Hana Šlachtová, Andrea Dalecká, Pavla Polaufová, Jiří Michalík, Ivan Tomášek, and Anna Šplíchalová. 2022. "Association between PM2.5 Exposure and Cardiovascular and Respiratory Hospital Admissions Using Spatial GIS Analysis" Atmosphere 13, no. 11: 1797. https://doi.org/10.3390/atmos13111797
APA StyleTomášková, H., Šlachtová, H., Dalecká, A., Polaufová, P., Michalík, J., Tomášek, I., & Šplíchalová, A. (2022). Association between PM2.5 Exposure and Cardiovascular and Respiratory Hospital Admissions Using Spatial GIS Analysis. Atmosphere, 13(11), 1797. https://doi.org/10.3390/atmos13111797






