Plant Organic Matter in Palsa and Khasyrei Type Mires: Direct Observations in West Siberian Sub-Arctic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of Field Sites
2.2. The Climate at Field Sites
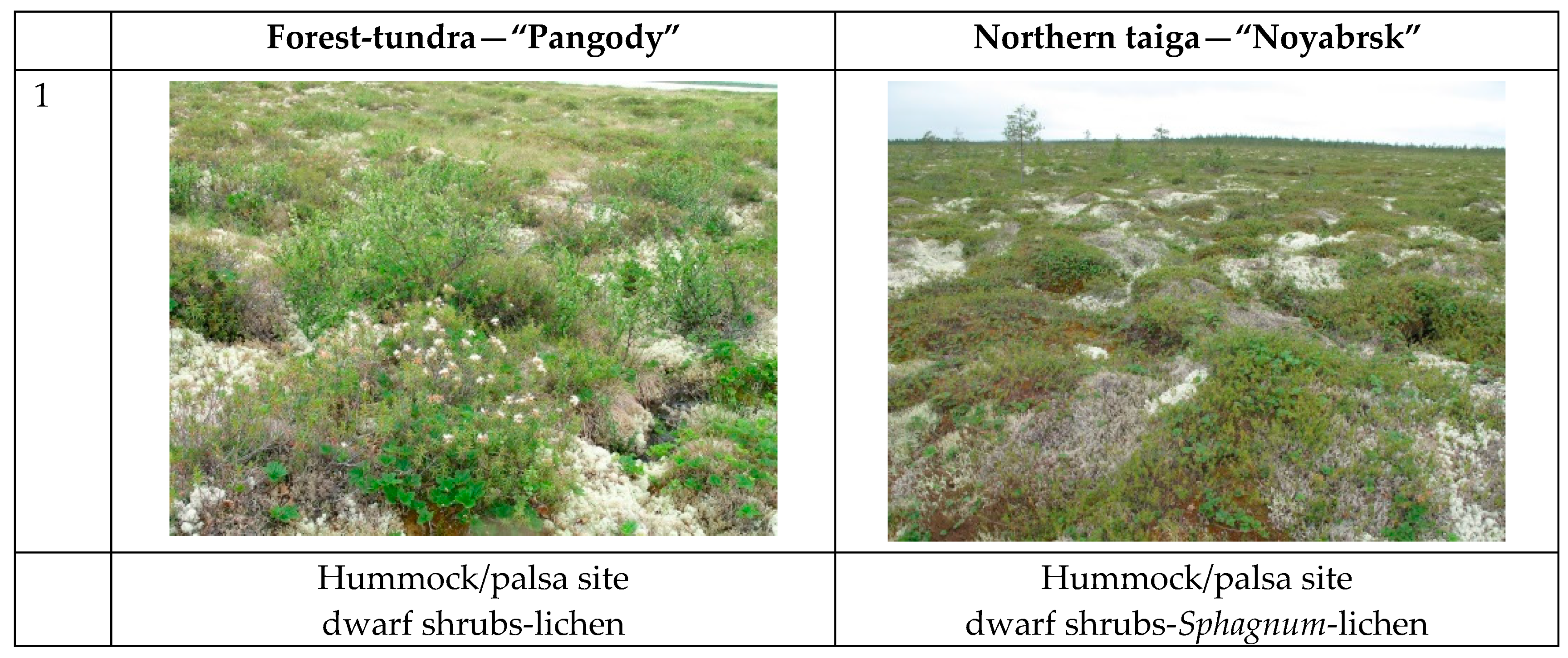
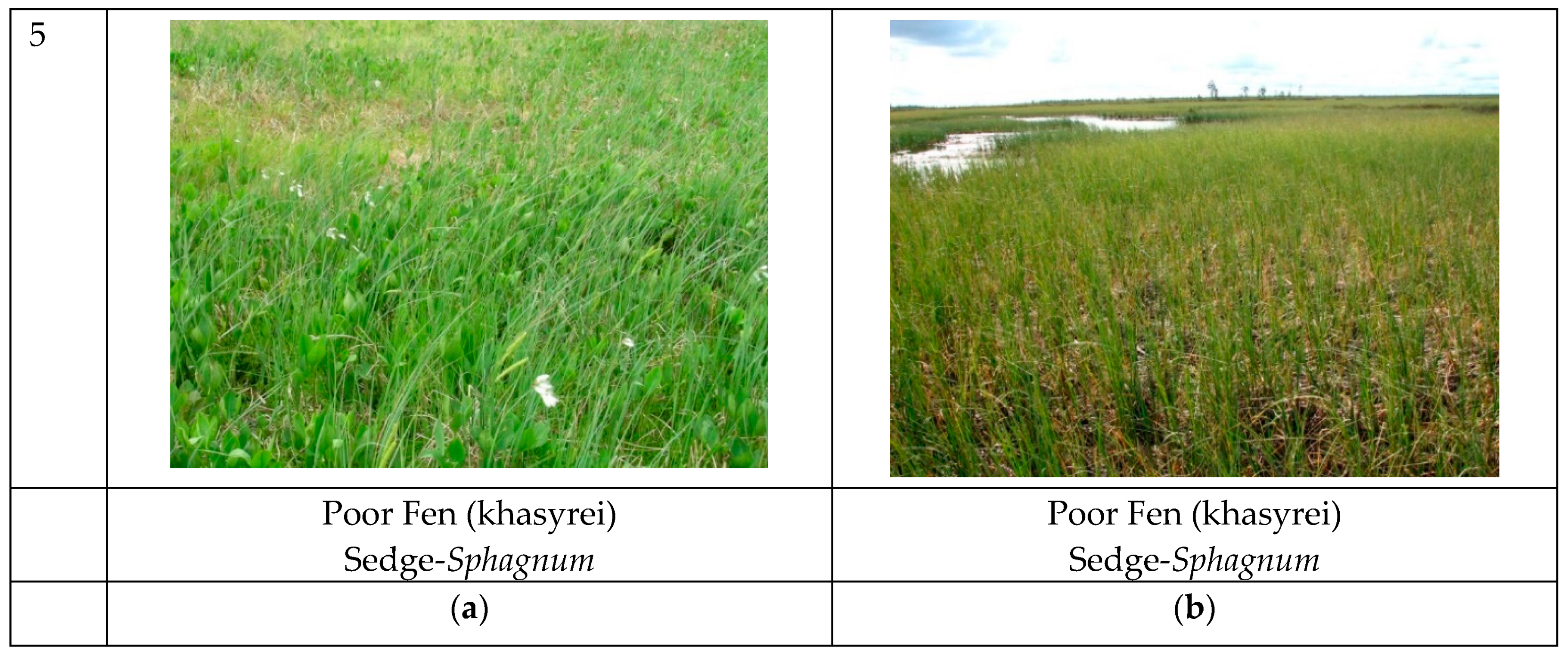
2.3. Land Cover/Plant Communities at Test Sites
2.4. Experimental Design
3. Results
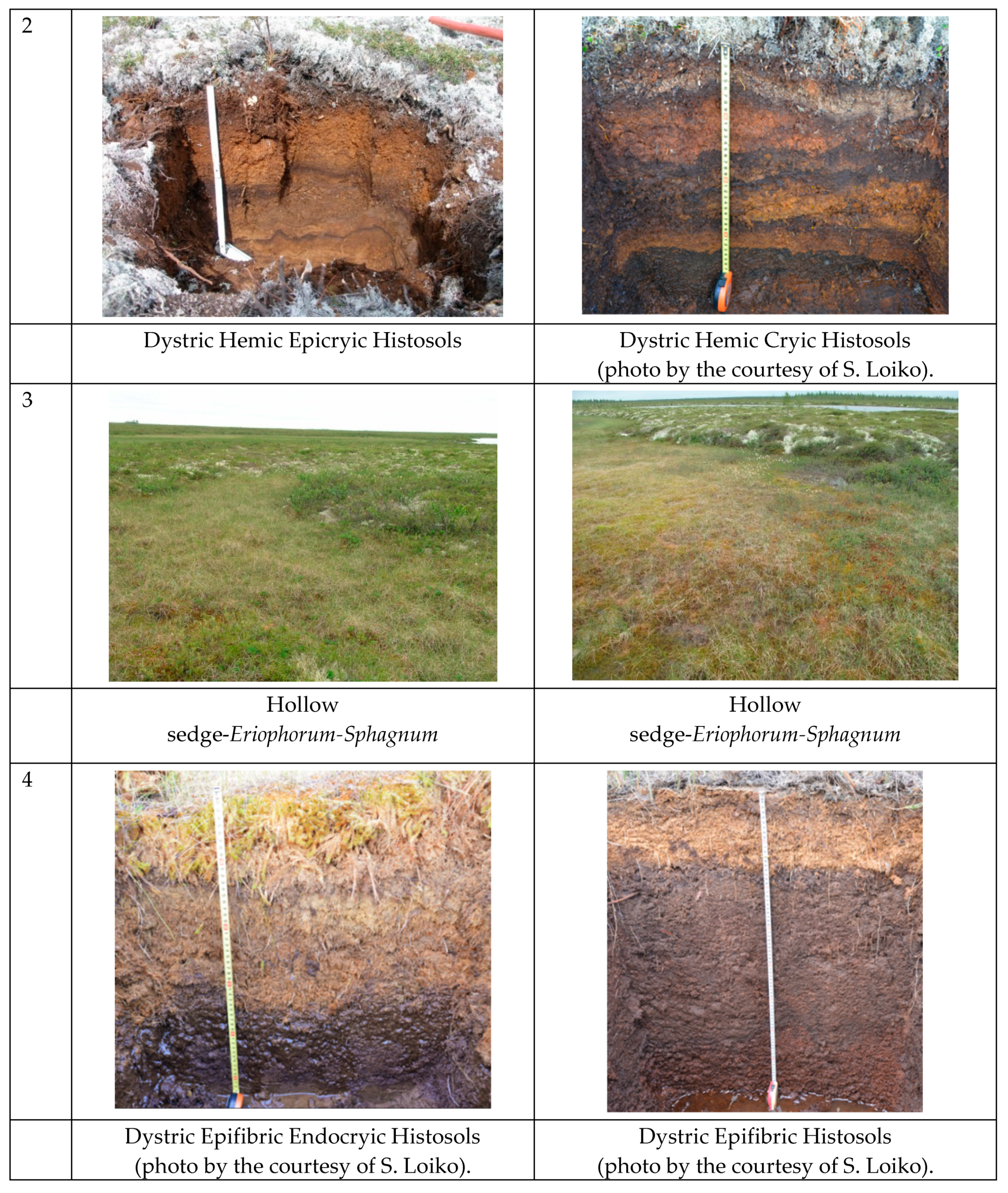
3.1. The Features of Soil Profiles and Soil Properties
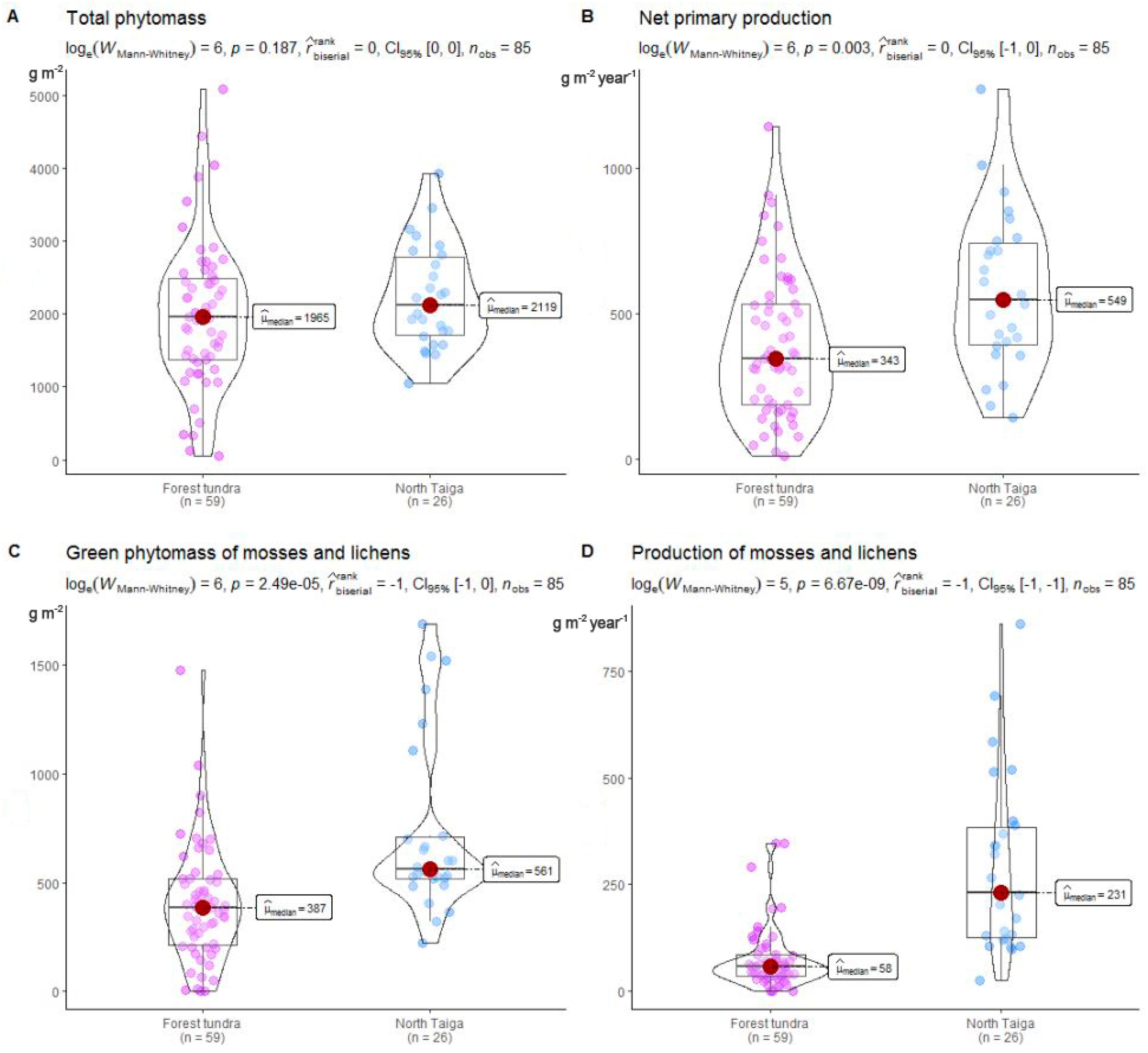
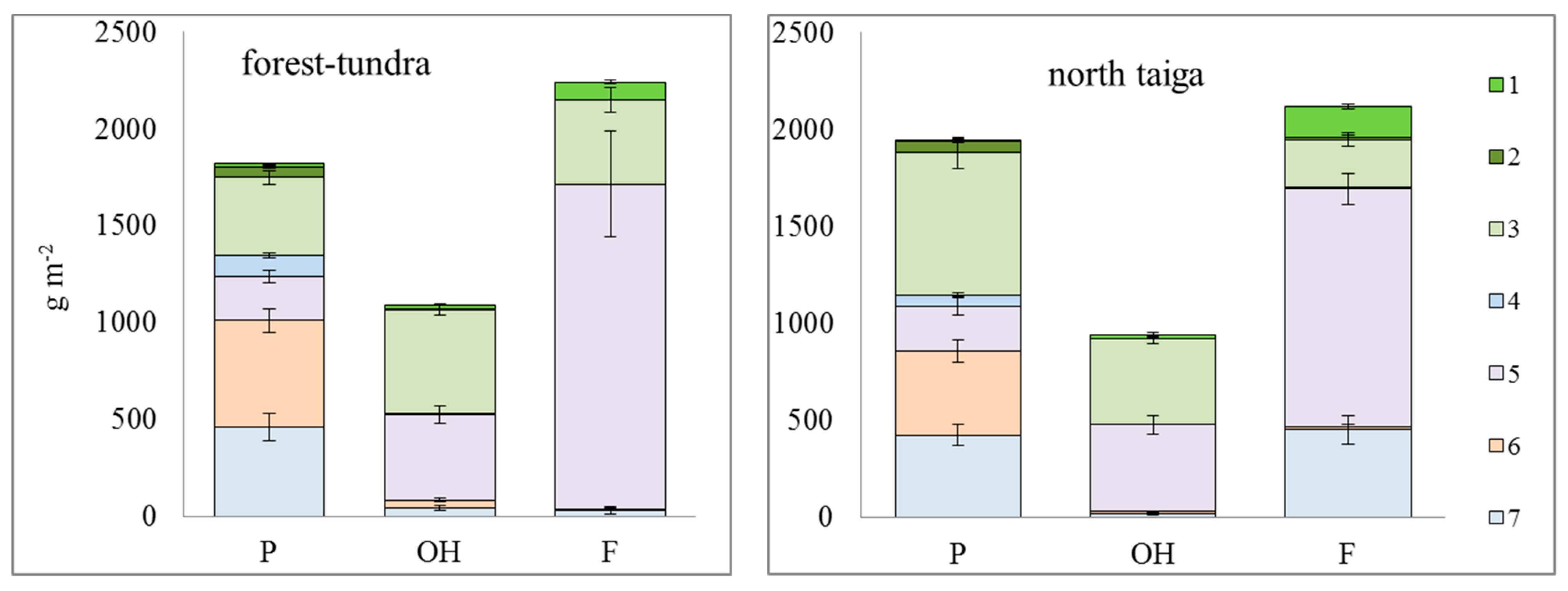
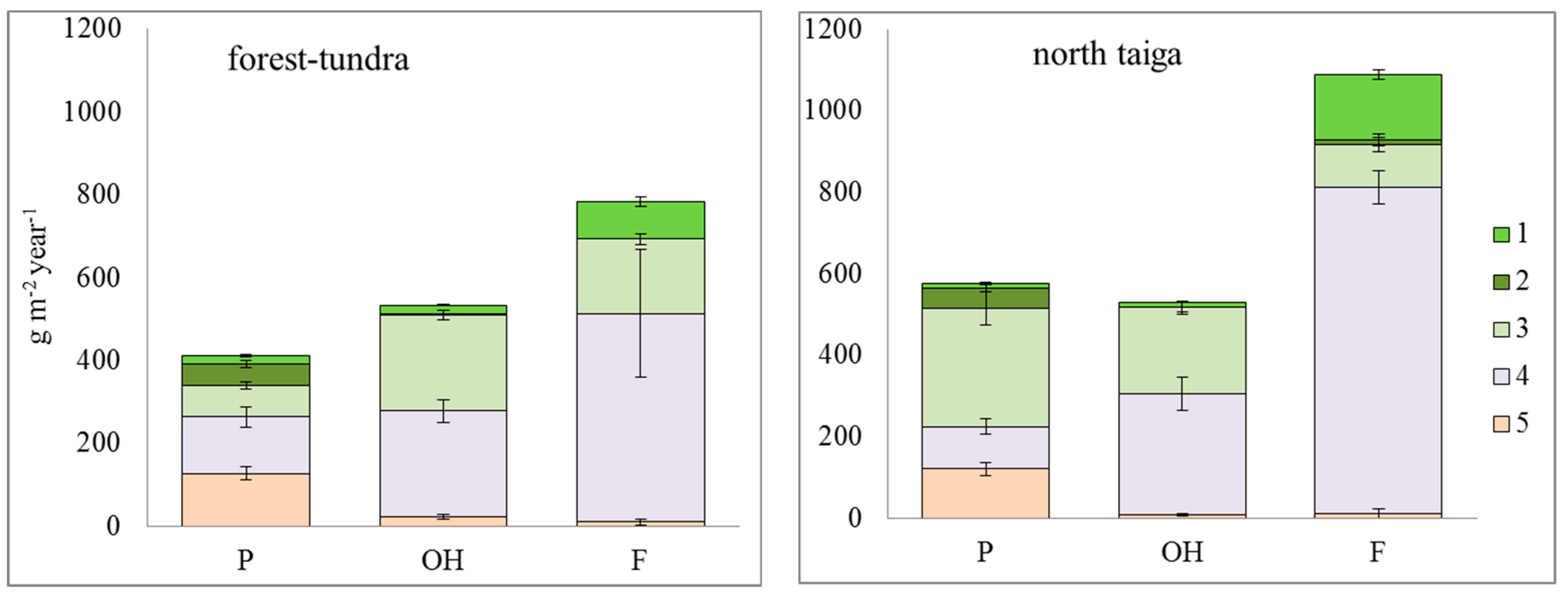
3.2. Plant Organic Matter
3.2.1. Biomass
3.2.2. Net Primary Production (NPP)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the Last Glacial Maximum. Geophys. Res. Lett. 2010, 37, L13402. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Turunen, J.; Tahvanainen, T.; Tolonen, K. Carbon accumulation in west Siberian mires, Russian. Glob. Biogeochem. Cycles 2001, 15, 285–296. [Google Scholar] [CrossRef]
- Botch, M.S.; Kobak, K.I.; Vinson, T.S.; Kolchugina, T.P. Carbon pools and accumulation in peatlands of the former Sovet Union. Glob. Biogeochem. Cycles 1995, 9, 37–46. [Google Scholar] [CrossRef]
- Loisel, J.; van Bellen, S.; Pelletier, L.; Talbot, J.; Hugelius, G.; Karran, D.; Yu, Z.; Nichols, J.; Holmquist, J. Insights and issues with estimating northern peatland carbon stocks and fluxes since the Last Glacial Maximum. Earth-Sci. Rev. 2017, 165, 59–80. [Google Scholar] [CrossRef]
- Smith, L.C.; MacDonald, G.M.; Velichko, A.A.; Beilman, D.W.; Borisova, O.K.; Frey, K.E.; Kremenetski, K.V.; Sheng, Y. Siberian Peatlands a Net Carbon Sink and Global Methane Source Since the Early Holocene. Science 2004, 303, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, Y.W.; Smith, L.C.; MacDonald, G.M.; Kremenetski, K.V.; Frey, K.E.; Velichko, A.A.; Lee, M.; Beilman, D.W.; Dubinin, P. A high-resolution GIS-based inventory of the west Siberian peat carbon pool. Glob. Biogeochem. Cycles 2004, 18, 14415–14428. [Google Scholar] [CrossRef]
- Efremov, S.P.; Efremova, T.T. Present stocks of peat and organic carbon in bog ecosystems of west Siberia. In Carbon Storage and Atmospheric Exchange by West Siberian Peatlands; Bleuten, W., Lapshina, E.D., Eds.; Utrecht University: Utrecht, The Netherlands, 2001; pp. 73–78. [Google Scholar]
- Vomperskiy, S.E. Biogeocenoticheskie Osobennosti Bolot i Ich Razional’noe Ispol’zovanie; Izdatel’stvo VZ SO RAN: Moskva, Russia, 1994; pp. 5–37. [Google Scholar]
- Efremov, S.P.; Efremova, T.T.; Melentyeva, N.V. Zapasy ugleroda v ecosystemah bolot. In Uglerod v Ecosystemah Lesov I Bolot Rossii; Alekseev, V.A., Berdsi, R.A., Eds.; Izdatel’stvo VZ SO RAN: Krasnoyarsk, Rossia, 1994; pp. 128–139. [Google Scholar]
- Titlianova, A.A.; Bulavko, G.I.; Kudryashova, S.Y.; Naumov, A.V.; Smirnov, V.V.; Tanasienko, A.A. Zapasi i poteri organicheskogo ugleroda v pochvach Sibiri. Pochvovedenie 1998, 1, 51–59. [Google Scholar]
- Novikov, S.M. Gydrologiya Zabolochennych Territoriy Zony Mnogoletney Merzloty Zapadnoi Sibiri; OOO Izdatel’stvo VVM: Saint-Petersburg, Russia, 2009; 536p. [Google Scholar]
- Romanova, E.A. Tipy bolotnych massivov i zakonomernoe raspredelenie ich na territorii Zapadnoi Sibiri. In Tipy Bolot SSSR I Principy Ich Klassifikazii; Nauka: Leningrad, Russia, 1974; pp. 167–174. [Google Scholar]
- Botch, M.S.; Mazing, V.V. Ecosystemy Bolot SSSR; Nauka: Leningrad, Russia, 1979; 188p. [Google Scholar]
- Seppälä, M. The term “palsa”. Z. Geomorphol. 1972, 16, 463. [Google Scholar]
- Luoto, M.; Heikkinen, R.K.; Carter, T.R. Loss of palsa mires in Europe and biological consequences. Environ. Conserv. 2004, 31, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Peregon, A.; Maksyutov, S.; Kosykh, N.; Mironysheva-Tokareva, N.P. Map based inventory of the wetland biomass and NPP in western Siberia. J. Geophys. Res. 2008, 113, G01007. [Google Scholar] [CrossRef]
- Bazilevich, N.I.; Titlyanova, A.A. Bioticheskiy Krugovorot na Pyati Kontinentah; Izdatel’stvo SO RAN: Novosibirsk, Russia, 2008; 380p. [Google Scholar]
- Bazilevich, N.I. Biologicheskaya Productivnost’ Ecosystem Severnoi Evrazii; Nauka: Moskva, Russia, 1993; 293p. [Google Scholar]
- Rodin, L.E.; Bazilevich, N.I. Dinamika Organicheskogo Veshestva i Biologicheskiy Krugovorot Zol’nych Elementov i Azota v Osnovnych Tipah Rastitel’nosti Zemnogo Schara; Nauka: Moskva, Russia; Leningrad, Russia, 1965; 253p. [Google Scholar]
- Karelin, D.V.; Zamolodchikov, D.G. Uglerodniy Obmen v Kriogennych Ecosystemah; Nauka: Moskva, Russia, 2008; 344p. [Google Scholar]
- Gould, W.A.; Raynolds, M.; Walker, D.A. Vegetation, plant biomass, and net primary productivity patterns in the Canadian Arctic. J. Geophys. Res. 2003, 108, 8167. [Google Scholar] [CrossRef]
- Prokushkin, A.S.; Hagedorn, F.; Pokrovsky, O.S.; Viers, J.; Kirdyanov, A.V.; Masyagina, O.V.; Prokushkina, M.P.; McDowell, W.H. Permafrost Regime Affects the Nutritional Status and Productivity of Larches in Central Siberia. Forests 2018, 9, 314. [Google Scholar] [CrossRef] [Green Version]
- Kosykh, N.P.; Koronatova, N.G.; Naumova, N.B.; Titlyanova, A.A. Above- and below-ground phytomass and net primary production in boreal mire ecosystems. Wetl. Ecol. Manag. 2008, 16, 139–153. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Lim, A.; Manasypov, R.M.; Shirokova, L.S.; Istigechev, G.I.; Kuzmina, D.M.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Permafrost thaw and climate warming may decrease the CO2, carbon, and metal concentration in peat soil waters of the Western Siberia Lowland. Sci. Total Environ. 2018, 634, 1004–1023. [Google Scholar] [CrossRef]
- Kirpotin, S.N.; Berezin, A.; Bazanov, V.; Polishchuk, Y.; Vorobiov, S.; Mironycheva-Tokoreva, N.; Kosykh, N.; Volkova, I.; Dupre, B.; Pokrovsky, O.; et al. Western Siberia wetlands as indicator and regulator of climate change on the global scale. Int. J. Environ. Stud. 2009, 66, 409–421. [Google Scholar] [CrossRef]
- Loiko, S.; Raudina, T.; Lim, A.; Kuzmina, D.; Kulizhskiy, S.; Pokrovsky, O. Microtopography controls of carbon and related elements distribution in the west siberian frozen bogs. Geosciences 2019, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Weltzin, J.F.; Harth, C.; Bridgham, S.D.; Pastor, J.; Vonderharr, M. Production and microtopography of bog bryophytes: Response to warming and water-table manipulations. Oecologia 2001, 128, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Vitt, D.H.; Halsey, L.A.; Zoltai, S.C. The bog landforms of continental Western Canada in relation to climate and permafrost patterns. Arct. Alp. Res. 1994, 26, 1–13. [Google Scholar] [CrossRef]
- Stepanova, V.A.; Pokrovsky, O.S.; Viers, J.; Mironycheva-Tokareva, N.P.; Kosykh, N.P.; Vishnyakova, E.K. Elemental composition of peat profiles in western Siberia: Effect of the micro-landscape, latitude position and permafrost coverage. Appl. Geochem. 2015, 53, 53–70. [Google Scholar] [CrossRef]
- Audry, S.; Pokrovsky, O.S.; Shirokova, L.S.; Kirpotin, S.N.; Dupré, B. Organic matter mineralization and trace element post-depositional redistribution in Western Siberia thermokarst lake sediments. Biogeoscience 2011, 8, 3341–3358. [Google Scholar] [CrossRef] [Green Version]
- Loiko, S.; Klimova, N.; Kuzmina, D.; Pokrovsky, O. Lake Drainage in Permafrost Regions Produces Variable Plant Communities of High Biomass and Productivity. Plants 2020, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Vaganov, E.A.; Vedrova, E.F.; Verhovets, S.V.; Efremov, S.P.; Efremova, T.T.; Kruglov, V.B.; Onuchin, A.A.; Sukhinin, A.I.; Shibistova, O.B. Lesa i bolota Sibiri v global’nom zikle ugleroda. Sibirskiy Ecol. Z. 2005, 4, 631–649. [Google Scholar]
- Meltzer, L.I. Tundrovaya rastitel’nost’. In Rastitel’nyi Pokrov Zapadno-Sibirskoi Ravniny; Nauka: Novosibirsk, Russia, 1985; pp. 41–54. [Google Scholar]
- Smolentsev, B.A. Struktura Pochvennogo Pokrova Sibirskich Uvalov; Izdatel’stvo SO RAN: Novosibirsk, Russia, 2002; 117p. [Google Scholar]
- Tonkonogov, V.D. Avtomorfnoe Pochvoobrazovanie v Tundrovoi i Taeshnoi Zonah Vostochno-Evropeiskoi i Zapadno-Sibirskoi Ravnin; Pochvenniy Institut V.V. Dokuchaeva: Moskva, Russia, 2010; 304p. [Google Scholar]
- Pavlov, A.V. Monitoring Kriolitozony; Akademicheskoe Izdatel’stvo “GEO”: Novosibirsk, Russia, 2008; 229p. [Google Scholar]
- Loiko, S.; Raudina, T.; Lim, A.; Kuzmina, D.; Kulizhskiy, S.; Pokrovsky, O. Dissolved organic carbon, macro- and microelements in solutions of peat soils in the cryolithozone of the West Siberian Plain. In Proceedings of the “Horizons of the Future” Conference, Soil Institute Named after V.V.Dokuchaev, Moscow, Russia, February 2017; Available online: https://www.researchgate.net/publication/337472993_Dissolved_organic_carbon_macro-_and_microelements_in_solutions_of_peat_soils_in_the_cryolithozone_of_the_West_Siberian_Plain?channel=doi&linkId=5dd9b175a6fdccdb4460010c&showFulltext=true (accessed on 30 September 2021).
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Parshina, E.K. Biologicheskaya productivnost’ bolot lesotundry Zapadnoi Sibiri. Vestnik TGPU Seriya Biol. Nauki 2008, 4, 53–57. [Google Scholar]
- Shishov, L.L.; Tonkonogov, V.D.; Lebedeva, I.I. Klassifikaziya i Diagnostika Pochv Rossii; Izdatel’stvo Oikumena: Moscow, Rossia, 2004; 342p. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006. A Framework for International Classification. No 103; FAO: Rome, Italy, 2006; 144p. [Google Scholar]
- Andreyashkina, N.I.; Andreiashkina, Y.G. Raznogodichnaya izmenchivost’ produkzii nadzemnoi biomassy tundrovych soobshestv. In Structura i Funkzionirovanie Biogeozenozov Priobskogo Severa; Publ. House of the UNC of the USSR AS: Sverdlovsk, Russia, 1981; pp. 12–19. [Google Scholar]
- Kosykh, N.P.; Koronatova, N.G.; Granath, G. Effect of Temperature and Precipitation on Linear Increment of Sphagnum fuscum and S. magellanicum in Western Siberia. Russ. J. Ecol. 2017, 48, 173–181. [Google Scholar] [CrossRef]
- Andreev, V.N. Prirost kormovych lishainikov i priyemy ego regulirovaniya. Trudy Bot. Inst. AN SSSR Geobotanika 1954, 3, 12–19. [Google Scholar]
- Vasilevskaya, V.D.; Ivanov, V.V.; Bogatyrev, L.G. Pochvy Severa Zapadnoi Sibiri; Izdatel’stvo Moscow State University: Moskva, Russia, 1986; 226p. [Google Scholar]
- Efremova, T.T.; Efremov, S.P.; Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Titlyanova, A.A. Biologicheskaya productivnost’ i pochvy bolot yushnogo Vasyuganiya. Sib. Ecol. Z. 1994, 3, 253–267. [Google Scholar]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Parshina, E.K. The carbon and macroelements budget in the bog ecosystems of the middle taiga in Western Siberia. Int. J. Environ. Stud. 2009, 66, 485–493. [Google Scholar] [CrossRef]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Bleuten, V. Productivnost’ bolot yushnoi taigi Zapadnoi Sibiri. Vestn. Tomsk. Uni. 2003, 7, 142–152. [Google Scholar]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Peregon, A.M.; Parshina, E.K. Net primary production in peatlands of middle taiga region in western Siberia. Rus. J. Ecol. 2008, 39, 466–474. [Google Scholar] [CrossRef]
- Titlyanova, A.A. Chistaya pervichnaya producziya travianych i bolotnych ecosystem. Sib. Ecolog. Z. 2007, 5, 763–770. [Google Scholar]
- Titlyanova, A.A.; Romanova, I.P.; Kosykh, N.P.; Mironycheva-Tokareva, N.P. Pattern and process in above-ground and below-ground components of grassland ecosystems. J. Veg. Sci. 1999, 10, 307–320. [Google Scholar] [CrossRef]
- Reader, R.J.; Stewart, J.M. The relationship between net primary production and accumulation for a peatland in southern-eastern Manitoba. Ecology 1972, 53, 1024–1037. [Google Scholar] [CrossRef]
- Moore, T.R.; Bubier, J.L.; Frolking, S.E.; LaFleur, P.M.; Roulet, N.T. Plant biomass and production and CO2 exchange in an ombrotrophic bog. J. Ecol. 2002, 90, 25–36. [Google Scholar] [CrossRef]
- Wallen, B. Methods for studing below-ground production in mire ecosystems. Suo 1992, 43, 155–162. [Google Scholar]
- Murray, K.J.; Tenhunen, J.D.; Nowak, R.S. Photoinhibition as a control on photosynthtsis and production of Sphagnum mosses. Oecologia 1993, 96, 200–207. [Google Scholar] [CrossRef]
- Nijp Jelmer, J.J.; Limpens, J.; Metselaar, K.; Van Der Zee, S.; Berendse, F.; Robroek, B.J.M. Can frequent precipitation moderate the impact of drought on peatmos uptake in northern peatlands. New Phytol. 2014, 203, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, T. Vascular Plants as Input of Carbon in Boreal Sedge Fens: Control of Production and Partitioning of Biomass; Publications in Botany, University of Helsinki 28: Yliopistopaino, Finland, 1999; p. 66. [Google Scholar]
- Beilman, D.W.; Yu, Z.; Vitt, D.H. Modelling regional variability in peat accumulation response to climate in continental Western Canada. In Proceedings of the West Siberian Peatlands and Carbon Cycle: Past and Present. International Field Symposium, Noyabrsk, Russia, 18–22 August 2001; pp. 15–18. [Google Scholar]


| Name of Test Site | Location | Ecosystem | Depth of Permafrost, cm | Number of Measurements |
|---|---|---|---|---|
| Pangody | 65°52′ N, 74°58′ E | palsa | 40–45 | 59 |
| oligotrophic hollow | 80 | 59 | ||
| poor fen (khasyrei) | - | 9 | ||
| Noyabrsk | 63°14′ N, 75°44′ E | palsa | 50–60 | 26 |
| oligotrophic hollow | - | 26 | ||
| poor fen (khasyrei) | - | 11 |
| Micro-Landscape | Element of Topography | Vegetation Community | Dominant Species |
|---|---|---|---|
| Forest Tundra (Pangody) | |||
| Palsa mire | Flat palsa site | Dwarf shrub-lichen | Ledum decumbens (Ait.) Lodd. ex Steud., Andromeda polifolia L., Oxycoccus microcarpus Turcz. ex Rupr., Cladonia stellaris (Opiz) Brodo, Cl. rangiferina (L.) F.H.Wigg, Sphagnum fuscum (Schimp.) Klinggr, S. lenense H.Lindb. ex L.I.Savicz. |
| Oligotrophic hollow | Sedge-Eriophorum-Sphagnum | Carex rotundata Wahlenb., Eriophorum russeolum Fries, Sphagnum lindbergii Schimp. ex Lindb., S. balticum (Russow) C.E.O. Jensen. | |
| Khasyrei | Hollow (poor fen) | Sedge-Eriophorum-Sphagnum | Carex rostrate Stokes, Carex lasiocarpa Ehrh., Eriophorum angustifolium Honck., Comarum palustre L., Sphagnum riparium Ångstr., S. squarrosum Crome. |
| North Taiga (Noyabrsk) | |||
| Palsa mire | Flat palsa site | Dwarf shrub-moss-lichen | Ledum decumbens, Andromeda polifolia, Oxycoccus microcarpus, Cladonia stellaris, Cl. rangiferina, Sphagnum fuscum |
| Oligotrophic hollow | Sedge-Eriophorum-Sphagnum | Carex rotundata, Eriophorum russeolum, S. balticum. | |
| Khasyrei | Hollow (poor fen) | Sedge-Eriophorum-Sphagnum | Carex lasiocarpa, Eriophorum angustifolium, Sphagnum riparium. |
| Soil Horizon | Soil Layer, cm | Description |
|---|---|---|
| Forest-tundra (Dystric Hemic Epicryic Histosols) | ||
| Tow | 0–7 | Color is brown and light brown. Wet layer of undecomposed peat. |
| T1 | 7–10 | Color is mainly brown. Wet peat at early stage of decomposition. |
| T2 | 10–25 | Homogeneous brown colored peat. Wet and moderately decomposed materials. |
| T3 | 25–40 | Inhomogeneous color, brown and dark brown. Well decomposed peat and very humid peat layer. |
| T3 | 40–50 | Homogeneous dark brown color. Well decomposed peat and very humid materials. |
| Northern taiga (Dystric Hemic Cryic Histosols) | ||
| Tow | 0–7 | Green and light grey color. The layer composed of dead parts of lichens and mosses that dry and mellow. Clear border with underlying peat horizons. |
| T1 | 7–10 | Wet horizon of ochre-colored moss-dwarf shrub peat deposits penetrated by roots and buried shrub trunks that are fresh and mellow. The border with underlying horizon is sharp and well distinguished by color. |
| T2 | 10–25 | Reddish-brown peat with a few plots of dark brown peat at a depth of 12–20 cm. There are no mineral particles; they are fresh and mellow materials. The border with underlying horizon is sharp and well distinguished by color. |
| T3 | 25–55 | Brown and dark brown with reddish-brown thin interlayers (stratified) peat deposit with the presence of mineral particles; they are well compacted and fresh. Presence of permafrost from 55 cm depth. |
| Soil Horizon | Soil Layer, cm | Description |
|---|---|---|
| Forest-tundra (Dystric Epifibric Endocryic Histosols) | ||
| Tow | 0–20 | The color is homogeneous light brown. Wet layer of undecomposed peat with residues of vascular plants: shrubs, sedges, and cotton grass. |
| T1 | 20–35 | Wet peat at early stage of decomposition: brown-colored. |
| T2 | 35–40 | Homogeneous dark brown well decomposed peat. Wet. Smeared in hands. |
| T3 | 40–50 | Dark brown well-decomposed peat. Wet. Smeared in hands. |
| Northern taiga (Dystric Epifibric Histosols) | ||
| Tow | 0–20 | Undecomposed residues of vascular plants (shrubs, sedges, cotton grass) and Sphagnum mosses. |
| T1 | 20–35 | Wet brown-colored horizon, moderately decomposed, and with plant residues of dwarf shrubs and cotton grass; some (minor) content of mineral particles. The border with underlying horizon is gradual in color. |
| T2 | 35–40 | Wet, brown, and moderately decomposed peat layer; minor content of mineral particles as compared with overlying deposits. |
| T3 | 40–50 | Dark brown and moderately decomposed peat; slightly smeared in hands. Large amount of mineral particles. |
| Soil Horizon | Depth | pH | Carbon (org.) | N | P | Ca2+ | Mg2+ | Carbon Storage |
|---|---|---|---|---|---|---|---|---|
| (cm) | (Water) | % | mg Per g of Soil | Mmol/100 g | gC m2 | |||
| Dystric Hemic Epicryic Histosols (frozen palsa site) | ||||||||
| Tow | 0–6 | 4.4 | 4.7 | 2.1 | 389 | |||
| At | 6–10 | 3.7 | 55.6 | 0.24 | 2.17 | 7.8 | 5.2 | 727 |
| T1 | 10–20 | 3.4 | 56.3 | 0.73 | 1.58 | 13.3 | 5.8 | 5450 |
| T2 | 20–30 | 3.5 | 55.9 | 0.46 | 1.42 | 9.0 | 2.2 | 5565 |
| T2 | 30–40 | 54.5 | 0.43 | 1.64 | ||||
| T3 | 30–40 | 3.4 | 55.9 | 0.37 | 1.89 | 11.4 | 4.2 | |
| Dystric Epifibric Histosols (hollow site) | ||||||||
| Tow | 0–10 | 4.7 | 53.2 | 0.13 | 1.86 | 7.3 | 4.9 | 829 |
| T1 | 10–20 | 4.1 | 57.5 | 0.19 | 1.67 | 14.3 | 7.7 | 846 |
| T2 | 20–30 | 4.6 | 55.0 | 0.19 | 1.54 | 4.5 | 0.8 | 6735 |
| T2 | 30–40 | 50.0 | 0.12 | 0.6 | ||||
| T3 | 40–50 | 56.4 | 0.18 | 1.24 | ||||
| Dystric Epifibric Endohemic Histosols (khasyrei) | ||||||||
| Tow | 0–10 | 4.2 | 50 * | 13.9 | 6.1 | 1043 | ||
| T1 | 10–20 | 4.3 | 50 * | 20.8 | 1.6 | 3732 | ||
| T2 | 20–30 | 4.5 | 50 * | 9.7 | 4088 | |||
| T2 | 30–40 | 4.6 | 9.0 | 2.4 | ||||
| T3 | 40–50 | 4.7 | 11.4 | 1.0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Vishnyakova, E.K.; Koronatova, N.G.; Stepanova, V.A.; Kolesnychenko, L.G.; Khovalyg, A.O.; Peregon, A.M. Plant Organic Matter in Palsa and Khasyrei Type Mires: Direct Observations in West Siberian Sub-Arctic. Atmosphere 2021, 12, 1612. https://doi.org/10.3390/atmos12121612
Kosykh NP, Mironycheva-Tokareva NP, Vishnyakova EK, Koronatova NG, Stepanova VA, Kolesnychenko LG, Khovalyg AO, Peregon AM. Plant Organic Matter in Palsa and Khasyrei Type Mires: Direct Observations in West Siberian Sub-Arctic. Atmosphere. 2021; 12(12):1612. https://doi.org/10.3390/atmos12121612
Chicago/Turabian StyleKosykh, Natalia P., Nina P. Mironycheva-Tokareva, Evgeniya K. Vishnyakova, Natalia G. Koronatova, Vera A. Stepanova, Larisa G. Kolesnychenko, Aldynai O. Khovalyg, and Anna M. Peregon. 2021. "Plant Organic Matter in Palsa and Khasyrei Type Mires: Direct Observations in West Siberian Sub-Arctic" Atmosphere 12, no. 12: 1612. https://doi.org/10.3390/atmos12121612
APA StyleKosykh, N. P., Mironycheva-Tokareva, N. P., Vishnyakova, E. K., Koronatova, N. G., Stepanova, V. A., Kolesnychenko, L. G., Khovalyg, A. O., & Peregon, A. M. (2021). Plant Organic Matter in Palsa and Khasyrei Type Mires: Direct Observations in West Siberian Sub-Arctic. Atmosphere, 12(12), 1612. https://doi.org/10.3390/atmos12121612






