The Toxic Effect of Water-Soluble Particulate Pollutants from Biomass Burning on Alveolar Lung Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Water-Soluble Particulate Pollutants from Biomass Burning
2.3. Measurements of Cytotoxicity
2.3.1. Real-Time Cell Analysis
2.3.2. Quantitative Phase Imaging
2.4. Statistical Analysis
3. Results
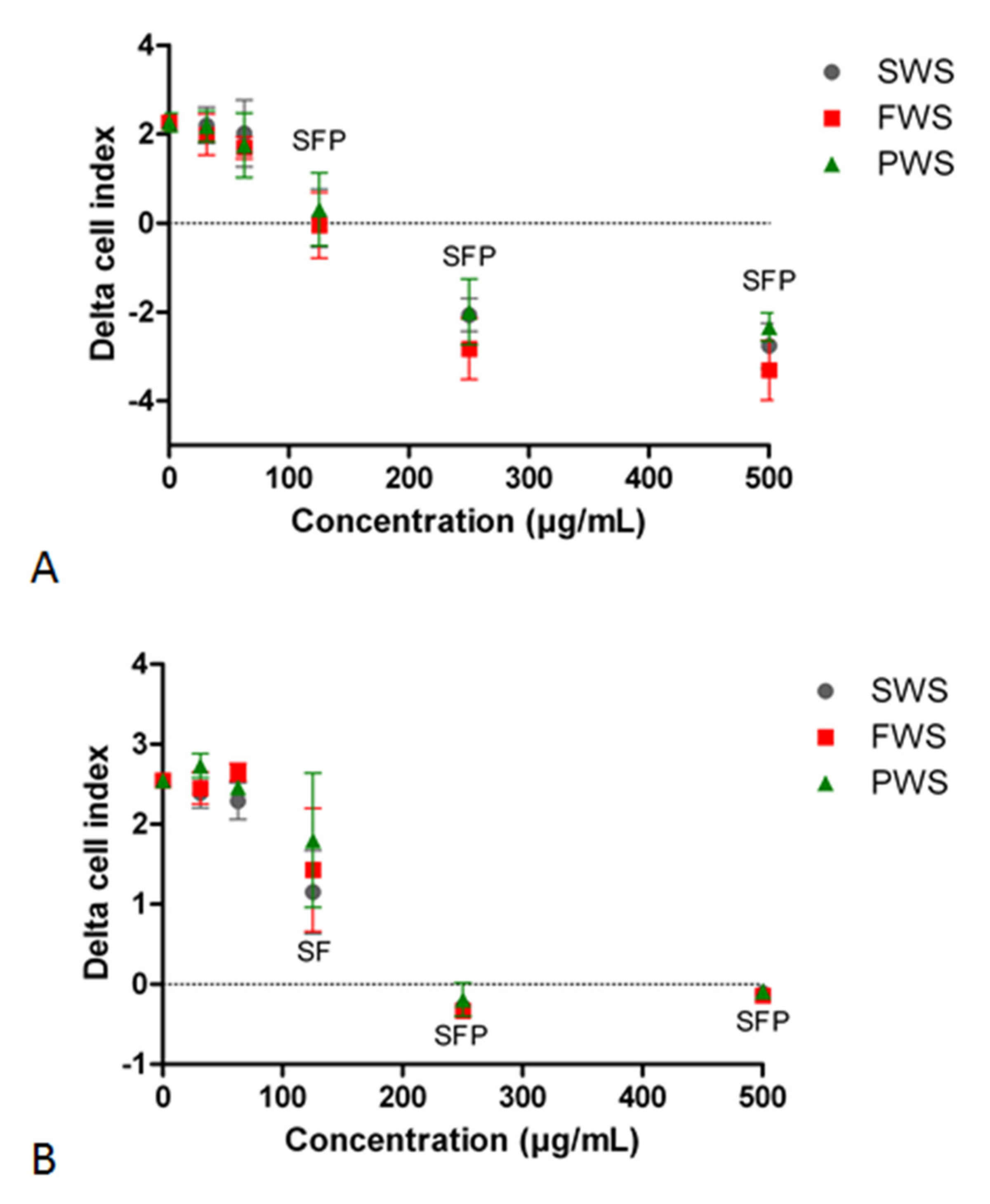
3.1. Effects of Water-Soluble Particulate Pollutants from Biomass Burning on A549 Cell Index
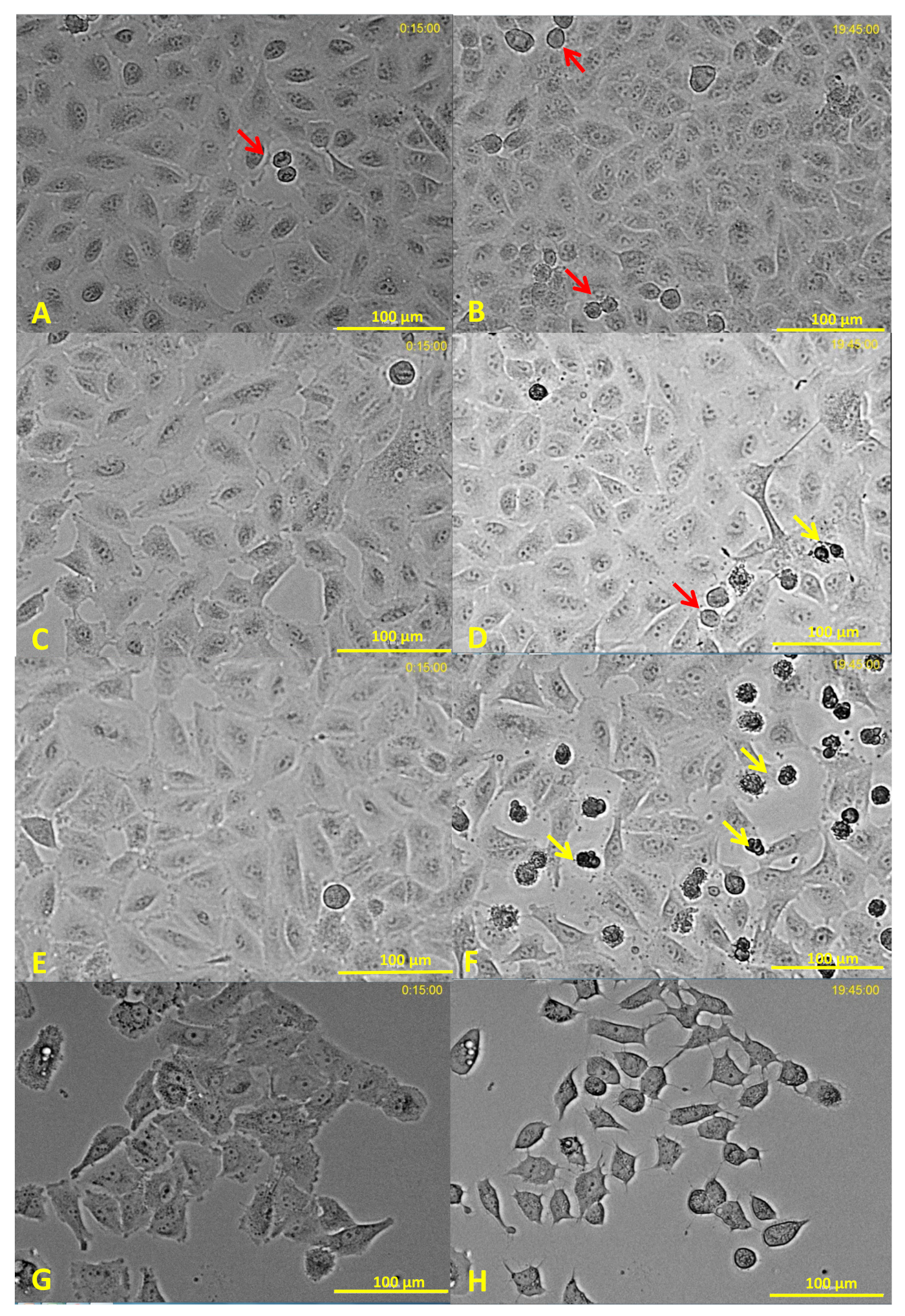
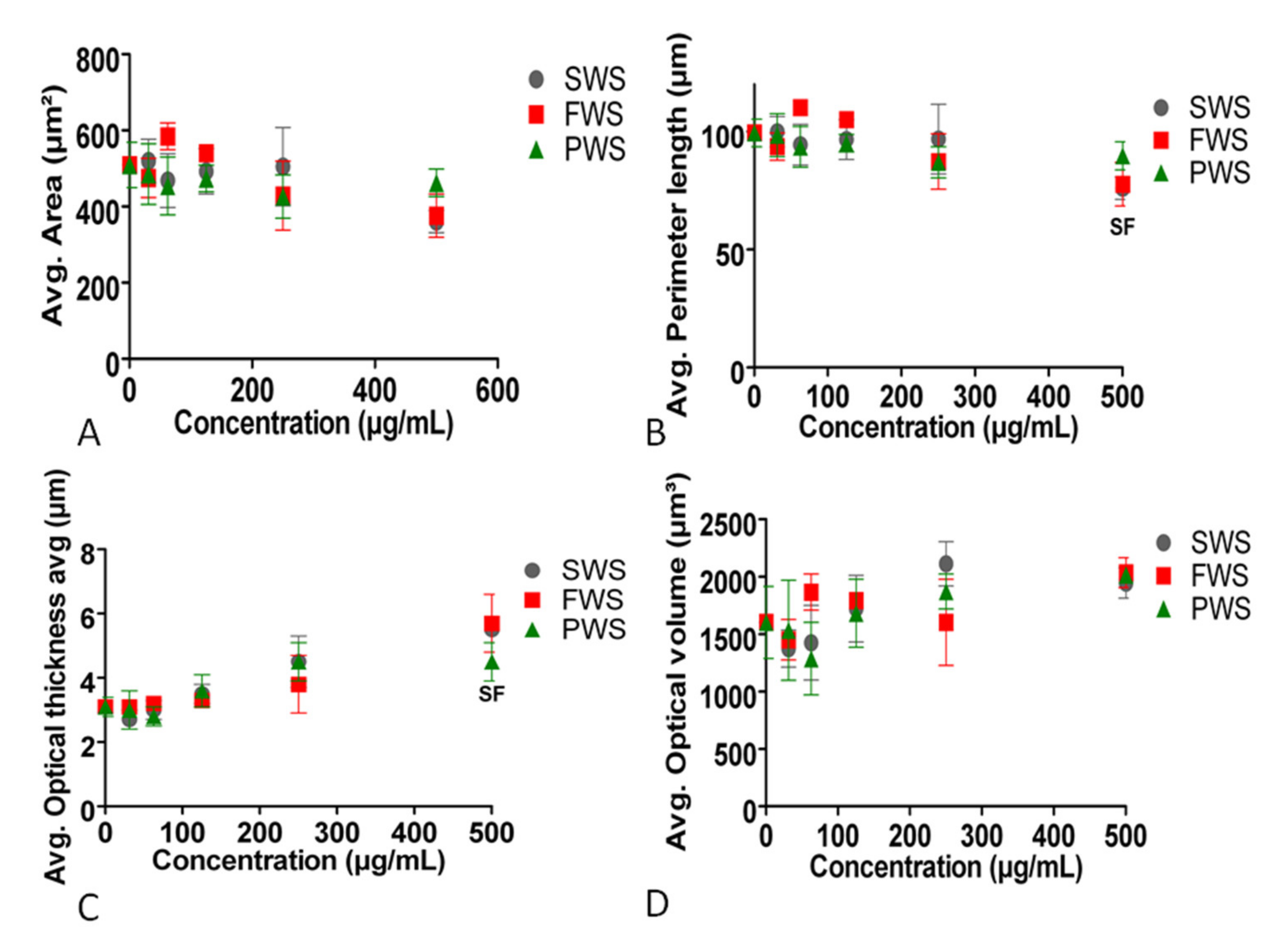
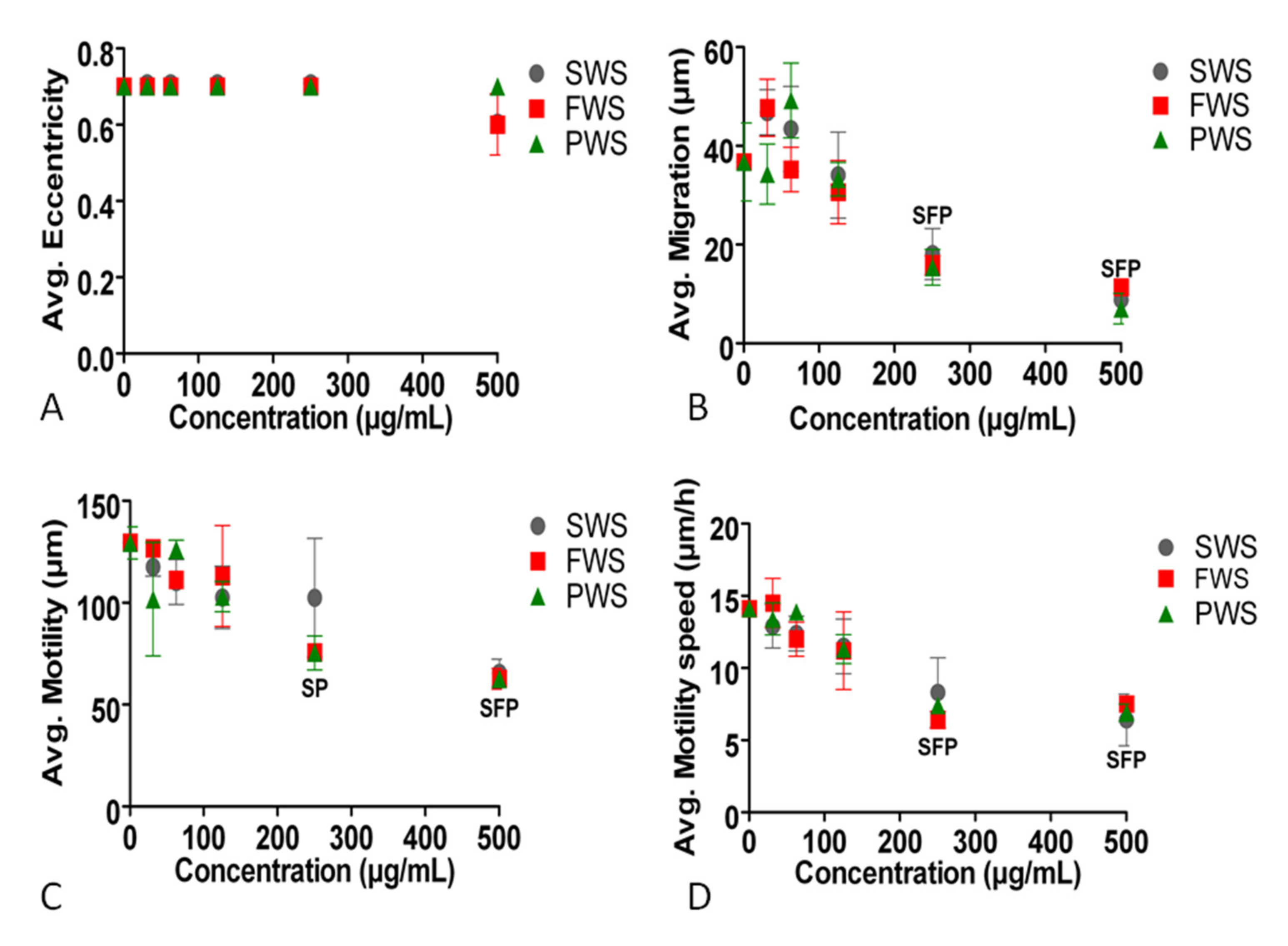
3.2. Effects of Water-Soluble Particulate Pollutants from Biomass Burning on A549 Behavioral and Structural Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Household Air Pollution and Health. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 7 July 2021).
- Straif, K.; Cohen, A.; Samet, J. Air Pollution and Cancer; IARC Scientific Publications; International Agency for Reseach on Cancer; WHO: Lyom, France, 2013; p. 161. [Google Scholar]
- Trojanowski, R.; Fthenakis, V. Nanoparticle emissions from residential wood combustion: A critical literature review, characterization, and recommendations. Renew. Sustain. Energy Rev. 2019, 103, 515–528. [Google Scholar] [CrossRef]
- European Comission. Clean Air Outlook. Available online: https://ec.europa.eu/environment/air/clean_air/outlook.htm (accessed on 7 July 2021).
- Saenz, J.L.; Wong, R.; Ailshire, J.A. Indoor air pollution and cognitive function among older Mexican adults. J. Epidemiol. Commun. Health 2017, 72, 21–26. [Google Scholar] [CrossRef]
- Thompson, L.M. Household Air Pollution from Cooking Fires Is a Global Problem. Am. J. Nurs. 2019, 119, 61–64. [Google Scholar] [CrossRef] [PubMed]
- McCall, C. Indonesian forest fires raise concerns about health. Lancet 2019, 394, 1699–1700. [Google Scholar] [CrossRef]
- Nobre, C.A. To save Brazil’s rainforest, boost its science. Nat. Cell Biol. 2019, 574, 455. [Google Scholar] [CrossRef] [Green Version]
- Rossiello, M.R.; Szema, A. Health Effects of Climate Change-induced Wildfires and Heatwaves. Cureus 2019, 11, e4771. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; He, Q.; Fang, Z.; Brown, S.S.; Laskin, A.; Cohen, S.R.; Rudich, Y. Laboratory Insights into the Diel Cycle of Optical and Chemical Transformations of Biomass Burning Brown Carbon Aerosols. Environ. Sci. Technol. 2020, 54, 11827–11837. [Google Scholar] [CrossRef]
- Desyaterik, Y.; Sun, Y.; Shen, X.; Lee, T.; Wang, X.; Wang, T.; Collett, J. Speciation of “brown” carbon in cloud water impacted by agricultural biomass burning in eastern China. J. Geophys. Res. Atmos. 2013, 118, 7389–7399. [Google Scholar] [CrossRef]
- Pardo, M.; Li, C.; He, Q.; Levin-Zaidman, S.; Tsoory, M.; Yu, Q.; Wang, X.; Rudich, Y. Mechanisms of lung toxicity induced by biomass burning aerosols. Part. Fibre Toxicol. 2020, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, M.; Li, C.; Fang, Z.; Levin-Zaidman, S.; Dezorella, N.; Czech, H.; Martens, P.; Käfer, U.; Gröger, T.; Rüger, C.P.; et al. Toxicity of Water- and Organic-Soluble Wood Tar Fractions from Biomass Burning in Lung Epithelial Cells. Chem. Res. Toxicol. 2021, 34, 1588–1603. [Google Scholar] [CrossRef] [PubMed]
- Atwi, K.; Mondal, A.; Pant, J.; Cheng, Z.; El Hajj, O.; Ijeli, I.; Handa, H.; Saleh, R. Physicochemical properties and cytotoxicity of brown carbon produced under different combustion conditions. Atmospheric Environ. 2021, 244, 117881. [Google Scholar] [CrossRef]
- Lee, T.; Sullivan, A.P.; Mack, L.; Jimenez, J.L.; Kreidenweis, S.M.; Onasch, T.B.; Worsnop, D.R.; Malm, W.; Wold, C.E.; Hao, W.M.; et al. Chemical Smoke Marker Emissions During Flaming and Smoldering Phases of Laboratory Open Burning of Wildland Fuels. Aerosol Sci. Technol. 2010, 44, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bathia, S.C. Advanced Renewed Energy Systems; Part 1; Woodhead Publishing India: New Delhi, India, 2014; p. 775. [Google Scholar]
- Kim, Y.H.; Warren, S.H.; Krantz, Q.T.; King, C.; Jaskot, R.; Preston, W.T.; George, B.J.; Hays, M.D.; Landis, M.; Higuchi, M.; et al. Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires. Environ. Health Perspect. 2018, 126, 017011. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; King, C.; Krantz, T.; Hargrove, M.M.; George, I.J.; McGee, J.; Copeland, L.; Hays, M.D.; Landis, M.; Higuchi, M.; et al. The role of fuel type and combustion phase on the toxicity of biomass smoke following inhalation exposure in mice. Arch. Toxicol. 2019, 93, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, M.M.; Kim, Y.H.; King, C.; Wood, C.E.; Gilmour, M.I.; Dye, J.A.; Gavett, S.H. Smoldering and flaming biomass wood smoke inhibit respiratory responses in mice. Inhal. Toxicol. 2019, 31, 236–247. [Google Scholar] [CrossRef]
- Santoso, M.A.; Christensen, E.G.; Yang, J.; Rein, G. Review of the Transition From Smouldering to Flaming Combustion in Wildfires. Front. Mech. Eng. 2019, 5, 49. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Dilger, M.; Orasche, J.; Zimmermann, R.; Paur, H.-R.; Diabaté, S.; Weiss, C. Toxicity of wood smoke particles in human A549 lung epithelial cells: The role of PAHs, soot and zinc. Arch. Toxicol. 2016, 90, 3029–3044. [Google Scholar] [CrossRef] [PubMed]
- Mölder, A.; Sebesta, M.; Gustafsson, M.; Gisselson, L.; Wingren, A.G.; Alm, K. Non-invasive, label-free cell counting and quantitative analysis of adherent cells using digital holography. J. Microsc. 2008, 232, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xCELLigence System for Real-Time and Label-Free Monitoring of Cell Viability. Assay Guid. Man. 2011, 740, 33–43. [Google Scholar] [CrossRef]
- De Albuquerque, Y.L.; Berger, E.; Tomaz, S.; George, C.; Géloën, A. Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols. Life 2021, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Kammen, D.M. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: An exposure-response study. Lancet 2001, 358, 619–624. [Google Scholar] [CrossRef]
- Alves, N.D.O.; Vessoni, A.; Quinet, A.; Fortunato, R.S.; Kajitani, G.S.; Peixoto, M.S.; Hacon, S.D.S.; Artaxo, P.; Saldiva, P.; Menck, C.F.; et al. Biomass burning in the Amazon region causes DNA damage and cell death in human lung cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Bølling, A.K.; Totlandsdal, A.I.; Sallsten, G.; Braun, A.; Westerholm, R.; Bergvall, C.; Boman, J.; Dahlman, H.J.; Sehlstedt, M.; Cassee, F.; et al. Wood smoke particles from different combustion phases induce similar pro-inflammatory effects in a co-culture of monocyte and pneumocyte cell lines. Part. Fibre Toxicol. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, N.K.; Saha, H.; Mukherjee, B.; Tyagi, N.; Ray, M.R. Inflammation, oxidative stress, and higher expression levels of Nrf2 and NQO1 proteins in the airways of women chronically exposed to biomass fuel smoke. Mol. Cell. Biochem. 2018, 447, 63–76. [Google Scholar] [CrossRef]
- Ko, E.; Kim, D.; Min, D.W.; Kwon, S.-H.; Lee, J.-Y. Nrf2 regulates cell motility through RhoA–ROCK1 signalling in non-small-cell lung cancer cells. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Biola-Clier, M.; Beal, D.; Caillat, S.; Libert, S.; Armand, L.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Comparison of the DNA damage response in BEAS-2B and A549 cells exposed to titanium dioxide nanoparticles. Mutagenesis 2016, 32, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Lankoff, A.; Brzóska, K.; Czarnocka, J.; Kowalska, M.; Lisowska, H.; Mruk, R.; Øvrevik, J.; Wegierek-Ciuk, A.; Zuberek, M.; Kruszewski, M. A comparative analysis of in vitro toxicity of diesel exhaust particles from combustion of 1st- and 2nd-generation biodiesel fuels in relation to their physicochemical properties—the FuelHealth project. Environ. Sci. Pollut. Res. 2017, 24, 19357–19374. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima de Albuquerque, Y.; Berger, E.; Li, C.; Pardo, M.; George, C.; Rudich, Y.; Géloën, A. The Toxic Effect of Water-Soluble Particulate Pollutants from Biomass Burning on Alveolar Lung Cells. Atmosphere 2021, 12, 1023. https://doi.org/10.3390/atmos12081023
Lima de Albuquerque Y, Berger E, Li C, Pardo M, George C, Rudich Y, Géloën A. The Toxic Effect of Water-Soluble Particulate Pollutants from Biomass Burning on Alveolar Lung Cells. Atmosphere. 2021; 12(8):1023. https://doi.org/10.3390/atmos12081023
Chicago/Turabian StyleLima de Albuquerque, Yuri, Emmanuelle Berger, Chunlin Li, Michal Pardo, Christian George, Yinon Rudich, and Alain Géloën. 2021. "The Toxic Effect of Water-Soluble Particulate Pollutants from Biomass Burning on Alveolar Lung Cells" Atmosphere 12, no. 8: 1023. https://doi.org/10.3390/atmos12081023
APA StyleLima de Albuquerque, Y., Berger, E., Li, C., Pardo, M., George, C., Rudich, Y., & Géloën, A. (2021). The Toxic Effect of Water-Soluble Particulate Pollutants from Biomass Burning on Alveolar Lung Cells. Atmosphere, 12(8), 1023. https://doi.org/10.3390/atmos12081023






