Patterns and Controls of the Latent and Sensible Heat Fluxes in the Brazilian Pampa Biome
Abstract
:1. Introduction
2. Materials and Methods
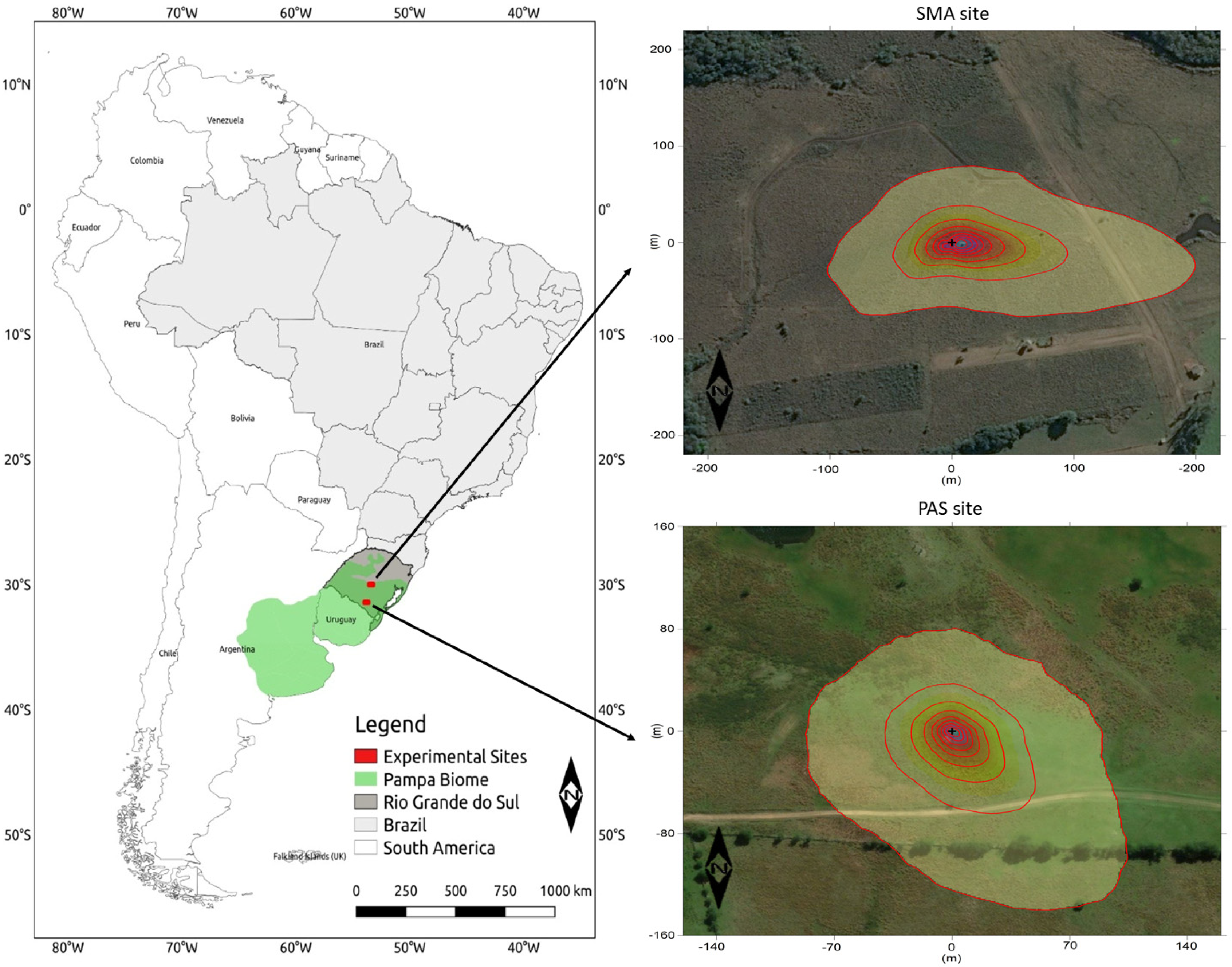
2.1. Site Description
2.2. Energy Fluxes and Meteorological Measurements
2.3. Components of the Energy Balance
- -
- For atmospheric instability:
- -
- For atmospheric stability:
3. Results and Discussion
3.1. Meteorological and Surface Conditions
3.2. Energy Balance Components
3.3. Environmental Variables That Control the H and LE Fluxes
3.4. Aerodynamic and Surface Conductances
3.4.1. Average Daily Cycles
3.4.2. Hysteresis Loops in the Surface Conductance
3.5. Biophysical Control of Evapotranspiration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sellers, P.J.; Bounoua, L.; Collatz, G.J.; Randall, D.A.; Dazlich, D.A.; Los, S.O.; Berry, J.A.; Fung, I.; Tucker, C.J.; Field, C.B.; et al. Comparison of Radiative and Physiological Effects of Doubled Atmospheric CO2 on Climate. Science 1996, 271, 1402–1406. [Google Scholar] [CrossRef]
- Zhang, Y.; Kadota, T.; Ohata, T.; Oyunbaatar, D. Environmental controls on evapotranspiration from sparse grassland in Mongolia. Hydrol. Process. 2007, 21, 2016–2027. [Google Scholar] [CrossRef]
- Woodward, F.I.; Smith, T.M. Global Photosynthesis and Stomatal Conductance: Modelling the Controls by Soil and Climate. Adv. Bot. Res. 1994, 20, 1–41. [Google Scholar]
- Adams, J.M.; Faure, H.; Faure-Denard, L.; McGlade, J.M.; Woodward, F.I. Increases in terrestrial carbon storage from the Last Glacial Maximum to the present. Nature 1990, 348, 711–714. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; Vieira, F.C.B.; Pereira, V.A.; Schünemann, A.L.; Teixeira, I.F.; Senna, A.J.T.; Stefenon, V.M. The Brazilian Pampa: A fragile biome. Diversity 2009, 1, 182–198. [Google Scholar] [CrossRef]
- Overbeck, G.; Muller, S.; Fidelis, A.; Pfadenhauer, J.; Pillar, V.; Blanco, C.; Boldrini, I.; Both, R.; Forneck, E. Brazil’s neglected biome: The South Brazilian Campos. Perspect. Plant Ecol. Evol. Syst. 2007, 9, 101–116. [Google Scholar] [CrossRef]
- Ruviaro, C.F.; de Léis, C.M.; Lampert, V.D.N.; Barcellos, J.O.J.; Dewes, H. Carbon footprint in different beef production systems on a southern Brazilian farm: A case study. J. Clean. Prod. 2015, 96, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Pillar, V.D.; Müller, S.C.; Castilhos, Z.M.; Jacques, A.V.Á. Campos Sulinos—Conservação E Uso Sustentável Da Biodiversidade; Ministério do Meio Ambiente: Brasília, Brazil, 2009; ISBN 978-85-7738-117-3.
- Oliveira, T.E.D.; Freitas, D.S.D.; Gianezini, M.; Ruviaro, C.F.; Zago, D.; Mércio, T.Z.; Dias, E.A.; Lampert, V.D.N.; Barcellos, J.O.J. Agricultural land use change in the Brazilian Pampa Biome: The reduction of natural grasslands. Land Use Policy 2017, 63, 394–400. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Lin, G.; Zhang, W.; Miao, H.; Wei, L.; Huang, J.; Han, X. Energy balance and partition in Inner Mongolia steppe ecosystems with different land use types. Agric. For. Meteorol. 2009, 149, 1800–1809. [Google Scholar] [CrossRef]
- Babel, W.; Biermann, T.; Coners, H.; Falge, E.; Seeber, E.; Ingrisch, J.; Schleuß, P.-M.; Gerken, T.; Leonbacher, J.; Leipold, T.; et al. Pasture degradation modifies the water and carbon cycles of the Tibetan highlands. Biogeosciences 2014, 11, 6633–6656. [Google Scholar] [CrossRef] [Green Version]
- Lan, X.; Li, Y.; Shao, R.; Chen, X.; Lin, K.; Cheng, L.; Gao, H.; Liu, Z. Vegetation controls on surface energy partitioning and water budget over China. J. Hydrol. 2021, 600, 125646. [Google Scholar] [CrossRef]
- Lang, Y.; Yang, X.; Cai, H. Assessing the degradation of grassland ecosystems based on the advanced local net production scaling method—The case of Inner Mongolia, China. Land Degrad. Dev. 2021, 32, 559–572. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, M.D. Variation Among Biomes in Temporal Dynamics of Aboveground Primary Production. Science 2001, 291, 481–484. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Westra, S.; Maier, H.R. Impact of evapotranspiration process representation on runoff projections from conceptual rainfall-runoff models. Water Resour. Res. 2017, 53, 435–454. [Google Scholar] [CrossRef]
- Rajan, N.; Maas, S.J.; Cui, S. Extreme drought effects on summer evapotranspiration and energy balance of a grassland in the Southern Great Plains. Ecohydrology 2015, 8, 1194–1204. [Google Scholar] [CrossRef]
- Wang, K.; Dickinson, R.E. A review of global terrestrial evapotranspiration: Observation, modeling, climatology, and climatic variability. Rev. Geophys. 2012, 50, RG2005. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [Green Version]
- Senay, G.B.; Leake, S.; Nagler, P.L.; Artan, G.; Dickinson, J.; Cordova, J.T.; Glenn, E.P. Estimating basin scale evapotranspiration (ET) by water balance and remote sensing methods. Hydrol. Process. 2011, 25, 4037–4049. [Google Scholar] [CrossRef]
- Forzieri, G.; Alkama, R.; Miralles, D.G.; Cescatti, A. Satellites reveal contrasting responses of regional climate to the widespread greening of Earth. Science 2017, 356, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Cheng, L.; Zhou, G.; Chen, X.; Lin, K.; Zhang, W.; Chen, X.; Zhou, P. Global Response of Evapotranspiration Ratio to Climate Conditions and Watershed Characteristics in a Changing Environment. J. Geophys. Res. Atmos. 2020, 125, e2020JD032371. [Google Scholar] [CrossRef]
- Zha, T.; Li, C.; Kellomäki, S.; Peltola, H.; Wang, K.-Y.; Zhang, Y. Controls of Evapotranspiration and CO2 Fluxes from Scots Pine by Surface Conductance and Abiotic Factors. PLoS ONE 2013, 8, e69027. [Google Scholar]
- Igarashi, Y.; Kumagai, T.; Yoshifuji, N.; Sato, T.; Tanaka, N.; Tanaka, K.; Suzuki, M.; Tantasirin, C. Environmental control of canopy stomatal conductance in a tropical deciduous forest in northern Thailand. Agric. For. Meteorol. 2015, 202, 1–10. [Google Scholar] [CrossRef]
- Hirano, T.; Suzuki, K.; Hirata, R. Energy balance and evapotranspiration changes in a larch forest caused by severe disturbance during an early secondary succession. Agric. For. Meteorol. 2017, 232, 457–468. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, B.; Chang, X.; Yang, Q.; Yang, Y.; Liu, Z.; Cleverly, J.; Eamus, D. Evapotranspiration partitioning, stomatal conductance, and components of the water balance: A special case of a desert ecosystem in China. J. Hydrol. 2016, 538, 374–386. [Google Scholar] [CrossRef]
- Yue, P.; Zhang, Q.; Zhang, L.; Li, H.; Yang, Y.; Zeng, J.; Wang, S. Long-term variations in energy partitioning and evapotranspiration in a semiarid grassland in the Loess Plateau of China. Agric. For. Meteorol. 2019, 278, 107671. [Google Scholar] [CrossRef]
- Baldocchi, D. A comparative study of mass and energy exchange rates over a closed C3 (wheat) and an open C4 (corn) crop: II. CO2 exchange and water use efficiency. Agric. For. Meteorol. 1994, 67, 291–321. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal Control of Transpiration: Scaling Up from Leaf to Region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar]
- Fraga, C.I.D.M.; Sanches, L.; Pinto Junior, O.B.; Curado, L.F.A.; Gaio, D.C. Canopy Conductance, Aerodynamic Conductance and the Decoupling Coefficient in the Vochysia Divergens Pohl (Vochysiaceae) Forest in the Brazilian Pantanal. Rev. Bras. Meteorol. 2015, 30, 275–284. [Google Scholar] [CrossRef]
- Cabral, O.M.R.; da Rocha, H.R.; Gash, J.H.; Freitas, H.C.; Ligo, M.A.V. Water and energy fluxes from a woodland savanna (cerrado) in southeast Brazil. J. Hydrol. Reg. Stud. 2015, 4, 22–40. [Google Scholar] [CrossRef] [Green Version]
- da Rocha, H.R.; Goulden, M.L.; Miller, S.D.; Menton, M.C.; Pinto, L.D.V.O.; de Freitas, H.C.; e Silva Figueira, A.M. Seasonality Of Water And Heat Fluxes Over A Tropical Forest In Eastern Amazonia. Ecol. Appl. 2004, 14, 22–32. [Google Scholar] [CrossRef]
- Eamus, D.; Cole, S. Diurnal and Seasonal Comparisons of Assimilation, Phyllode Conductance and Water Potential of Three Acacia and One Eucalyptus Species in the Wet-Dry Tropics of Australia. Aust. J. Bot. 1997, 45, 275. [Google Scholar] [CrossRef]
- Wilson, K.B.; Baldocchi, D.D. Seasonal and interannual variability of energy fluxes over a broadleaved temperate deciduous forest in North America. Agric. For. Meteorol. 2000, 100, 1–18. [Google Scholar] [CrossRef]
- Yu, G.-R.; Nakayama, K.; Lu, H.-Q. Responses of Stomatal Conductance in Field-grown Maize Leaves to Certain Environmental Factors over a Long Term. J. Agric. Meteorol. 1996, 52, 311–320. [Google Scholar] [CrossRef]
- Wright, I.R.; Manzi, A.O.; da Rocha, H.R. Surface conductance of Amazonian pasture: Model application and calibration for canopy climate. Agric. For. Meteorol. 1995, 75, 51–70. [Google Scholar] [CrossRef]
- Sellers, P.J.; Randall, D.A.; Collatz, G.J.; Berry, J.A.; Field, C.B.; Dazlich, D.A.; Zhang, C.; Collelo, G.D.; Bounoua, L. A Revised Land Surface Parameterization (SiB2) for Atmospheric GCMS. Part I: Model Formulation. J. Clim. 1996, 9, 676–705. [Google Scholar] [CrossRef]
- Villalobos, F.J.; Testi, L.; Orgaz, F.; García-Tejera, O.; Lopez-Bernal, A.; González-Dugo, M.V.; Ballester-Lurbe, C.; Castel, J.R.; Alarcón-Cabañero, J.J.; Nicolás-Nicolás, E.; et al. Modelling canopy conductance and transpiration of fruit trees in Mediterranean areas: A simplified approach. Agric. For. Meteorol. 2013, 171–172, 93–103. [Google Scholar] [CrossRef]
- Polhamus, A.; Fisher, J.B.; Tu, K.P. What controls the error structure in evapotranspiration models? Agric. For. Meteorol. 2013, 169, 12–24. [Google Scholar] [CrossRef]
- Rubert, G.C.; Roberti, D.R.; Pereira, L.S.; Quadros, F.L.F.; Velho, H.F.D.C.; Moraes, O.L.L.D. Evapotranspiration of the Brazilian Pampa Biome: Seasonality and Influential Factors. Water 2018, 10, 1864. [Google Scholar] [CrossRef] [Green Version]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- IBGE. Mapas. Available online: http://mapas.ibge.gov.br/tematicos/solos (accessed on 26 June 2021).
- dos Santos, A.B.; de Quadros, F.L.F.; Confortin, A.C.C.; Seibert, L.; Ribeiro, B.S.M.R.; Severo, P.D.O.; Casanova, P.T.; Machado, G.K.G. Morfogênese de gramíneas nativas do Rio Grande do Sul (Brasil) submetidas a pastoreio rotativo durante primavera e verão. Ciência Rural 2014, 44, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Quadros, F.L.F.; Pillar, V.D.P. Dinâmica vegetacional em pastagem natural submetida a tratamentos de queima e pastejo. Ciência Rural 2001, 31, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Timm, A.U.; Roberti, D.R.; Streck, N.A.; de Gonçalves, L.G.G.; Acevedo, O.C.; Moraes, O.L.; Moreira, V.S.; Degrazia, G.A.; Ferlan, M.; Toll, D.L. Energy Partitioning and Evapotranspiration over a Rice Paddy in Southern Brazil. J. Hydrometeorol. 2014, 15, 1975–1988. [Google Scholar] [CrossRef]
- Diaz, M.B.; Roberti, D.R.; Carneiro, J.V.; Souza, V.D.A.; de Moraes, O.L.L. Dynamics of the superficial fluxes over a flooded rice paddy in southern Brazil. Agric. For. Meteorol. 2019, 276–277, 107650. [Google Scholar] [CrossRef]
- Acevedo, O.C.; Moraes, O.L.L.; Degrazia, G.A.; Medeiros, L.E. Intermittency and the exchange of scalars in the nocturnal surface layer. Bound.-Layer Meteorol. 2006, 119, 41–55. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Gash, J.H.C.; Culf, A.D. Applying a linear detrend to eddy correlation data in realtime. Bound.-Layer Meteorol. 1996, 79, 301–306. [Google Scholar] [CrossRef]
- Moncrieff, J.B.; Massheder, J.M.; de Bruin, H.; Elbers, J.; Friborg, T.; Heusinkveld, B.; Kabat, P.; Scott, S.; Soegaard, H.; Verhoef, A. A system to measure surface fluxes of momentum, sensible heat, water vapour and carbon dioxide. J. Hydrol. 1997, 188–189, 589–611. [Google Scholar] [CrossRef]
- Moncrieff, J.; Clement, R.; Finnigan, J.; Meyers, T. Averaging, Detrending, and Filtering of Eddy Covariance Time Series. In Handbook of Micrometeorology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 7–31. [Google Scholar]
- Mauder, M.; Foken, T. Impact of post-field data processing on eddy covariance flux estimates and energy balance closure. Meteorol. Z. 2006, 15, 597–609. [Google Scholar] [CrossRef]
- Nakai, T.; Shimoyama, K. Ultrasonic anemometer angle of attack errors under turbulent conditions. Agric. For. Meteorol. 2012, 162–163, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Vickers, D.; Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean. Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Wilson, K.B.; Baldocchi, D.D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Dolman, H.; Falge, E.; Field, C.; Goldstein, A.; Granier, A.; et al. Energy partitioning between latent and sensible heat flux during the warm season at FLUXNET sites. Water Resour. Res. 2002, 38, 30-1–30-11. [Google Scholar] [CrossRef] [Green Version]
- Leuning, R.; Cleugh, H.A.; Zegelin, S.J.; Hughes, D. Carbon and water fluxes over a temperate Eucalyptus forest and a tropical wet/dry savanna in Australia: Measurements and comparison with MODIS remote sensing estimates. Agric. For. Meteorol. 2005, 129, 151–173. [Google Scholar] [CrossRef]
- Foken, T. The energy balance closure problem: An overview. Ecol. Appl. 2008, 18, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines For Computing Crop Water Requirements—FAO Irrigation And Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer: New York, NY, USA, 1998; ISBN 978-0-387-94937-6. [Google Scholar]
- Lyra, G.B.; Pereira, A.R. Dificuldades de estimativa dos parâmetros de rugosidade aerodinâmica pelo perfil logarítmico do vento sobre vegetação esparsa em região semi-árida. Rev. Bras. Geofísica 2007, 25. [Google Scholar] [CrossRef]
- Yunusa, I.A.M.; Eamus, D.; Taylor, D.; Whitley, R.; Gwenzi, W.; Palmer, A.R.; Li, Z. Partitioning of turbulent flux reveals contrasting cooling potential for woody vegetation and grassland during heat waves. Q. J. R. Meteorol. Soc. 2015, 141, 2528–2537. [Google Scholar] [CrossRef]
- Trepekli, A.; Loupa, G.; Rapsomanikis, S. Seasonal evapotranspiration, energy fluxes and turbulence variance characteristics of a Mediterranean coastal grassland. Agric. For. Meteorol. 2016, 226–227, 13–27. [Google Scholar] [CrossRef]
- Kuplich, T.M.; Moreira, A.; Fontana, D.C. Série temporal de índice de vegetação sobre diferentes tipologias vegetais no Rio Grande do Sul. Rev. Bras. Eng. Agrícola E Ambient. 2013, 17, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Guerini Filho, M.; Kuplich, T.M.; Quadros, F.L.F. De Estimating natural grassland biomass by vegetation indices using Sentinel 2 remote sensing data. Int. J. Remote Sens. 2020, 41, 2861–2876. [Google Scholar] [CrossRef]
- Wilson, K.; Goldstein, A.; Falge, E.; Aubinet, M.; Baldocchi, D.; Berbigier, P.; Bernhofer, C.; Ceulemans, R.; Dolman, H.; Field, C.; et al. Energy balance closure at FLUXNET sites. Agric. For. Meteorol. 2002, 113, 223–243. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, T.; Buligon, L.; de Arruda Souza, V.; Romio, L.C.; Roberti, D.R. Influence of clearness index and soil moisture in the soil thermal dynamic in natural pasture in the Brazilian Pampa biome. Geoderma 2020, 378, 114582. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Corti, T.; Davin, E.L.; Hirschi, M.; Jaeger, E.B.; Lehner, I.; Orlowsky, B.; Teuling, A.J. Investigating soil moisture–climate interactions in a changing climate: A review. Earth-Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Majozi, N.P.; Mannaerts, C.M.; Ramoelo, A.; Mathieu, R.; Nickless, A.; Verhoef, W. Analysing surface energy balance closure and partitioning over a semi-arid savanna FLUXNET site in Skukuza, Kruger National Park, South Africa. Hydrol. Earth Syst. Sci. 2017, 21, 3401–3415. [Google Scholar] [CrossRef] [Green Version]
- Alves, I.; Santos Pereira, L. Modelling surface resistance from climatic variables? Agric. Water Manag. 2000, 42, 371–385. [Google Scholar] [CrossRef]
- Goulden, M.L.; Miller, S.D.; da Rocha, H.R.; Menton, M.C.; de Freitas, H.C.; de Silva Figueira, A.M.; de Sousa, C.A.D. Diel and seasonal patterns of tropical forest CO2 exchange. Ecol. Appl. 2004, 14, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, P.; Meyers, T.P.; Scott, R.L.; Kennedy, L.; Heuer, M. Energy exchange and evapotranspiration over two temperate semi-arid grasslands in North America. Agric. For. Meteorol. 2012, 153, 31–44. [Google Scholar] [CrossRef]
- Huizhi, L.; Jianwu, F. Seasonal and Interannual Variations of Evapotranspiration and Energy Exchange over Different Land Surfaces in a Semiarid Area of China. J. Appl. Meteorol. Climatol. 2012, 51, 1875–1888. [Google Scholar] [CrossRef]
- Wang, H.; Guan, H.; Deng, Z.; Simmons, C.T. Optimization of canopy conductance models from concurrent measurements of sap flow and stem water potential on Drooping Sheoak in South Australia. Water Resour. Res. 2014, 50, 6154–6167. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, T.R.; Vourlitis, G.L.; Lobo, F.D.A.; Santanna, F.B.; de Arruda, P.H.Z.; Nogueira, J.D.S. Modeling canopy conductance under contrasting seasonal conditions for a tropical savanna ecosystem of south central Mato Grosso, Brazil. Agric. For. Meteorol. 2016, 218–219, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.-H.; Zhao, J.-F.; Wang, G.-Z.; Chen, M.-P.; Yang, L.-Y.; He, C.-S.; Restrepo-Coupe, N.; Peng, S.-S.; Liu, X.-Y.; da Rocha, H.R.; et al. Surface conductance for evapotranspiration of tropical forests: Calculations, variations, and controls. Agric. For. Meteorol. 2019, 275, 317–328. [Google Scholar] [CrossRef]
- Marques, T.V.; Mendes, K.; Mutti, P.; Medeiros, S.; Silva, L.; Perez-Marin, A.M.; Campos, S.; Lúcio, P.S.; Lima, K.; dos Reis, J.; et al. Environmental and biophysical controls of evapotranspiration from Seasonally Dry Tropical Forests (Caatinga) in the Brazilian Semiarid. Agric. For. Meteorol. 2020, 287, 107957. [Google Scholar] [CrossRef]
- Groh, J.; Pütz, T.; Gerke, H.H.; Vanderborght, J.; Vereecken, H. Quantification and Prediction of Nighttime Evapotranspiration for Two Distinct Grassland Ecosystems. Water Resour. Res. 2019, 55, 2961–2975. [Google Scholar] [CrossRef]
- Phillips, N.; Oren, R. A comparison of daily representations of canopy conductance based on two conditional time-averaging methods and the dependence of daily conductance on environmental factors. Ann. Des. Sci. For. 1998, 55, 217–235. [Google Scholar] [CrossRef] [Green Version]
- Moffat, A.M.; Papale, D.; Reichstein, M.; Hollinger, D.Y.; Richardson, A.D.; Barr, A.G.; Beckstein, C.; Braswell, B.H.; Churkina, G.; Desai, A.R.; et al. Comprehensive comparison of gap-filling techniques for eddy covariance net carbon fluxes. Agric. For. Meteorol. 2007, 147, 209–232. [Google Scholar] [CrossRef]
- Grelle, A.; Lindroth, A.; Mölder, M. Seasonal variation of boreal forest surface conductance and evaporation. Agric. For. Meteorol. 1999, 98–99, 563–578. [Google Scholar] [CrossRef]
- Souza Filho, J.D.D.C.; Ribeiro, A.; Costa, M.H.; Cohen, J.C.P. Mecanismos de controle da variação sazonal da transpiração de uma floresta tropical no nordeste da amazônia. Acta Amaz. 2005, 35, 223–229. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [Green Version]
- Streck, N.A. Stomatal response to water vapor pressure deficit: An unsolved issue. Rev. Bras. Agrociência 2003, 09, 314–322. [Google Scholar]
- Kelliher, F.M.; Leuning, R.; Raupach, M.R.; Schulze, E.-D. Maximum conductances for evaporation from global vegetation types. Agric. For. Meteorol. 1995, 73, 1–16. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Li, Z.; Tang, J.; Caldwell, P.; Zhang, W. Biophysical control of whole tree transpiration under an urban environment in Northern China. J. Hydrol. 2011, 402, 388–400. [Google Scholar] [CrossRef]
- Lu, P.; Yunusa, I.A.M.; Walker, R.R.; Müller, W.J. Regulation of canopy conductance and transpiration and their modelling in irrigated grapevines. Funct. Plant Biol. 2003, 30, 689. [Google Scholar] [CrossRef]
- Unsworth, M.; Phillips, N.; Link, T.; Bond, B.; Falk, M.; Harmon, M.; Hinckley, T.; Marks, D.; Paw, U. Components and Controls of Water Flux in an Old-growth Douglas-fir?Western Hemlock Ecosystem. Ecosystems 2004, 7, 468–481. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, G.; Su, Y.; Zhang, K.; Han, T.; Ma, J.; Wang, W.; Ma, T.; Feng, L. Hysteresis loops between canopy conductance of grapevines and meteorological variables in an oasis ecosystem. Agric. For. Meteorol. 2015, 214–215, 319–327. [Google Scholar] [CrossRef]
- Tuzet, A.; Perrier, A.; Leuning, R. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant. Cell Environ. 2003, 26, 1097–1116. [Google Scholar] [CrossRef]
- Zhang, Q.; Manzoni, S.; Katul, G.; Porporato, A.; Yang, D. The hysteretic evapotranspiration-Vapor pressure deficit relation. J. Geophys. Res. Biogeosciences 2014, 119, 125–140. [Google Scholar] [CrossRef]
- Mallick, K.; Trebs, I.; Boegh, E.; Giustarini, L.; Schlerf, M.; Drewry, D.T.; Hoffmann, L.; von Randow, C.; Kruijt, B.; Araùjo, A.; et al. Canopy-scale biophysical controls of transpiration and evaporation in the Amazon Basin. Hydrol. Earth Syst. Sci. 2016, 20, 4237–4264. [Google Scholar] [CrossRef] [Green Version]







| Variable | Sensor Model and Manufacturer/Sensor Type | Position (m)-Sites | Frequency |
|---|---|---|---|
| Wind speed components and air temperature | CSAT3, Campbell Scientifific Inc., Logan, UT, USA/3D sonic anemometer | 2.5-PAS | 10 Hz |
| Wind Master Pro; Gill Instruments, Hampshire, UK/3D sonic anemometer | 3.0-SMA (until 25 June 2016) | 10 Hz | |
| IRGASON, Campbell Scientific Inc., Logan, UT, USA/Integrate 3D sonic anemometer and open path gas analyzer | 3.0-SMA (after 25 June 2016) | 10 Hz | |
| H2O concentration | LI7500, LI-COR Inc., Lincoln, NE, USA/Open path gas analyzer | 2.5-PAS 3.0-SMA (until 25 June 2016) | 10 Hz |
| IRGASON, Campbell Scientific Inc., Logan, UT, USA/Integrate 3D sonic anemometer and open path gas analyzer | 3.0-SMA (after 25 June 2016) | 10 Hz | |
| Air temperature (Temp) and relative humidity (RH) | HMP155, Vaisala, Finland/Thermohygrometer | 2.5-PAS 3.0-SMA | 1 min |
| Precipitation | TR525USW, Texas Electronics, Dallas, TX, USA/Pluviometer | 2.5-SMA 2.0-SMA | 1 min |
| Net radiation (Rn) | CNR4, Kipp & Zonen, Delft, The Netherlands/Net Radiometer | 3.0-SMA | 1 min |
| CNR2, Campbell Scientific Inc., Logan, UT, USA/Net Radiometer | 2.5-PAS | 1 min | |
| Global Radiation (Rg) | CNR4, Kipp & Zonen, Delft, The Netherlands/Net Radiometer | 3-SMA | 1 min |
| Li 200S Pyranometer—LI-COR, Lincoln, NE, USA/Pyranometer | 2.5-PAS | 1 min | |
| Ground heat flux (G) | HFP01, Hukseflux Thermal Sensors B.V., Delft, The Netherlands/Thermopile | −0.10-PAS −0.10-SMA | 5 min |
| Soil moisture ( | CS616, Campbell Scientific Inc., Logan, UT, USA/Water Content Reflectometer | −0.10-PAS −0.10-SMA | 1 min |
| Soil Temperature (Tsoil) | T108, Campbell Scientific Inc., Logan, UT, USA/Thermometer | −0.05-PAS −0.05-SMA | 1 min |
| Site | Temp (°C) | Rg (Wm−2) | Prec (mm) | θ (m3 m−3) | Tsoil (°C) | Rn (Wm−2) | LE (Wm−2) | H (Wm−2) | G (Wm−2) | β | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AW | SMA | 16.2 | 127.2 | 1813 | 0.24 | 16.6 | 69.7 | 48.4 | 26.1 | −4.5 | 0.54 | |
| PAS | 13.9 | 123.9 | 1359 | 0.20 | 15.7 | 57.2 | 44.1 | 19.0 | −4.6 | 0.43 | ||
| SS | SMA | 22.6 | 226.3 | 2036 | 0.17 | 22.1 | 148.4 | 105.5 | 42.1 | 0.6 | 0.40 | |
| PAS | 19.9 | 243.4 | 1919 | 0.20 | 23.2 | 145.1 | 108.8 | 35.2 | 2.2 | 0.33 | ||
| Annual | 2014/2015 | SMA | 19.8 | 183.1 | 1824 | 0.23 | 19.8 | 114.1 | 80.8 | 34.9 | −1.6 | 0.43 |
| PAS | 17.6 | 192.4 | 1723 | 0.17. | 19.8 | 105.9 | 78.4 | 28.9 | −0.2 | 0.37 | ||
| 2015/2016 | SMA | 18.7 | 171.0 | 2025 | 0.21 | 18.2 | 104.4 | 73.2 | 33.2 | −2.1 | 0.45 | |
| PAS | 16.2 | 176.7 | 1555 | 0.19 | 18.7 | 97.2 | 75.6 | 25.3 | −2.1 | 0.33 | ||
| Entire period | SMA | 19.4 | 177.1 | 3849 | 0.22 | 19.3 | 109.4 | 77.1 | 34.1 | −1.8 | 0.44 | |
| PAS | 16.9 | 184.5 | 3278 | 0.18 | 19.3 | 101.6 | 77.1 | 27.0 | −1.1 | 0.35 | ||
| Pearson’s Correlation | PAS | SMA |
|---|---|---|
| LE vs. Rg | 0.97 | 0.86 |
| LE vs. VPD | 0.66 | 0.65 |
| LE vs. Temp | 0.50 | 0.48 |
| LE vs. RH | −0.59 | −0.64 |
| H vs. Rg | 0.92 | 0.90 |
| H vs. VPD | 0.48 | 0.44 |
| H vs. Temp | 0.36 | 0.30 |
| H vs. RH | −0.47 | −0.50 |
| Variables | Site | Max Value (Pick) | Hour | Values at Max Cs |
|---|---|---|---|---|
| Cs | SMA | 11.9 mm s−1 | 10 h 30 min | 11.9 mm s−1 |
| PAS | 17.5 mm s−1 | 9 h | 17.5 mm s−1 | |
| Rn | SMA | 424.9 W m−2 | 12 h 30 min | 181.0 W m−2 |
| PAS | 429.1 W m−2 | 12 h 30 min | 328.4 W m−2 | |
| VPD | SMA | 1.182 kPa | 15 h 30 min | 0.40 kPa |
| PAS | 0.96 kPa | 15 h 30 min | 0.52 kPa | |
| Temp | SMA | 23.94 °C | 15 h 30 min | 16.5 °C |
| PAS | 20.92 °C | 15 h | 18.1 °C |
| Variables | P1 (Morning) | P2 (Afternoon) | |||
|---|---|---|---|---|---|
| Cs | Cs | ||||
| SMA | PAS | SMA | PAS | ||
| P1 (morning) | Rn | 0.71 | 0.90 | −0.98 | −0.98 |
| VPD | 0.65 | 0.82 | −0.99 | −1 | |
| Temp | 0.70 | 0.87 | −0.98 | −0.99 | |
| P2 (afternoon) | Rn | −0.49 | −0.73 | 0.98 | 0.98 |
| VPD | 0.96 | 0.92 | −0.63 | −0.56 | |
| Temp | 0.97 | 0.85 | −0.61 | −0.42 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubert, G.C.D.; de Arruda Souza, V.; Zimmer, T.; Veeck, G.P.; Mergen, A.; Bremm, T.; Ruhoff, A.; de Gonçalves, L.G.G.; Roberti, D.R. Patterns and Controls of the Latent and Sensible Heat Fluxes in the Brazilian Pampa Biome. Atmosphere 2022, 13, 23. https://doi.org/10.3390/atmos13010023
Rubert GCD, de Arruda Souza V, Zimmer T, Veeck GP, Mergen A, Bremm T, Ruhoff A, de Gonçalves LGG, Roberti DR. Patterns and Controls of the Latent and Sensible Heat Fluxes in the Brazilian Pampa Biome. Atmosphere. 2022; 13(1):23. https://doi.org/10.3390/atmos13010023
Chicago/Turabian StyleRubert, Gisele Cristina Dotto, Vanessa de Arruda Souza, Tamíres Zimmer, Gustavo Pujol Veeck, Alecsander Mergen, Tiago Bremm, Anderson Ruhoff, Luis Gustavo Gonçalves de Gonçalves, and Débora Regina Roberti. 2022. "Patterns and Controls of the Latent and Sensible Heat Fluxes in the Brazilian Pampa Biome" Atmosphere 13, no. 1: 23. https://doi.org/10.3390/atmos13010023
APA StyleRubert, G. C. D., de Arruda Souza, V., Zimmer, T., Veeck, G. P., Mergen, A., Bremm, T., Ruhoff, A., de Gonçalves, L. G. G., & Roberti, D. R. (2022). Patterns and Controls of the Latent and Sensible Heat Fluxes in the Brazilian Pampa Biome. Atmosphere, 13(1), 23. https://doi.org/10.3390/atmos13010023






