Evaluation of a Novel Poultry Litter Amendment on Greenhouse Gas Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Treatments
2.2. Flux Measurements and Litter Analyses
2.3. Data Analysis
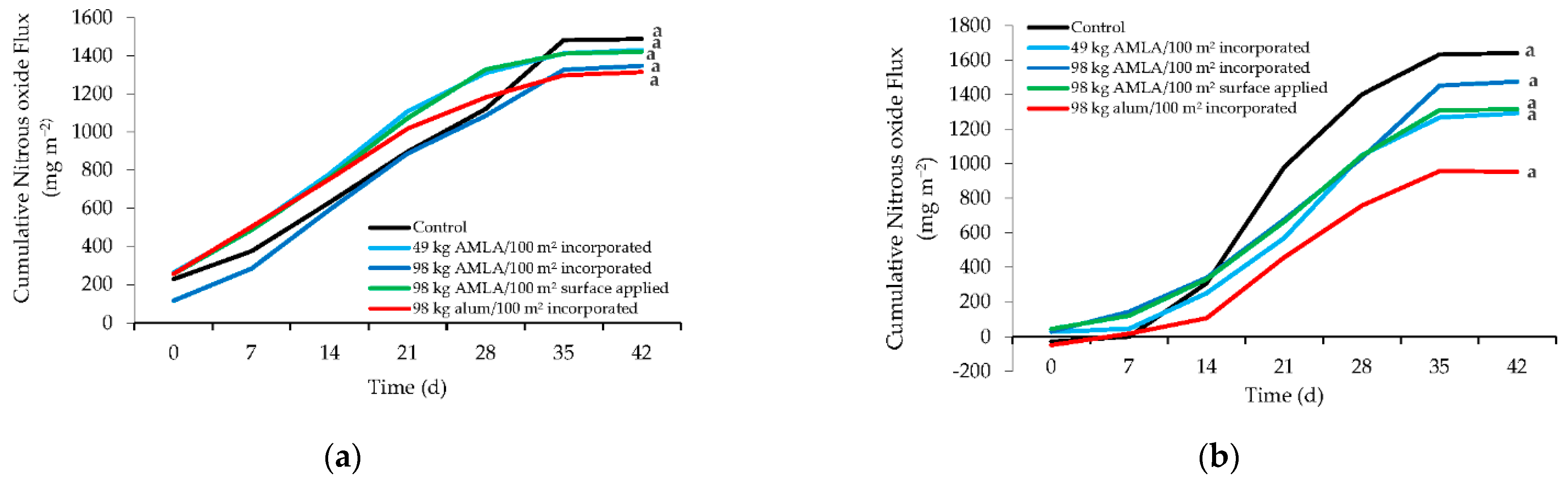
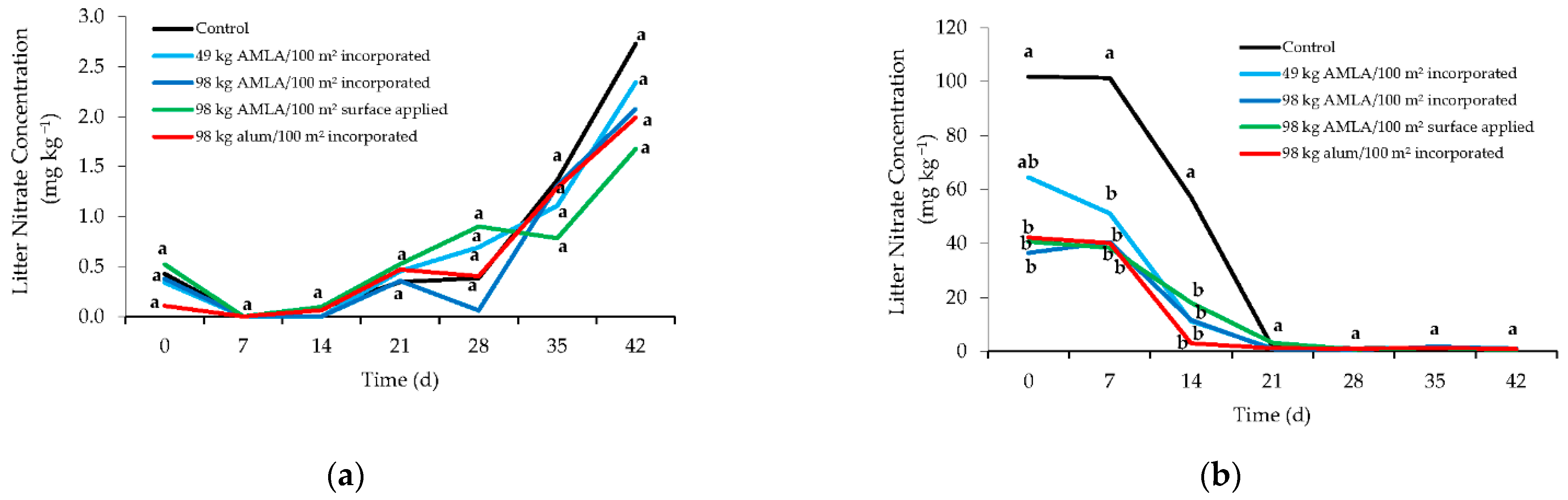
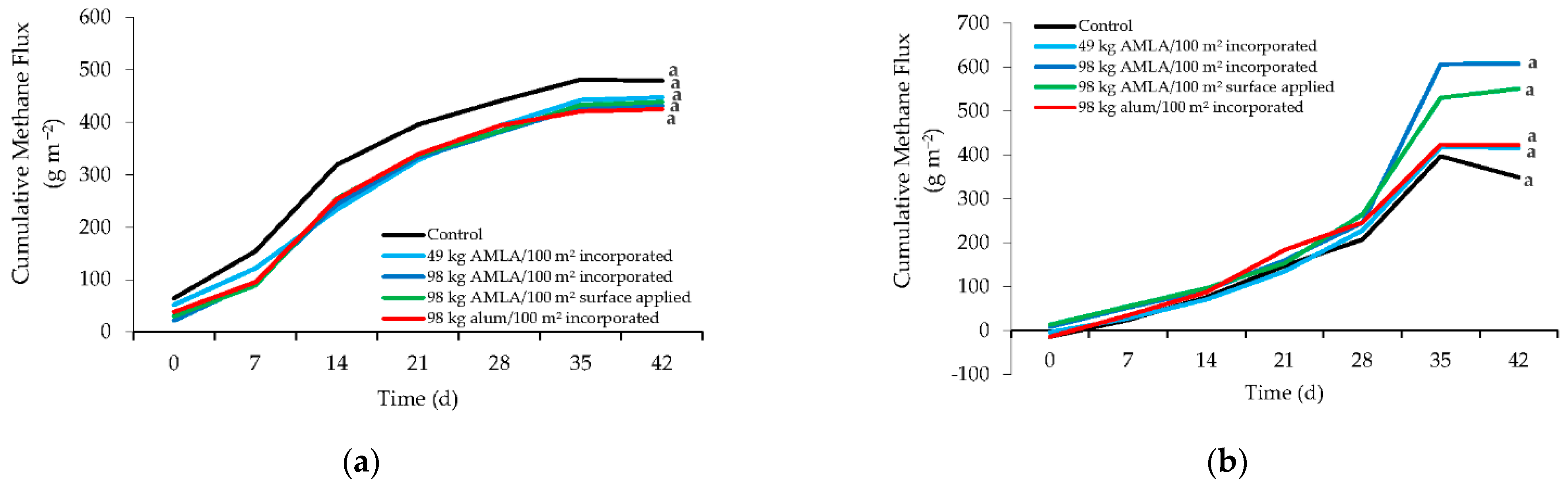
3. Results and Discussion
3.1. Nitrous Oxide Emissions and Litter Nitrate Concentrations
3.2. Methane Emissions
3.3. Carbon Dioxide Emissions
3.4. Effect of Litter Accuulation on Greenhouse Gas Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashworth, A.J.; Chastain, J.P.; Moore, P.A., Jr. Nutrient characteristics of poultry manure and litter. In Animal Manure: Production, Characteristics, Environmental Concerns and Management; Waldrip, H., Pagliari, P.H., He, Z., Eds.; Soil Science Society of America Monograph: Madison, WI, USA, 2019; pp. 63–88. [Google Scholar] [CrossRef]
- USEPA. National Emission Inventory: Ammonia Emissions from Animal Husbandry; Revised Draft Report; USEPA: Washington, DC, USA, 2004. [Google Scholar]
- Eggelston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) 2006 ICPP Guidelines for National Greenhouse Gas Inventories Hayama; Institute for Global Environmental Strategies: Hayama, Japan, 2006. [Google Scholar]
- USEPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2018; USEPA: Washington, DC, USA, 2020. [Google Scholar]
- Meda, B.; Hassouna, M.; Aubert, C.; Robin, P.; Dourmad, J.Y. Influence of rearing conditions and manure management practices on ammonia and greenhouse gas emissions from poultry houses. World Poul. Sci. J. 2011, 67, 441–456. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC, 2013: Cimate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambrigdge University Preee Cambridge: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Moore, P.A., Jr.; Miles, D.; Burns, R.; Pote, D.; Berg, K.; Choi, I. Ammonia emission factors from broiler litter in barns, in storage, and after land application. J. Environ. Qual. 2011, 40, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.M.; Owens, P.R.; Rowe, D.E. Spatial variability of litter gaseous flux within a commercial broiler house: Ammonia, nitrous oxide, carbon dioxide, and methane. Poult. Sci. 2006, 85, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Vergé, X.P.C.; Dryer, J.A.; Desjardins, R.L.; Worth, D. Long-term trends in greenhouse gas emission from the Canadian poultry industry. J. Appl. Poult. Res. 2009, 18, 210–222. [Google Scholar] [CrossRef]
- Wathes, C.M.; Holden, M.R.; Sneath, R.P.; White, R.P.; Phillips, V.R. Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses. Br. Poult. Sci. 1997, 38, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, D. Assessment of greenhouse gas emissions from poultry enteric fermentation. Asian Australas. J. Anim. Sci. 2005, 18, 873–878. [Google Scholar] [CrossRef]
- Sommer, S.G.; Dahl, P. Nutrient and carbon balance during the composting of deep litter. J. Argic. Eng. Res. 1999, 74, 145–153. [Google Scholar] [CrossRef]
- Malomo, G.A.; Bolu, S.A.; Madugu, A.S.; Usman, Z.S. Nitrogen Emissions and Mitigation Strategies in Chicken Production. In Animal Husbandry and Nutrition; Yücel, B., Taşkin, T., Eds.; Intech Open: London, UK, 2018; pp. 43–62. [Google Scholar]
- Carlile, F.S. Ammonia in poultry houses: A literature review. Worlds Poult. Sci. J. 1984, 40, 99–113. [Google Scholar] [CrossRef]
- Calvet, S.; Cambra-López, M.; Estellés, F.; Torres, A.G. Characterization of gas emissions from a Mediterranean broiler farm. Poult. Sci. 2011, 90, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Eugene, B.; Moore, P.A., Jr.; Li, H.; Miles, D.; Trabue, S.; Burns, R.; Buser, M. Effects of alum additions to poultry litter on in-house ammonia and greenhouse gas concentrations and emissions. J. Environ. Qual. 2015, 44, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A., Jr. Composition and Methods of Treating Animal Manure. US Patent 9,301,440, 5 April 2016. [Google Scholar]
- Adak, A.; Mallik, D.; Chaudhuri, S.K. Alum mud: Phase identification and catalytic potential for aquepis-phase decomposition of hydrogen peroxide. Clays Clay Miner. 1999, 47, 234–238. [Google Scholar] [CrossRef]
- Moore, P.A., Jr. Development of a new manure amendment for reducing ammonia volatilization and phosphorus runoff from poultry litter. J. Environ. Qual. 2016, 45, 1412–1429. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Moore, P.A., Jr.; Martin, J.; Ashworth, A.J. Effect of a new manure amendment on ammonia emissions from poultry litter. Atmosphere 2020, 11, 257. [Google Scholar] [CrossRef]
- Choi, I.H.; Moore, P.A., Jr. Effects of liquid aluminum chloride additions to poultry litter on broiler performance, ammonia emissions, soluble phosphorus, total volatile fatty acids, and nitrogen contents of litter. Poult. Sci. 2008, 87, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Self-Davis, M.L.; Moore, P.A., Jr. Method of determining water soluble phosphorus in animal manure. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Water; Southern Cooperative Series Bulletin No. 396, SERA-IEG 17; Pierzynski, G.M., Ed.; North Carolina State University: Raleigh, NC, USA, 2000; pp. 74–77. ISBN 1-58161-396-2. [Google Scholar]
- USEPA. Methods for Chemical Analysis of Water and Wastes; USEPA Rep. 600/4-79-020. USEPA, Environ; Monitoring and Support Lab.: Cincinnati, OH, USA, 1983; USEPA Rep. 600/4-79-020. USEPA, Environ. [Google Scholar]
- SAS Institute. SAS User’s Guide: Statistics; SAS Inst.: Cary, NC, USA, 2014. [Google Scholar]
- Moore, P.A., Jr.; Miles, D.M.; Burns, R.; Pote, D.; Berg, K. Evaluation and Management of Ammonia Emissions from Poultry Litter. In Best Management Practices, Proceedings of Workshop on Agricultural Air Quality: State of Science, Potomac, MA, USA, 5–8 June 2006; Aneja, V.P., Schlesinger, W.H., Knighton, R., Jennings, G., Niyogi, D., Gilliam, W., Duke, C.S., Eds.; North Carolina State University: Raleigh, NC, USA, 2006; pp. 304–310. [Google Scholar]
- Chastain, J.P.; Camberato, J.J.; Skewes, P. Poultry Manure Production and Nutrient Content. Chapter 3b. In Confined Animal Manure Managers Certification Program Manual Poultry Version; Clemson University Extension: Clemson, SC, USA, 2001. [Google Scholar]
- USEPA. Nitrification; USEPA: Washington, DC, USA, 2002. [Google Scholar]
- Burns, R.T.; Li, H.; Xin, H.; Gates, R.S.; Overhults, D.G.; Earnest, J.; Moody, L. Greenhouse Gas (GHG) Emissions from Broiler Houses in the Southeastern United States. In Proceedings of the ASABE Annual International Meeting, St. Joseph, MI, USA, 29 June–2 July 2008. Paper No. 084649. [Google Scholar]

| Treatment | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | ||||||||
| Control | 0.43a† | 0.28a | 0.48a | 0.50a | 0.42a | 0.68a | 0.08a | 0.41a |
| 49 kg AMLA/100 m2 incorporated | 0.50a | 0.44a | 0.54a | 0.62a | 0.38a | 0.19a | 0.23a | 0.41a |
| 98 kg AMLA/100 m2 incorporated | 0.22a | 0.32a | 0.58a | 0.56a | 0.37a | 0.46a | 0.28a | 0.40a |
| 98 kg AMLA/100 m2 surface applied | 0.48a | 0.44a | 0.52a | 0.59a | 0.48a | 0.16a | 0.15a | 0.40a |
| 98 kg alum/100 m2 incorporated | 0.48a | 0.47a | 0.47a | 0.50a | 0.31a | 0.22a | 0.22a | 0.38a |
| Flock 3 | ||||||||
| Control | −0.05a | 0.05a | 0.59a | 1.27a | 0.79a | 0.44a | 0.10a | 0.46a |
| 49 kg AMLA/100 m2 incorporated | 0.05a | 0.03a | 0.39a | 0.60a | 0.91a | 0.41a | 0.13a | 0.36a |
| 98 kg AMLA/100 m2 incorporated | 0.05a | 0.22a | 0.37a | 0.64a | 0.67a | 0.79a | 0.29a | 0.43a |
| 98 kg AMLA/100 m2 surface applied | 0.08a | 0.14a | 0.40a | 0.63a | 0.72a | 0.50a | 0.06a | 0.36a |
| 98 kg alum/100 m2 incorporated | −0.09a | 0.13a | 0.17a | 0.67a | 0.57a | 0.38a | −0.06a | 0.25a |
| Flock | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | 36.0 | 30.6 | 30.1 | 30.0 | 37.9 | 43.0 | 43.4 | 35.9 |
| Flock 3 | 16.4 | 15.4 | 26.8 | 37.4 | 40.9 | 47.7 | 50.2 | 33.5 |
| Treatment | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | ||||||||
| Control | 32.0a† | 44.4a | 81.8a | 38.1a | 22.2a | 20.4a | −7.48b | 33.1a |
| 49 kg AMLA/100 m2 incorporated | 25.7a | 34.5ab | 55.5a | 47.1a | 32.3a | 24.4a | 17.5a | 33.9a |
| 98 kg AMLA/100 m2 incorporated | 10.8a | 36.1ab | 73.9a | 44.3a | 23.9a | 23.6a | 8.12a | 31.5a |
| 98 kg AMLA/100 m2 surface applied | 14.8a | 29.3b | 82.0a | 41.3a | 22.5a | 27.2a | 19.3a | 33.9a |
| 98 kg alum/100 m2 incorporated | 18.9a | 28.3b | 78.4a | 42.7a | 27.0a | 13.6a | 14.2a | 31.9a |
| Flock 3 | ||||||||
| Control | −7.15b | 19.0a | 25.5a | 35.5a | 29.7a | 94.1a | −167.2a | 4.21a |
| 49 kg AMLA/100 m2 incorporated | −2.04ab | 15.7a | 21.4a | 31.6a | 46.4a | 94.1a | −8.81a | 28.3a |
| 98 kg AMLA/100 m2 incorporated | 4.03a | 21.8a | 19.5a | 33.7a | 42.6a | 178.9a | 3.97a | 43.5a |
| 98 kg AMLA/100 m2 surface applied | 6.47a | 20.8a | 20.5a | 28.0a | 55.3a | 131.7a | 72.4a | 47.9a |
| 98 kg alum/100 m2 incorporated | −7.00b | 24.1a | 26.0a | 47.7a | 31.1a | 87.7a | −1.98a | 29.7a |
| Treatment | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | ||||||||
| Control | 29.1a† | 12.6a | 23.4a | 19.8a | 33.0a | 47.3a | 18.9a | 26.3a |
| 49 kg AMLA/100 m2 incorporated | 27.5a | 13.2a | 24.2a | 21.9a | 41.7a | 41.0a | 18.9a | 26.9a |
| 98 kg AMLA/100 m2 incorporated | 28.2a | 11.3a | 21.6a | 22.8a | 39.1a | 35.4a | 23.2a | 26.0a |
| 98 kg AMLA/100 m2 surface applied | 29.0a | 10.1a | 19.9a | 21.7a | 32.0a | 37.1a | 22.5a | 25.0a |
| 98 kg alum/100 m2 incorporated | 26.8a | 10.2a | 20.0a | 18.5a | 35.4a | 41.1a | 18.5a | 24.4a |
| Flock 3 | ||||||||
| Control | 0.61a | 1.25a | 18.3a | 42.9a | 40.4a | 29.7a | 21.6a | 22.1a |
| 49 kg AMLA/100 m2 incorporated | 0.46a | 0.69a | 20.1a | 39.8a | 39.6a | 25.7a | 17.6a | 20.6a |
| 98 kg AMLA/100 m2 incorporated | 0.25a | 0.50a | 19.8a | 45.0a | 50.5a | 25.5a | 17.5a | 22.7a |
| 98 kg AMLA/100 m2 surface applied | 0.61a | 0.46a | 16.8a | 36.0a | 39.5a | 22.4a | 14.4a | 18.6a |
| 98 kg alum/100 m2 incorporated | 0.18a | 0.79a | 19.0a | 35.5a | 43.4a | 25.3a | 12.4a | 19.5a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, K.; Moore, P.A., Jr.; Martin, J.; Ashworth, A.J. Evaluation of a Novel Poultry Litter Amendment on Greenhouse Gas Emissions. Atmosphere 2021, 12, 563. https://doi.org/10.3390/atmos12050563
Anderson K, Moore PA Jr., Martin J, Ashworth AJ. Evaluation of a Novel Poultry Litter Amendment on Greenhouse Gas Emissions. Atmosphere. 2021; 12(5):563. https://doi.org/10.3390/atmos12050563
Chicago/Turabian StyleAnderson, Kelsey, Philip A. Moore, Jr., Jerry Martin, and Amanda J. Ashworth. 2021. "Evaluation of a Novel Poultry Litter Amendment on Greenhouse Gas Emissions" Atmosphere 12, no. 5: 563. https://doi.org/10.3390/atmos12050563
APA StyleAnderson, K., Moore, P. A., Jr., Martin, J., & Ashworth, A. J. (2021). Evaluation of a Novel Poultry Litter Amendment on Greenhouse Gas Emissions. Atmosphere, 12(5), 563. https://doi.org/10.3390/atmos12050563






