Air Pollution in New Delhi during Late Winter: An Overview of a Group of Campaign Studies Focusing on Composition and Sources
Abstract
:1. Introduction
2. Sites and Instruments Used in Past Studies
2.1. High-Resolution Time-of-Flight Aerosol Mass Spectrometer (HR-ToF-AMS)
2.2. Time-of-Flight Aerosol Chemical Speciation Monitor
2.3. Proton-Transfer-Reaction Time-of-Flight Mass Spectrometer (PTR-ToF-MS)
2.4. Aethalometer (Model AE-33)
2.5. PILS-LWCC-TOC Analyzer
2.6. Single-Particle Soot Photometer (SP2)
2.7. Xact 625i® Ambient Metals Monitor
2.8. EC-OC Analyzer, TOC-L Analyzer, and Ion Chromatography
2.9. Real-Time Oxidative Potential (OP) Instrument
3. Overview of Groups’ Results and Discussion
3.1. Types of Fine Pollutants in the Environment of Delhi Megacity
3.2. Source Apportionment of Different Types of Aerosols Using a PMF Model
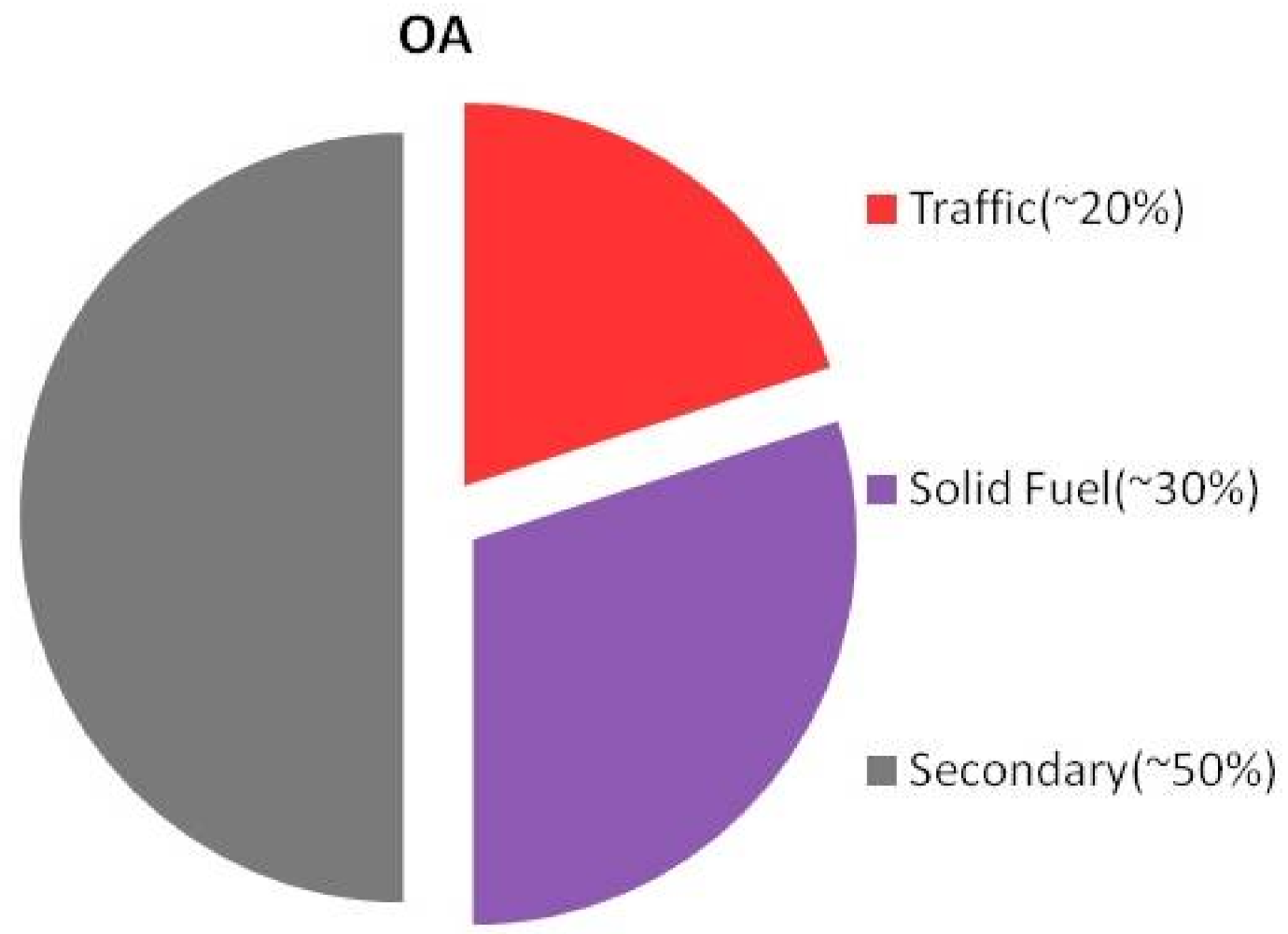
3.2.1. Source Apportionment of Organic Aerosols (OAs)
3.2.2. Primary and Secondary Fraction of OAs from Offline Analysis
3.2.3. Source Apportionment of BC
3.2.4. Source Apportionment of WS-BrC
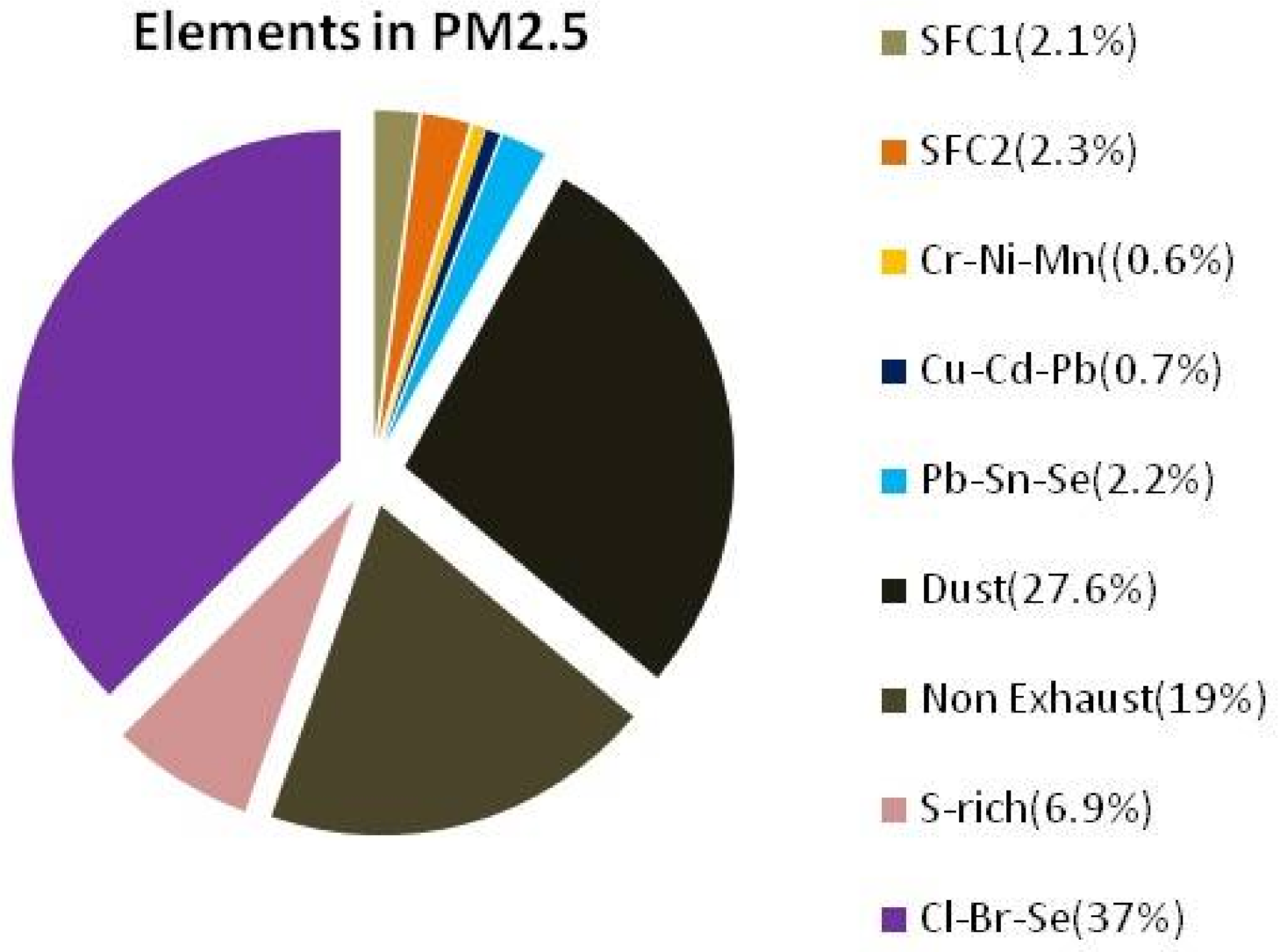
3.2.5. Source Apportionment of Elements in PM
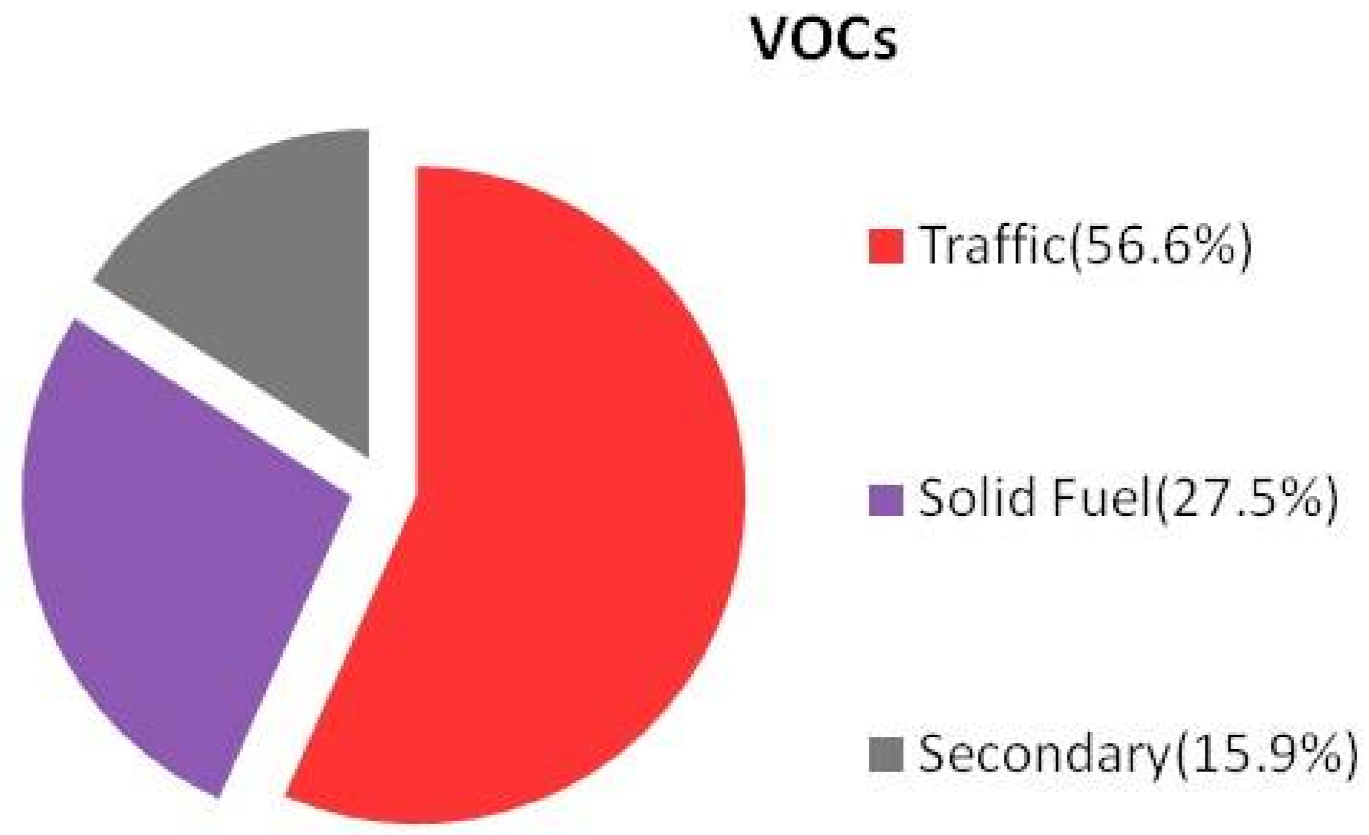
3.2.6. Source Apportionment of VOCs
3.3. Case Study: Analysis of Changes in All Pollutants during a Two-Week Span
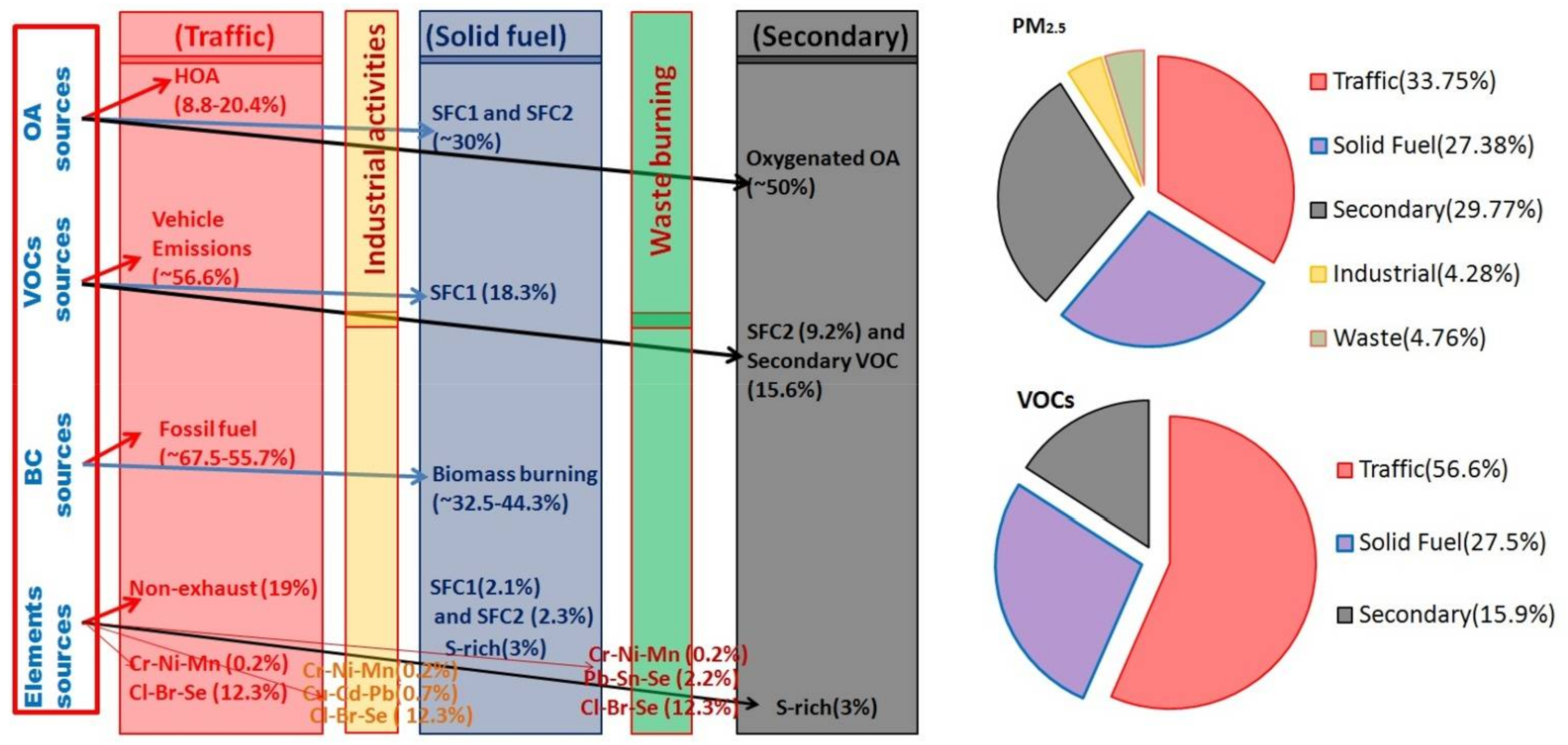
3.4. Categorization of the Sources of PM2.5 and VOCs
3.5. PM2.5 Pollution Scenario as Reported from Some Important Urban Sites in Asia
3.6. Potential Health Impacts of Offline Particulate Matters Using Oxidative Potential
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Black carbon |
| BrC | Brown carbon |
| CMB | Chemical mass balance |
| DTT | Dithiothreitol |
| EC | Elemental carbon |
| HR-ToF-AMS | High-resolution time-of-flight aerosol mass spectrometer |
| HOA | Hydrocarbon-like organic aerosol |
| ICP-MS | Inductively coupled plasma mass spectroscopy |
| L-ToF-AMS | High-resolution long-time-of-flight aerosol mass spectrometer |
| LVOOA | Low-volatile oxygenated organic aerosol |
| ME | Multilinear engine |
| NCR | National Capital Region |
| NMOG | Nonmethane organic gases |
| NR | Nonrefractory |
| OC | Organic carbon |
| OA | Organic aerosol |
| OM/OC | Organic matter to organic carbon ratio |
| OP | Oxidative potential |
| PAN | Peroxyacetyl nitrate |
| PM | Particulate matter |
| PMF | Positive matrix factorization |
| PBL | Planetary boundary layer |
| PTR-ToF-MS | Proton-transfer-reaction time-of-flight mass spectrometer |
| POA | Primary organic aerosol |
| ROS | Reactive oxygen species |
| SDD | Silicon drift detector |
| SoFi | Source Finder |
| SOA | Secondary organic aerosol |
| SFC | Solid fuel combustion |
| SVOOA | Semivolatile oxygenated organic aerosol |
| ToF-ACSM | Time-of-flight aerosol chemical speciation monitor |
| TOC | Total organic carbon |
| VOCs | Volatile organic compounds |
| WS-BrC | Water-soluble brown carbon |
| XRF | X-ray fluorescence |
References
- Singh, V.; Singh, S.; Biswal, A. Exceedances and trends of particulate matter (PM2.5) in five Indian megacities. Sci. Total Environ. 2021, 750, 141461. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Shukla, A.; Kohl, S.D.; Chow, J.C.; Watson, J.; Harrison, R.M. Characterization of ambient PM2.5 at a pollution hotspot in New Delhi, India and inference of sources. Atmos. Environ. 2015, 109, 178–189. [Google Scholar] [CrossRef]
- Tiwari, S.; Thomas, A.; Rao, P.; Chate, D.; Soni, V.; Singh, S.; Ghude, S.; Singh, D.; Hopke, P.K. Pollution concentrations in Delhi India during winter 2015–2016: A case study of an odd-even vehicle strategy. Atmos. Pollut. Res. 2018, 9, 1137–1145. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Dey, S.; Gupta, T.; Dhaliwal, R.S.; Brauer, M.; Cohen, A.J.; Stanaway, J.D.; Beig, G.; Joshi, T.K.; Aggarwal, A.N.; et al. The Impact of Air Pollution on Deaths, Disease Burden, and Life Expectancy across the States of India: The Global Burden of Disease Study 2017. Lancet Planet. Health 2019, 3, 26−39. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, X.-L.; Dai, D.-W.; Huang, Z.-Y.; Ma, Z.; Guan, Y.-J. Air pollution and temperature are associated with increased COVID-19 incidence: A time series study. Int. J. Infect. Dis. 2020, 97, 278–282. [Google Scholar] [CrossRef]
- Lolli, S.; Chen, Y.-C.; Wang, S.-H.; Vivone, G. Impact of meteorological conditions and air pollution on COVID-19 pandemic transmission in Italy. Sci. Rep. 2020, 10, 16213. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, S.; Jain, V. Winter-time size distribution and source apportionment of total suspended particulate matter and associated metals in Delhi. Atmos. Res. 2009, 92, 88–99. [Google Scholar] [CrossRef]
- Tiwari, S.; Srivastava, A.; Chate, D.; Safai, P.; Bisht, D.; Beig, G. Impacts of the high loadings of primary and secondary aerosols on light extinction at Delhi during wintertime. Atmos. Environ. 2014, 92, 60–68. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mandal, T.K.; Jain, S.; Saraswati; Sharma, A.; Saxena, M. Source Apportionment of PM2.5 in Delhi, India Using PMF Model. Bull. Environ. Contam. Toxicol. 2016, 97, 286–293. [Google Scholar] [CrossRef]
- Lalchandani, V.; Kumar, V.; Tobler, A.; Thamban, N.M.; Mishra, S.; Slowik, J.G.; Bhattu, D.; Rai, P.; Satish, R.; Ganguly, D.; et al. Real-time characterization and source apportionment of fine particulate matter in the Delhi megacity area during late winter. Sci. Total Environ. 2021, 770, 145324. [Google Scholar] [CrossRef] [PubMed]
- Tobler, A.; Bhattu, D.; Canonaco, F.; Lalchandani, V.; Shukla, A.; Thamban, N.M.; Mishra, S.; Srivastava, A.K.; Bisht, D.S.; Tiwari, S.; et al. Chemical characterization of PM2.5 and source apportionment of organic aerosol in New Delhi, India. Sci. Total Environ. 2020, 745, 140924. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Furger, M.; El Haddad, I.; Kumar, V.; Wang, L.; Singh, A.; Dixit, K.; Bhattu, D.; Petit, J.-E.; Ganguly, D.; et al. Real-time measurement and source apportionment of elements in Delhi’s atmosphere. Sci. Total Environ. 2020, 742, 140332. [Google Scholar] [CrossRef]
- Wang, L.; Slowik, J.G.; Tripathi, N.; Bhattu, D.; Rai, P.; Kumar, V.; Vats, P.; Satish, R.; Baltensperger, U.; Ganguly, D.; et al. Source characterization of volatile organic compounds measured by PTR-ToF-MS in Delhi, India. Atmos. Chem. Phys. 2020, 20, 9753–9770. [Google Scholar] [CrossRef]
- Bhowmik, H.S.; Naresh, S.; Bhattu, D.; Rastogi, N.; Prévôt, A.S.; Tripathi, S.N. Temporal and spatial variability of carbonaceous species (EC; OC.; WSOC and SOA) in PM2.5 aerosol over five sites of Indo-Gangetic Plain. Atmos. Pollut. Res. 2021, 12, 375–390. [Google Scholar] [CrossRef]
- Singh, A.; Rastogi, N.; Kumar, V.; Slowik, J.G.; Satish, R.; Lalchandani, V.; Thamban, N.M.; Rai, P.; Bhattu, D.; Vats, P.; et al. Sources and characteristics of light-absorbing fine particulates over Delhi through the synergy of real-time optical and chemical measurements. Atmos. Environ. 2021, 252, 118338. [Google Scholar] [CrossRef]
- Rastogi, N.; Satish, R.; Singh, A.; Kumar, V.; Thamban, N.; Lalchandani, V.; Shukla, A.; Vats, P.; Tripathi, S.; Ganguly, D.; et al. Diurnal variability in the spectral characteristics and sources of water-soluble brown carbon aerosols over Delhi. Sci. Total Environ. 2021, 794, 148589. [Google Scholar] [CrossRef] [PubMed]
- Puthussery, J.V.; Singh, A.; Rai, P.; Bhattu, D.; Kumar, V.; Vats, P.; Furger, M.; Rastogi, N.; Slowik, J.G.; Ganguly, D.; et al. Real-Time Measurements of PM2.5 Oxidative Potential Using a Dithiothreitol Assay in Delhi, India. Environ. Sci. Technol. Lett. 2020, 7, 504–510. [Google Scholar] [CrossRef]
- Fröhlich, R.; Cubison, M.J.; Slowik, J.G.; Bukowiecki, N.; Prévôt, A.S.H.; Baltensperger, U.; Schneider, J.; Kimmel, J.R.; Gonin, M.; Rohner, U.; et al. The ToF-ACSM: A portable aerosol chemical speciation monitor with TOFMS detection. Atmos. Meas. Technol. 2013, 6, 3225–3241. [Google Scholar] [CrossRef] [Green Version]
- DeCarlo, P.F.; Kimmel, J.R.; Trimborn, A.; Northway, M.J.; Jayne, J.T.; Aiken, A.C.; Gonin, M.; Fuhrer, K.; Horvath, T.; Docherty, K.S.; et al. Field-Deployable, High-Resolution, Time-of-Flight Aerosol Mass Spectrometer. Anal. Chem. 2006, 78, 8281–8289. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Märk, L.; Seehauser, H.; Schottkowsky, R.; Sulzer, P.; Märk, T. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Drinovec, L.; Močnik, G.; Zotter, P.; Prévôt, A.S.H.; Ruckstuhl, C.; Coz, E.; Rupakheti, M.; Sciare, J.; Müller, T.; Wiedensohler, A.; et al. The “dual-spot” Aethalometer: An improved measurement of aerosol black carbon with real-time loading compensation. Atmos. Meas. Technol. 2015, 8, 1965–1979. [Google Scholar] [CrossRef] [Green Version]
- Thamban, N.M.; Tripathi, S.N.; Moosakutty, S.P.; Kuntamukkal, P.; Kanawade, V.P. Internally mixed black carbon in the Indo-Gangetic Plain and its effect on absorption enhancement. Atmos. Res. 2017, 197, 211–223. [Google Scholar] [CrossRef]
- Stephens, M.; Turner, N.; Sandberg, J. Particle identification by laser-induced incandescence in a solid-state laser cavity. Appl. Opt. 2003, 42, 3726. [Google Scholar] [CrossRef] [PubMed]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Canonaco, F.; Crippa, M.; Slowik, J.G.; Baltensperger, U.; Prévôt, A.S.H. SoFi, an IGOR-based interface for the efficient use of the generalized multilinear engine (ME-2) for the source apportionment: ME-2 application to aerosol mass spectrometer data. Atmos. Meas. Technol. 2013, 6, 3649–3661. [Google Scholar] [CrossRef] [Green Version]
- Paatero, P.; Hopke, P.K. Rotational tools for factor analytic models. J. Chemom. 2009, 23, 91–100. [Google Scholar] [CrossRef]
- Ulbrich, I.M.; Canagaratna, M.R.; Zhang, Q.; Worsnop, D.R.; Jimenez, J.L. Interpretation of organic components from Positive Matrix Factorization of aerosol mass spectrometric data. Atmos. Chem. Phys. Discuss. 2009, 9, 2891–2918. [Google Scholar] [CrossRef] [Green Version]
- Canonaco, F.; Slowik, J.G.; Baltensperger, U.; Prévôt, A.S.H. Seasonal differences in oxygenated organic aerosol composition: Implications for emissions sources and factor analysis. Atmos. Chem. Phys. Discuss. 2015, 15, 6993–7002. [Google Scholar] [CrossRef] [Green Version]
- Crippa, M.; Canonaco, F.; Lanz, V.A.; Äijälä, M.; Allan, J.D.; Carbone, S.; Capes, G.; Ceburnis, D.; Dall’Osto, M.; Day, D.A.; et al. Organic aerosol components derived from 25 AMS data sets across Europe using a consistent ME-2 based source apportionment approach. Atmos. Chem. Phys. Discuss. 2014, 14, 6159–6176. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.L.; Charlson, R.J.; Winker, D.M.; Ogren, J.; Holmén, K. Mesoscale Variations of Tropospheric Aerosols*. J. Atmos. Sci. 2003, 60, 119–136. [Google Scholar] [CrossRef]
- Campbell, J.R.; Ge, C.; Wang, J.; Welton, E.J.; Bucholtz, A.; Hyer, E.; Reid, E.A.; Chew, B.N.; Liew, S.C.; Salinas, S.V.; et al. Applying Advanced Ground-Based Remote Sensing in the Southeast Asian Maritime Continent to Characterize Regional Proficiencies in Smoke Transport Modeling. J. Appl. Meteorol. Clim. 2016, 55, 3–22. [Google Scholar] [CrossRef]
- Canagaratna, M.; Jayne, J.; Jimenez, J.; Allan, J.; Alfarra, M.; Zhang, Q.; Onasch, T.; Drewnick, F.; Coe, H.; Middlebrook, A.; et al. Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer. Mass Spectrom. Rev. 2007, 26, 185–222. [Google Scholar] [CrossRef]
- Ng, N.L.; Canagaratna, M.R.; Zhang, Q.; Jimenez, J.L.; Tian, J.; Ulbrich, I.M.; Kroll, J.H.; Docherty, K.S.; Chhabra, P.S.; Bahreini, R.; et al. Organic aerosol components observed in Northern Hemispheric datasets from Aerosol Mass Spectrometry. Atmos. Chem. Phys. 2010, 10, 4625–4641. [Google Scholar] [CrossRef] [Green Version]
- Kirchstetter, T.W.; Novakov, T.; Hobbs, P.V. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. Atmos. 2004, 109, 1−12. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Rastogi, N.; Patel, A.; Satish, R.V.; Singh, D. Size-segregated characteristics of carbonaceous aerosols over the north western Indo-Gangetic Plain: Year-round temporal behavior. Aerosol. Air Qual. Res. 2016, 16, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, S.; Rupakheti, M.; Lawrence, M.G. Black carbon dominates the aerosol absorption over the Indo-Gangetic Plain and the Himalayan foothills. Environ. Int. 2020, 142, 105814. [Google Scholar] [CrossRef]
- de Gouw, J.; Warneke, C. Measurements of volatile organic compounds in the earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 2007, 26, 223–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.-C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. Discuss. 2013, 13, 7053–7074. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.D.; Wang, G.F.; Zhang, R.J.; Zhang, L.M.; Song, Y.; Wu, B.B.; Li, X.F.; An, K.; Chu, J.H. Characterization an source apportionment of PM2.5 in an urban environment in Beijing. Aerosol. Air Qual. Res. 2013, 13, 574–583. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, T.; Yang, J.; Wu, J.; Wang, J.; Gao, J.; Bi, X.; Feng, Y.; Zhang, Y.; Yang, H. Source apportionment and a novel approach of estimating regional contributions to ambient PM2.5 in Haikou, China. Environ. Pollut. 2017, 223, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.-B.; Hopke, P.K.; Yi, S.-M. Source apportionment of PM2.5 in Seoul, Korea. Atmos. Chem. Phys. Discuss. 2009, 9, 4957–4971. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Shon, Z.H.; Kang, M.; Song, S.K.; Kim, Y.K.; Park, J.; Kim, H. Comparison of source apportionment of PM2.5 using receptor models in the main hub port city of East Asia: Busan. Atmos. Environ. 2017, 148, 115–127. [Google Scholar] [CrossRef]
- Yau, P.S.; Lee, S.C.; Cheng, Y.; Huang, Y.; Lai, S.C.; Xu, X.H. Contribution of ship emissions to the fine particulate in the community near an internationalport in Hong Kong. Atmos. Res. 2013, 124, 61–72. [Google Scholar] [CrossRef]
- Cheng, Y.; Lee, S.; Gu, Z.; Ho, K.; Zhang, Y.; Huang, Y.; Chowa, J.C.; Watson, J.G.; Cao, J.; Zhang, R. PM2.5 and PM10-2.5 chemical composition and source apportionment near a Hong Kong roadway. Particuology 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Wimolwattanapun, W.; Hopke, P.K.; Pongkiatkul, P. Source apportionment and potential source locations of PM2.5 and PM2.5–10 at residential sites in metropolitan Bangkok. Atmos. Pollut. Res. 2011, 2, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Jayarathne, T.; Simpson, I.J.; Werden, B.; Maben, J.; Gilbert, A.; Praveen, P.S.; Adhikari, S.; Panday, A.K.; Rupakheti, M.; et al. Ambient air quality in the Kath-mandu Valley, Nepal during the pre-monsoon: Concentrations and sources of particulate matter and trace gases. Atmos. Chem. Phys. 2020, 20, 2927–2951. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A. Neurological Disorders from Ambient (Urban) Air Pollution Emphasizing UFPM and PM2.5. Curr. Pollut. Rep. 2016, 2, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 782462. [Google Scholar] [CrossRef] [Green Version]


| Instruments | Site of Sample Collection | Sampling Period | Type of Samples and Volume | Parameters |
|---|---|---|---|---|
| High-resolution time-of-flight aerosol mass spectrometer (HR-ToF-AMS) | MRIIRS Faridabad IITDelhi | 27 January–15 March 2018 17–19 January 2018 and 5 February–11 March 2018 | Ambient air 0.0.08 L/min | Real-time nonrefractory and size-resolved PM2.5 speciation |
| Time-of-flight aerosol chemical speciation monitor (ToF-ACSM) | IITMDelhi | 17 February–15 March 2018 | ambient air (0.1 L/min) | Real-time bulk nonrefractory PM2.5 speciation |
| Proton-transfer-reaction time-of-flight mass spectrometer (PTR-ToF-MS) | IITDelhi MRIIRS Faridabad | 18 January–10 March 2018 16 January–8 March 2018 | ambient air (0.06 L/min) | High time- and mass-resolved online measurements of volatile organic compounds |
| Aethalometer (AE 33) | IITDelhi IITMDelhi | 17–19 January 2018 and 5 February–11 March 2018 17 February–15 March 2018 | Ambient air sampled on a quartz filter tape (3 L/min) | Black carbon mass concentration |
| PILS-LWCC-TOC analyzer | IITDelhi | 9 January–3 March 2018 | PILS liquid, sample flow rate (0.7 mL/min) and air sampling flow rate (16.7 L/min) | Ambient aerosol extracts at wavelengths ranging from 300 to 600 nm and water-soluble BrC |
| Single-particle soot photometer (SP2) | MRIIRS Faridabad | 27 January–15 March 2018 | Ambient air | Black carbon (BC) mass concentration, BC number concentration, BC size distribution, BC mixing state |
| Xact 625i® ambient metals monitor | IITDelhi | 20 January–11 March in 2018 15 January–9 February 2019 | Ambient air Sampled on Teflon filter tape (16.7 L/min) | Equipped to report the following 44 elements—Al, Si, P, S, Cl, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Rb, Sr, Y, Zr, Mo, Pd, Ag, Cd, In, Sn, Sb, Te, Cs, Ba, La, Ce, W, Pt, Au, Hg, Tl, Pb, and Bi |
| High-volume sampler | IITDelhi | January–December 2018 | Ambient air sampled on quartz filter media | PM2.5 |
| EC-OC analyzer, TOC-L analyzer and ion chromatography, inductively coupled plasma mass spectroscopy (ICP-MS) | IITDelhi IITMDelhi MRIIRS Faridabad | January–December 2018 | Offline analysis of quartz filter media | Elemental carbon (EC), organic carbon (OC), and total organic carbon (TOC), and major anions and cations (Na+, K+, Ca2+, Cl− and SO42−) |
| Real-time oxidative potential (OP) instrument | IITDelhi | 3–9 February 2019 | Ambient air | Measures the ability of particles to consume antioxidants and estimates the toxicity of particulate matters |
| IITDelhi | IITMDelhi | MRIIRS, Delhi | |
|---|---|---|---|
| Organics | 65.3 ± 51.68 | 55 ± 37.4 | 32.4 ± 20.5 |
| NO3 | 13.3 ± 12.1 | 11.1 ± 8 | 8 ± 6.2 |
| SO4 | 12 ± 10.1 | 12.5 ± 5.3 | 8.9 ± 6.2 |
| NH4 | 14.3 ± 13.5 | 13.6 ± 11.4 | 9 ± 6.9 |
| Cl | 20.4 ± 30.3 | 22.3 ± 27.6 | 7.7 ± 10 |
| BC | 24.5 ± 20.6 | 14.4 ± 9.9 | 3.3 ± 2.4 |
| * PM2.5elements | 32.6 | ||
| VOCs | 27.6 | 19.4 |
| Site (Sampling Year) | Traffic Emissions | Biomass Burning/SFC | Secondary Aerosols |
|---|---|---|---|
| Delhi (2018) | 33.75 | 27.38 | 29.77 |
| Beijing (2010) | 17.1 | 11.2 | 26.5 |
| Haikou (2013–2015) | 10.5–20.2 | ---- | 9.9–21.4 |
| Seoul (2009) | 27.9 | 13.6 ± 11.4 | 41.4 |
| Hong Kong (2014) | 29 | 23 | 27 |
| Bangkok (2011) | 7.65 ± 4.42 | 4.51 ± 4.17 | 2.39 ± 2.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talukdar, S.; Tripathi, S.N.; Lalchandani, V.; Rupakheti, M.; Bhowmik, H.S.; Shukla, A.K.; Murari, V.; Sahu, R.; Jain, V.; Tripathi, N.; et al. Air Pollution in New Delhi during Late Winter: An Overview of a Group of Campaign Studies Focusing on Composition and Sources. Atmosphere 2021, 12, 1432. https://doi.org/10.3390/atmos12111432
Talukdar S, Tripathi SN, Lalchandani V, Rupakheti M, Bhowmik HS, Shukla AK, Murari V, Sahu R, Jain V, Tripathi N, et al. Air Pollution in New Delhi during Late Winter: An Overview of a Group of Campaign Studies Focusing on Composition and Sources. Atmosphere. 2021; 12(11):1432. https://doi.org/10.3390/atmos12111432
Chicago/Turabian StyleTalukdar, Shamitaksha, Sachchida Nand Tripathi, Vipul Lalchandani, Maheswar Rupakheti, Himadri Sekhar Bhowmik, Ashutosh K. Shukla, Vishnu Murari, Ravi Sahu, Vaishali Jain, Nidhi Tripathi, and et al. 2021. "Air Pollution in New Delhi during Late Winter: An Overview of a Group of Campaign Studies Focusing on Composition and Sources" Atmosphere 12, no. 11: 1432. https://doi.org/10.3390/atmos12111432
APA StyleTalukdar, S., Tripathi, S. N., Lalchandani, V., Rupakheti, M., Bhowmik, H. S., Shukla, A. K., Murari, V., Sahu, R., Jain, V., Tripathi, N., Dave, J., Rastogi, N., & Sahu, L. (2021). Air Pollution in New Delhi during Late Winter: An Overview of a Group of Campaign Studies Focusing on Composition and Sources. Atmosphere, 12(11), 1432. https://doi.org/10.3390/atmos12111432







