The Role of Aging and Wind in Inducing Death and/or Growth Reduction in Korean Fir (Abies Koreana Wilson) on Mt. Halla, Korea
Abstract
:1. Introduction
2. Materials and Methods
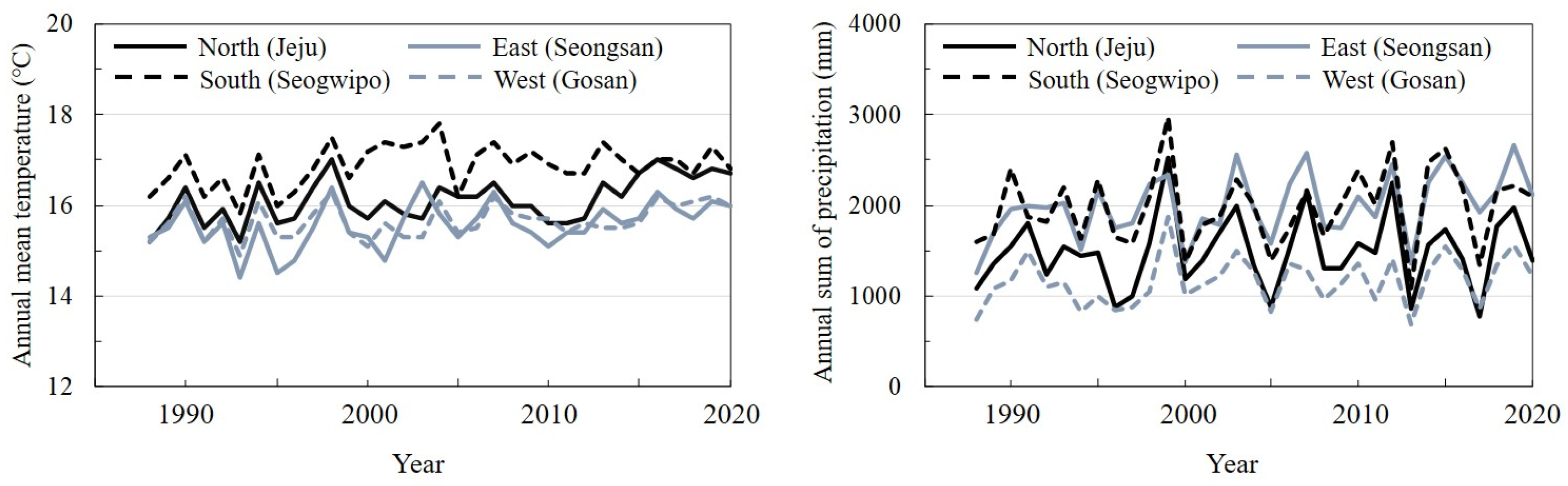
2.1. Study Sites and Sampling in the Field
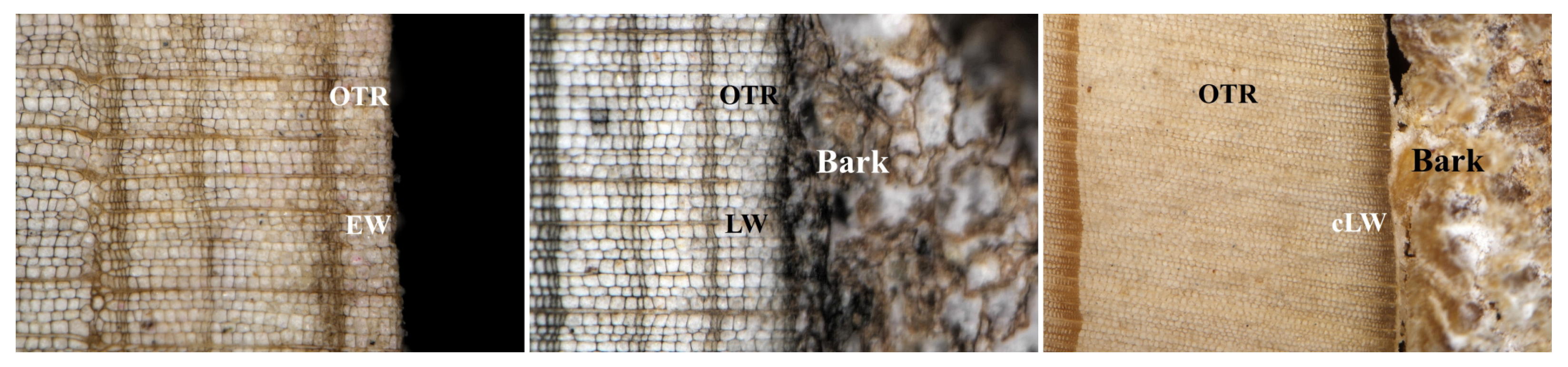
2.2. Tree-Ring Measurement and Cross-Dating
- If () > 0, = +1/2, () > 0, = +1/2,
- () = 0, = 0, () = 0, = 0,
- () < 0, = –1/2, () < 0, = –1/2
2.3. Death of Trees According to Season
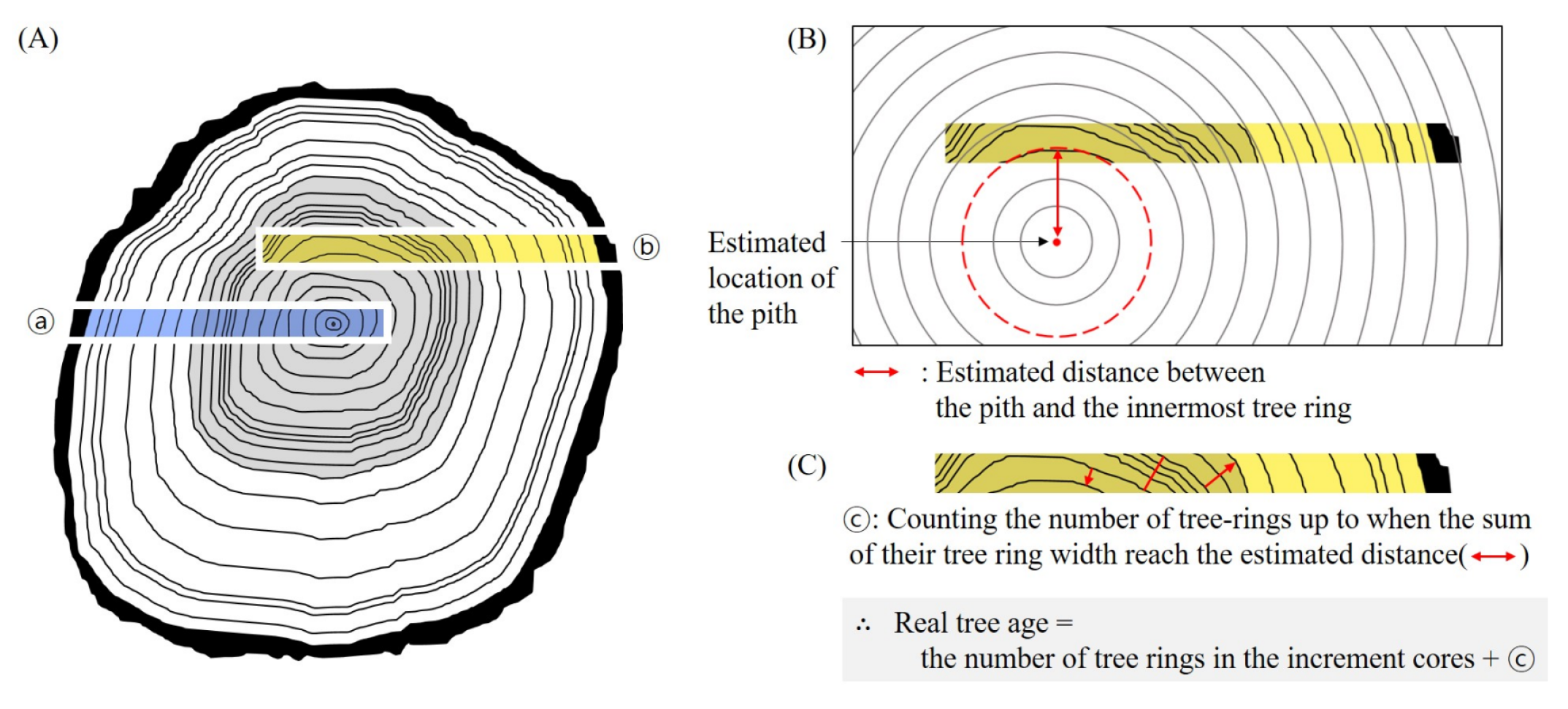
2.4. Age Analysis
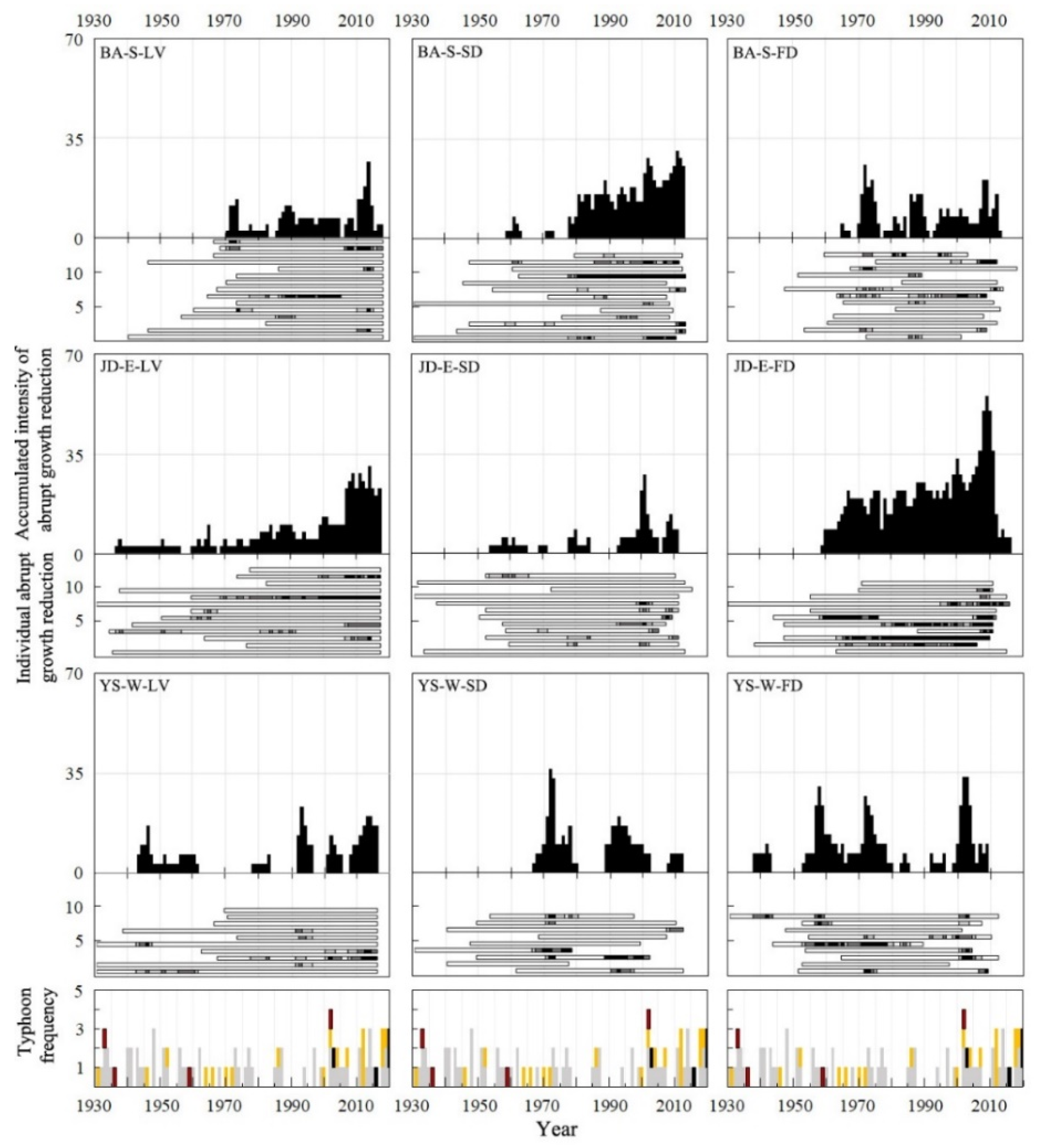
2.5. Monitoring of Abrupt Growth Reduction
3. Results and Discussion
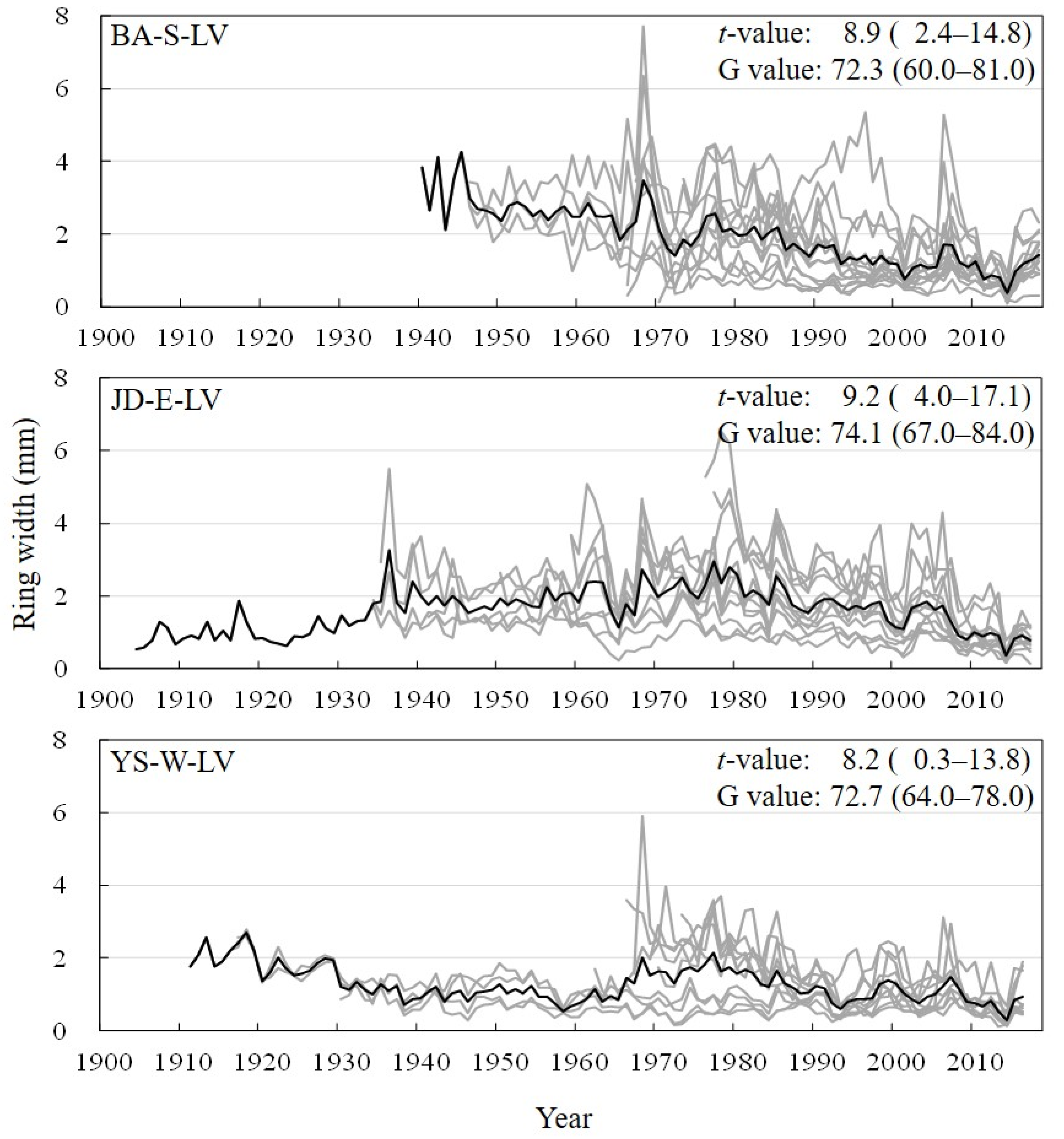
3.1. Site Chronologies and Their Synchronization
3.2. Tree Ages
3.3. Year and Season of Death
3.4. Abrupt Growth Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diez, J.M.; Boone, R.; Bohner, T.; Godoy, O. Frequency-dependent tree growth depends on climate. Ecology 2021, 102, e03284. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.K.; Ritland, K.; et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrens, C.W.; Andrew, M.E.; Mazanec, R.A.; Ruthrof, K.X.; Challis, A.; Hardy, G.; Byrne, M.; Tissue, D.T.; Rymer, P.D. Plant functional traits differ in adaptability and are predicted to be differentially affected by climate change. Ecol. Evol. 2020, 10, 232–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweingruber, F.H. Tree Rings and Environment Dendroecology; Paul Haupt: Berne, Switzerland, 1996. [Google Scholar]
- Splechtna, B.E.; Dobrys, J.; Klinka, K. Tree-ring characteristics of subalpine fir (Abies lasiocarpa (Hook.) Nutt.) in relation to elevation and climatic fluctuations. Ann. For. Sci. 2000, 57, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Eilmann, B.; Zweifel, R.; Buchmann, N. Fonti, P.; Rigling, A.; Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol. 2009, 29, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Koo, K.A.; Kong, W.-S.; Park, S.U.; Lee, J.H.; Kim, J. Sensitivity of Korean fir (Abies koreana Wils.), a threatened climate relict species, to increasing temperature at an island subalpine area. Ecol. Model. 2017, 353, 5–16. [Google Scholar] [CrossRef]
- Fang, O.; Alfaro, R.I.; Zhang, Q.-B. Tree rings reveal a major episode of forest mortality in the late 18th century on the Tibetan Plateau. Glob. Planet. Chang. 2018, 163, 44–50. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Bell, D.M.; Bradford, J.B.; Lauenroth, W.K. Mountain landscapes offer few opportunities for high-elevation tree species migration. Glob. Chang. Biol. 2014, 20, 1441–1451. [Google Scholar] [CrossRef]
- Koo, K.A.; Kim, J.; Kong, W.-S.; Jung, H.; Kim, G. Projecting the potential distribution of Abies koreana in Korea under the climate change based on RCP scenarios. J. Korean Environ. Res. Tech. 2016, 19, 19–30. [Google Scholar]
- Shin, S.; Kim, J.-H.; Dang, J.-H.; Seo, I.-S.; Lee, B.Y. Elevational distribution ranges of vascular plant species in the Baekdudaegan mountain range, South Korea. J. Ecol. Environ. 2021, 45, 7. [Google Scholar] [CrossRef]
- National Institute of Forest Science (NIFS). Status and Conservation Plan of Endangered Coniferous Forest in Republic of Korea; NIFS: Seoul, Korea, 2019. (In Korean) [Google Scholar]
- Kim, N.-S.; Lee, H.-C. A study on changes and distributions of Korean fir in sub-alpine zone. J. Korean Environ. Res. Tech. 2013, 16, 49–57, (In Korean with English abstract). [Google Scholar]
- Koh, J.G.; Kim, D.S.; Kim, J.G.; Ko, Y.J. Growth dynamics of Korean fir in Mt. Hallasan. Hallasan Res. Rep. 2015, 14, 9–26, (In Korean with English abstract). [Google Scholar]
- National Institute of Forest Science (NIFS). Korean Fir in Hallasan Mountain. The Tale of Tee: The Life and Death of Korean Fir; NIFS: Seoul, Korea, 2016. (In Korean) [Google Scholar]
- Park, W.-K.; Seo, J.-W. A dendroclimatic analysis on Abies koreana in Cheonwang-bong area of Mt. Chiri, Korea. Korean J. Quarter. Res. 1999, 13, 25–33, (In Korean with English abstract). [Google Scholar]
- Kim, J.-K.; Koh, J.-G.; Yim, H.-T.; Kim, D.-S. Changes of spatial distribution of Korean fir forest in Mt. Hallasan. Korean J. Environ. Ecol. 2017, 31, 549–556, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Kim, N.-S.; Han, D.U.; Cha, J.-Y.; Park, Y.-S.; Cho, H.-J.; Kwon, H.-J.; Cho, Y.-C.; Oh, S.-H.; Lee, C.-S. A detection of novel habitats of Abies Koreana by using species distribution models(SDMs) and its application for plan conservation. J. Korean Environ. Res. Tech. 2015, 18, 135–149, (In Korean with English abstract). [Google Scholar]
- Lee, K.-J.; Ryu, C.-H.; Choi, S.-H. The study of plant community on Orimok, Yongsil and Donnaeko area in Mt. Halla. Ecol. Resil. Infrastruct. 1992, 6, 25–43, (In Korean with English abstract). [Google Scholar]
- Kong, W.-S.; Watts, P. The Plant Geography of Korea: With an Emphasis on the Alpine Zones; Springer Science+Science & Business Media: Berlin, Germany, 2012; Volume 19. [Google Scholar]
- Kong, W.-S. Biogeography of native Korean Pinaceae. J. Korean Geograph. Soc. 2006, 41, 73–93, (In Korean with English abstract). [Google Scholar]
- Kim, E.-S.; Lee, J.-W.; Choi, I.-J.; Lim, W.; Choi, J.; Oh, C.H.; Lee, S.-H.; Kim, Y.-S. Disturbance in seedling development of Korean fir (Abies koreana Wilson) tree species on higher altitude forests of Mt. Hallasan National Park, the central part of Jeju Island, Korea. J. Ecol. Environ. 2017, 41, 22. [Google Scholar] [CrossRef] [Green Version]
- Koo, K.-A.; Park, W.-K.; Kong, W.-S. Dendrochronological analysis of Abies koreana W. at Mt. Halla, Korea: Effects of climate change on the growths. J. Ecol. Environ. 2001, 24, 281–288, (In Korean with English abstract). [Google Scholar]
- Seo, J.-W.; Kim, Y.-J.; Choi, E.-B.; Park, J.-H.; Kim, J.-H. Investigation of death years and inter-annual growth reduction of Korean firs (Abies koreana) at Yeongsil in Mt. Halla. J. Korean Environ. Res. Tech. 2019, 22, 1–14, (In Korean with English abstract). [Google Scholar]
- Chung, C.-H. Vegetation response to climate change on Jeju Island, South Korea, during the last deglaciation based on pollen record. Geosci. J. 2007, 11, 147–155. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, K.-O.; You, C.-H.; Uyeda, H.; Lee, D.-I. Microphysical characteristics of a convective precipitation system observed on July 04, 2012, over Mt. Halla in South Korea. Atmos. Res. 2019, 222, 74–87. [Google Scholar] [CrossRef]
- Ahn, U.S.; Yun, Y.S. Causes of decline in the Korean fir based on spatial distribution in the Mt. Halla region in Korea: A meta-analysis. Forest 2020, 11, 391. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.B. Tree characteristics related to stem breakage of Picea glehnii and Abies sachalinensis. For. Ecol. Manag. 2005, 215, 295–306. [Google Scholar] [CrossRef]
- Kamimura, K.; Shiraishi, N. A review of strategies for wind damage assessment in Japanese forest. J. For. Res. 2007, 12, 162–176. [Google Scholar] [CrossRef]
- Han, A.R.; Jung, J.B.; Park, P.S. Effects of micro-topography on the crown growth of Picea jezoensis under different wind conditions on Mt. Deokyu, Korea. Korean J. Agric. For. Meteorol. 2019, 21, 277–285, (In Korean with English abstract). [Google Scholar]
- Park, W.-K. Increasing atmospheric carbon dioxide and growth trends of Korean subalpine conifers. J. Korean For. Soc. 1993, 82, 17–25, (In Korean with English abstract). [Google Scholar]
- Lim, J.-H.; Woo, S.-Y.; Kwon, M.J.; Chun, J.H.; Shin, J.H. Photosynthetic capacity and water use efficiency under different temperature regimes on healthy and declining Korean fir in Mt. Halla. J. Korean For. Soc. 2006, 95, 705–710, (In Korean with English abstract). [Google Scholar]
- Cho, M.-G.; Chung, J.-M.; Kim, T.-W.; Kim, C.-Y.; Noh, I.; Moon, H.-S. Ecological characteristics of Abies koreana forest on Seseok in Mt. Jiri. J. Clim. Chang. Res. 2015, 6, 379–388, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Seo, J.-W.; Jeong, H.-M.; Sano, M.; Choi, E.-B.; Park, J.-H.; Lee, K.-H.; Kim, Y.-J.; Park, H.-C. Establishing tree ring δ18O chronologies for principle tree species (T. cuspidata, P. koraiensis, A. koreana, Q. mongolica) at subalpine zone in Jiri National Park and their correlations with the corresponding climate. J. Korean Wood Sci. Technol. 2017, 45, 661–670, (In Korean with English abstract). [Google Scholar]
- Seo, J.-W.; Jeong, H.-M.; Lee, K.-H.; Park, H.-C. Dating the dead years of Korean fir (Abies koreana E.H. Wilson) at Imgeollyeong in Jirisan National Park. J. Natl. Park Res. 2019, 10, 219–223, (In Korean with English abstract). [Google Scholar]
- Jacobs, B.F.; Werth, C.R.; Guttman, S.I. Genetic relationships in Abies (fir) of eastern United States: An electrophoretic study. Can. J. Bot. 1984, 62, 609–616. [Google Scholar] [CrossRef]
- Gärtner, H.; Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Baillie, M.G.L.; Pilcher, J.R. A simple cross-dating program for tree-ring research. Tree Ring Bull. 1973, 33, 7–14. [Google Scholar]
- Eckstein, D.; Bauch, J. Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss. Cent. 1969, 88, 230–250. [Google Scholar] [CrossRef]
- Choi, E.-B.; Kim, Y.-J.; Park, J.-H.; Pakr, C.-R.; Seo, J.-W. Reconstruction of resin collection history of pine forests in Korea from tree-ring dating. Sustainability 2020, 12, 9118. [Google Scholar] [CrossRef]
- Larson, P.R. The Vascular Cambium. Development and Structure; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Park, J.-H.; Choi, E.-B.; Park, H.-C.; Lee, N.-Y.; Seo, J.-W. Intra-annual dynamics of cambial and xylem phenology in subalpine conifers at Deogyusan National Park in the republic of Korea. J. Wood. Sci. 2021, 67, 22. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Tree Rings: Basic and Applications of Dendrochronology; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Pannatier, E.G.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef] [Green Version]
- Obojes, N.; Meurer, A.; Newesely, C.; Tasser, E.; Oberhuber, W.; Mayr, S.; Tappeiner, U. Water stress limits transpiration and growth of European larch up to the lower subalpine belt in an inner-alpine dry valley. New Phytol. 2018, 220, 460–475. [Google Scholar] [CrossRef] [Green Version]
- Lange, J.; Carrer, M.; Pisaric, M.F.J.; Porter, T.J.; Seo, J.-W.; Trouillier, M.; Wilmking, M. Moisture-driven shift in the climate sensitivity of white spruce xylem anatomical traits is coupled to large-scale oscillation patterns across northern treeline in northwest North America. Glob. Chang. Biol. 2020, 26, 1842–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolezal, J.; Jandova, V.; Macek, M.; Mudrak, O.; Altman, J.; Schweingruber, F.H.; Liancourt, P. Climate warming drives Himalayan alpine plant growth and recruitment dynamics. J. Ecol. 2021, 109, 179–190. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.J.; Wu, Z.; Du, H.; Zong, S.; Ma, S. Potential distribution shifts of plant species under climate change in Changbai Mountains, China. Forests 2019, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Leonelli, G.; Pelfini, M.; di Cella, U.M.; Garavaglia, V. Climate warming and the recent treeline shift in the European Alps; the role of geomorphological factors in high-altitude sites. Ambio 2011, 40, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.P.; Panigrahy, S.; Thapliyal, A.; Kimothi, M.M.; Soni, P.; Parihar, J.S. Monitoring the alpine treeline shift in parts of the Indian Himalayas using remote sensing. Curr. Sci. 2012, 102, 559–562. [Google Scholar]
- Gatti, R.C.; Callaghan, T.; Velichevskaya, A.; Dudko, A.; Fabbio, L.; Battipaglia, G.; Ligng, J. Accelerating upward treeline shift in the Altai Mountains under last-century climate change. Sci. Rep. 2019, 9, 7678. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Zhang, Y.; Zhang, K.; Jiang, M.; Zhang, Q. Age structure and regeneration of subalpine fir (Abies fargesii) forests across an altitudinal rage in the Qinling Mountains, China. For. Ecol. Manag. 2010, 259, 547–554. [Google Scholar] [CrossRef]
- Adams, T.P.; Purves, D.W.; Pacala, S.W. Understanding height-structured competition in forests: Is there an R* for light? Proc. Biol. Sci. 2007, 274, 3039–3048. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forest. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.D.; Macalady, A.K.; Breshears, D.D.; Allen, C.D.; Stephenson, N.L.; Saleska, S.R.; Huxman, T.E.; McDowell, N.G. Climate-induced tree mortality: Earth system consequences. Eos Trans. Am. Geophys. Union 2010, 91, 153–154. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichsein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Csilléry, K.; Kunstler, G.; Courbaud, B.; Allard, D.; Lassègues, P.; Haslinger, K.; Gardiner, B. Coupled effects of wind-storms and drought on tree mortality across 115 forest stands from the Western Alps and the Jura mountains. Glob. Chang. Biol. 2017, 23, 5092–5107. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.M.; Varner, J.M.; van Mantgem, P.; Cansler, C.A. Fire and tree death: Understanding and improving modeling of fire-induced tree mortality. Environ. Res. Lett. 2018, 13, 113004. [Google Scholar] [CrossRef]
- Tzeng, H.-Y.; Wang, W.; Tseng, Y.-H.; Chiu, C.-A.; Kuo, C.-C.; Tsai, S.-T. Tree mortality in response to typhoon-induced floods and mudslides is determined by tree species, size, and position in a riparian Formosan gum forest in subtropical Taiwan. PLoS ONE 2018, 13, e0190832. [Google Scholar] [CrossRef] [Green Version]
- Tei, S.; Sugimoto, A.; Yonenobu, H.; Kotani, A.; Maximov, T.C. Effects of extreme drought and wet events for tree mortality: Insights from tree-ring width and carbon isotope ratio in a Siberian larch forest. Ecohydrology 2019, 12, e2143. [Google Scholar] [CrossRef]
- Altman, J.; Treydte, K.; Pejcha, V.; Cerny, T.; Petrik, P.; Srutek, M.; Song, J.-S.; Trouet, V.; Dolezal, J. Tree growth response to recent warming of two endemic species in Northeast Asia. Clim. Chang. 2020, 162, 1345–1364. [Google Scholar] [CrossRef]
- Kim, T.-H. Rates and factors of path widening in Seongpanak hiking trail of Mount Halla, Jeju Island. Korean Geogr. Soc. 2008, 43, 296–311, (In Korean with English abstract). [Google Scholar]
- Kueppers, L.M.; Conlisk, E.; Castanha, C.; Moyes, A.B.; Germino, M.J.; de Valpine, P.; Torn, M.S.; Mitton, J.B. Warming and provenance limit tree recruitment across and beyond the elevation range of subalpine forest. Glob. Chang. Biol. 2017, 23, 2383–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, A.R.; Lee, S.K.; Suh, G.U.; Park, Y.M.; Park, P.S. Wind and topography influence the crown growth of Picea jezoensis in a subalpine forest on Mt. Deoyu, Korea. Agric. For. Meteorol. 2012, 166–167, 207–214. [Google Scholar] [CrossRef]
- Kashian, D.M.; Turner, M.G.; Romme, W.H.; Lorimer, C.G. Variability and convergence in stand structural development on a fire-dominated subalpine landscape. Ecology 2005, 86, 643–654. [Google Scholar] [CrossRef] [Green Version]
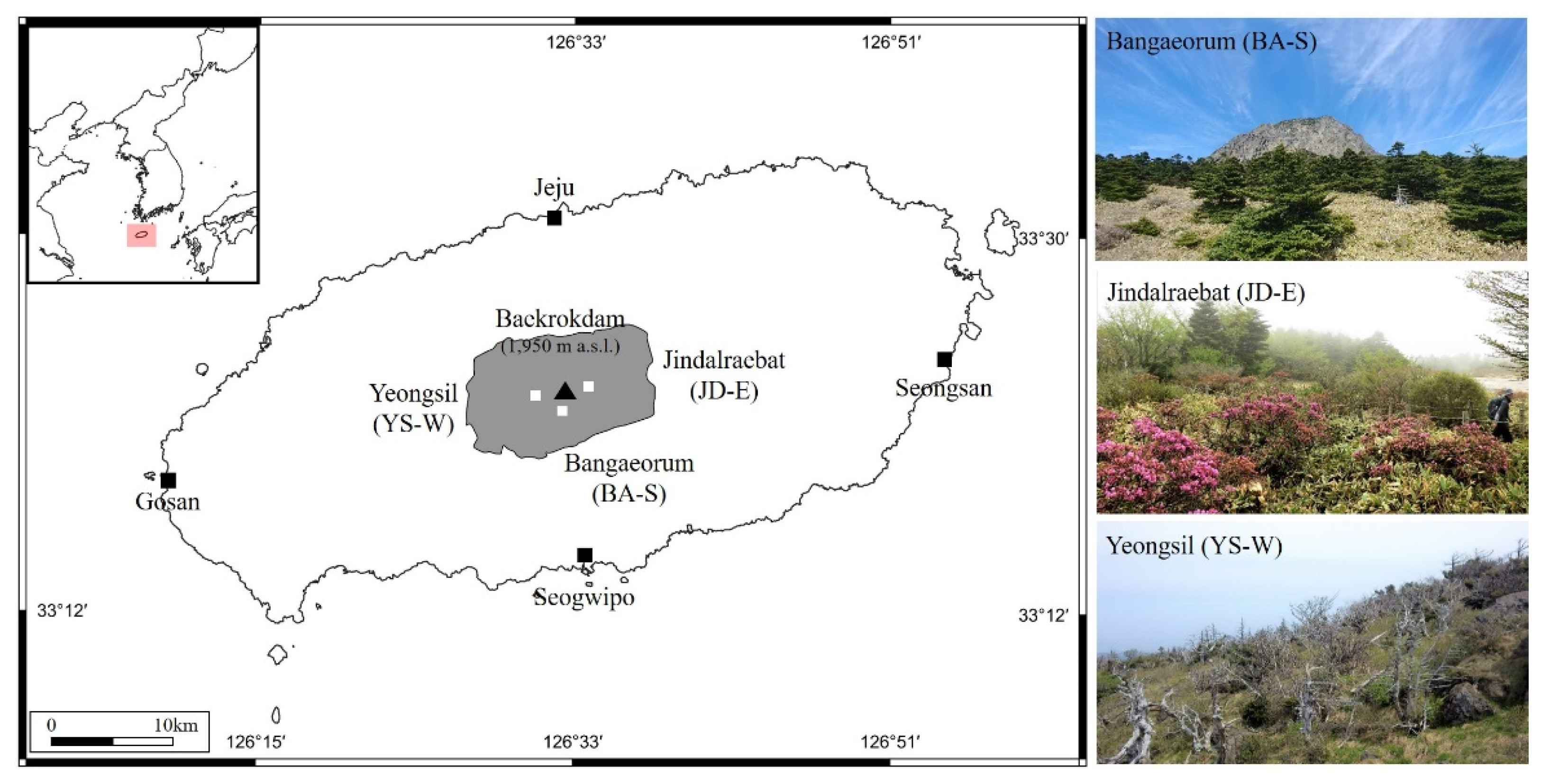


| Site | Y | Tree Condition | ID | N | DBH cm (Min.–Max.) | Long. (N)/Lat. €/m a.s.l. |
|---|---|---|---|---|---|---|
| Bangae -orum | 2019 | Living | BA-S-LV | 18 | 23.1 (12.0–35.0) | 126°31′/33°21′/1700 |
| (BA-S) | Standing dead | BA-S-SD | 15 | 21.9 (11.0–27.5) | 126°31′/33°21′/1694 | |
| Fallen dead | BA-S-FD | 15 | 19.3 (13.0–29.0) | 126°31′/33°21′/1692 | ||
| Jindalrae (JD-E) | 2018 | Living | JD-E-LV | 13 | 28.2 (21.0–33.0) | 126°32′/33°21′/1672 |
| Standing dead | JD-E-SD | 14 | 22.4 (16.0–31.0) | 126°32′/33°21′/1674 | ||
| Fallen dead | JD-E-FD | 15 | 21.1 (16.0–34.0) | 126°32′/33°21′/1683 | ||
| Youngsil (YS-W) | 2017 | Living | YS-W-LV | 10 | 22.7 (18.0–26.0) | 126°30′/33°21′/1610 |
| Standing dead | YS-W-SD | 10 | 21.2 (16.0–24.5) | 126°30′/33°21′/1611 | ||
| Fallen dead | YS-W-FD | 10 | 21.9 (19.0–25.5) | 126°30′/33°21′/1613 |
| Site | ID | N | Mean No. of Tree Rings (Min.–Max.) | Mean Estimated Age (Min.–Max.) |
|---|---|---|---|---|
| Bangaeorum | BA-S-LV | 16/18 | 55 (37–9) | 58 (37–85) |
| (BA-S) | BA-S-SD | 14/15 | 55 (25–89) | 58 (25–100) |
| BA-S-FD | 15/15 | 45 (33–68) | 49 (33–70) | |
| Jindalrae | JD-E-LV | 12/13 | 66 (40–114) | 70 (40–114) |
| (JD-E) | JD-E-SD | 10/14 | 74 (59–111) | 80 (59–131) |
| JD-E-FD | 8/15 | 54 (30–69) | 61 (30–83) | |
| Youngsil | YS-W-LV | 9/10 | 69 (51–106) | 73 (51–109) |
| (YS-W) | YS-W-SD | 8/10 | 53 (38–73) | 54 (38–73) |
| YS-W-FD | 9/10 | 56 (46–93) | 59 (46–96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.-W.; Choi, E.-B.; Park, J.-H.; Kim, Y.-J.; Lim, H.-I. The Role of Aging and Wind in Inducing Death and/or Growth Reduction in Korean Fir (Abies Koreana Wilson) on Mt. Halla, Korea. Atmosphere 2021, 12, 1135. https://doi.org/10.3390/atmos12091135
Seo J-W, Choi E-B, Park J-H, Kim Y-J, Lim H-I. The Role of Aging and Wind in Inducing Death and/or Growth Reduction in Korean Fir (Abies Koreana Wilson) on Mt. Halla, Korea. Atmosphere. 2021; 12(9):1135. https://doi.org/10.3390/atmos12091135
Chicago/Turabian StyleSeo, Jeong-Wook, En-Bi Choi, Jun-Hui Park, Yo-Jung Kim, and Hyo-In Lim. 2021. "The Role of Aging and Wind in Inducing Death and/or Growth Reduction in Korean Fir (Abies Koreana Wilson) on Mt. Halla, Korea" Atmosphere 12, no. 9: 1135. https://doi.org/10.3390/atmos12091135
APA StyleSeo, J.-W., Choi, E.-B., Park, J.-H., Kim, Y.-J., & Lim, H.-I. (2021). The Role of Aging and Wind in Inducing Death and/or Growth Reduction in Korean Fir (Abies Koreana Wilson) on Mt. Halla, Korea. Atmosphere, 12(9), 1135. https://doi.org/10.3390/atmos12091135





