Effect of Densification on Biomass Combustion and Particulate Matter Emission Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Fuel Property and Ash Composition
2.2. Densification Process
2.3. Thermogravimetric Analysis (TG−DTG)
2.4. Kinetic Analysis
2.5. Collection and Analysis of Particulate Matter
3. Results and Discussion
3.1. Thermogravimetric Analysis and Combustion Behavior
3.2. Combustion Kinetics
3.3. PM Emission Characteristics
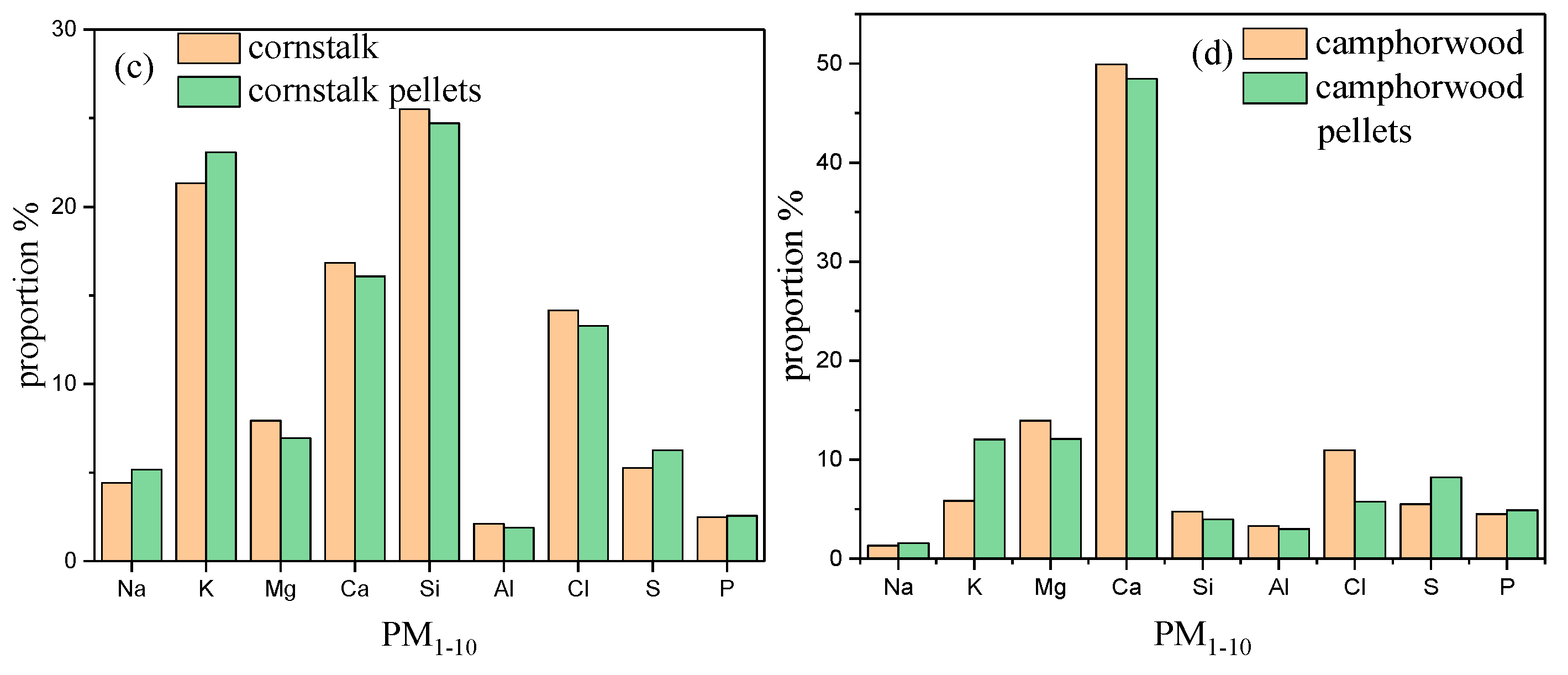
3.4. Chemical Composition of Particulate Matters
3.5. Discussion on the Effect of Densification on PM
4. Conclusions
- (1)
- The TG−DTG results show that the biomass densification increases the ignition, burnout, and composite combustion indexes, leading to a better performance of biomass combustion.
- (2)
- The results of the kinetic analysis suggest that the main reaction mechanism of cornstalk pellets can be well-expressed with the chemical reaction series model, whereas the diffusion mechanism and chemical reaction series models can be applied to the combustion of camphorwood pellets.
- (3)
- The influence of biomass densification on PM emission is related to the biomass properties. The densification significantly reduces PM emission for agricultural biomass residues such as cornstalk but significantly increases the yield of PM1 for woody biomass such as camphorwood.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, Y.; Yang, Q.; Bartocci, P.; Wei, H.; Liu, S.S.; Wu, Z.; Zhou, H.; Yang, H.; Fantozzi, F.; Chen, H. Bioenergy in China: Evaluation of domestic biomass resources and the associated greenhouse gas mitigation potentials. Renew. Sustain. Energy Rev. 2020, 127, 109842. [Google Scholar] [CrossRef]
- Králík, T.; Knápek, J.; Dvořáček, L.; Vávrová, K. Impact of pelleting cost on competitiveness of intentionally grown biomass for local space heating: Case example of the Czech Republic. Energy Rep. 2020, 6, 732–737. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhang, Y.; Wang, Y.; Yu, Y.; Ji, F.; Ahmad, R.; Dong, R. A comprehensive review on densified solid biofuel industry in China. Renew. Sustain. Energy Rev. 2016, 54, 1412–1428. [Google Scholar] [CrossRef]
- Mian, I.; Li, X.; Dacres, O.D.; Wang, J.; Wei, B.; Jian, Y.; Zhong, M.; Liu, J.; Ma, F.; Rahman, N. Combustion kinetics and mechanism of biomass pellet. Energy 2020, 205, 117909. [Google Scholar] [CrossRef]
- Horvat, I.; Dović, D.; Filipović, P. Numerical and experimental methods in development of the novel biomass combustion system concept for wood and agro pellets. Energy 2021, 231, 120929. [Google Scholar] [CrossRef]
- Gao, X.; Rahim, M.U.; Chen, X.; Wu, H. Inorganic PM10 emission from the combustion of individual mallee components and whole-tree biomass. Proc. Combust. Inst. 2017, 36, 3313–3319. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Cheng, W.; Sang, H.; Yang, H.; Chen, H. Characteristics of Particulate Matter Emitted from Agricultural Biomass Combustion. Energy Fuels 2017, 31, 7493–7501. [Google Scholar] [CrossRef]
- Wang, K.; Nakao, S.; Thimmaiah, D.; Hopke, P.K. Emissions from in-use residential wood pellet boilers and potential emissions savings using thermal storage. Sci. Total Environ. 2019, 676, 564–576. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Cheng, W.; Sang, H.; Xu, H.; Yang, H.; Chen, H. Effect of minerals and binders on particulate matter emission from biomass pellets combustion. Appl. Energy 2018, 215, 106–115. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, Y.; Shao, J.A.; Zhang, W.; Wu, G.; Jiang, H.; Hu, J.; Huang, Z.; Yang, H.; Chen, H. Mitigation of ultrafine particulate matter emission from agricultural biomass pellet combustion by the additive of phosphoric acid modified kaolin. Renew. Energy 2021, 172, 177–187. [Google Scholar] [CrossRef]
- Si, Y.; Hu, J.; Wang, X.; Yang, H.; Chen, Y.; Shao, J.; Chen, H. Effect of Carboxymethyl Cellulose Binder on the Quality of Biomass Pellets. Energy Fuels 2016, 30, 5799–5808. [Google Scholar] [CrossRef]
- Li, X.G.; Lv, Y.; Ma, B.G.; Jian, S.W.; Tan, H.B. Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Bioresour. Technol. 2011, 102, 9783–9787. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, S.; Liu, L.; Li, X.; Chen, H.; Yang, H.; Wang, X. Combustion behaviours of tobacco stem in a thermogravimetric analyser and a pilot-scale fluidized bed reactor. Bioresour. Technol. 2012, 110, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; He, Y.; Hassanpour, A.; Gardy, J.; Zhong, Z. Thermogravimetric analysis on the co-combustion of biomass pellets with lignite and bituminous coal. Energy 2020, 197, 117147. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Magalhães, D.; Kazanç, F.; Riaza, J.; Erensoy, S.; Kabaklı, Ö.; Chalmers, H. Combustion of Turkish lignites and olive residue: Experiments and kinetic modelling. Fuel 2017, 203, 868–876. [Google Scholar] [CrossRef]
- Málek, J. The kinetic analysis of non-isothermal data. Thermochim. Acta 1992, 200, 257–269. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Li, Y.; Cheng, W.; Zhang, W.; Yang, H.; Tan, Z.; Chen, H. Mitigation of particulate matter emissions from co-combustion of rice husk with cotton stalk or cornstalk. Renew. Energy 2022, 190, 893–902. [Google Scholar] [CrossRef]
- Gao, W.; Chen, K.; Xiang, Z.; Yang, F.; Zeng, J.; Li, J.; Yang, R.; Rao, G.; Tao, H. Kinetic study on pyrolysis of tobacco residues from the cigarette industry. Ind. Crops Prod. 2013, 44, 152–157. [Google Scholar] [CrossRef]
- Ding, K.; Wang, Y.; Liu, S.; Lin, G.; Syed-Hassan, S.S.A.; Li, B.; Hu, X.; Huang, Y.; Zhang, S.; Zhang, H. Volatile-char interactions during biomass pyrolysis: Insight into the activity of chars derived from three major components. J. Anal. Appl. Pyrol. 2021, 159, 105320. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Liu, Z.; Zhang, G.; Xu, T.; Guo, J.; Wang, H.; Xu, R.; Lin, H. Thermal behavior and kinetic analysis of co-combustion of waste biomass/low rank coal blends. Energy Convers. Manag. 2016, 124, 414–426. [Google Scholar] [CrossRef]
- Guo, F.; Zhong, Z. Optimization of the co-combustion of coal and composite biomass pellets. J. Clean. Prod. 2018, 185, 399–407. [Google Scholar] [CrossRef]
- Xinjie, L.; Shihong, Z.; Xincheng, W.; Jinai, S.; Xiong, Z.; Xianhua, W.; Haiping, Y.; Hanping, C. Co-combustion of wheat straw and camphor wood with coal slime: Thermal behaviour, kinetics, and gaseous pollutant emission characteristics. Energy 2021, 234, 121292. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.-P.; Qin, S.-N.; Guo, J.-R.; Zhang, Y.-Q.; Wang, J.-C.; Zhang, J.-C.; Fan, B.-G.; Jin, Y. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef]
- Liu, L.; Pang, Y.; Lv, D.; Wang, K.; Wang, Y. Thermal and kinetic analyzing of pyrolysis and combustion of self-heating biomass particles. Process Saf. Environ. 2021, 151, 39–50. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, W.; Fan, J.; Kan, T.; Zhang, W.; Liu, H.; Cheng, W.; Haiping, Y.; Wu, X.; Chen, H. Effect of sodium carboxymethyl cellulose addition on particulate matter emissions during biomass pellet combustion. Appl. Energy 2018, 230, 925–934. [Google Scholar] [CrossRef]
- Elbl, P.; Sitek, T.; Lachman, J.; Lisý, M.; Baláš, M.; Pospíšil, J. Sewage sludge and wood sawdust co-firing: Gaseous emissions and particulate matter size distribution. Energy 2022, 256, 124680. [Google Scholar] [CrossRef]
- Yang, W.; Pudasainee, D.; Gupta, R.; Li, W.; Wang, B.; Sun, L. Particulate matter emission during municipal solid waste combustion: Submicron particulates formation mechanism. Fuel 2022, 310, 122271. [Google Scholar] [CrossRef]
- Li, X.; Gong, X.; Zhang, C.; Liu, T.; Wang, W.; Zhang, Y. Occurrence characteristics of ash-forming elements in sea rice waste and their effects on particulate matter emission during combustion. Fuel 2020, 273, 117769. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, G.; Niu, Y.; Wen, L.; Lei, Y.; Wang, D.; Hui, S.E. Characteristics of particulate emissions from coal char combustion: Char fragmentation and ash coalescence behaviors. Fuel 2022, 310, 122283. [Google Scholar] [CrossRef]
- Zhai, M.; Li, X.; Yang, D.; Ma, Z.; Dong, P. Ash fusion characteristics of biomass pellets during combustion. J. Clean. Prod. 2022, 336, 130361. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, K.; He, Y.; Wang, Z.; Xia, J.; Cen, K. Experimental study of potassium release during biomass-pellet combustion and its interaction with inhibitive additives. Fuel 2020, 260, 116346. [Google Scholar] [CrossRef]
- Clery, D.S.; Mason, P.E.; Rayner, C.M.; Jones, J.M. The effects of an additive on the release of potassium in biomass combustion. Fuel 2018, 214, 647–655. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S.E. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
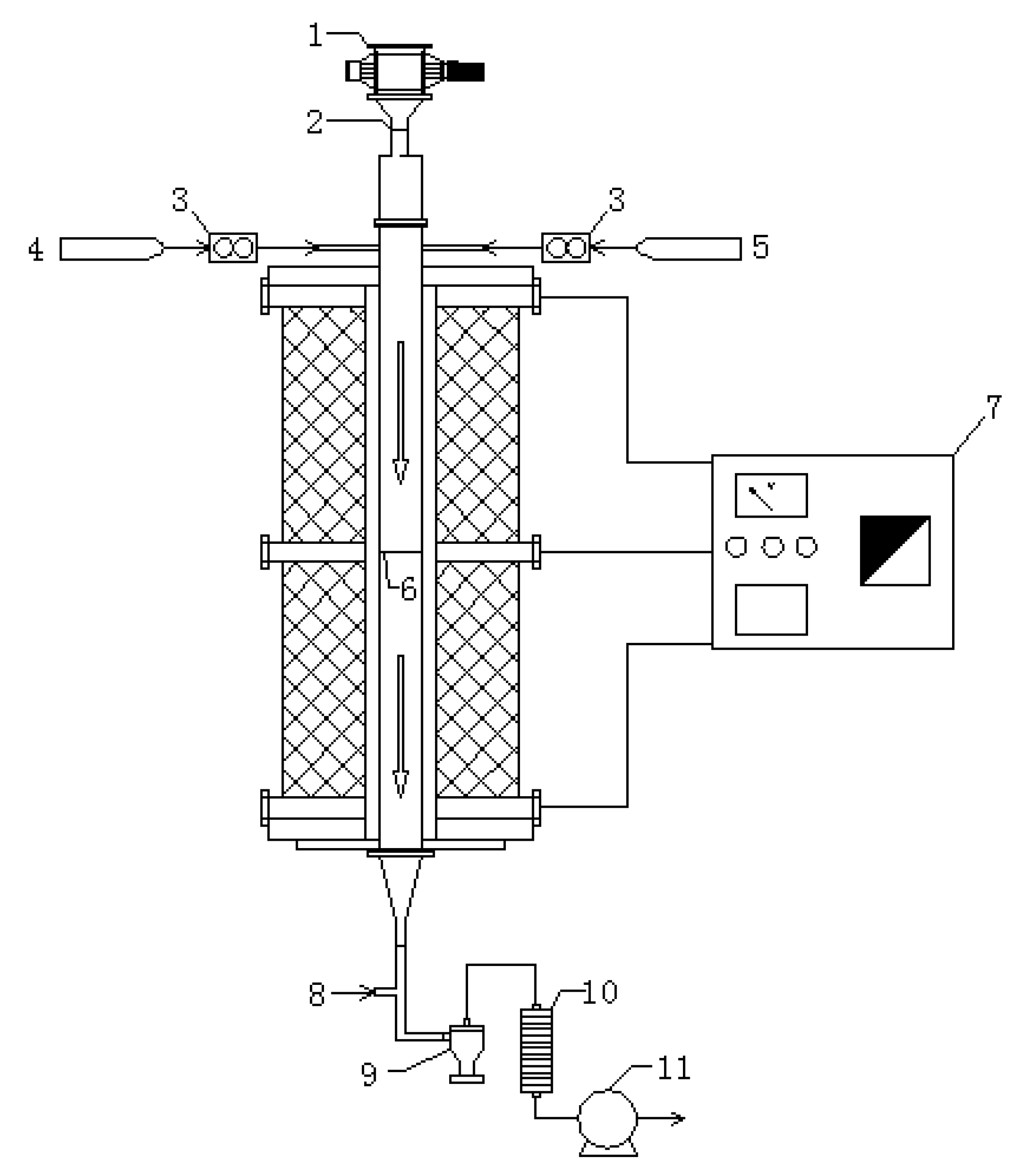
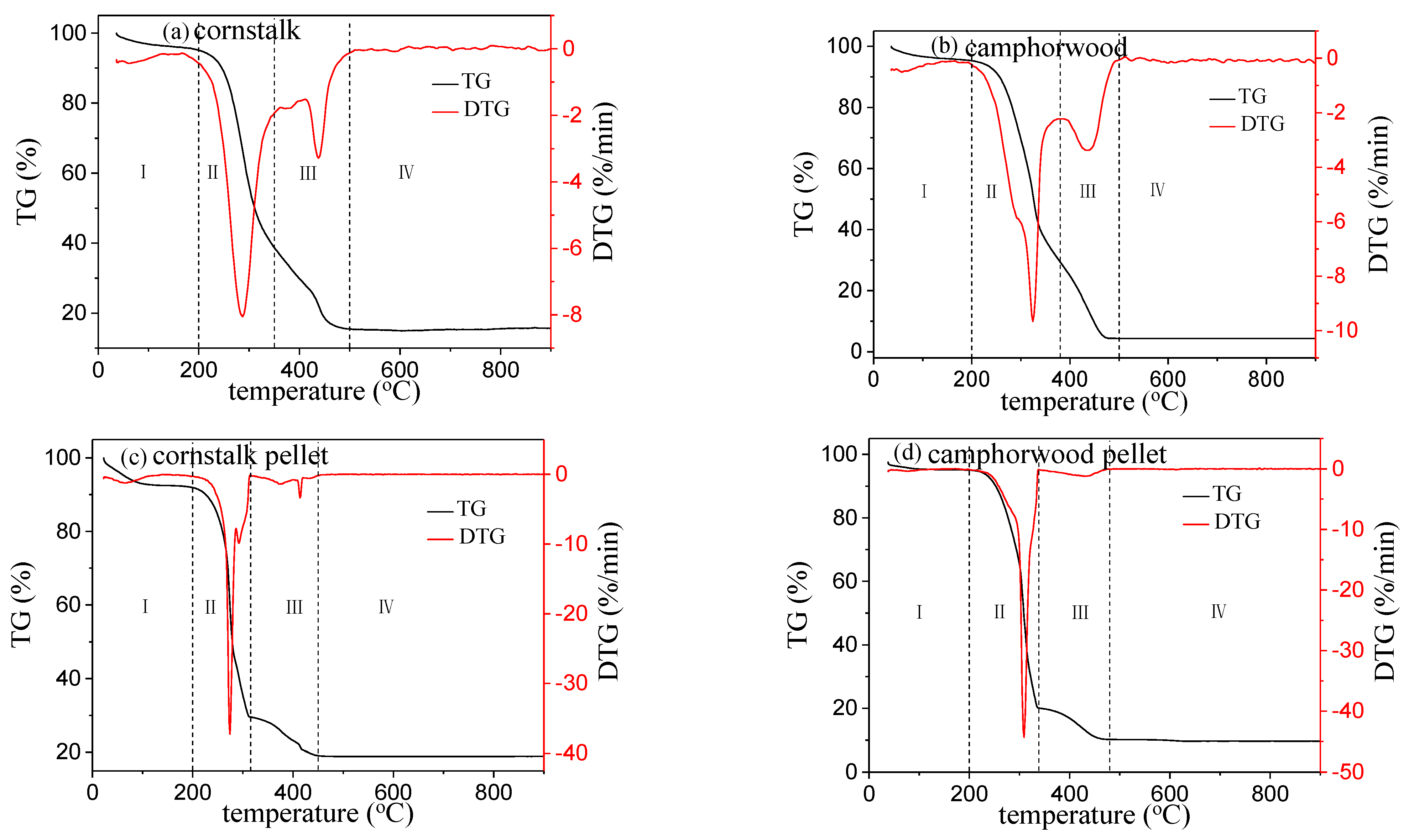
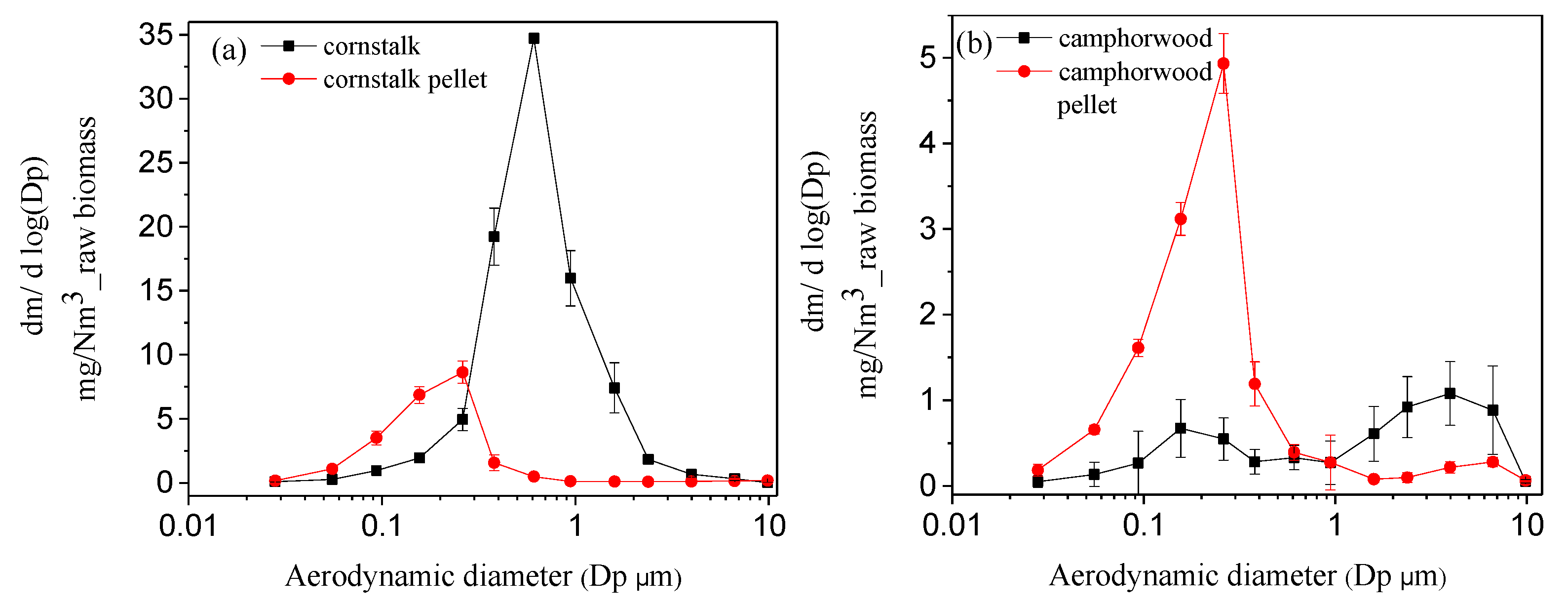
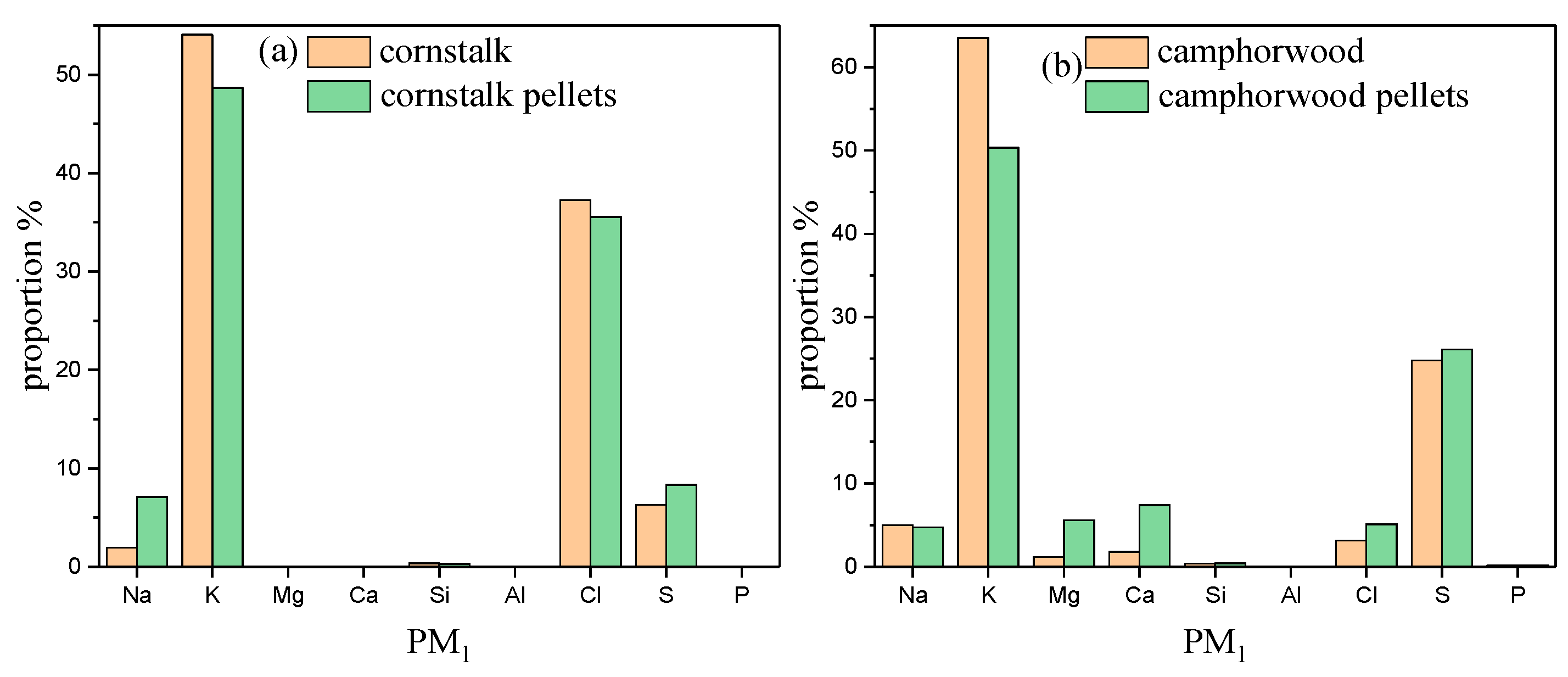
| Samples | Cornstalk | Camphorwood |
|---|---|---|
| Proximate analysis (wt%) | ||
| Mad | 2.86 | 4.03 |
| Adb | 6.58 | 0.89 |
| Vdb | 64.49 | 87.93 |
| FCdb | 28.93 | 11.18 |
| Ultimate analysis (wt%) | ||
| Cdb | 39.81 | 48.7 |
| Hdb | 5.08 | 5.93 |
| Ndb | 0.94 | 0.51 |
| Sdb | 0.23 | 0.17 |
| Cldb | 0.61 | 0.11 |
| Odb | 46.75 | 43.69 |
| LHV (MJ/kg) | 13.49 | 17.56 |
| Ash composition (wt%) | ||
| MgO | 4.59 | 12.96 |
| SiO2 | 18.18 | 10.38 |
| P2O5 | 7.55 | 8.72 |
| SO3 | 2.51 | 5.32 |
| Cl2O | 12.68 | - |
| K2O | 46.73 | 7.99 |
| CaO | 7.12 | 53.77 |
| Fe2O3 | 0.39 | 0.59 |
| Molar ratio of Si/(Mg + Ca) | 1.25 | 0.13 |
| Molar ratio of(Si + P + K)/(Mg + Ca) | 5.80 | 0.36 |
| Models | Reaction Order | f(α) | g(α) |
|---|---|---|---|
| Chemical reaction Series model | F1 | 1 − α | −ln(1 − α) |
| F3/2 | (1 − α)1.5 | 2(1 − α)−0.5 − 2 | |
| F2 | (1 − α)2 | (1 − α)−1 | |
| Phase interface model | R1 | 1 | a |
| R2 | 2(1 − α)0.5 | 1 − (1 − α)0.5 | |
| R3 | 3(1 − α)1/3 | 1 − (1 − α)1/3 | |
| Diffusion mechanism model | D1 | 1/2α | α2 |
| D2 | [−ln(1 − α)]1/3 | (1 − α)ln(1 − α) + α | |
| D3 | 3(1 − α)2/3/2[1 − (1 − α)1/3] | [1 − (1 − α)1/3]2 | |
| D4 | 3/2[(1 − α)1/3 − 1] | (1 − 2α/3) − (1 − α)2/3 | |
| Random nucleation model | A2 | 2(1 − α)[−ln(1 − α)]1/2 | [−ln(1 − α)]1/2 |
| A3 | 3(1 − α)[−ln(1 − α)]2/3 | [−ln(1 − α)]1/3 |
| Samples | Ti °C | Tb °C | DTGmax %/min | Tmax°C | Di × 10−3 | Db × 10−4 | S × 1013 |
|---|---|---|---|---|---|---|---|
| Cornstalk | 250 | 459 | 8.0525 | 287 | 14.83 | 12.97 | 39.13 |
| Cornstalk pellet | 260 | 420 | 37.2953 | 274 | 56.50 | 443.15 | 157.35 |
| Camphorwood | 273 | 654 | 9.6609 | 324 | 13.88 | 8.92 | 40.17 |
| Camphorwood pellet | 286 | 445 | 44.3195 | 309 | 54.99 | 440.19 | 183.10 |
| Samples | Stages | Temperature °C | Activation Energy kJ/mol | Pre-Exponential Factor | Correlation Coefficient | Models |
|---|---|---|---|---|---|---|
| Cornstalk | 1 | 200–350 | 104.62 | 4.07 × 107 | 0.9724 | D3 |
| 2 | 350–425 | 23.90 | 0.9117 | 0.9990 | D3 | |
| 3 | 425–459 | 74.42 | 1.35 × 105 | 0.9940 | F1 | |
| Cornstalk pellet | 1 | 220–270 | 87.76 | 9.23 × 106 | 0.9609 | D1 |
| 2 | 270–280 | 135.71 | 9.71 × 1012 | 0.9950 | F2 | |
| 3 | 280–315 | 49.79 | 1.00 × 104 | 0.9992 | F1 | |
| Camphorwood | 1 | 200–380 | 96.54 | 2.62 × 106 | 0.9742 | D3 |
| 2 | 380–480 | 16.04 | 0.4308 | 0.9813 | D1 | |
| Camphorwood pellet | 1 | 230–300 | 78.62 | 6.51 × 105 | 0.9847 | D1 |
| 2 | 300–335 | 132.00 | 8.12 × 1011 | 0.9986 | F2 |
| Samples | PM0.3 | PM1 | PM1–10 | PM10 | Ratio of PM0.3 to PM1, % | Ratio of PM1 to PM10, % |
|---|---|---|---|---|---|---|
| mg/Nm3 | ||||||
| Cornstalk | 8.22 ± 0.94 | 78.12 ± 1.22 | 10.23 ± 2.67 | 88.35 ± 3.89 | 10.52 | 88.42 |
| Cornstalk pellets | 20.30 ± 2.10 | 22.47 ± 2.90 | 0.65 ± 0.33 | 23.12 ± 2.59 | 90.34 | 97.20 |
| Camphorwood | 1.67 ± 0.55 | 2.55 ± 0.73 | 3.54 ± 0.29 | 6.10 ± 0.62 | 65.49 | 41.80 |
| Camphorwood pellets | 10.50 ± 0.19 | 12.36 ± 0.60 | 0.74 ± 0.18 | 13.10 ± 0.57 | 84.95 | 94.36 |
| Samples | (Na + K)/Cl | (Na + K)/2/S | (Na + K)/(Cl + 2S) | |||
|---|---|---|---|---|---|---|
| PM1 | PM1–10 | PM1 | PM1–10 | PM1 | PM1–10 | |
| Cornstalk | 1.50 | 1.82 | 4.44 | 2.45 | 1.12 | 1.04 |
| Cornstalk pellet | 1.57 | 2.12 | 3.35 | 2.25 | 1.07 | 1.09 |
| Camphorwood | 21.70 | 0.65 | 1.38 | 0.65 | 1.30 | 0.33 |
| Camphorwood pellets | 10.74 | 2.37 | 1.06 | 0.83 | 0.96 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Lv, L.; Han, Y.; Li, Y.; Liu, H.; Zhu, Y.; Zhang, W.; Yang, H. Effect of Densification on Biomass Combustion and Particulate Matter Emission Characteristics. Atmosphere 2022, 13, 1582. https://doi.org/10.3390/atmos13101582
Yang W, Lv L, Han Y, Li Y, Liu H, Zhu Y, Zhang W, Yang H. Effect of Densification on Biomass Combustion and Particulate Matter Emission Characteristics. Atmosphere. 2022; 13(10):1582. https://doi.org/10.3390/atmos13101582
Chicago/Turabian StyleYang, Wei, Leida Lv, Yong Han, Yu Li, Huihui Liu, Youjian Zhu, Wennan Zhang, and Haiping Yang. 2022. "Effect of Densification on Biomass Combustion and Particulate Matter Emission Characteristics" Atmosphere 13, no. 10: 1582. https://doi.org/10.3390/atmos13101582
APA StyleYang, W., Lv, L., Han, Y., Li, Y., Liu, H., Zhu, Y., Zhang, W., & Yang, H. (2022). Effect of Densification on Biomass Combustion and Particulate Matter Emission Characteristics. Atmosphere, 13(10), 1582. https://doi.org/10.3390/atmos13101582





