Electric Field-Driven Air Purification Filter for High Efficiency Removal of PM2.5 and SO2: Local Electric Field Induction and External Electric Field Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Copper-Mesh-Supported CuO Nanowire Arrays
2.3. Synthesis of Copper-Mesh-Supported CuO@NH2-MIL-53(Al) NWAs
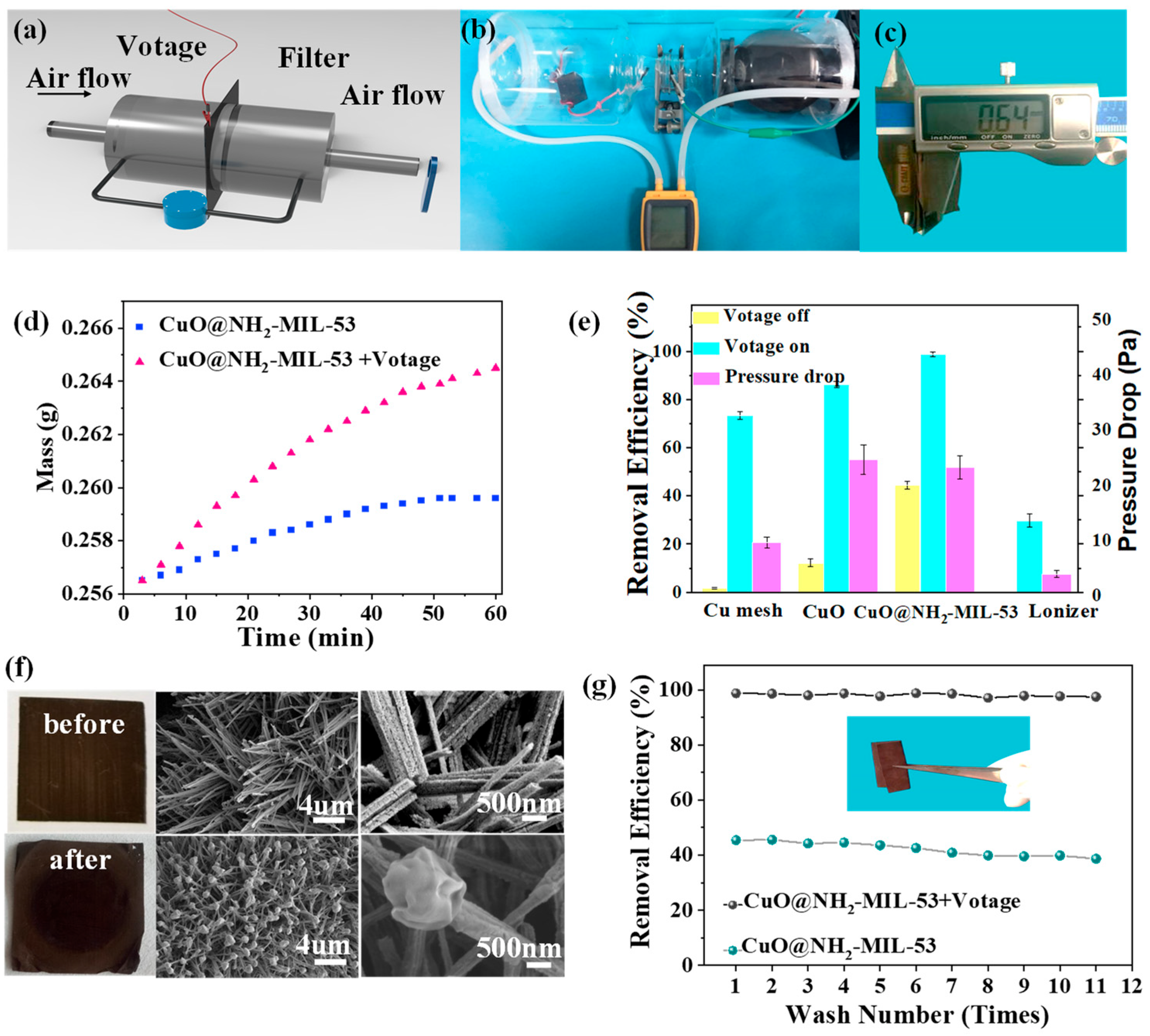
2.4. PM2.5 Filtration Test
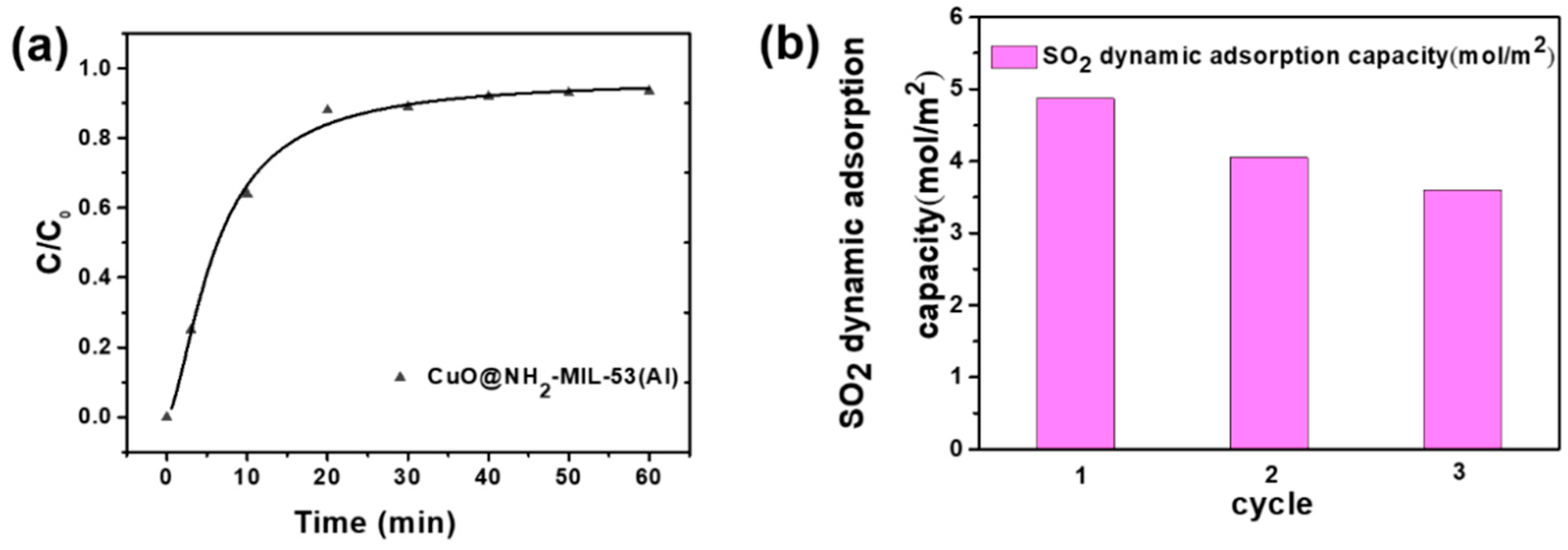
2.5. SO2 Filtration Test
2.6. Characterizations
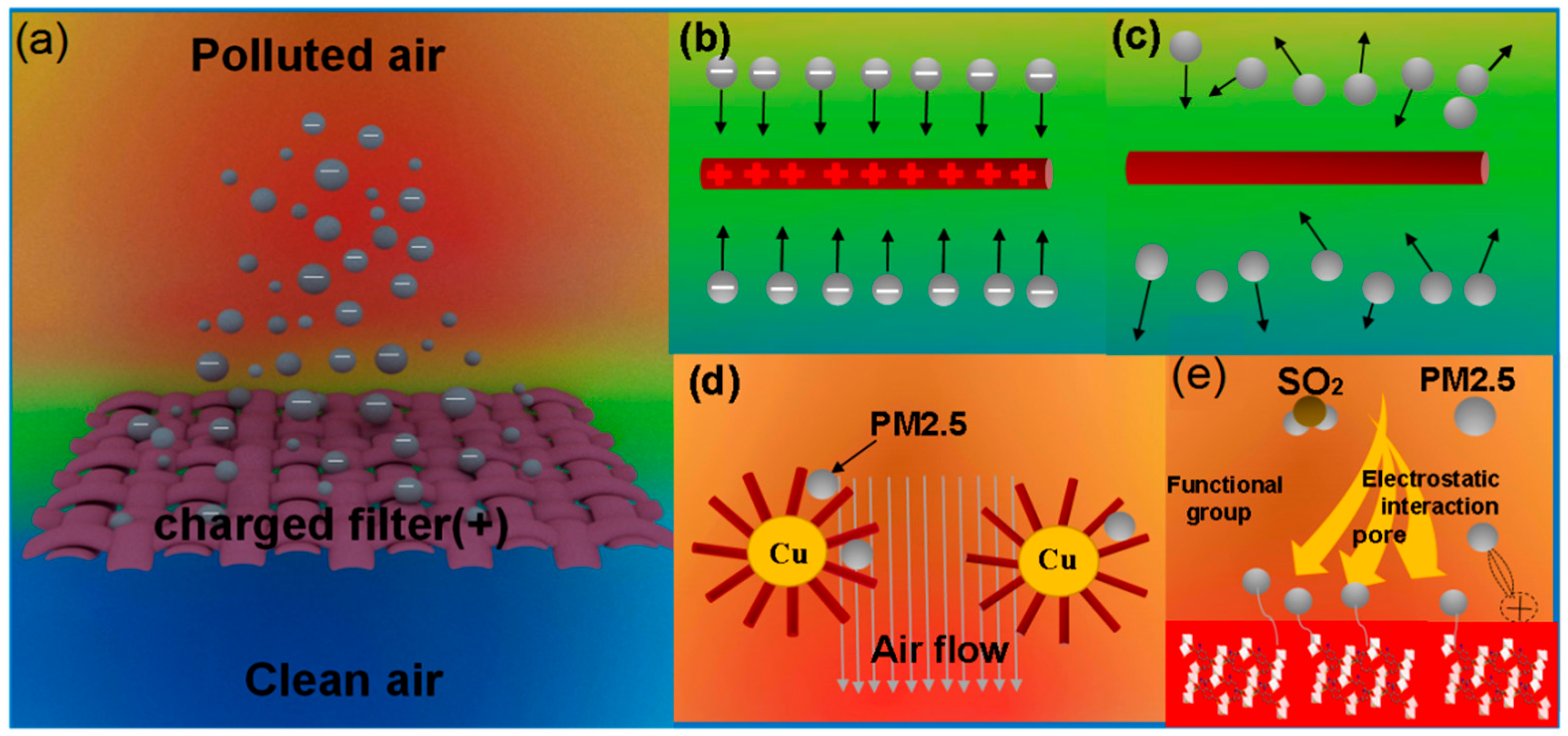
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.; Thurston, G. A hybrid satellite and land use regression model of source-specific PM2.5 and PM2.5 constituents. Environ. Int. 2022, 163, 107233. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Liu, J.; Zhang, Q.; Geng, G.; Zheng, Y.; Tong, D.; Liu, Z.; Guan, D.; Bo, Y.; Zhu, T.; et al. Rapid Improvement of PM2.5 Pollution and Associated Health Benefits in China During 2013–2017. Sci. China Earth Sci. 2019, 62, 1847–1856. [Google Scholar] [CrossRef]
- Azevedo, R.S.S.; de Sousa, J.R.; Araujo, M.T.F.; Martins Filho, A.J.; de Alcantara, B.N.; Araujo, F.M.C.; Queiroz, M.G.L.; Cruz, A.C.R.; Vasconcelos, B.H.B.; Chiang, J.O.; et al. In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by zika virus. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.C.; Shi, Q.; Wang, H.; Sun, K.Z.; Lu, Y.X.; Di, K.X. Multi-modal image feature fusion-based PM2.5 concentration estimation. Atmos. Pollut. Res. 2022, 13, 101345. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Evans, J.; van Donkelaar, A.; Martin, R.V.; Burnett, R.; Rainham, D.G.; Birkett, N.J.; Krewski, D. Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environ. Res. 2013, 120, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, J.S.; Streit, G.E.; Spall, W.D.; Hall, J.H. Beyond Acid Rain. Do Soluble Oxidants and Organic Toxinsinteract with SO2 and NOx to Increase Ecosystem Effects ? Environ. Sci. Technol. 1987, 21, 519–524. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Pope, C.A., 3rd; Chen, Y.; Gapstur, S.M.; Thun, M.J. Long-Term Ambient Fine Particulate Matter Air Pollution and Lung Cancer in a Large Cohort of Never-Smokers. Am. J. Respir. Crit Care Med. 2011, 184, 1374–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jin, X.; Johnson, A.C.; Giesy, J.P. Hazard Posed by Metals and As in PM2.5 in Air of Five Megacities in the Beijing-Tianjin-Hebei Region of China During APEC. Environ. Sci. Pollut. Res. Int. 2016, 23, 17603–17612. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Liang, J.; Zhang, C.; Tao, Y.; Ling, G.-W.; Yang, Q.-H. Advanced Materials for Capturing Particulate Matter: Progress and Perspectives. Small Methods 2018, 2, 1800012. [Google Scholar] [CrossRef]
- Trakumas, S.; Willeke, K.; Reponen, T.; Grinshpun, S.A.; Friedman, W. Comparison of Filter Bag, Cyclonic, and Wet Dust Collection Methods in Vacuum Cleaners. AIHAJ-Am. Ind. Hyg. Assoc. 2001, 62, 573–583. [Google Scholar] [CrossRef]
- Grass, N.; Hartmann, W.; Klockner, M. Application of Different Types of High-Voltage Supplies on Industrial Electrostatic Precipitators. IEEE Trans. Ind. Appl. 2004, 40, 1513–1520. [Google Scholar] [CrossRef]
- Park, J.-S. Electrospinning and its applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 1, 043002. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ren, B.; Wang, Y.; Lu, Y.; Wang, L.; Chen, Y.; Yang, J.; Huang, Y. Hierarchical Porous Ceramics with 3D Reticular Architecture and Efficient Flow-Through Filtration Towards High-Temperature Particulate Matter Capture. Chem. Eng. J. 2019, 362, 504–512. [Google Scholar] [CrossRef]
- Bai, Y.; Han, C.B.; He, C.; Gu, G.Q.; Nie, J.H.; Shao, J.J.; Xiao, T.X.; Deng, C.R.; Wang, Z.L. Washable Multilayer Triboelectric Air Filter for Efficient Particulate Matter PM2.5Removal. Adv. Funct. Mater. 2018, 28, 1706680. [Google Scholar] [CrossRef]
- Khalid, B.; Bai, X.; Wei, H.; Huang, Y.; Wu, H.; Cui, Y. Direct Blow-Spinning of Nanofibers on a Window Screen for Highly Efficient PM2.5 Removal. Nano Lett. 2017, 17, 1140–1148. [Google Scholar] [CrossRef]
- Jeong, S.; Cho, H.; Han, S.; Won, P.; Lee, H.; Hong, S.; Yeo, J.; Kwon, J.; Ko, S.H. High Efficiency, Transparent, Reusable, and Active PM2.5 Filters by Hierarchical Ag Nanowire Percolation Network. Nano Lett. 2017, 17, 4339–4346. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.R.; He, Z.; Wang, J.L.; Liu, J.W.; Yu, S.H. Mass Production of Nanowire-Nylon Flexible Transparent Smart Windows for PM2.5 Capture. iScience 2019, 12, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Zhang, X.; Yu, X.; Wang, S.; Qiu, J.; Tang, W.; Li, L.; Liu, H.; Wang, Z.L. Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers. ACS Nano 2016, 10, 5086–5095. [Google Scholar] [CrossRef]
- Zhang, P.; Wan, D.; Zhang, Z.; Wang, G.; Hu, J.; Shao, G. RGO-Functionalized Polymer Nanofibrous Membrane with Exceptional Surface Activity and Ultra-Low Airflow Resistance for PM2.5 Filtration. Environ. Sci. Nano 2018, 5, 1813–1820. [Google Scholar] [CrossRef]
- Glover, T.G.; Peterson, G.W.; Schindler, B.J.; Britt, D.; Yaghi, O. MOF-74 Building Unit has a Direct Impact on Toxic Gas Adsorption. Chem. Eng. Sci. 2011, 66, 163–170. [Google Scholar] [CrossRef]
- Xu, J.; Liu, C.; Hsu, P.C.; Liu, K.; Zhang, R.; Liu, Y.; Cui, Y. Roll-to-Roll Transfer of Electrospun Nanofiber Film for High-Efficiency Transparent Air Filter. Nano Lett. 2016, 16, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, C.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Progress on Particulate Matter Filtration Technology: Basic Concepts, Advanced Materials, and Performances. Nanoscale 2020, 12, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.Q.; Han, C.B.; Lu, C.X.; He, C.; Jiang, T.; Gao, Z.L.; Li, C.J.; Wang, Z.L. Triboelectric Nanogenerator Enhanced Nanofiber Air Filters for Efficient Particulate Matter Removal. ACS Nano 2017, 11, 6211–6217. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal-Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Accounts Chem. Res. 2019, 52, 1461–1470. [Google Scholar]
- Ma, S.; Zhang, M.; Nie, J.; Tan, J.; Yang, B.; Song, S. Design of Double-Component Metal-Organic Framework Air Filters with PM2.5 Capture, Gas Adsorption and Antibacterial Capacities. Carbohydr. Polym. 2019, 203, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, X.; Zhao, S.; Hu, Y.; Zhong, Z.; Xing, W.; Wang, H. Multifunctional Metal Organic Framework and Carbon Nanotube-Modified Filter for Combined Ultrafine Dust Capture and SO2 Dynamic Adsorption. Environ. Sci. Nano 2018, 5, 3023–3031. [Google Scholar] [CrossRef]
- Peterson, G.W.; Mahle, J.J.; DeCoste, J.B.; Gordon, W.O.; Rossin, J.A. Extraordinary NO2 Removal by the Metal-Organic Framework UiO-66-NH2. Angew. Chem. 2016, 55, 6235–6238. [Google Scholar] [CrossRef]
- Peterson, G.W.; DeCoste, J.B.; Fatollahi-Fard, F.; Britt, D.K. Engineering UiO-66-NH2 for Toxic Gas Removal. Ind. Eng. Chem. Res. 2014, 53, 701–707. [Google Scholar] [CrossRef]
- Zhao, J.; Nunn, W.T.; Lemaire, P.C.; Lin, Y.; Dickey, M.D.; Oldham, C.J.; Walls, H.J.; Peterson, G.W.; Losego, M.D.; Parsons, G.N. Facile Conversion of Hydroxy Double Salts to Metal-Organic Frameworks Using Metal Oxide Particles and Atomic Layer Deposition Thin-Film Templates. J. Am. Chem. Soc. 2015, 137, 13756–13759. [Google Scholar] [CrossRef] [PubMed]
- Wisser, D.; Wisser, F.M.; Raschke, S.; Klein, N.; Leistner, M.; Grothe, J.; Brunner, E.; Kaskel, S. Biological Chitin-MOF Composites with Hierarchical Pore Systems for Air-Filtration Application. Angew. Chem. Int. Ed. 2015, 54, 12588–12591. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yuan, S.; Feng, X.; Li, H.; Zhou, J.; Wang, B. Preparation of Nanofibrous Metal-Organic Framework Filters for Efficient Air Pollution Control. J. Am. Chem. Soc. 2016, 138, 5785–5788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Hua, T.; Jiang, P.; Yin, X.; Yu, J.; Ding, B. Low-Resistance Dual-Purpose Air Filter Releasing Negative Ions and Effectively Capturing PM2.5. ACS Appl. Mater. Interfaces 2017, 9, 12054–12063. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, Q.; Ping, Z.; Gao, Y.; Chen, P.; Huang, F. Electric Field-Driven Air Purification Filter for High Efficiency Removal of PM2.5 and SO2: Local Electric Field Induction and External Electric Field Enhancement. Atmosphere 2022, 13, 1260. https://doi.org/10.3390/atmos13081260
Li J, Sun Q, Ping Z, Gao Y, Chen P, Huang F. Electric Field-Driven Air Purification Filter for High Efficiency Removal of PM2.5 and SO2: Local Electric Field Induction and External Electric Field Enhancement. Atmosphere. 2022; 13(8):1260. https://doi.org/10.3390/atmos13081260
Chicago/Turabian StyleLi, Jian, Qingyun Sun, Zhongxin Ping, Yihong Gao, Peiyu Chen, and Fangzhi Huang. 2022. "Electric Field-Driven Air Purification Filter for High Efficiency Removal of PM2.5 and SO2: Local Electric Field Induction and External Electric Field Enhancement" Atmosphere 13, no. 8: 1260. https://doi.org/10.3390/atmos13081260
APA StyleLi, J., Sun, Q., Ping, Z., Gao, Y., Chen, P., & Huang, F. (2022). Electric Field-Driven Air Purification Filter for High Efficiency Removal of PM2.5 and SO2: Local Electric Field Induction and External Electric Field Enhancement. Atmosphere, 13(8), 1260. https://doi.org/10.3390/atmos13081260





