Air Quality Assessment in the Central Mediterranean Sea (Tyrrhenian Sea): Anthropic Impact and Miscellaneous Natural Sources, including Volcanic Contribution, on the Budget of Volatile Organic Compounds (VOCs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scope and Outline of the Study
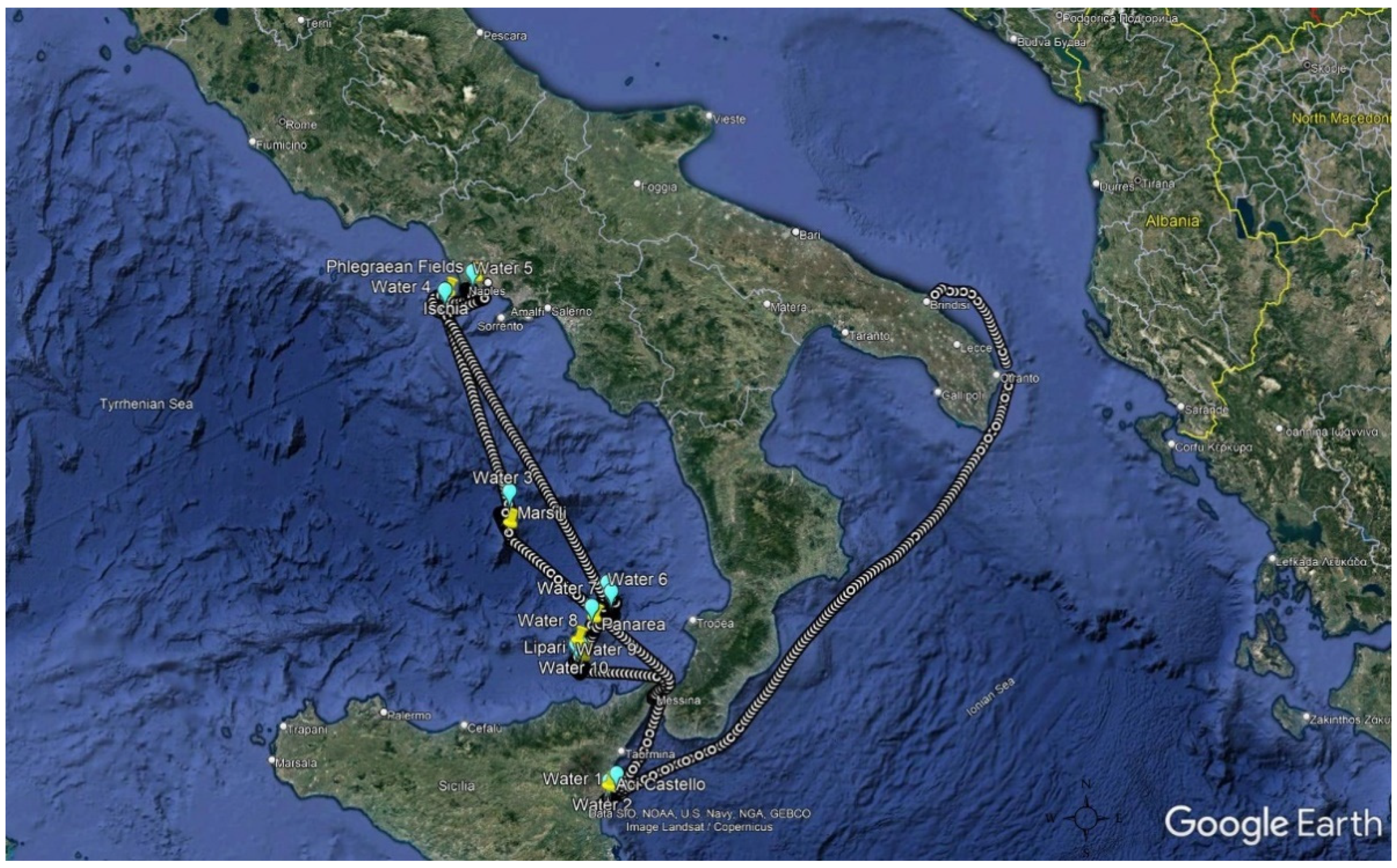
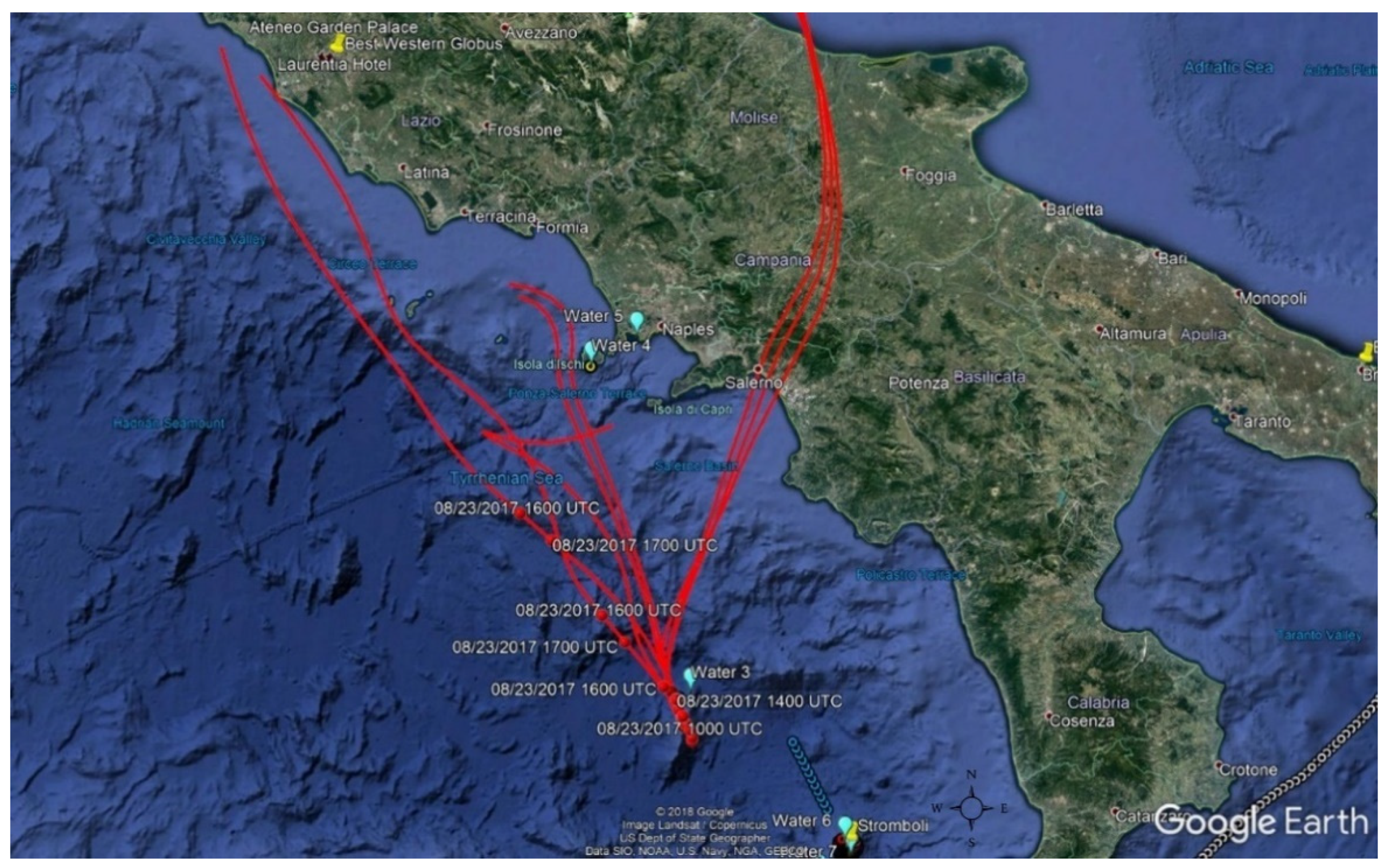
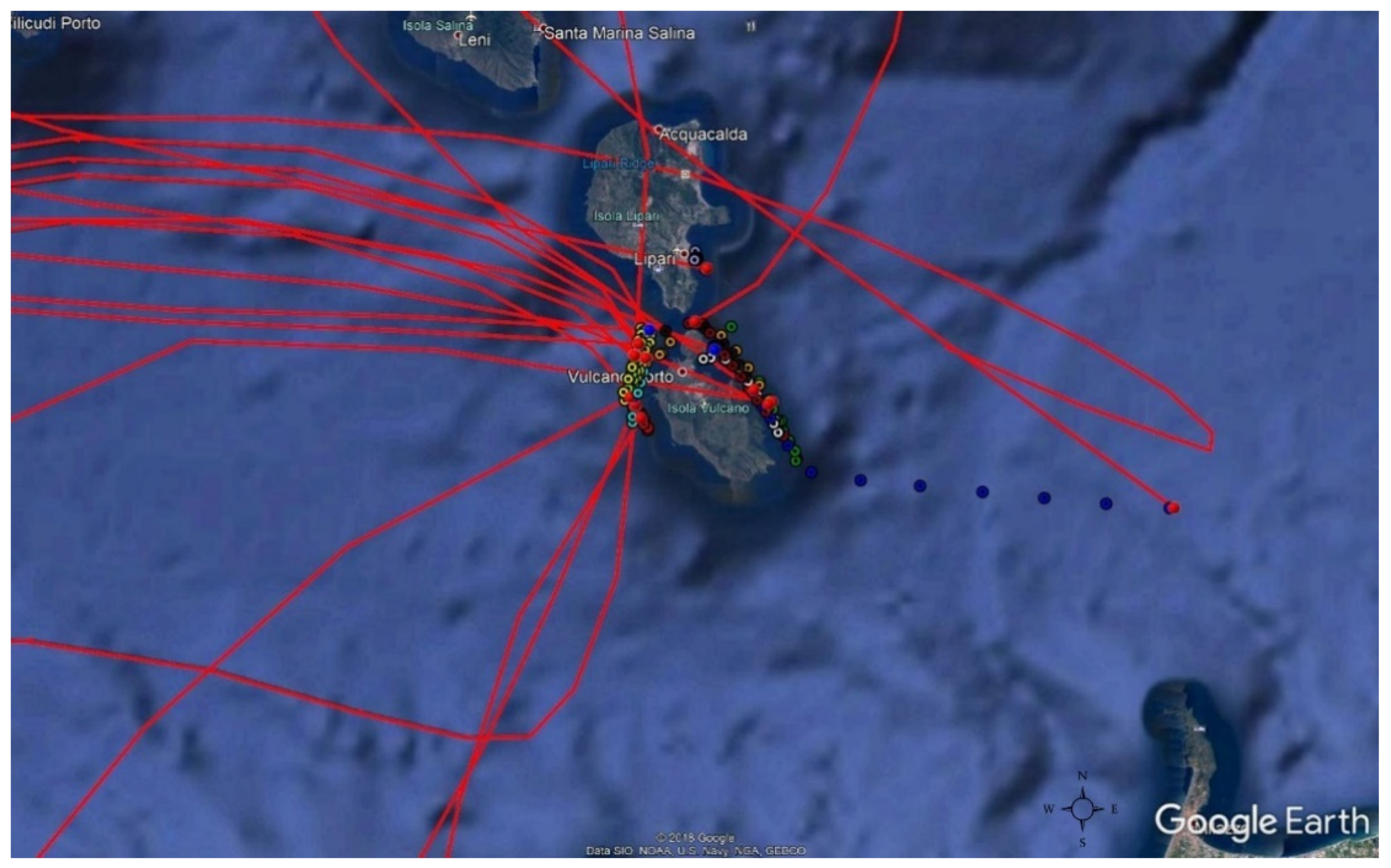
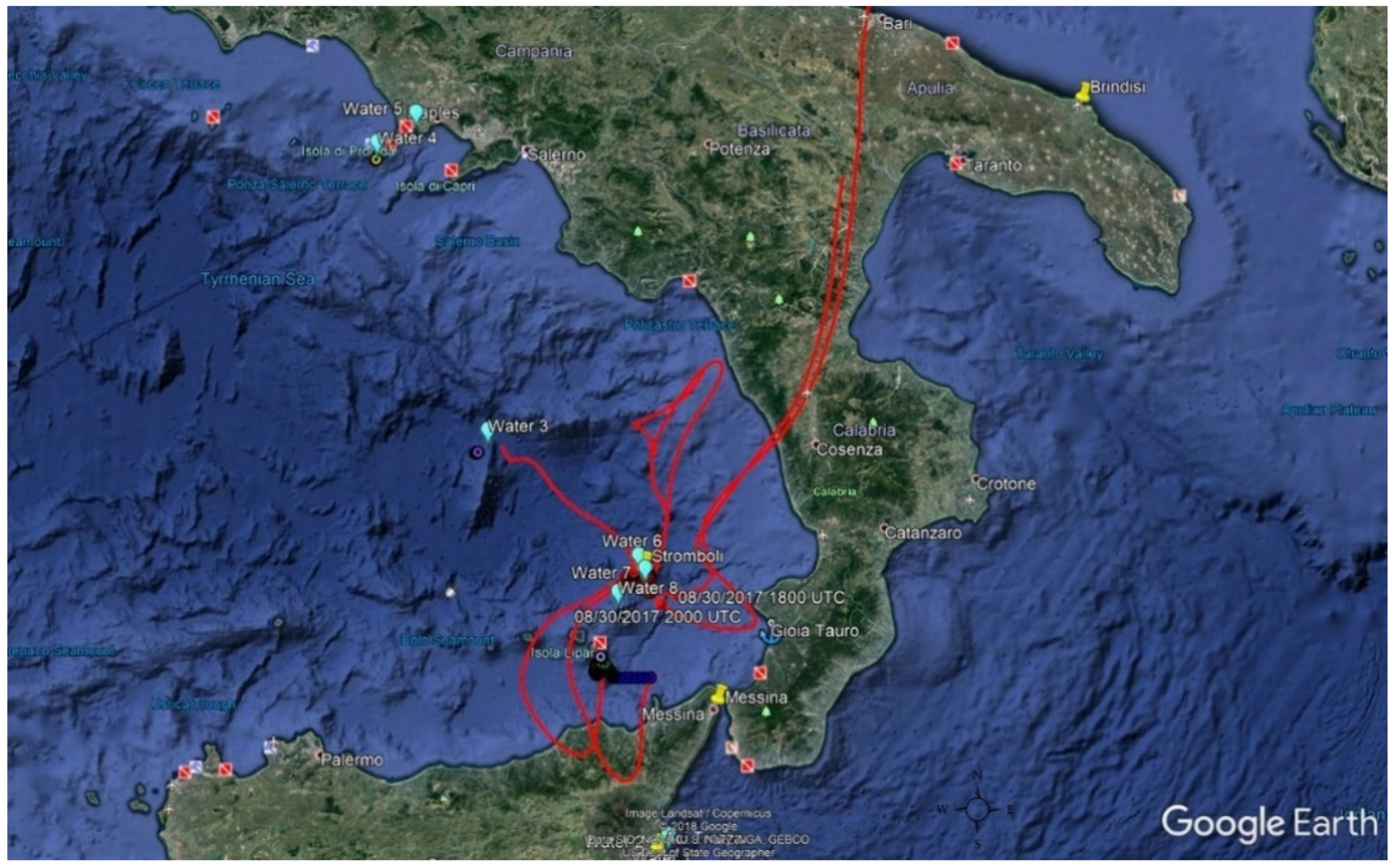
2.2. Sampling
2.2.1. Air Samples Collection
2.2.2. Water Samples Collection
2.3. Analysis
2.3.1. Air Samples Analysis
2.3.2. Water Samples Analysis
Carbonyl Compounds Analysis
Acids
Anions & Cations
Metals
2.4. Continuous Inorganic Gaseous Pollutants Measurements
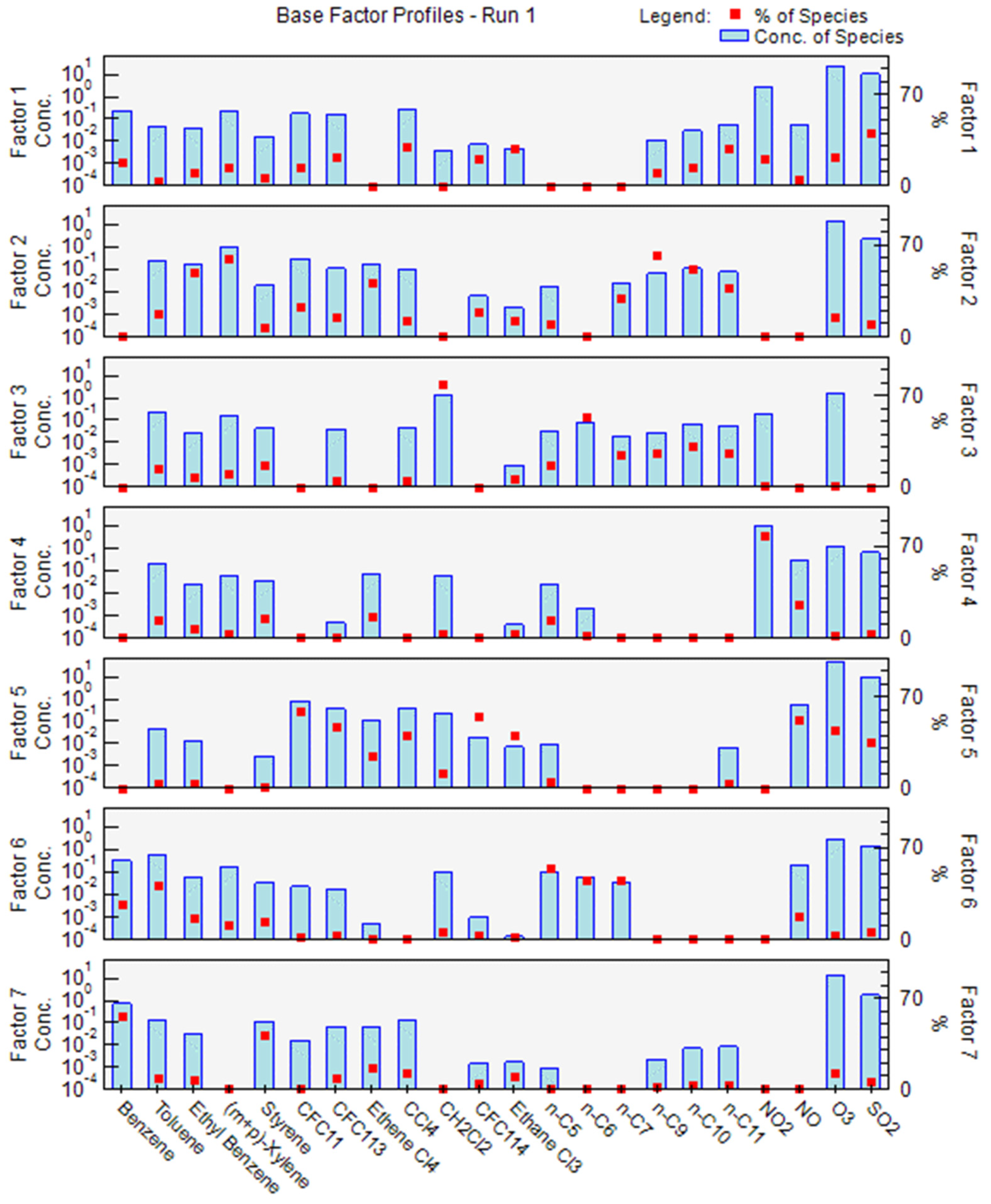
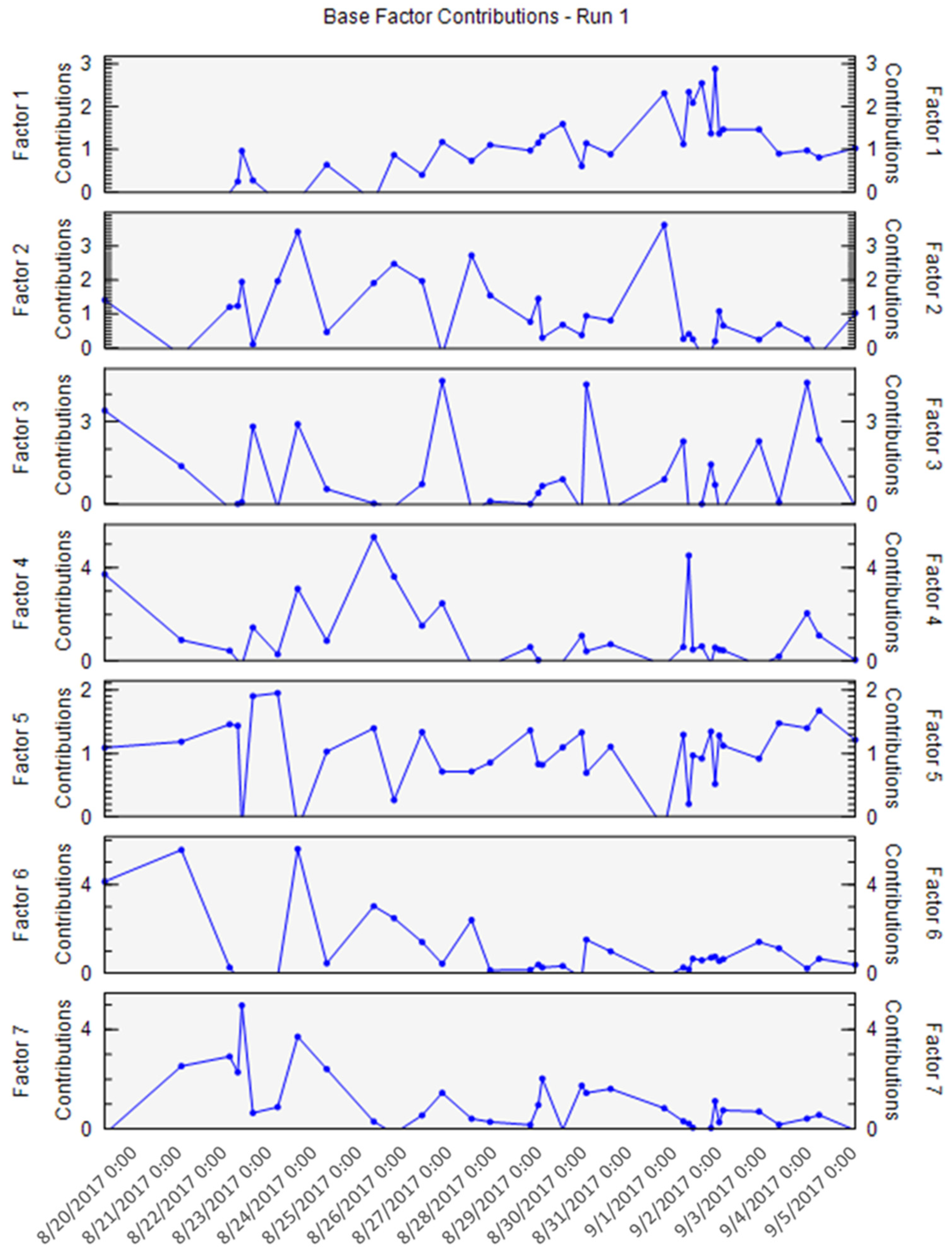
2.5. Statistical Treatment on the Dataset by PMF Model
3. Results and Discussion
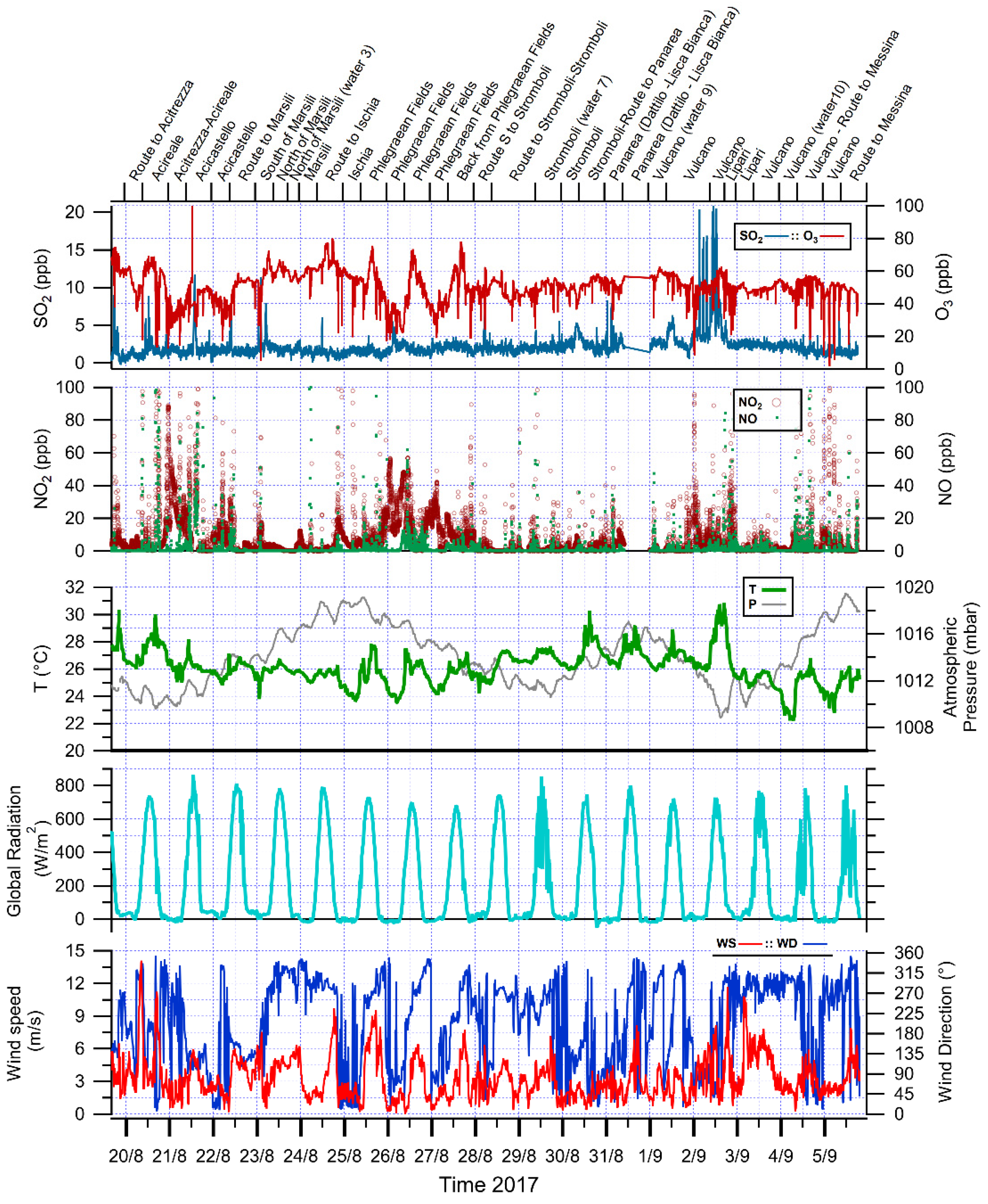
3.1. Meteorology of the Period
3.2. General Observations on Gaseous Pollutants Dataset
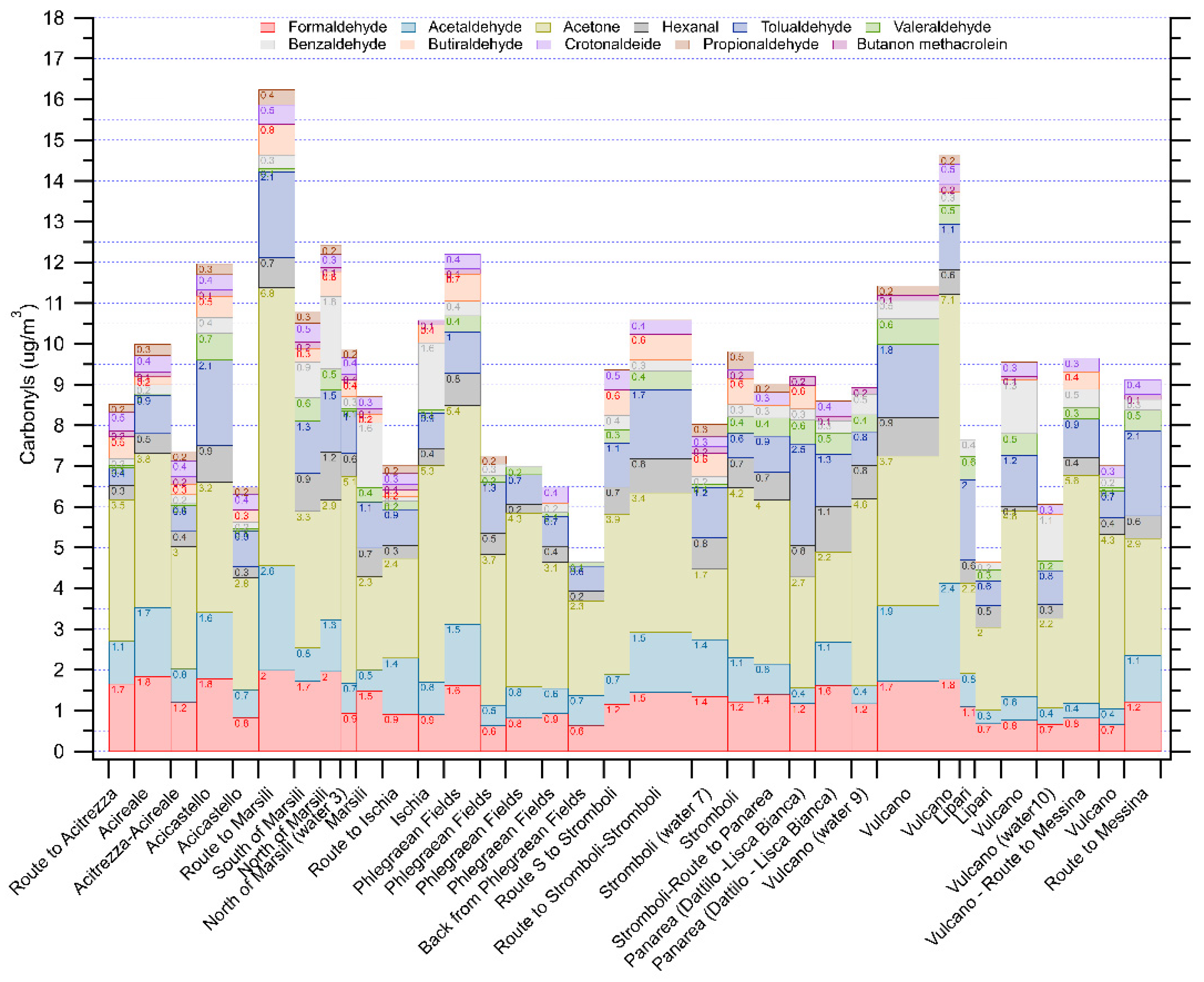
3.3. Volatile Organic Compounds Concentration Distribution over the Area
3.4. Water Samples Measurements
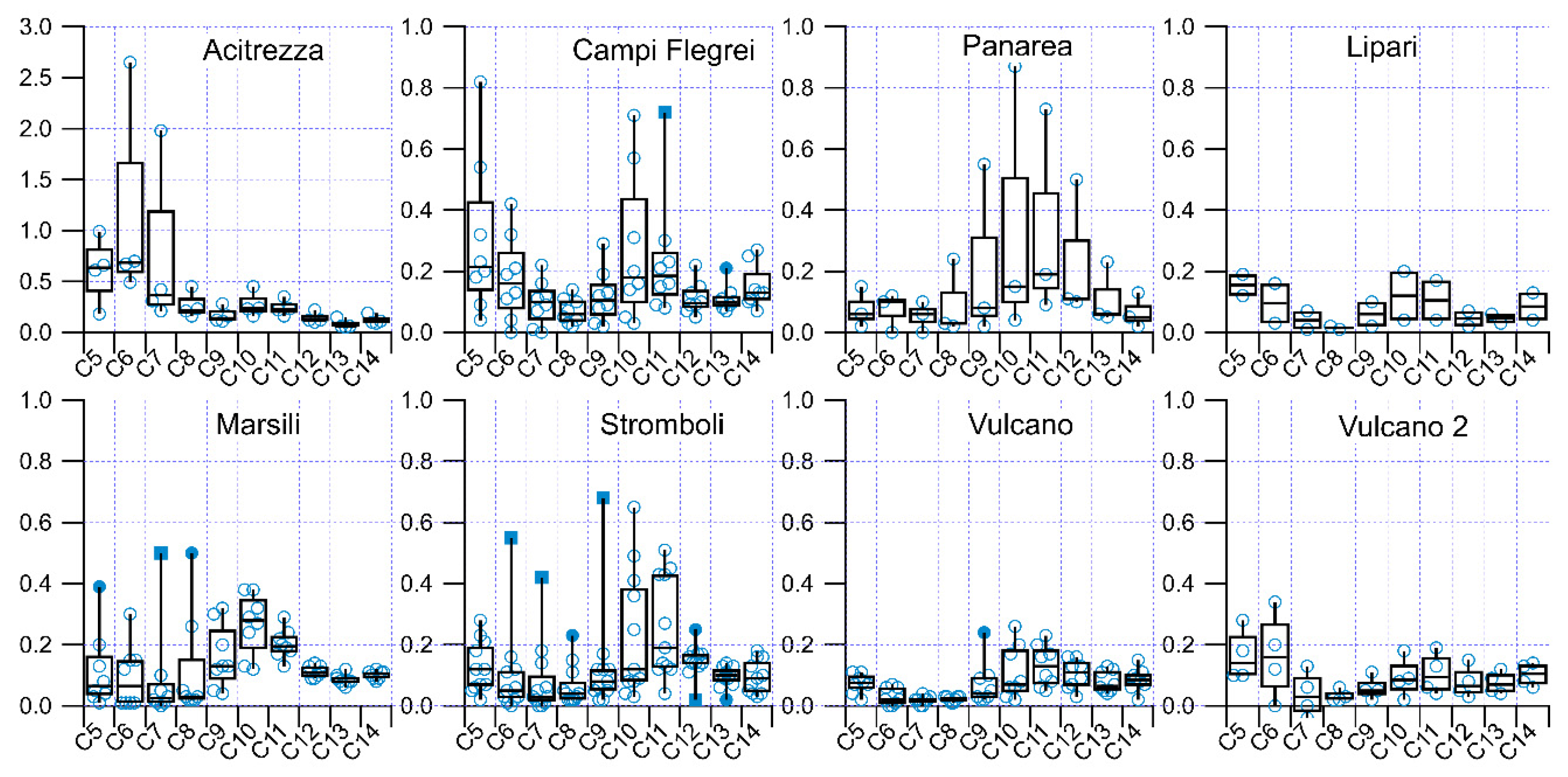
3.5. Statistical Treatment on the Dataset
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lelieveld, J.; Berresheim, H.; Borrmann, S.; Crutzen, P.J.; Dentener, F.J.; Fischer, H.; Feichter, J.; Flatau, P.J.; Heland, J.; Holzinger, R. Global air pollution crossroads over the Mediterranean. Science 2002, 298, 794–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanakidou, M.; Mihalopoulos, N.; Kindap, T.; Im, U.; Vrekoussis, M.; Gerasopoulos, E.; Dermitzaki, E.; Unal, A.; Koçak, M.; Markakis, K. Megacities as hot spots of air pollution in the East Mediterranean. Atmos. Environ. 2011, 45, 1223–1235. [Google Scholar] [CrossRef]
- Debevec, C.; Sauvage, S.; Gros, V.; Sciare, J.; Pikridas, M.; Stavroulas, I.; Salameh, T.; Leonardis, T.; Gaudion, V.; Depelchin, L. Origin and variability in volatile organic compounds observed at an Eastern Mediterranean background site (Cyprus). Atmos. Chem. Phys. 2017, 17, 11355–11388. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Juráň, S.; Grace, J.; Urban, O. Temporal changes in ozone concentrations and their impact on vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Kaser, L.; Peron, A.; Graus, M.; Striednig, M.; Wohlfahrt, G.; Juráň, S.; Karl, T. Interannual variability of BVOC emissions in an alpine city. Atmos. Chem. Phys. 2021. in review. [Google Scholar] [CrossRef]
- Derstroff, B.; Hüser, I.; Bourtsoukidis, E.; Crowley, J.N.; Fischer, H.; Gromov, S.; Harder, H.; Janssen, R.H.H.; Kesselmeier, J.; Lelieveld, J. Volatile organic compounds (VOCs) in photochemically aged air from the eastern and western Mediterranean. Atmos. Chem. Phys. 2017, 17, 9547–9566. [Google Scholar] [CrossRef] [Green Version]
- Castagna, J.; Bencardino, M.; D’Amore, F.; Esposito, G.; Pirrone, N.; Sprovieri, F. Atmospheric mercury species measurements across the Western Mediterranean region: Behaviour and variability during a 2015 research cruise campaign. Atmos. Environ. 2018, 173, 108–126. [Google Scholar] [CrossRef]
- Vichi, F.; Imperiali, A.; Frattoni, M.; Perilli, M.; Benedetti, P.; Esposito, G.; Cecinato, A. Air pollution survey across the western Mediterranean Sea: Overview on oxygenated volatile hydrocarbons (OVOCs) and other gaseous pollutants. Environ. Sci. Pollut. Res. 2019, 26, 16781–16799. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Salmatonidis, A.; Querol, X.; Tassone, A.; Andreoli, V.; Bencardino, M.; Pirrone, N.; Sprovieri, F.; Naccarato, A. Contribution of volcanic and fumarolic emission to the aerosol in marine atmosphere in the Central Mediterranean Sea: Results from med-oceanor 2017 cruise campaign. Atmosphere 2020, 11, 149. [Google Scholar] [CrossRef] [Green Version]
- Frazzetta, G.; Gillot, P.Y.; La Volpe, L.; Sheridan, M.F. Volcanic hazards at Fossa of Vulcano: Data from the last 6000 years. Bull. Volcanol. 1984, 47, 105–124. [Google Scholar] [CrossRef]
- Capaccioni, B.; Giannini, L.; Martini, M.; Mangani, F.; Nappi, G.; Prati, F. Light hydrocarbons in gas-emissions from geothermal fields volcanic areas and geothermal fields. Geochem. J. 1993, 27, 7–17. [Google Scholar] [CrossRef]
- Tassi, F.; Capaccioni, B.; Capecchiacci, F.; Vaselli, O. Non–methane Volatile Organic Compounds (VOCs) at El Chichón volcano (Chiapas, México): Geochemical features, origin and behavior. Geofís. Int. 2009, 48, 85–95. [Google Scholar] [CrossRef]
- Tassi, F.; Capecchiacci, F.; Cabassi, J.; Calabrese, S.; Vaselli, O.; Rouwet, D.; Pecoraino, G.; Chiodini, G. Geogenic and atmospheric sources for volatile organic compounds in fumarolic emissions from Mt. Etna and Vulcano Island (Sicily, Italy). J. Geophys. Res. 2012, 117, 1010. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.A.; Zenkevich, I.G.; Ioffe, B.V. Volatile organic compounds in solfataric gases. J. Atmos. Chem. 1990, 10, 329–340. [Google Scholar] [CrossRef]
- Schwandner, F.M.; Seward, T.M.; Gize, A.P.; Hall, P.A.; Dietrich, V.J. Diffuse emission of organic trace gases from the flank and crater of a quiescent active volcano (Vulcano, Aeolian Islands, Italy). J. Geophys. Res. 2004, 109, D04301. [Google Scholar] [CrossRef] [Green Version]
- Schwandner, F.; Seward, T.M.; Gize, A.; Hall, K.; Dietrich, V. Halocarbons and other trace heteroatomic organic compounds in volcanic gases from Vulcano (Aeolian Islands, Italy). Geochim. Cosmochim. Acta 2013, 101, 191–221. [Google Scholar] [CrossRef]
- Frische, M.; Garofalo, K.; Hansteen, T.H.; Borchers, R.; Harnisch, J. The origin of stable halogenated compounds in volcanic gases. Environ. Sci. Pollut. Res. 2006, 13, 406. [Google Scholar] [CrossRef]
- Tassi, F.; Venturi, S.; Cabassi, J.; Capecchiacci, F.; Nisi, B.; Vaselli, O. Volatile organic compounds (VOCs) in soil gases from Solfatara crater (Campi Flegrei, southern Italy): Geogenic source(s) vs. biogeochemical processes. Appl. Geochem. 2015, 56, 37–49. [Google Scholar] [CrossRef]
- Butler, J.H.; Battle, M.; Bender, M.L.; Montzka, S.A.; Clarke, A.D.; Saltzman, E.S.; Sucher, C.M.; Severinghaus, J.P.; Elkins, J.W. A record of atmospheric halocarbons during the twentieth century from polar firn air. Nature 1999, 399, 749–755. [Google Scholar] [CrossRef]
- Mather, T.A.; Witt, M.L.I.; Pyle, D.M.; Quayle, B.M.; Aiuppa, A.; Bagnato, E.; Martin, R.S.; Sims, K.W.W.; Edmonds, M.; Sutton, A.J. Halogens and trace metal emissions from the ongoing 2008 summit eruption of Kīlauea volcano, Hawaii. Geochim. Cosmochim. Acta 2012, 83, 292–323. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Optics of UV-B radiation in natural water. Arch. Hydrobiol. Beih. 1994, 43, 1–16. [Google Scholar]
- Pullin, M.J.; Bertilsson, S.; Goldstone, J.V.; Voelker, B.M. Effects of sunlight and hydroxyl radical on dissolved organic matter: Bacterial growth efficiency and production of carboxylic acids and other substrates. Limnol. Oceanogr. 2004, 49, 2011–2022. [Google Scholar] [CrossRef]
- Kester, A.S.; Foster, J.W. Diterminal oxidation of long-chain alkanes by bacteria. J. Bacteriol. 1963, 85, 859–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedetti, M.; Kawamura, K.; Charrière, B.; Chevalier, N.; Sempéré, R. Determination of low molecular weight dicarboxylic and ketocarboxylic acids in seawater samples. Anal. Chem. 2006, 78, 6012–6018. [Google Scholar] [CrossRef]
- Saxena, P.; Hildemann, L.M.; Mcmurry, P.H.; Seinfeld, J.H. Organics alter hygroscopic behavior of atmospheric particles. J. Geophys. Res. Atmos. 1995, 100, 18755–18770. [Google Scholar] [CrossRef]
- Yu, S.C. Role of organic acids (formic, acetic, pyruvic and oxalic) in the formation of cloud condensation nuclei (CCN): A review. Atmos. Res. 2000, 53, 185–217. [Google Scholar] [CrossRef]
- Kumar, P.P.; Broekhuizen, K.; Abbatt, J.P.D. Organic acids as cloud condensation nuclei: Laboratory studies of highly soluble and insoluble species. Atmos. Chem. Phys. 2003, 3, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Sempéré, R.; Charrière, B.; Castro Jimenez, J.; Kawamura, K.; Panagiotopoulos, C. Occurrence of α, ω-dicarboxylic acids and ω-oxoacids in surface waters of the Rhone River and fluxes into the Mediterranean Sea. Prog. Oceanogr. 2018, 163, 136–146. [Google Scholar] [CrossRef]
- Obernosterer, I.; Kraay, G.; de Ranitz, E.; Herndl, G.J. Concentrations of low molecular weight carboxylic acids and carbonyl compounds in the Aegean Sea (Eastern Mediterranean) and the turnover of pyruvate. Aquat. Microb. Ecol. 1999, 20, 147–156. [Google Scholar] [CrossRef] [Green Version]
- U.S. EPA. Compendium method TO-11A: Determination of formaldehyde in ambient air using adsorbent cartridge followed by High Performance Liquid Chromatography (HPLC). In Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, 2nd ed.; EPA/625/R-96/010b; U.S. Environmental Protection Agency, Center for Environmental Research Information, Office of Research and Development: Washington, DC, USA, 1999. [Google Scholar]
- ASTM. Method D5197-97, standard test method for determination of formaldehyde and other carbonyl compounds in air (active sampler methodology). In Atmospheric Analysis, Occupational Health and Safety, Protective Clothing, 1103; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- International Organization for Standardization. Water Quality—Sampling—Part 3: Preservation and Handling of Water Samples; ISO 5667-3:2018; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Gillett, R.W.; Ayers, G.P. The use of thymol as a biocide in rainwater samples. Atmos. Environ. Gen. Top. 1991, 25, 2677–2681. [Google Scholar] [CrossRef]
- Dabek-Zlotorzynska, E.; McGrath, M. Determination of low-molecular-weight carboxylic acids in the ambient air and vehicle emissions: A review. Fresenius J. Anal. Chem. 2000, 367, 507–518. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Srivastava, D.; Xu, J.; Vu, T.V.; Liu, D.; Li, L.; Fu, P.; Hou, S.; Moreno Palmerola, N.; Shi, Z.; Harrison, R.M. Insight into PM2.5 sources by applying positive matrix factorization (PMF) at urban and rural sites of Beijing. Atmos. Chem. Phys. 2021, 21, 14703–14724. [Google Scholar] [CrossRef]
- Pernov, J.B.; Bossi, R.; Lebourgeois, T.; Nøjgaard, J.K.; Holzinger, R.; Hjorth, J.L.; Skov, H. Atmospheric VOC measurements at a High Arctic site: Characteristics and source apportionment. Atmos. Chem. Phys. 2021, 21, 2895–2916. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Zhang, Y.; Tan, Q.; Feng, M.; Qu, Y.; An, J.; Deng, Y.; Zhai, R.; Wang, Z. Characteristics, source apportionment and chemical conversions of VOCs based on a comprehensive summer observation experiment in Beijing. Atmos. Pollut. Res. 2021, 12, 230–241. [Google Scholar] [CrossRef]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Doche, C.; Dufour, G.; Foret, G.; Eremenko, M.; Cuesta, J.; Beekmann, M.; Kalabokas, P. Summertime tropospheric-ozone variability over the Mediterranean basin observed with IASI. Atmos. Chem. Phys. 2014, 14, 10589–10600. [Google Scholar] [CrossRef] [Green Version]
- Velchev, K.; Cavalli, F.; Hjorth, J.; Marmer, E.; Vignati, E.; Dentener, F.; Raes, F. Ozone over the Western Mediterranean Sea—Results from two years of shipborne measurements. Atmos. Chem. Phys. 2011, 11, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Liakakou, E.; Bonsang, B.; Williams, J.; Kalivitis, N.; Kanakidou, M.; Mihalopoulos, N. C2–C8 NMHCs over the Eastern Mediterranean: Seasonal variation and impact on regional oxidation chemistry. Atmos. Environ. 2009, 43, 5611–5621. [Google Scholar] [CrossRef]
- Sartin, J.H.; Halsall, C.J.; Robertson, L.A.; Gonard, R.G.; MacKenzie, A.R. Temporal patterns, sources, and sinks of C8-C16 hydrocarbons in the atmosphere of Mace Head, Ireland. J. Geophys. Res. 2002, 107, 8099. [Google Scholar] [CrossRef]
- De Gouw, J.A.; Gilman, J.B.; Kim, S.-W.; Lerner, B.M.; Isaacman-VanWertz, G.; McDonald, B.C.; Warneke, C.; Kuster, W.C.; Lefer, B.L.; Griffith, S.M. Chemistry of volatile organic compounds in the Los Angeles basin: Nighttime removal of alkenes and determination of emission ratios. J. Geophys Res. Atmos. 2017, 122, 11843–11861. [Google Scholar] [CrossRef]
- Han, L.; Siekmann, F.; Zetzsch, C. Rate constants for the reaction of OH radicals with hydrocarbons in a smog chamber at low atmospheric temperatures. Atmosphere 2018, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Edtbauer, A.; Stönner, C.; Pozzer, C.; Bourtsoukidis, E.; Ernle, L.; Dienhart, D.; Hottmann, B.; Fischer, H.; Schuladen, J. Measurements of carbonyl compounds around the Arabian Peninsula: Overview and model comparison. Atmos. Chem. Phys. 2020, 20, 10807–10829. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Louie, P.K.K.; Zou, S.C. Seasonal variation of carbonyl compound concentrations in urban area of Hong Kong. Atmos. Environ. 2002, 36, 1259–1265. [Google Scholar] [CrossRef]
- Duan, J.; Guo, S.; Tan, J.; Wang, S.; Chai, F. Characteristics of atmospheric carbonyls during haze days in Beijing, China. Atmos. Res. 2012, 114, 17–27. [Google Scholar] [CrossRef]
- Possanzini, M.; Di Palo, V.; Petricca, M.; Fratarcangeli, R.; Brocco, D. Measurement of formaldehyde and acetaldehyde in the urban ambient air. Atmos. Environ. 1996, 30, 3757–3764. [Google Scholar] [CrossRef]
- Shepson, P.B.; Sirju, A.P.; Hopper, J.F.; Barrie, L.A.; Young, V.; Niki, H.; Dryfhout, H. Sources and sinks of carbonyl compounds in the Arctic Ocean boundary layer: Polar Ice Floe Experiment. J. Geophys. Res. 1996, 101, 21081–21089. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dutton, G.S.; Yu, P. An unexpected and persistent increase in global emissions of ozone-depleting CFC-11. Nature 2018, 557, 413–417. [Google Scholar] [CrossRef]
- Millero, F.J.; Morse, J.; Chen, C.T. The carbonate system in the western Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 1979, 26, 1395–1404. [Google Scholar] [CrossRef]
- Esposito, V.; Andaloro, F.; Canese, S.; Bortoluzzi, G.; Bo, M.; Di Bella, M.; Italiano, F.; Sabatino, G.; Battaglia, P.; Consoli, P. Exceptional discovery of a shallow-water hydrothermal site in the SW area of Basiluzzo islet (Aeolian archipelago, South Tyrrhenian Sea): An environment to preserve. PLoS ONE 2018, 13, e0190710. [Google Scholar] [CrossRef]
- Boatta, F.; D’Alessandro, W.; Gagliano, A.L.; Liotta, M.; Milazzo, M.; Rodolfo-Metalpa, R.; Hall-Spencer, J.M.; Parello, F. Geochemical survey of Levante Bay, Vulcano Island (Italy), a natural laboratory for the study of ocean acidification. Mar. Pollut. Bull. 2013, 73, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resing, J.A.; Sansone, F.J. The chemistry of lava-seawater interactions: The generation of acidity. Geochim. Cosmochim. Acta 1999, 63, 2183–2198. [Google Scholar] [CrossRef]
- Stefánsson, A.; Stefánsdóttir, G.; Keller, N.S.; Barsotti, S.; Sigurdsson, Á.; Thorláksdóttir, S.B.; Pfeffer, M.A.; Eiríksdóttir, E.S.; Jónasdóttir, E.B.; von Löwis, S.; et al. Major impact of volcanic gases on the chemical composition of precipitation in Iceland during the 2014–2015 Holuhraun eruption: Impact of volcanic gas on precipitation. J. Geophys. Res. Atmos. 2017, 122, 1971–1982. [Google Scholar] [CrossRef]
- Elderfield, H.; Schultz, A. Mid-Ocean Ridge hydrothermal fluxes and the chemical composition of the Ocean. Annu. Rev. Earth Planet. Sci. 1996, 24, 191–224. [Google Scholar] [CrossRef]
- Zhou, X.L.; Mopper, K. Photochemical production of low molecular weight carbonyl compounds in seawater and surface microlayer and their air-sea exchange. Mar. Chem. 1997, 56, 201–213. [Google Scholar] [CrossRef]
- Largiuni, O.; Becagli, S.; Innocenti, M.; Stortini, A.M.; Traversi, R.; Udisti, R. Formaldehyde determination in seawater. Preliminary application to coastal samples at Terra Nova Bay (Antarctica). J. Environ. Monit. 2005, 7, 1299–1304. [Google Scholar]
- Nassiri, M.; Kaykhaii, M.; Hashemi, S.H.; Sepahi, M. Spectrophotometric determination of formaldehyde in seawater samples after in-situ derivatization and dispersive liquid-liquid microextraction. Iran. J. Chem. Chem. Eng. 2018, 37, 89–98. [Google Scholar]
- Beale, R.; Dixon, J.L.; Arnold, S.R.; Liss, P.S.; Nightingale, P.D. Methanol, acetaldehyde, and acetone in the surface waters of the Atlantic Ocean. J. Geophys. Res. Ocean. 2013, 118, 5412–5425. [Google Scholar] [CrossRef] [Green Version]
- Schlundt, C.; Tegtmeier, S.; Lennartz, S.T.; Bracher, A.; Cheah, W.; Krüger, K.; Quack, B.; Marandino, C.A. Oxygenated volatile organic carbon in the western Pacific convective center: Ocean cycling, air-sea gas exchange and atmospheric. Atmos. Chem. Phys. 2017, 17, 10837–10854. [Google Scholar] [CrossRef] [Green Version]
- Sempéré, R.; Kawamura, K. Trans-hemispheric contribution of C2-C10 α, ω-dicarboxylic acids, and related polar compounds to water-soluble organic carbon in the western Pacific aerosols in relation to photochemical oxidation reactions transport. Glob. Biogeochem. Cycles 2003, 17, 1069. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Jing, S.-A.; Lou, S.-R.; Hu, Q.-Y.; Li, L.; Tao, S.-K.; Huang, C.; Qiao, L.-P.; Chen, C.-H. Volatile organic compounds (VOCs) source profiles of on-road vehicle emissions in China. Sci. Total Environ. 2017, 607–608, 253–261. [Google Scholar]
- Tan, Y.; Han, S.; Chen, Y.; Zhang, Z.; Li, H.; Li, W.; Yuan, Q.; Li, X.; Wang, T.; Lee, S.C. Characteristics and source apportionment of volatile organic compounds (VOCs) at a coastal site in Hong Kong. Sci. Total Environ. 2021, 777, 146241. [Google Scholar] [CrossRef]
- Verreyken, B.; Amelynck, C.; Schoon, N.; Müller, J.F.; Brioude, J.; Kumps, N.; Hermans, C.; Metzger, J.M.; Colomb, A.; Stavrakou, T. Measurement report: Source apportionment of volatile organic compounds at the remote high-altitude Maïdo observatory. Atmos. Chem. Phys. 2021, 21, 12965–12988. [Google Scholar] [CrossRef]
| Pollutant | Mean | SD | Variance | RSD | Min | Median | Max |
|---|---|---|---|---|---|---|---|
| NO2 | 13.7 | 13.4 | 179.4 | 1.0 | 1.0 | 8.8 | 51.7 |
| NO | 1.8 | 1.7 | 2.9 | 0.9 | 0.6 | 1.3 | 7.1 |
| O3 | 103 | 10.4 | 108 | 0.10 | 77.4 | 104 | 126 |
| SO2 | 25.9 | 5.30 | 28.0 | 0.20 | 20.6 | 25.2 | 46.6 |
| Pollutant | N Tot | Mean | SD | Variance | RSD | Min | Median | Max |
|---|---|---|---|---|---|---|---|---|
| Benzene | 48 | 1.47 | 1.32 | 1.74 | 0.89 | 0.34 | 1.11 | 7.26 |
| Toluene | 48 | 1.79 | 1.85 | 3.42 | 1.03 | 0.44 | 1.07 | 9.07 |
| Ethyl Bz | 48 | 0.44 | 0.43 | 0.18 | 0.97 | 0.11 | 0.32 | 2.88 |
| (m+p)-Xylene | 48 | 1.60 | 1.09 | 1.18 | 0.68 | 0.46 | 1.21 | 4.90 |
| o-Xylene | 48 | 0.54 | 0.49 | 0.24 | 0.91 | 0.14 | 0.36 | 2.93 |
| Styrene | 48 | 0.32 | 0.28 | 0.08 | 0.89 | 0.02 | 0.22 | 1.12 |
| CFC11 | 48 | 1.13 | 0.64 | 0.41 | 0.57 | 0.12 | 1.01 | 3.06 |
| CFC113 | 48 | 0.73 | 0.20 | 0.04 | 0.28 | 0.25 | 0.78 | 1.18 |
| C2Cl4 | 48 | 0.39 | 0.34 | 0.12 | 0.88 | 0.01 | 0.28 | 1.85 |
| C2HCl3 | 48 | 0.09 | 0.05 | 0.00 | 0.61 | 0.02 | 0.07 | 0.21 |
| CCl4 | 48 | 0.90 | 0.28 | 0.08 | 0.31 | 0.00 | 0.94 | 1.62 |
| CHCl3 | 44 | 0.71 | 0.84 | 0.70 | 1.17 | 0.02 | 0.34 | 3.28 |
| CH2Cl2 | 45 | 1.66 | 2.03 | 4.12 | 1.22 | 0.02 | 0.56 | 6.81 |
| CFC114 | 48 | 0.03 | 0.02 | 0.00 | 0.57 | 0.00 | 0.03 | 0.10 |
| C2H3Cl3 | 48 | 0.02 | 0.01 | 0.00 | 0.35 | 0.00 | 0.02 | 0.03 |
| n-C5 | 48 | 0.21 | 0.23 | 0.05 | 1.10 | 0.01 | 0.12 | 0.99 |
| n-C6 | 48 | 0.38 | 0.86 | 0.74 | 2.28 | 0.00 | 0.11 | 4.37 |
| n-C7 | 48 | 0.15 | 0.33 | 0.11 | 2.14 | 0.00 | 0.05 | 1.98 |
| n-C8 | 48 | 0.09 | 0.11 | 0.01 | 1.29 | 0.01 | 0.03 | 0.50 |
| n-C9 | 48 | 0.12 | 0.13 | 0.02 | 1.05 | 0.02 | 0.10 | 0.68 |
| n-C10 | 48 | 0.23 | 0.22 | 0.05 | 0.98 | 0.02 | 0.17 | 1.11 |
| n-C11 | 48 | 0.21 | 0.15 | 0.02 | 0.73 | 0.04 | 0.18 | 0.73 |
| n-C12 | 48 | 0.12 | 0.07 | 0.01 | 0.61 | 0.02 | 0.12 | 0.50 |
| n-C13 | 48 | 0.09 | 0.04 | 0.00 | 0.46 | 0.02 | 0.09 | 0.23 |
| n-C14 | 48 | 0.10 | 0.05 | 0.00 | 0.49 | 0.02 | 0.10 | 0.27 |
| N Tot | Mean | SD | Variance | RSD | Min | Median | Max | |
|---|---|---|---|---|---|---|---|---|
| Formaldehyde | 34 | 1.23 | 0.43 | 0.18 | 0.35 | 0.63 | 1.20 | 1.99 |
| Acetaldehyde | 34 | 0.99 | 0.56 | 0.32 | 0.57 | 0.33 | 0.81 | 2.57 |
| Acetone | 34 | 3.66 | 1.32 | 1.75 | 0.36 | 1.72 | 3.45 | 7.09 |
| Acrolein | 0 | -- | -- | -- | -- | -- | -- | -- |
| Propionaldehyde | 16 | 0.26 | 0.07 | 0.01 | 0.29 | 0.19 | 0.23 | 0.46 |
| Crotonaldehyde | 24 | 0.37 | 0.07 | 0.01 | 0.20 | 0.25 | 0.37 | 0.51 |
| 2-Butanon-methacrolein | 22 | 0.13 | 0.05 | 0.00 | 0.41 | 0 | 0.14 | 0.23 |
| Butiraldehyde | 19 | 0.46 | 0.18 | 0.03 | 0.39 | 0.16 | 0.52 | 0.75 |
| Benzaldehyde | 33 | 0.48 | 0.46 | 0.22 | 0.96 | 0 | 0.31 | 1.78 |
| Valeraldehyde | 34 | 0.31 | 0.20 | 0.04 | 0.66 | 0.04 | 0.31 | 0.66 |
| Tolualdehyde | 34 | 1.16 | 0.52 | 0.27 | 0.45 | 0.43 | 1.03 | 2.49 |
| Hexaldehyde | 34 | 0.60 | 0.26 | 0.07 | 0.44 | 0.11 | 0.57 | 1.18 |
| OVOC Sample | Sampling Area | C1/C2 | C2/C3 |
|---|---|---|---|
| 1 | Route to Acitrezza | 1.58 | 5.39 |
| 2 | Acireale (water 1) | 1.09 | 6.13 |
| 3 | Acitrezza-Acireale | 1.48 | 3.61 |
| 4 | Acicastello (water 2) | 1.10 | 6.10 |
| 5 | Acicastello (water 2) | 1.22 | 3.57 |
| 6 | Route to Marsili | 0.77 | 6.90 |
| 7 | South of Marsili | 2.09 | 2.77 |
| 8 | North of Marsili | 1.53 | 5.80 |
| 9 | North of Marsili (water 3) | 1.27 | 3.45 |
| 10 | Marsili | 2.91 | |
| 11 | Ischia | 0.68 | 7.28 |
| 12 | Ischia western coast (water 4) | 1.14 | |
| 13 | Phlegraean Fields Pozzuoli Gulf (water 5) | 1.09 | |
| 14 | Phlegraean Fields Pozzuoli Gulf (water 5) | 1.23 | 2.48 |
| 15 | Phlegraean Fields Pozzuoli Gulf (water 5) | 1.05 | |
| 16 | Phlegraean Fields Pozzuoli Gulf (water 5) | 1.50 | |
| 17 | Backward Route from Phlegraean Fields | 0.87 | |
| 18 | Route southwards to Stromboli | 1.59 | |
| 19 | Stromboli (water 6) | 0.99 | |
| 20 | Stromboli | ||
| 21 | Stromboli (water 7) | 0.96 | 4.53 |
| 22 | Stromboli 1 | 1.12 | 2.37 |
| 23 | Stromboli-Route to Panarea | 1.86 | 3.43 |
| 24 | Panarea (between Dattilo and Lisca Bianca) | 3.07 | |
| 25 | Panarea (between Dattilo and Lisca Bianca)(water 8) | 1.51 | |
| 26 | Vulcano (water 9) | 2.65 | |
| 27 | Lipari | 0.93 | 8.16 |
| 28 | Lipari | ||
| 29 | Lipari | 0.75 | 11.23 |
| 30 | Lipari | 1.36 | |
| 31 | Vulcano | 2.07 | |
| 32 | Vulcano (water 10) | 1.34 | |
| 33 | Vulcano—Route to Messina | 1.67 |
| Sample | pH | F#x2212; | Cl− | Br− | SO42− | F#x2212;/Cl#x2212; (·10^−5) | Na+ | Mg2+ | K+ | Ca2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| water 1 | 8.60 | 0.03 | 688.74 | 0.81 | 30.77 | 4.09 | 476.03 | 53.74 | 9.53 | 9.37 |
| water 2 | 8.62 | 0.04 | 298.39 | 0.36 | 12.86 | 12.19 | 522.22 | 59.37 | 10.30 | 10.20 |
| water 3 | 8.52 | 0.09 | 591.69 | 0.72 | 25.30 | 15.33 | 523.86 | 60.61 | 10.81 | 10.83 |
| water 4 | 8.55 | 0.05 | 561.93 | 0.68 | 25.08 | 9.67 | 523.44 | 59.68 | 10.34 | 10.53 |
| water 5 | 8.59 | 0.03 | 767.80 | 0.95 | 35.04 | 4.07 | 565.67 | 64.46 | 11.12 | 10.62 |
| water 6 | 8.59 | 0.04 | 448.30 | 0.58 | 19.28 | 8.00 | 513.39 | 58.91 | 15.29 | 9.72 |
| water 7 | 8.57 | 0.03 | 450.12 | 0.57 | 18.87 | 6.72 | 510.97 | 58.89 | 10.35 | 9.67 |
| water 8 | 7.70 | 0.11 | 223.99 | 0.26 | 9.55 | 48.73 | 494.45 | 55.99 | 10.32 | 9.35 |
| water 9 | 8.58 | 0.08 | 117.41 | 0.18 | 5.08 | 72.02 | 476.09 | 54.10 | 9.65 | 9.31 |
| water 10 | 6.92 | 0.08 | 302.93 | 0.43 | 12.73 | 25.38 | 524.09 | 59.37 | 10.22 | 10.40 |
| average | 8.32 | 0.06 | 445.13 | 0.55 | 19.46 | 20.62 | 513.02 | 58.51 | 10.79 | 10.00 |
| Samples | Li | Be | Al | V | Cr | Mn | Fe | Co | Ni | Cu | As | Rb | Sr | Cs | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| water 1 | 34.8 | 0.3 | 2.1 | 0.6 | 0.1 | 0.1 | 8.0 | 0.1 | 0.2 | 0.2 | 0.8 | 1.6 | 103.7 | 0.02 | 0.01 |
| water 2 | 35.4 | 0.3 | 2.7 | 0.6 | 0.1 | 0.1 | 10.2 | 0.1 | 0.2 | 0.2 | 0.7 | 1.6 | 103.3 | 0.02 | 0.01 |
| water 3 | 36.4 | 0.2 | 1.8 | 0.6 | 0.1 | 0.1 | 12.5 | 0.1 | 0.2 | 0.2 | 0.6 | 1.7 | 104.5 | 0.02 | 0.01 |
| water 4 | 36.5 | 0.3 | 2.1 | 0.6 | 0.1 | 0.1 | 13.9 | 0.1 | 0.2 | 0.2 | 0.6 | 1.7 | 105.7 | 0.02 | 0.01 |
| water 5 | 38.0 | 0.3 | 2.9 | 0.6 | 0.2 | 0.2 | 16.3 | 0.1 | 0.2 | 0.2 | 0.5 | 1.8 | 107.6 | 0.03 | 0.01 |
| water 6 | 37.1 | 0.1 | 1.0 | 0.6 | 0.1 | 0.1 | 17.4 | 0.0 | 0.2 | 0.1 | 0.4 | 1.7 | 107.2 | 0.02 | 0.00 |
| water 7 | 39.3 | 0.3 | 2.5 | 0.6 | 0.2 | 0.1 | 15.8 | 0.1 | 0.2 | 0.1 | 0.5 | 1.7 | 105.7 | 0.02 | 0.01 |
| water 8 | 31.0 | 0.0 | 1.0 | 0.7 | 0.2 | 0.1 | 37.0 | 0.0 | 0.1 | 0.2 | 0.3 | 1.6 | 97.9 | 0.01 | 0.01 |
| water 9 | 30.4 | 0.0 | 1.3 | 0.7 | 0.2 | 0.1 | 35.6 | 0.0 | 0.2 | 0.2 | 0.3 | 1.6 | 95.2 | 0.01 | 0.01 |
| water 10 | 35.0 | 0.0 | 2.6 | 0.8 | 0.2 | 1.4 | 43.3 | 0.0 | 0.2 | 0.2 | 0.3 | 2.0 | 104.1 | 0.03 | 0.00 |
| average | 35.4 | 0.2 | 2.0 | 0.6 | 0.2 | 0.2 | 21.0 | 0.1 | 0.2 | 0.2 | 0.5 | 1.7 | 103.5 | 0.02 | 0.01 |
| Sample | Formal- dehyde | Acetal- dehyde | Acetone | Benzal- dehyde | Valeral- dehyde | Tolual- dehyde | Hexal- dehyde |
|---|---|---|---|---|---|---|---|
| water 1 | 8.77 | 8.03 | 25.22 | 0.04 | 1.03 | 3.87 | 0.43 |
| water 2 | 6.17 | 13.14 | 22.84 | 0.09 | 0.53 | 4.37 | 0.00 |
| water 3 | 4.29 | 1.88 | 15.87 | 0.00 | 0.15 | 5.20 | 0.00 |
| water 4 | 6.31 | 10.69 | 25.91 | 0.00 | 0.88 | 4.45 | 0.00 |
| water 5 | 5.96 | 27.78 | 5.84 | 0.04 | 0.76 | 6.48 | 0.00 |
| water 6 | 5.55 | 14.69 | 12.76 | 0.00 | 0.37 | 5.29 | 0.00 |
| water 7 | 10.87 | 22.61 | 31.49 | 0.00 | 2.77 | 1.77 | 0.00 |
| water 8 | 1.62 | 25.80 | 13.49 | 0.00 | 2.06 | 0.97 | 0.00 |
| water 9 | 17.33 | 30.38 | 32.26 | 0.00 | 0.68 | 5.97 | 3.34 |
| water 10 | 24.48 | 27.17 | 23.21 | 0.00 | 0.62 | 6.14 | 0.68 |
| average | 9.14 | 18.22 | 20.89 | 0.02 | 0.98 | 4.45 | 0.45 |
| Sample | Formic Acid | Acetic Acid | Oxalic Acid | Succinic Acid | Malic Acid | Maleic Acid | Lactic Acid | Methane- Sulphonic Acid |
|---|---|---|---|---|---|---|---|---|
| water 1 | 7.717 | 32.73 | 281.2 | 0.000 | 4.567 | 0.207 | 3.297 | 34.56 |
| water 2 | 9.196 | 56.83 | 270.6 | 0.000 | 5.261 | 0.198 | 3.364 | 24.01 |
| water 3 | 2.848 | 16.53 | 231.6 | 0.000 | 0.955 | 0.172 | 7.527 | 55.91 |
| water 4 | 12.45 | 0.65 | 229.1 | 0.000 | 0.918 | 0.190 | 3.552 | 32.60 |
| water 5 | 6.978 | 0.33 | 217.0 | 0.000 | 3.418 | 0.422 | 3.841 | 16.16 |
| water 6 | 4.652 | 17.22 | 264.3 | 3.229 | 11.10 | 0.216 | 7.582 | 51.79 |
| water 7 | 38.76 | 13.80 | 258.3 | 11.49 | 4.888 | 0.224 | 3.297 | 32.80 |
| water 8 | 7.935 | 14.57 | 232.9 | 24.07 | 0.373 | 0.259 | 1.077 | 44.42 |
| water 9 | 15.23 | 28.02 | 258.8 | 0.000 | 10.93 | 1.509 | 3.974 | 36.31 |
| water 10 | 7.674 | 53.88 | 258.5 | 0.000 | 3.799 | 0.000 | 4.263 | 9.52 |
| average | 11.35 | 23.46 | 250.2 | 3.879 | 4.621 | 0.340 | 4.177 | 33.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vichi, F.; Ianniello, A.; Frattoni, M.; Imperiali, A.; Esposito, G.; Tomasi Scianò, M.C.; Perilli, M.; Cecinato, A. Air Quality Assessment in the Central Mediterranean Sea (Tyrrhenian Sea): Anthropic Impact and Miscellaneous Natural Sources, including Volcanic Contribution, on the Budget of Volatile Organic Compounds (VOCs). Atmosphere 2021, 12, 1609. https://doi.org/10.3390/atmos12121609
Vichi F, Ianniello A, Frattoni M, Imperiali A, Esposito G, Tomasi Scianò MC, Perilli M, Cecinato A. Air Quality Assessment in the Central Mediterranean Sea (Tyrrhenian Sea): Anthropic Impact and Miscellaneous Natural Sources, including Volcanic Contribution, on the Budget of Volatile Organic Compounds (VOCs). Atmosphere. 2021; 12(12):1609. https://doi.org/10.3390/atmos12121609
Chicago/Turabian StyleVichi, Francesca, Antonietta Ianniello, Massimiliano Frattoni, Andrea Imperiali, Giulio Esposito, Maria Concetta Tomasi Scianò, Mattia Perilli, and Angelo Cecinato. 2021. "Air Quality Assessment in the Central Mediterranean Sea (Tyrrhenian Sea): Anthropic Impact and Miscellaneous Natural Sources, including Volcanic Contribution, on the Budget of Volatile Organic Compounds (VOCs)" Atmosphere 12, no. 12: 1609. https://doi.org/10.3390/atmos12121609
APA StyleVichi, F., Ianniello, A., Frattoni, M., Imperiali, A., Esposito, G., Tomasi Scianò, M. C., Perilli, M., & Cecinato, A. (2021). Air Quality Assessment in the Central Mediterranean Sea (Tyrrhenian Sea): Anthropic Impact and Miscellaneous Natural Sources, including Volcanic Contribution, on the Budget of Volatile Organic Compounds (VOCs). Atmosphere, 12(12), 1609. https://doi.org/10.3390/atmos12121609






