Estimation of the Size Distribution of Suspended Particulate Matters in the Urban Atmospheric Surface Layer and Its Influence on Bronchopulmonary Pathology
Abstract
:1. Introduction
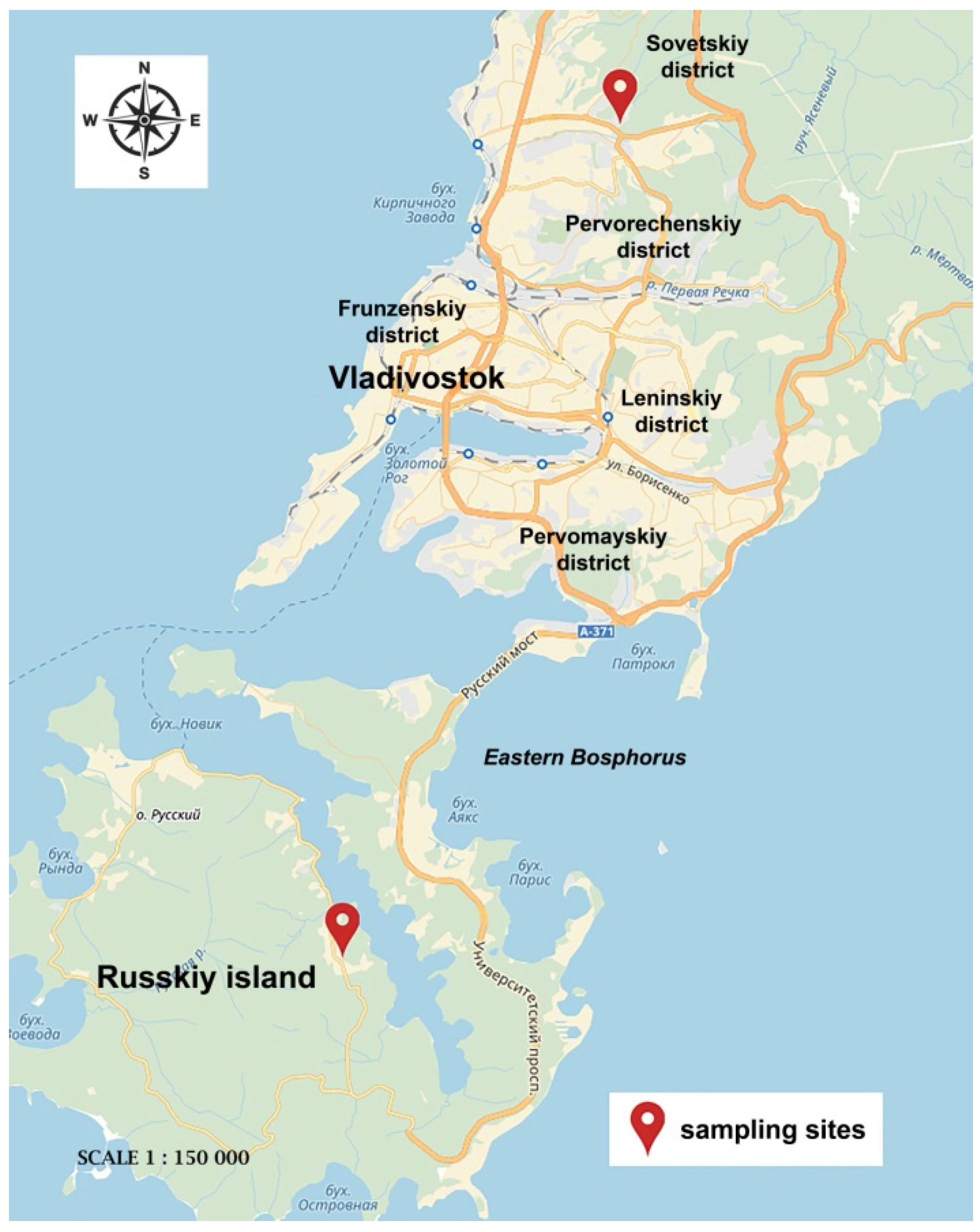
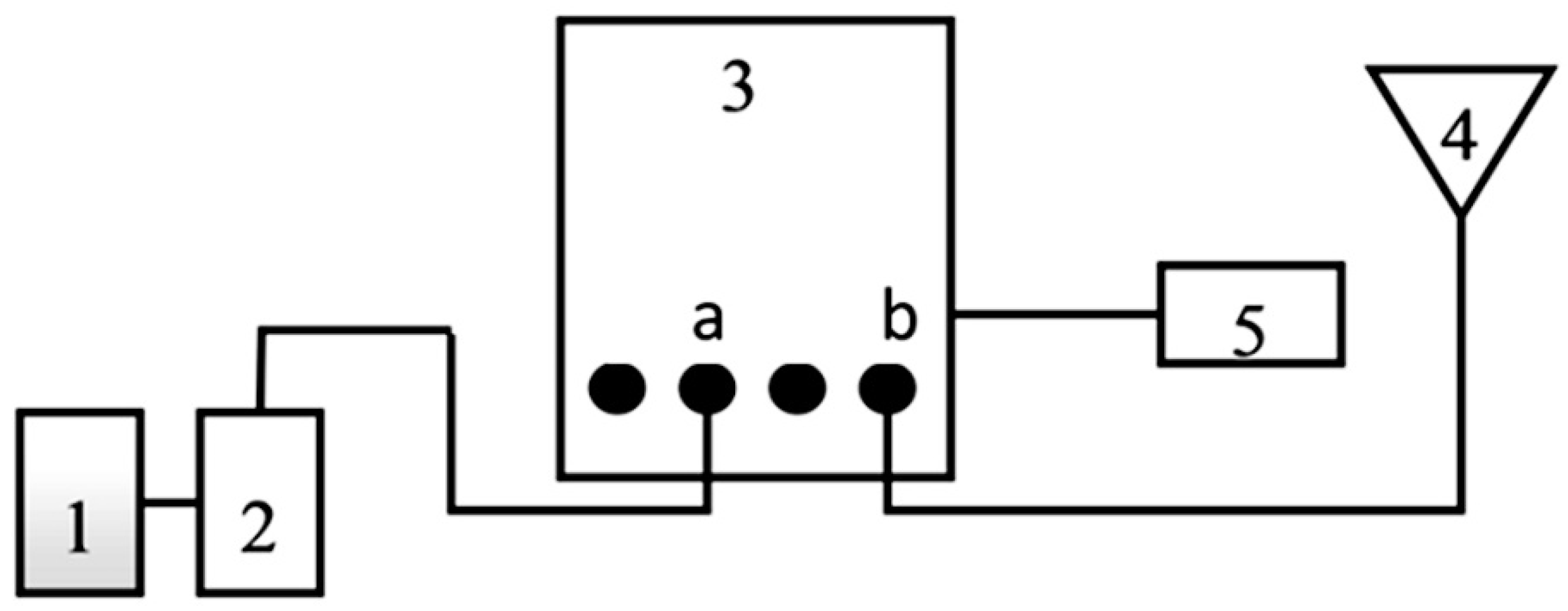
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014, 348, f7412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Fang, B.; Wang, C.; Xia, T.; Bottai, M.; Fang, F.; Cao, Y. Relationship between fine particulate matter, weather condition and daily non-accidental mortality in Shanghai, China: A Bayesian approach. PLoS ONE 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta 2016, 1860, 2844–2855. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Biryukov, S.; Brauer, M.; Cercy, K.; Anderson, H.R.; Bhutta, Z.A.; BurnNet, R.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- De Marco, A.; Proietti, C.; Anav, A.; Ciancarella, L.; D’Elia, I.; Fares, S.; Fornasier, M.F.; Fusaro, L.; Gualtieri, M.; Manes, F.; et al. Impacts of air pollution on human and ecosystem health, and implications for the National Emission Ceilings Directive: Insights from Italy. Environ. Int. 2019, 125, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulin, L.; Hansel, N. Particulate air pollution and impaired lung function. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Wei, T.; Tang, M. Biological effects of airborne fine particulate matter (PM2.5) exposure on pulmonary immune system. Environ. Toxicol. Pharmacol. 2018, 60, 195–201. [Google Scholar] [CrossRef]
- Veremchuk, L.V.; Tsarouhas, K.; Vitkina, T.I.; Mineeva, E.E.; Gvozdenko, T.A.; Antonyuk, M.V.; Rakitskii, V.N.; Sidletskaya, K.A.; Tsatsakis, A.M.; Golokhvast, K.S. Impact evaluation of environmental factors on respiratory function of asthma patients living in urban territory. Environ. Pollut. 2018, 235, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.; Arce, I. Antioxidants in respiratory diseases: Basic science research and therapeutic alternatives. Clin. Res. Trials 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Veremchuk, L.V.; Yankova, V.I.; Vitkina, T.I.; Nazarenko, A.V.; Golokhvast, K.S. Urban air pollution, climate and its impact on asthma morbidity. Asian Pac. J. Trop. Biomed. 2016, 6, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Veremchuk, L.V.; Mineeva, E.E.; Vitkina, T.I.; Gvozdenko, T.A.; Golokhvast, K.S. Impact of atmospheric microparticles and heavy metals on external respiration function of urbanized territory population. ROMJ 2017, 6. Available online: http://www.romj.org/2017-0402 (accessed on 4 August 2021). [CrossRef]
- Robinson, D.L. Composition and oxidative potential of PM2.5 pollution and health. J. Thorac. Dis. 2017, 9, 444–447. [Google Scholar] [CrossRef] [Green Version]
- Caramori, G.; Casolari, P.; Barczyk, A.; Durham, A.L.; Di Stefano, A.; Adcock, I. COPD immunopathology. Semin. Immunopathol. 2016, 38, 497–515. [Google Scholar] [CrossRef] [Green Version]
- Yan’kova, V.I.; Gvozdenko, T.A.; Golokhvast, K.S.; Chaika, V.V.; Gorodnoy, V.A. Granulometric analysis of atmospheric suspensions of ecologically favorable and unfavorable districts of Vladivostok. Health Med. Ecology. Sci. 2014, 2, 62–66. (In Russian) [Google Scholar]
- Waste Incineration Plant Poisons the Air of Vladivostok; Information Agency Vostok-Media. Available online: http://www.vostokmedia.com/n225329.html (accessed on 10 February 2015). (In Russian).
- Mayanskiy, D.N.; Shcherbakov, V.I.; Makarova, O.P. Comprehensive Assessment of the Function of Phagocytes in Inflammatory Diseases Guidelines; Rotaprint Section of the Siberian Branch of the USSR Academy of Medical Sciences: Novosibirsk, Russia, 1985; p. 31. (In Russian) [Google Scholar]
- Shmelev, E.V.; Bumagina, G.K.; Miterov, P.P. Modification of the Park method. Lab. Sci. 1979, 9, 13–15. (In Russian) [Google Scholar]
- Abbasov, P.A.; Odintsova, E.Y.; Shatrova, N.E. Self-cleaning potential of the air basin of the city of Vladivostok. Acad. Archit. Constr. 2009, 5, 132–135. (In Russian) [Google Scholar]
- Levanchuk, A.V. Environmental pollution by products of wear and tear automobile-road complex. Gig. Sanit. 2014, 93, 17–21. (In Russian) [Google Scholar] [PubMed]
- World Health Organization. WHO Global Ambient Air Quality Database (Update 2018). Available online: http://www.who.int/airpollution/data/cities/en/ (accessed on 13 June 2020).
- Beier, C.M.; Caputo, J.; Lawrence, G.B.; Sullivan, T.J. Loss of ecosystem services due to chronic pollution of forests and surface waters in the Adirondack region (USA). J. Environ. Manag. 2017, 191, 19–27. [Google Scholar] [CrossRef]
- Schiffer, J.M.; Mael, L.E.; Prather, K.A.; Amaro, R.E.; Grassian, V.H. Sea Spray Aerosol: Where Marine Biology Meets Atmospheric Chemistry. ACS Cent. Sci. 2018, 26, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Marignani, M.; Bruschi, D.; Astiaso, G.D.; Frondoni, R.; Carli, E.; Pinna, M.S.; Cumo, F.; Gugliermetti, F.; Saatkamp, A.; Doxa, A.; et al. Identification and prioritization of areas with high environmental risk in Mediterranean coastal areas: A flexible approach. Sci. Total Environ. 2017, 590, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.S.; Duan, F.K.; He, K.B.; Ma, Y.L. Review on recent progress in observations, source identifications and countermeasures of PM2.5. Environ. Int. 2016, 86, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Golokhvast, K.S. Atmospheric Suspensions in the Cities of the Far East. of Russia; Far Eastern Federal University: Vladivostok, Russia, 2013; p. 178. (In Russian) [Google Scholar]
- Longhin, E.; Gualtieri, M.; Capasso, L.; Bengalli, R.; Mollerup, S.; Holme, J.A.; Øvrevik, J.; Casadei, S.; Di Benedetto, C.; Parenti, P.; et al. Physico-chemical properties and biological effects of diesel and biomass particles. Environ. Pollut. 2016, 215, 366–375. [Google Scholar] [CrossRef]
- Pardo, M.; Porat, Z.; Rudich, A.; Schauer, J.J.; Rudich, Y. Repeated exposures to roadside particulate matter extracts suppresses pulmonary defense mechanisms, resulting in lipid and protein oxidative damage. Environ. Pollut. 2015, 210, 227–237. [Google Scholar] [CrossRef]
- Azarov, V.K.; Saykin, A.M.; Kutenev, V.F.; Malkin, M.A. Tires and road surface as a source of air pollution from motor vehicles. Proc. Cent. Res. Dev. Automob. Engine Inst. 2014, 256, 72–84. (In Russian) [Google Scholar]
- Vitkina, T.I.; Barskova, L.S.; Zyumchenko, N.E.; Tokmakova, N.P.; Gvozdenko, T.A.; Golokhvast, K.S. Balance of glutathione-related processes in alveolar macrophages under exposure to suspended particulate matter of atmospheric air in of Wistar rats. Gig. Sanit. 2020, 99, 200–205. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Jose Chong-Neto, H.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Ge, D.-D.; Zhou, L.-F.; Hou, L.-Y.; Zhou, Y.; Li, Q.-Y. Effects of particulate matter on allergic respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Vitkina, T.I.; Sidletskaya, K.A. Diagnostic criteria for the progression of the chronic obstructive pulmonary disease under a high technogenic load. Gig. Sanit. 2020, 99, 140–144. (In Russian) [Google Scholar] [CrossRef]
- Mills, N.L.; Amin, N.; Robinson, S.D.; Anand, A.; Davies, J.; Patel, D.; de la Fuente, J.M.; Cassee, F.R.; Boon, N.A.; Macnee, W.; et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am. J. Respir. Crit. Care Med. 2006, 173, 426–431. [Google Scholar] [CrossRef] [PubMed]
| SPMs (%) | Russkiy Island | Continental Part of the City p—Significance of Difference |
|---|---|---|
| <0.1 μm | 0.044 ± 0.005 | 0.764 ± 0.029 (p = 0.002) |
| 0.1–1 μm | 0.612 ± 0.065 | 1.577 ± 0.262 (p = 0.014) |
| 1–2.5 μm | 7.388 ± 0.713 | 11.955 ± 0.533 (p = 0.037) |
| 2.5–10 μm | 26.188 ± 2.513 | 45.721 ± 1.872 (p = 0.021) |
| 10–50 μm | 34.889 ± 3.869 | 11.034 ± 2.713 (p = 0.046) |
| 50–100 μm | 0.878 ± 0.045 | 0 (p = 0.009) |
| 100–400 μm | 4.276 ± 0.236 | 0.688 ± 0.028 (p = 0.003) |
| 400–700 μm | 3.652 ± 0.605 | 8.873 ± 0.310 (p = 0.024) |
| 700–2000 μm | 21.669 ± 1.988 | 19.383 ± 1.111 |
| Size Range of SPMs (μm) | Island Part of the City | Continental Part of the City | ||
|---|---|---|---|---|
| LPO-AOD System | Immune System | LPO-AOD System | Immune System | |
| Control group | ||||
| 0–1 | 0.28 | 0.32 | 0.72 | 0.43 |
| 1–10 | 0.29 | 0.36 | 0.92 | 0.41 |
| 10–50 | 0.39 | 0.31 | ||
| 50–100 | 0.32 | 0.23 | 0.54 | 0.37 |
| 100–400 | 0.21 | |||
| 400–700 | 0.19 | |||
| >700 | 0.24 | |||
| Total | 1.53 | 0.91 | 2.57 | 1.52 |
| Patients with bronchopulmonary pathology | ||||
| 0–1 | 0.29 | 0.38 | 0.72 | 0.23 |
| 1–10 | 0.29 | 0.26 | 0.45 | 0.34 |
| 10–50 | 0.37 | 0.44 | 0.27 | |
| 50–100 | 0.31 | 0.32 | 0.28 | 0.22 |
| 100–400 | 0.28 | 0.28 | ||
| 400–700 | 0.29 | |||
| >700 | ||||
| Total | 1.83 | 0.96 | 2.02 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veremchuk, L.V.; Vitkina, T.I.; Barskova, L.S.; Gvozdenko, T.A.; Mineeva, E.E. Estimation of the Size Distribution of Suspended Particulate Matters in the Urban Atmospheric Surface Layer and Its Influence on Bronchopulmonary Pathology. Atmosphere 2021, 12, 1010. https://doi.org/10.3390/atmos12081010
Veremchuk LV, Vitkina TI, Barskova LS, Gvozdenko TA, Mineeva EE. Estimation of the Size Distribution of Suspended Particulate Matters in the Urban Atmospheric Surface Layer and Its Influence on Bronchopulmonary Pathology. Atmosphere. 2021; 12(8):1010. https://doi.org/10.3390/atmos12081010
Chicago/Turabian StyleVeremchuk, Lyudmila V., Tatyana I. Vitkina, Lyudmila S. Barskova, Tatyana A. Gvozdenko, and Elena E. Mineeva. 2021. "Estimation of the Size Distribution of Suspended Particulate Matters in the Urban Atmospheric Surface Layer and Its Influence on Bronchopulmonary Pathology" Atmosphere 12, no. 8: 1010. https://doi.org/10.3390/atmos12081010
APA StyleVeremchuk, L. V., Vitkina, T. I., Barskova, L. S., Gvozdenko, T. A., & Mineeva, E. E. (2021). Estimation of the Size Distribution of Suspended Particulate Matters in the Urban Atmospheric Surface Layer and Its Influence on Bronchopulmonary Pathology. Atmosphere, 12(8), 1010. https://doi.org/10.3390/atmos12081010






