Air Concentrations of Gaseous Elemental Mercury and Vegetation–Air Fluxes within Saltmarshes of the Tagus Estuary, Portugal
Abstract
1. Introduction
2. Materials and Methods
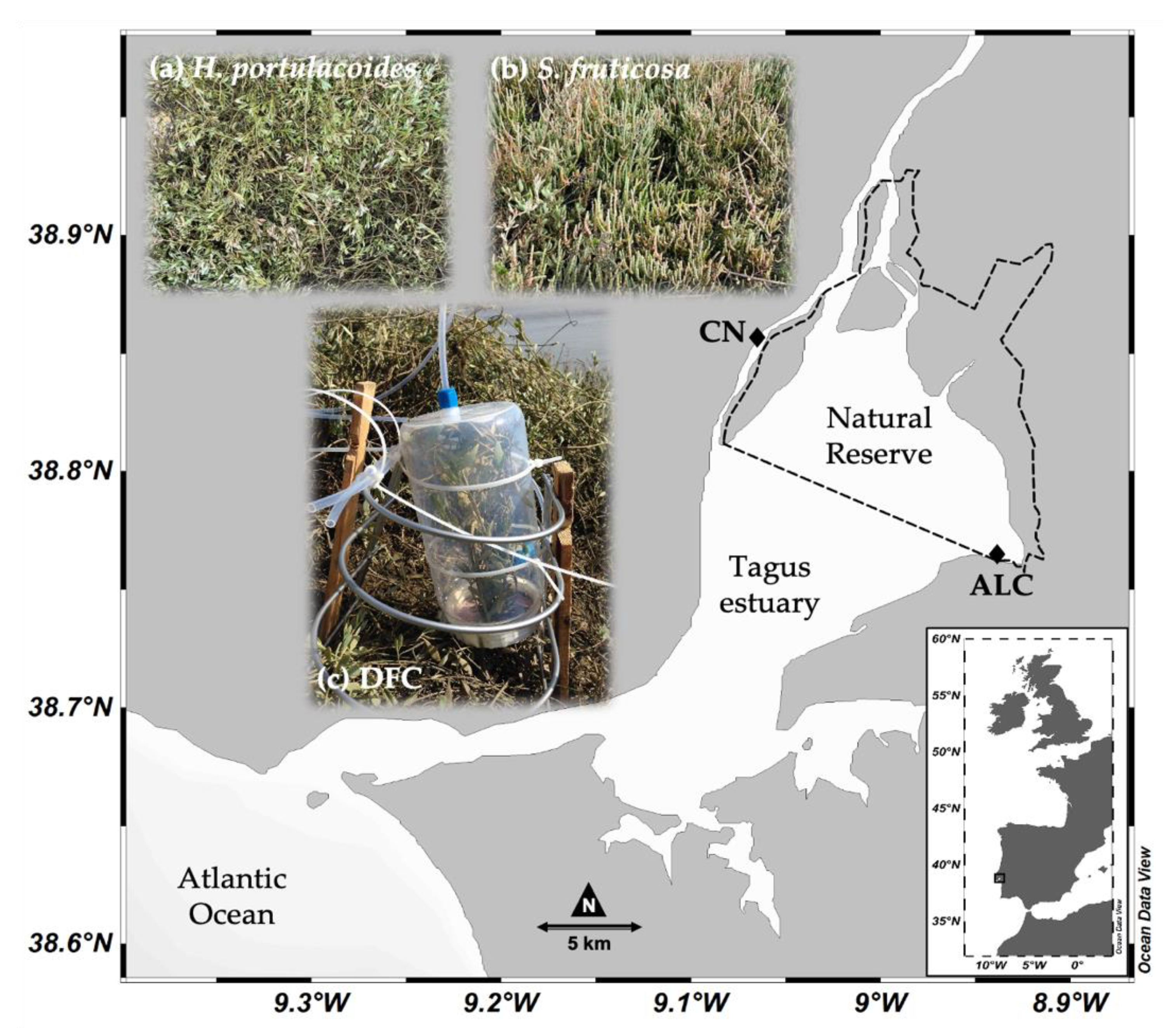
2.1. Site Description
2.2. Fieldwork
2.3. Instrumentation and Analysis
2.3.1. Meteorological Parameters Measured In Situ
2.3.2. Atmospheric Mercury Analyzer
2.3.3. In Situ Vegetation-To-Air Mercury Flux Measurements
2.3.4. Total Hg Concentration in Plants and Soils
2.3.5. Statistical Analysis and Data Processing
3. Results and Discussion
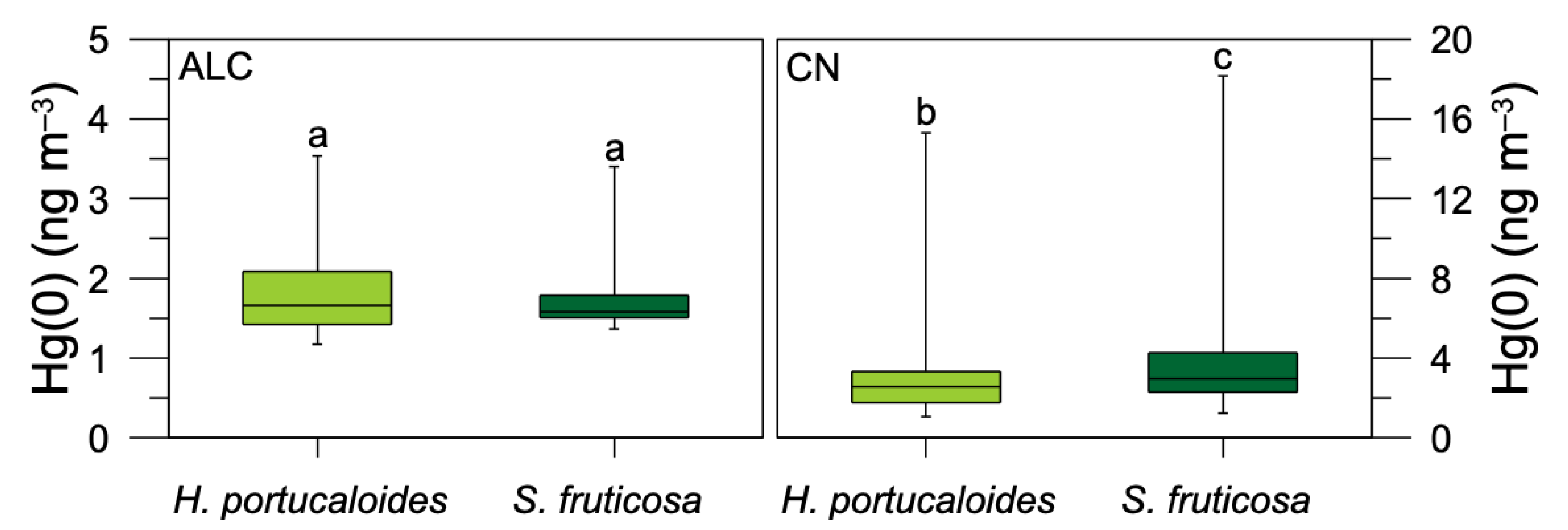
3.1. Air Hg(0) Concentrations in Saltmarsh Vegetated Areas
3.2. The Role of Meteorological Parameters in Air Hg(0) Concentrations
3.2.1. “Background Area”—ALC
3.2.2. Industrial Area—CN
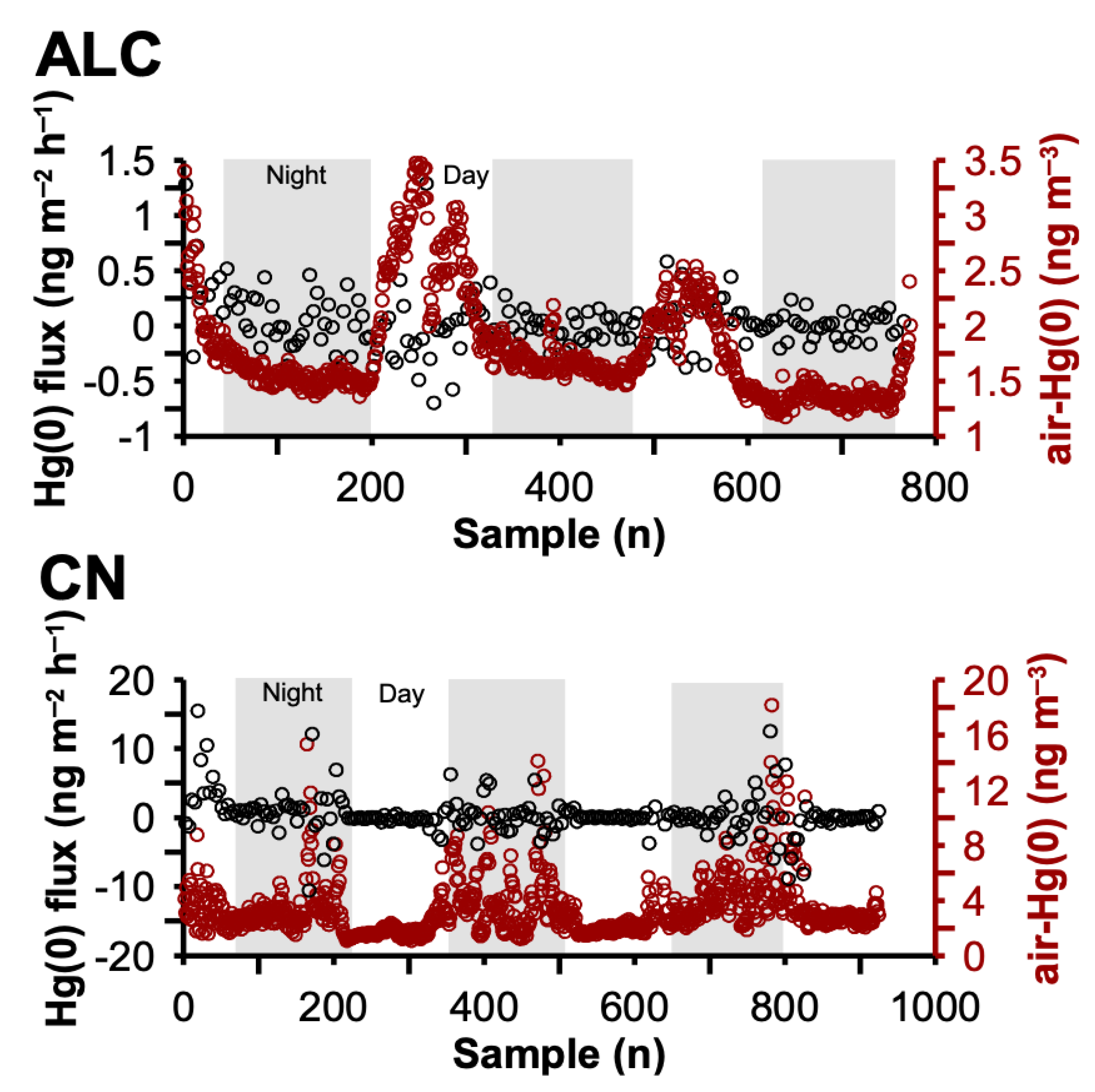
3.3. Daily Variation on Vegetation–Air Hg(0) Fluxes
3.4. Total Mercury in Aboveground Plant Parts and Sediments
4. Scientific Uncertainties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, E.D.; Cohen, Y.; Winer, A.M. Environmental distribution and transformation of mercury compounds. Crit. Rev. Environ. Sci. Technol. 1996, 26, 1–43. [Google Scholar] [CrossRef]
- Ariya, P.A.; Amyot, M.; Dastoor, A.; Deeds, D.; Feinberg, A.; Kos, G.; Poulain, A.; Ryjkov, A.; Semeniuk, K.; Subir, M.; et al. Mercury Physicochemical and Biogeochemical Transformation in the Atmosphere and at Atmospheric Interfaces: A Review and Future Directions. Chem. Rev. 2015, 115, 3760–3802. [Google Scholar] [CrossRef]
- Krabbenhoft, D.P.; Sunderland, E.M. Global change and mercury. Science 2013, 341, 1457–1458. [Google Scholar] [CrossRef]
- Qureshi, A.; O`Driscoll, N.J.; Macleod, M.; Neuhold, Y.M.; Hungerbühler, K. Photoreactions of mercury in surface ocean water: Gross reaction kinetics and possible pathways. Environ. Sci. Technol. 2010, 44, 644–649. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Ma, M.; Sun, T.; Du, H.; Wang, D. A two-year study on mercury fluxes from the soil under different vegetation cover in a subtropical region, South China. Atmosphere 2018, 9, 30. [Google Scholar] [CrossRef]
- Khan, T.R.; Obrist, D.; Agnan, Y.; Selin, N.E.; Perlinger, J.A. Atmosphere-terrestrial exchange of gaseous elemental mercury: Parameterization improvement through direct comparison with measured ecosystem fluxes. Environ. Sci. Process. Impacts 2019, 21, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.E.; Dong, W.; Chanton, J.; Qualls, R.G.; Meyers, T. A mechanism for bimodal emission of gaseous mercury from aquatic macrophytes. Atmos. Environ. 2005, 39, 1289–1301. [Google Scholar] [CrossRef]
- Smith, L.M.; Reinfelder, J.R. Mercury volatilization from salt marsh sediments. J. Geophys. Res. Biogeosci. 2009, 114, 1–12. [Google Scholar] [CrossRef]
- Canário, J.; Poissant, L.; Pilote, M.; Caetano, M.; Hintelmann, H.; O’Driscoll, N.J. Salt-marsh plants as potential sources of Hg0 into the atmosphere. Atmos. Environ. 2017, 152, 458–464. [Google Scholar] [CrossRef]
- Liu, G.; Cai, Y.; O’Driscoll, N.J.; Feng, X. Overview of mercury. Environ. Chem. Toxicol. Mercur. 2012, 1–12. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Q.; Pan, X. Photochemical behaviors of mercury (Hg) species in aquatic systems: A systematic review on reaction process, mechanism, and influencing factor. Sci. Total Environ. 2020, 720, 137540. [Google Scholar] [CrossRef]
- Siddiqi, Z.M. Transport and Fate of Mercury (Hg) in the Environment: Need for Continuous Monitoring BT. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–20. ISBN 978-3-319-58538-3. [Google Scholar]
- Vost, E.E.; Amyot, M.; O’Driscoll, N.J. Photoreactions of Mercury in Aquatic Systems. Environ. Chem. Toxicol. Mercur. 2012, 193–218. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Hanson, P.J.; Meyers, T.P.; Kim, K.H. Air/surface exchange of mercury vapor over forests - The need for a reassessment of continental biogenic emissions. Atmos. Environ. 1998, 32, 895–908. [Google Scholar] [CrossRef]
- Zhang, H.H.; Piossant, L.; Xu, X.; Pilote, M.; Beauvais, C.; Amyot, M.; Garcia, E.; Laroulandie, J. Air-water gas exchange of mercury in the Bay Saint François wetlands: Observation and model parameterization. J. Geophys. Res. Atmos. 2006, 111, 1–14. [Google Scholar] [CrossRef]
- Poissant, L.; Pilote, M.; Yumvihoze, E.; Lean, D. Mercury concentrations and foliage/atmosphere fluxes in a maple forest ecosystem in Québec, Canada. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Gustin, M.S.; Lindberg, S.E.; Weisberg, P.J. An update on the natural sources and sinks of atmospheric mercury. Appl. Geochemistry 2008, 23, 482–493. [Google Scholar] [CrossRef]
- Eckley, C.S.; Gustin, M.; Lin, C.J.; Li, X.; Miller, M.B. The influence of dynamic chamber design and operating parameters on calculated surface-to-air mercury fluxes. Atmos. Environ. 2010, 44, 194–203. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32. [Google Scholar] [CrossRef]
- Canário, J.; Vale, C. Rapid Release of Mercury from Intertidal Sediments Exposed to Solar Radiation: A Field Experiment. Environ. Sci. Technol. 2004, 38, 3901–3907. [Google Scholar] [CrossRef]
- Frescholtz, T.F.; Gustin, M.S. Soil and foliar mercury emission as a function of soil concentration. Water. Air. Soil Pollut. 2004, 155, 223–237. [Google Scholar] [CrossRef]
- Schwesig, D.; Matzner, E. Pools and fluxes of mercury and methylmercury in two forested catchments in Germany. Sci. Total Environ. 2000, 260, 213–223. [Google Scholar] [CrossRef]
- Cizdziel, J.V.; Zhang, Y.; Nallamothu, D.; Brewer, J.S.; Gao, Z. Air/surface exchange of gaseous elemental mercury at different landscapes in Mississippi, USA. Atmosphere 2019, 10, 538. [Google Scholar] [CrossRef]
- Sizmur, T.; McArthur, G.; Risk, D.; Tordon, R.; O’Driscoll, N.J. Gaseous mercury flux from salt marshes is mediated by solar radiation and temperature. Atmos. Environ. 2017, 153, 117–125. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, W.; Zhang, H.; Sommar, J.; Yu, B.; Yang, X.; Wang, X.; Lin, C.-J.; Feng, X. Depletion of atmospheric gaseous elemental mercury by plant uptake at Mt. Changbai, Northeast China. Atmos. Chem. Phys. 2016, 16, 12861–12873. [Google Scholar] [CrossRef]
- Fritsche, J.; Obrist, D.; Zeeman, M.J.; Conen, F.; Eugster, W.; Alewell, C. Elemental mercury fluxes over a sub-alpine grassland determined with two micrometeorological methods. Atmos. Environ. 2008, 42, 2922–2933. [Google Scholar] [CrossRef]
- Enrico, M.; Roux, G.L.; Marusczak, N.; Heimbürger, L.E.; Claustres, A.; Fu, X.; Sun, R.; Sonke, J.E. Atmospheric Mercury Transfer to Peat Bogs Dominated by Gaseous Elemental Mercury Dry Deposition. Environ. Sci. Technol. 2016, 50, 2405–2412. [Google Scholar] [CrossRef]
- Obrist, D.; Agnan, Y.; Jiskra, M.; Olson, C.L.; Colegrove, D.P.; Hueber, J.; Moore, C.W.; Sonke, J.E.; Helmig, D. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution. Nature 2017, 547, 201–204. [Google Scholar] [CrossRef]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef]
- Jiskra, M.; Wiederhold, J.G.; Skyllberg, U.; Kronberg, R.M.; Hajdas, I.; Kretzschmar, R. Mercury Deposition and Re-emission Pathways in Boreal Forest Soils Investigated with Hg Isotope Signatures. Environ. Sci. Technol. 2015, 49, 7188–7196. [Google Scholar] [CrossRef]
- Agnan, Y.; Le Dantec, T.; Moore, C.W.; Edwards, G.C.; Obrist, D. New Constraints on Terrestrial Surface-Atmosphere Fluxes of Gaseous Elemental Mercury Using a Global Database. Environ. Sci. Technol. 2016, 50, 507–524. [Google Scholar] [CrossRef]
- Lee, X.; Benoit, G.; Hu, X. Total gaseous mercury concentration and flux over a coastal saltmarsh vegetation in Connecticut, USA. Atmos. Environ. 2000, 34, 4205–4213. [Google Scholar] [CrossRef]
- Zhang, H.H.; Poissant, L.; Xu, X.; Pilote, M. Explorative and innovative dynamic flux bag method development and testing for mercury air-vegetation gas exchange fluxes. Atmos. Environ. 2005, 39, 7481–7493. [Google Scholar] [CrossRef]
- Converse, A.D.; Riscassi, A.L.; Scanlon, T.M. Seasonal variability in gaseous mercury fluxes measured in a high-elevation meadow. Atmos. Environ. 2010, 44, 2176–2185. [Google Scholar] [CrossRef]
- Cesário, R.; Poissant, L.; Pilote, M.; O’Driscoll, N.J.; Mota, A.M.; Canário, J. Dissolved gaseous mercury formation and mercury volatilization in intertidal sediments. Sci. Total Environ. 2017, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.T.; Duarte, B.; Cesário, R.; Mendes, R.; Hintelmann, H.; Eckey, K.; Dimock, B.; Caçador, I.; Canário, J. Mercury mobility and effects in the salt-marsh plant Halimione portulacoides: Uptake, transport, and toxicity and tolerance mechanisms. Sci. Total Environ. 2019, 650, 111–120. [Google Scholar] [CrossRef]
- Taborda, R.; Freire, P.; Silva, A.N.; Andrade, C.; Freitas, M.D.C. Origin and evolution of Tagus estuarine beaches. J. Coast. Res. 2009, 2009, 213–217. [Google Scholar]
- Vaz, N.; Mateus, M.; Dias, J.M. Semidiurnal and spring-neap variations in the Tagus estuary: Application of a process-oriented hydro-biogeochemical model. J. Coast. Res. 2011, SI, 1619–1623. [Google Scholar]
- Canário, J.; Vale, C.; Nogueira, M. The pathway of mercury in contaminated waters determined by association with organic carbon (Tagus Estuary, Portugal). Appl. Geochem. 2008, 23, 519–528. [Google Scholar] [CrossRef]
- Cesário, R.; Mota, A.M.; Caetano, M.; Nogueira, M.; Canário, J. Mercury and methylmercury transport and fate in the water column of Tagus estuary (Portugal). Mar. Pollut. Bull. 2018, 127. [Google Scholar] [CrossRef] [PubMed]
- Canário, J.; Vale, C.; Caetano, M. Distribution of monomethylmercury and mercury in surface sediments of the Tagus Estuary (Portugal). Mar. Pollut. Bull. 2005, 50, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Canário, J.; Caetano, M.; Vale, C.; Cesário, R. Evidence for elevated production of methylmercury in salt marshes. Environ. Sci. Technol. 2007, 41, 7376–7382. [Google Scholar] [CrossRef]
- Canário, J.; Vale, C.; Poissant, L.; Nogueira, M.; Pilote, M.; Branco, V. Mercury in sediments and vegetation in a moderately contaminated salt marsh (Tagus Estuary, Portugal). J. Environ. Sci. 2010, 22, 1151–1157. [Google Scholar] [CrossRef]
- Monteiro, C.E.; Cesário, R.; O’Driscoll, N.J.; Nogueira, M.; Válega, M.; Caetano, M.; Canário, J. Seasonal variation of methylmercury in sediment cores from the Tagus Estuary (Portugal). Mar. Pollut. Bull. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cesário, R.; Hintelmann, H.; Mendes, R.; Eckey, K.; Dimock, B.; Araújo, B.; Mota, A.M.; Canário, J. Evaluation of mercury methylation and methylmercury demethylation rates in vegetated and non-vegetated saltmarsh sediments from two Portuguese estuaries. Environ. Pollut. 2017, 226. [Google Scholar] [CrossRef] [PubMed]
- Cesário, R.; Hintelmann, H.; O’Driscoll, N.J.; Monteiro, C.E.; Caetano, M.; Nogueira, M.; Mota, A.M.; Canário, J. Biogeochemical Cycle of Mercury and Methylmercury in Two Highly Contaminated Areas of Tagus Estuary (Portugal). Water. Air. Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Cesário, R.; Monteiro, C.E.; Nogueira, M.; O’Driscoll, N.J.; Caetano, M.; Hintelmann, H.; Mota, A.M.; Canário, J. Mercury and Methylmercury Dynamics in Sediments on a Protected Area of Tagus Estuary (Portugal). Water. Air. Soil Pollut. 2016. [Google Scholar] [CrossRef]
- Zhang, H.; Lindberg, S.E.; Barnett, M.O.; Vette, A.F.; Gustin, M.S. Dynamic flux chamber measurement of gaseous mercury emission fluxes over soils: Part 2 - Effect of flushing flow rate and verification of a two-resistance exchange interface simulation model. Atmos. Environ. 2002, 36, 847–859. [Google Scholar] [CrossRef]
- Costley, C.T.; Mossop, K.F.; Dean, J.R.; Garden, L.M.; Marshall, J.; Carroll, J. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal. Chim. Acta 2000, 405, 179–183. [Google Scholar] [CrossRef]
- Caçador, I.; Caetano, M.; Duarte, B.; Vale, C. Stock and losses of trace metals from salt marsh plants. Mar. Environ. Res. 2009, 67, 75–82. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: London, UK, 2010; ISBN 978-0-273-73042-2. [Google Scholar]
- Polyzou, C.; Loupa, G.; Trepekli, A.; Rapsomanikis, S. Fluxes of gaseous elemental mercury on a Mediterranean coastal grassland. Atmosphere 2019, 10, 485. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Meyers, T.P. Development of an automated micrometeorological method for measuring the emission of mercury vapor from wetland vegetation. Wetl. Ecol. Manag. 2001, 9, 333–347. [Google Scholar] [CrossRef]
- Ericksen, J.A.; Gustin, M.S.; Xin, M.; Weisberg, P.J.; Fernandez, G.C.J. Air-soil exchange of mercury from background soils in the United States. Sci. Total Environ. 2006, 366, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Travnikov, O.; Angot, H.; Artaxo, P.; Bencardino, M.; Bieser, J.; D’Amore, F.; Dastoor, A.; De Simone, F.; DIéguez, M.C.; Dommergue, A.; et al. Multi-model study of mercury dispersion in the atmosphere: Atmospheric processes and model evaluation. Atmos. Chem. Phys. 2017, 17, 5271–5295. [Google Scholar] [CrossRef]
- Danzon, M.A.; Van Leeuwen, F.X.R.; Krzyzanowski, M. Air Quality Guidelines: For Europe; WHO Regional Office for Europe: Copenhagen, Denmark, 2001; pp. 1–496. [Google Scholar]
- Osawa, T.; Ueno, T.; Fu, F.F. Sequential variation of atmospheric mercury in Tokai-mura, seaside area of eastern central Japan. J. Geophys. Res. Atmos. 2007, 112, 1–9. [Google Scholar] [CrossRef]
- Jen, Y.H.; Chen, W.H.; Hung, C.H.; Yuan, C.S.; Ie, I.R. Field measurement of total gaseous mercury and its correlation with meteorological parameters and criteria air pollutants at a coastal site of the Penghu Islands. Aerosol Air Qual. Res. 2014, 14, 364–375. [Google Scholar] [CrossRef]
- Weiss-Penzias, P.; Gustin, M.S.; Lyman, S.N. Observations of speciated atmospheric mercury at three sites in Nevada: Evidence for a free tropospheric source of reactive gaseous mercury. J. Geophys. Res. Atmos. 2009, 114, 1–11. [Google Scholar] [CrossRef]
- Wallschläger, D.; Herbert Kock, H.; Schroeder, W.H.; Lindberg, S.E.; Ebinghaus, R.; Wilken, R.D. Mechanism and significance of mercury volatilization from contaminated floodplains of the German river Elbe. Atmos. Environ. 2000, 34, 3745–3755. [Google Scholar] [CrossRef]
- Cha, S.H.; Han, Y.J.; Jeon, J.W.; Kim, Y.H.; Kim, H.; Noh, S.; Kwon, M.H. Development and field application of a passive sampler for atmospheric mercury. Asian J. Atmos. Environ. 2020, 14, 14–27. [Google Scholar] [CrossRef]
- Zhang, L.; Brook, J.R.; Vet, R. On ozone dry deposition - With emphasis on non-stomatal uptake and wet canopies. Atmos. Environ. 2002, 36, 4787–4799. [Google Scholar] [CrossRef]
- Gustin, M.S. Exchange of Mercury between the Atmosphere and Terrestrial Ecosystems. Environ. Chem. Toxicol. Mercur. 2012, 423–451. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Dong, W.; Meyers, T. Transpiration of gaseous elemental mercury through vegetation in a subtropical wetland in Florida. Atmos. Environ. 2002, 36, 5207–5219. [Google Scholar] [CrossRef]
- Bash, J.O.; Miller, D.R.; Meyer, T.H.; Bresnahan, P.A. Northeast United States and Southeast Canada natural mercury emissions estimated with a surface emission model. Atmos. Environ. 2004, 38, 5683–5692. [Google Scholar] [CrossRef]
- Bash, J.O.; Miller, D.R. Growing season total gaseous mercury (TGM) flux measurements over an Acer rubrum L. stand. Atmos. Environ. 2009, 43, 5953–5961. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Meyers, T.P.; Taylor, G.E., Jr.; Turner, R.R.; Schroeder, W.H. Atmosphere-surface exchange of mercury in a forest: Results of modeling and gradient approaches. J. Geophys. Res. Atmos. 1992, 97, 2519–2528. [Google Scholar] [CrossRef]
- Hanson, P.J.; Lindberg, S.E.; Tabberer, T.A.; Owens, J.G.; Kim, K.-H. Foliar exchange of mercury vapor: Evidence for a compensation point. Water. Air. Soil Pollut. 1995, 80, 373–382. [Google Scholar] [CrossRef]
- Bigham, G.N.; Murray, K.J.; Masue-Slowey, Y.; Henry, E.A. Biogeochemical controls on methylmercury in soils and sediments: Implications for site management. Integr. Environ. Assess. Manag. 2017, 13, 249–263. [Google Scholar] [CrossRef]
- Pannu, R.; Siciliano, S.D.; O’Driscoll, N.J. Quantifying the effects of soil temperature, moisture and sterilization on elemental mercury formation in boreal soils. Environ. Pollut. 2014, 193, 138–146. [Google Scholar] [CrossRef]
- Elfving, D.C.; Kaufmann, M.R.; Hall, A.E. Interpreting Leaf Water Potential Measurements with a Model of the Soil-Plant-Atmosphere Continuum. Physiol. Plant. 1972, 27, 161–168. [Google Scholar] [CrossRef]
- Wunderground. Available online: https://www.wunderground.com/ (accessed on 6 January 2021).
- Marumoto, K.; Hayashi, M.; Takami, A. Atmospheric mercury concentrations at two sites in the Kyushu Islands, Japan, and evidence of long-range transport from East Asia. Atmos. Environ. 2015, 117, 147–155. [Google Scholar] [CrossRef]
- Xu, X.; Akhtar, U.; Clark, K.; Wang, X. Temporal variability of atmospheric total gaseous mercury in Windsor, ON, Canada. Atmosphere 2014, 5, 536–556. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, J.E.; Kim, P.R.; Kim, W.J.; Yi, S.M.; Seo, Y.S.; Kim, S.H. General trends of atmospheric mercury concentrations in urban and rural areas in Korea and characteristics of high-concentration events. Atmos. Environ. 2014, 94, 754–764. [Google Scholar] [CrossRef]
- Leonard, T.L.; Taylor, G.E.; Gustin, M.S.; Fernandez, G.C.J. Mercury and plants in contaminated soils: 1. Uptake, partitioning, and emission to the atmosphere. Environ. Toxicol. Chem. 1998, 17, 2063–2071. [Google Scholar] [CrossRef]
- Windham, L.; Weis, J.S.; Weis, P. Patterns and processes of mercury release from leaves of two dominant salt marsh macrophytes, Phragmites australis and Spartina alterniflora. Estuaries 2001, 24, 787–795. [Google Scholar] [CrossRef]
- Osterwalder, S.; Bishop, K.; Alewell, C.; Fritsche, J.; Laudon, H.; Åkerblom, S.; Nilsson, M.B. Mercury evasion from a boreal peatland shortens the timeline for recovery from legacy pollution. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lindberg, S.E.; Meyers, T.P. Micrometeorological measurements of mercury vapor fluxes over background forest soils in eastern Tennessee. Atmos. Environ. 1995, 29, 267–282. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Qiu, G.; Hou, Y.; Tang, S. Total gaseous mercury emissions from soil in Guiyang, Guizhou, China. J. Geophys. Res. D Atmos. 2005, 110, 1–12. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011. [Google Scholar] [CrossRef]
- Clackett, S.P.; Porter, T.J.; Lehnherr, I. 400-Year Record of Atmospheric Mercury from Tree-Rings in Northwestern Canada. Environ. Sci. Technol. 2018, 52, 9625–9633. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Ahmad, I.; Válega, M.; Pacheco, M.; Figueira, E.; Duarte, A.C.; Pereira, E. Impact of seasonal fluctuations on the sediment-mercury, its accumulation and partitioning in Halimione portulacoides and Juncus maritimus collected from Ria de Aveiro coastal lagoon (Portugal). Water. Air. Soil Pollut. 2011, 222, 1–15. [Google Scholar] [CrossRef]
- Castro, R.; Pereira, S.; Lima, A.; Corticeiro, S.; Válega, M.; Pereira, E.; Duarte, A.; Figueira, E. Accumulation, distribution and cellular partitioning of mercury in several halophytes of a contaminated salt marsh. Chemosphere 2009, 76, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Válega, M.; Lillebø, A.I.; Caçador, I.; Pereira, M.E.; Duarte, A.C.; Pardal, M.A. Mercury mobility in a salt marsh colonised by Halimione portulacoides. Chemosphere 2008, 72, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Laacouri, A.; Nater, E.A.; Kolka, R.K. Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, U.S.A. Environ. Sci. Technol. 2013, 47, 10462–10470. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, Y.; Richards, J.; Cai, Y. Investigating uptake and translocation of mercury species by sawgrass (Cladium jamaicense) using a stable isotope tracer technique. Environ. Sci. Technol. 2013, 47, 9678–9684. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Poissant, L.; Pilote, M.; Constant, P.; Beauvais, C.; Zhang, H.H.; Xu, X. Mercury gas exchanges over selected bare soil and flooded sites in the bay St. François wetlands (Québec, Canada). Atmos. Environ. 2004, 38, 4205–4214. [Google Scholar] [CrossRef]
- Ericksen, J.A.; Gustin, M.S.; Schorran, D.E.; Johnson, D.W.; Lindberg, S.E.; Coleman, J.S. Accumulation of atmospheric mercury in forest foliage. Atmos. Environ. 2003, 37, 1613–1622. [Google Scholar] [CrossRef]
- Fleck, J.A.; Grigal, D.F.; Nater, E.A. Mercury uptake by trees: An observational experiment. Water. Air. Soil Pollut. 1999, 115, 513–523. [Google Scholar] [CrossRef]
- Fay, L.; Gustin, M. Assessing the influence of different atmospheric and soil mercury concentrations on foliar mercury concentrations in a controlled environment. Water. Air. Soil Pollut. 2007, 181, 373–384. [Google Scholar] [CrossRef]
- Huang, S.; Jiang, R.; Song, Q.; Zhang, Y.; Huang, Q.; Su, B.; Chen, Y.; Huo, Y.; Lin, H. Study of mercury transport and transformation in mangrove forests using stable mercury isotopes. Sci. Total Environ. 2020, 704, 135928. [Google Scholar] [CrossRef] [PubMed]
- Wallschläger, D.; Kock, H.H.; Schroeder, W.H.; Lindberg, S.E.; Ebinghaus, R.; Wilken, R.D. Estimating gaseous mercury emissions from contaminated floodplain soils to the atmosphere with simple field measurement techniques. Water. Air. Soil Pollut. 2002, 135, 39–54. [Google Scholar] [CrossRef]
- Ericksen, J.A.; Gustin, M.S. Foliar exchange of mercury as a function of soil and air mercury concentrations. Sci. Total Environ. 2004, 324, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Nacht, D.M.; Gustin, M.S. Mercury emmision from background and altered geologic units throughout Nevada. Water. Air. Soil Pollut. 2004, 151, 179–193. [Google Scholar] [CrossRef]
- Wang, S.; Feng, X.; Qiu, G.; Wei, Z.; Xiao, T. Mercury emission to atmosphere from Lanmuchang Hg-Tl mining area, Southwestern Guizhou, China. Atmos. Environ. 2005, 39, 7459–7473. [Google Scholar] [CrossRef]
- Poissant, L.; Constant, P.; Pilote, M.; Canário, J.; O’Driscoll, N.; Ridal, J.; Lean, D. The ebullition of hydrogen, carbon monoxide, methane, carbon dioxide and total gaseous mercury from the Cornwall Area of Concern. Sci. Total Environ. 2007, 381, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.M.; Moore, C.W.; Wohlfahrt, G.; Hörtnagl, L.; Kljun, N.; Obrist, D. Eddy Covariance Flux Measurements of Gaseous Elemental Mercury Using Cavity Ring-Down Spectroscopy. Environ. Sci. Technol. 2015, 49, 1559–1568. [Google Scholar] [CrossRef] [PubMed]






| Sampling Sites | Plant Species | THg-Sed (ng g−1, Dry Weight) | THg-Stems (ng g−1, Dry Weight) | THg-Leaves (ng g−1, Dry Weight) | Air (Hg(0)) (ng Hg m−3) | Hg(0) Flux (ng Hg m−2 h−1) |
|---|---|---|---|---|---|---|
| ALC | H. portulacoides | 620 ± 90 (n = 5) | 20 ± 3 (n = 4) | 47 ± 13 (n = 4) | 1.18–3.53 1.84 ± 0.52 (n = 354) | −0.76–1.52 0.01 ± 0.27 (n = 554) |
| S. fruticosa | 406 ± 33 (n = 5) | 16 ± 2 (n = 4) | 24 ± 4 (n = 4) | 1.36–3.40 1.73 ± 0.37 (n = 221) | −0.40–1.28 0.09 ± 0.29 (n = 221) | |
| CN | H. portulacoides | 3720 ± 960 (n = 5) | 31 ± 9 (n = 8) | 173 ± 70 (n = 8) | 1.08–15.3 2.86 ± 1.64 (n = 354) | −9.90–15.5 0.64 ± 2.76 (n = 354) |
| S. fruticosa | 2770 ± 350 (n = 5) | 24 ± 4 (n = 4) | 53 ± 14 (n = 4) | 1.22–18.2 3.82 ± 2.34 (n = 455) | −8.93–12.6 0.13 ± 2.62 (n = 455) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesário, R.; O’Driscoll, N.J.; Justino, S.; Wilson, C.E.; Monteiro, C.E.; Zilhão, H.; Canário, J. Air Concentrations of Gaseous Elemental Mercury and Vegetation–Air Fluxes within Saltmarshes of the Tagus Estuary, Portugal. Atmosphere 2021, 12, 228. https://doi.org/10.3390/atmos12020228
Cesário R, O’Driscoll NJ, Justino S, Wilson CE, Monteiro CE, Zilhão H, Canário J. Air Concentrations of Gaseous Elemental Mercury and Vegetation–Air Fluxes within Saltmarshes of the Tagus Estuary, Portugal. Atmosphere. 2021; 12(2):228. https://doi.org/10.3390/atmos12020228
Chicago/Turabian StyleCesário, Rute, Nelson J. O’Driscoll, Sara Justino, Claire E. Wilson, Carlos E. Monteiro, Henrique Zilhão, and João Canário. 2021. "Air Concentrations of Gaseous Elemental Mercury and Vegetation–Air Fluxes within Saltmarshes of the Tagus Estuary, Portugal" Atmosphere 12, no. 2: 228. https://doi.org/10.3390/atmos12020228
APA StyleCesário, R., O’Driscoll, N. J., Justino, S., Wilson, C. E., Monteiro, C. E., Zilhão, H., & Canário, J. (2021). Air Concentrations of Gaseous Elemental Mercury and Vegetation–Air Fluxes within Saltmarshes of the Tagus Estuary, Portugal. Atmosphere, 12(2), 228. https://doi.org/10.3390/atmos12020228








