1. Introduction
In the global pandemic situation, the prevention and mitigation of pathogenic microorganisms in indoor air spaces and on various surfaces attract significant attention. While conventional disinfection methods may be limited by reliance on the operator, the use of ‘no-touch’ automated disinfection systems provides a promising alternative [
1]. The disinfectants used for these systems should not only be effective in reducing the microorganisms present in the air but should also not be toxic for humans, allowing their continuous application.
Essential oils (EOs) are complex mixtures of volatile liquid and lipophilic compounds obtained by parts of plants by extraction, effleurage, drag steam vapour, extraction with organic solvents, pressing, or supercritical fluid extraction [
2]. EOs have been shown to have bactericidal, fungicidal, virucidal, and phagocidal activities. The antimicrobial effects of EOs are explained by their composition and cytotoxic effects, which cause cell membrane damage.
Australian endemic plants produce a variety of essential oils such as tea tree oil (oil of Melaleuca alternifolia), eucalyptus oil (oil of Eucalyptus polybractea) and lemon myrtle oil (oil of Backhousia citriodora) which are used in health products, aromatherapy, perfumes, bushfoods, food flavourings and herbal teas [
3].
The potential use of
Melaleuca alternifolia (Tea tree) oil as a disinfectant has been clearly shown in the literature for treating bacteria [
4,
5,
6], fungi [
7,
8] and viruses [
9,
10]. The antimicrobial activity of Tea Tree Oil (TTO) is attributed mainly to terpinen-4-ol (35–45%) and 1,8-cineole (1–6%); however, other components such as a-terpineol, terpinolene and a- and c-terpinene are also often present and potentially contribute to microbial disinfection [
11].
Eucalyptus oil obtained from Eucalyptus corresponds to one of the principal genera of the Myrtaceae family. Eucalyptus oil is usually rich in monoterpenes and, in some cases, sesquiterpenes. Eucalyptus oil was evaluated against several Gram-positive and Gram-negative bacterial strains as well as against various fungal and viral species and showed different degrees of efficiency [
12,
13]. Both tea tree oil and eucalyptus oil were challenged as aerosols and vapours and demonstrated very promising capabilities on the inactivation of pathogenic airborne microbes over short periods of time [
14].
The major component of Backhousia citriodora oil is citral (>85%), with two main isomeric aldehydes: neral and geranial with minor amounts of (E)-β-ocimene,
p-cymene, citronellal, linalool, β-elemene, β-caryophyllene, nerol, geraniol, globulol and spathulenol [
15]. Lemon myrtle oil was shown to possess significant antimicrobial activity against bacteria, including
Escherichia coli and methicillin-resistant
S. aureus, and fungi including
Aspergillus niger [
16]. The antimicrobial activity of Lemon myrtle EO was found to be greater than that of citral alone and often superior to
Melaleuca alternifolia essential oil [
17].
A number of studies reported a greater antimicrobial effect of EO vapour compared with EOs in liquid form applied by direct contact [
18,
19]. The use of combinations of EOs is capable of increasing the efficacy of EOs, taking advantage of their synergistic and additive effects [
20]. Although various approaches for the evaluation of microbial inactivation by EOs in the vapour phase are provided in the literature, no standard assay has been developed.
The growth of methicillin-resistant
Staphylococcus aureus (MRSA) on seeded agar plates was reduced by 38% after 20 h of exposure to a blend of geranium and lemongrass Eos, dispersed via a generator that releases vapours into the air by means of negative and Venturi airflow in a sealed box environment. In an office environment, the same disinfection method achieved an 89% reduction of airborne bacteria in 15 h when operated at a constant output of 100% [
21]. A significant reduction in viable numbers of
E. coli and
S. aureus inoculated on agar plates was obtained when the bacteria were exposed to a flame of candles containing orange oil in a large air-tight chamber for 3 h. As the aerial concentration of the volatiles was increased, the viability of
E. coli and
S. aureus declined [
22].
Inouye et al. [
19] showed that the rapid evaporation of essential oils is more effective than slow evaporation in a small (1.3 L) enclosure. Antibacterial activity of tea tree, eucalyptus and lemongrass oils by gaseous contact against
E. coli was achieved at the minimal inhibitory dose (MIC) of 50, >1600 and 100 mg/L air, respectively. These high concentrations seem hardly achievable with a domestic oil diffusor.
Chao et al. [
23] investigated the antibacterial activity of an oil blend including cinnamon, clove, rosemary, eucalyptus and lemon EOs against bacterial bioaerosols into a 0.4 m
3 enclosed fume hood. An 82% reduction in
M. luteus bioaerosol, a 96% reduction in the
P. aeruginosa bioaerosol and a 44% reduction in the
S. aureus bioaerosol were detected following 10 min of exposure.
The dispersion of a mixture of Citrus limon EO and Abies alba EO using commercially available ultrasonic vaporisers in hospital rooms resulted in a 40% reduction in bacteria, and a 30–60% reduction in fungal contamination was observed in the first two hours [
24]. In another study, terpineol, an important compound of tea tree oil, showed an average reduction of the germ count in a testing room of 59.4% after 5 h of spreading when vaporised with a room diffuser [
25].
Reductions in both bacterial and fungal contamination were observed between rooms cleaned using standard sanitisation alone or in combination with a mixture of essential oils containing Lavender, Cajeput, Siberian Fir, Myrtle and Geranium bourbon EOs dispersed using commercially available ultrasound vaporisers in a residential health care house [
26]. The potential reduction of bacterial and fungal contamination in unventilated lab spaces and unventilated real cultural heritage assets when tea tree and thyme EOs are cold diffused over 24 h has recently been evaluated. Tea tree EO vaporisation showed an up to 77.3% fungal and 95.0% bacterial air contamination reduction [
27]. Lavender essential oil dispersed by automatic diffusers significantly reduced the number of bacteria in hospital areas [
28].
Evaluation of the antiviral activity of tea tree and eucalyptus EOs aerosolised via a nebuliser showed strong virucidal activity against airborne influenza virus A strain [
13] and phagocidal activity against
E. coli phage M13 [
14].
The aim of this study is the determination of antimicrobial activities of a mixture of Melaleuca alternifolia, Eucalyptus polybractea and Backhousia citriodora essential oils dispersed via a novel oil atomizer VaxiPod (Model: #ALNEBVP01; Linfield Pacific P/L, Gladesville, NSW, Australia). The EO blend was formulated, taking into account the chemical composition and specific activity of each. The antimicrobial activity of the oil blend against two bacterial (Escherichia coli and Bacillus subtilis), one fungal (Aspergillus niger) and one viral (bacteriophage MS2) strain was considered on a porous and nonporous surface as well as in aerosolised form.
2. Materials and Methods
2.1. Microorganisms
The working culture of E. coli was prepared from the stock culture on nutrient agar (NA) and incubated at 37 °C. After 24 h, the culture was aseptically transferred to 10 mL of nutrient broth and incubated for 19 h at 37 °C. Cells were washed twice by centrifugation in sterile distilled water. The resulting pellet was resuspended in sterile Phosphate-Buffered Saline (PBS), and the number of cells in the suspension was adjusted to achieve a number of colony-forming units (CFU) between 107 and 109 CFU/mL. The working culture of B. subtilis was prepared in the same way.
The working culture of Aspergillus niger was prepared from the stock culture on malt extract agar (MEA) and incubated at 25 °C. After 48 h, a second subculture was prepared in the same way and grown for 7 days. A bowl with water was placed in the incubator to keep the relative humidity at 55–65%. 10 mL of sterile PBS was placed on the surface of the working culture. The conidiospores were disclosed using a sterile glass spatula and the suspension was transferred into a conical flask and shaken gently for 1 min. The number of spores in suspension was adjusted to achieve the number of CFU between 106 and 107 CFU/mL.
MS2 bacteriophage was recovered from a freeze-dried state and propagated on plates with soft-agar/host overlay. Host strain of E. coli was cultured in 2YT medium broth (Bacto tryptone 16 g; yeast extract 10 g; NaCl 5 g; distilled water 1 L). To prepare viral stock, a log-stage host cells culture was infected with phage from a single plaque and incubated overnight at 37 °C with constant agitation. Overnight phage culture was centrifuged twice at high speed to remove cells and then chloroform to prevent microbial growth was added. Decimal dilutions of phage stock were made in PBS to achieve the number of plaque-forming units (PFU) between 108 and 109 PFU/mL.
All preparations and experiments were conducted in a Class II biological safety cabinet (Model BH 2000, Biolab, Australia) to prevent microbial release into the laboratory environment.
2.2. Essential Oil and VaxiPod Atomiser
Melaleuca alternifolia (Tea tree), Eucalyptus polybractea (Eucalyptus) and Backhousia citriodora (Lemon myrtle) EOs acquired from Bosisto’s and NATIO (Australia) and produced by Australian endemic plants were used for the experiments. The EO blend was formulated, taking into account the chemical composition and specific activity of each and included a mixture of Tea tree, Eucalyptus and Lemon myrtle in ratio 4.5:4.5:1. This ratio has been proposed to expand the range of microbes that are affected by different, but not overlapping, chemical constituents of the oils. In addition, the Lemon Myrtle also served to impart a pleasant scent to the oil mixture. The oil blend was dispersed via the VaxiPod oil atomiser, providing rate of the oil mixture release of ~1.0 mL/h.
2.3. Inactivation of Microorganisms on Porous Filter Surface
The experimental setup used for this part of investigation is presented in
Figure 1. Such arrangement (four parallel filters) was convenient for direct comparison of a time-related microbial decay results within each particular run, as all initial experimental conditions, including number of microbes on the surface as well as physical parameters of the airflow, were identical. Sterile polypropylene fibre filters were used as carriers in this investigation. The carriers were placed in sterile Petri dishes and contaminated by depositing 0.05 mL = 50 μL of microbial suspension in the centre of the carrier. The inoculum was spread evenly on the carrier, covering the area of 3.0 ± 0.5 cm
2. Then, the carriers were placed in the filter holder for experimental use.
Two experimental runs were undertaken for each microorganism used in this work. The first run did not involve any disinfecting substances that can have an impact on the survivability of test microorganisms (Vaxipod was not operating and no oils were added to the air carrier); only natural microbial inactivation on the filter surface resulting from interaction with the airflow was evaluated. In the second part of the experiment, the oil atomiser, containing a mixture of tea tree oil, eucalyptus oil and lemon myrtle, was placed at the entry of the aerosol chamber. After passing through the filter stage, the stream was HEPA-filtered and discharged to the atmosphere. The temperature and RH were kept at levels of 25 °C and 50 to 55%, respectively, throughout all experimental runs.
The following procedure was used for all experimental runs. A filter holder with four filters contaminated with inoculum was stationary fixed and hermetically sealed in the chamber to avoid any possible air bypass. For the first part of the experiment, the filters were removed after at 15-, 30-, 60- and 120-minute intervals of the sampling pump operation. Then, another four filters similarly contaminated with inoculum were placed in the filter holder, stationary fixed and hermetically sealed in the chamber, and the oil atomiser was switched on. The filters were removed at 15-, 30-, 60- and 120-minute intervals. Immediately upon removal from the holder, each filter was placed into the glass bottle containing 50 mL of sterile PBS and sonicated in a sonic bath (Biolab, Clayton, VIC, Australia) for a period of 1 min to ensure that the majority of particles were transmitted from the filter to the liquid.
Then, an aliquot of 0.1 mL of an appropriate 10-fold dilution of the liquid was spread on the surface of the NA for
E. coli and
B. sublilis and MEA medium for
A. niger. NA plates were placed in an incubator set at 37 °C for 24 h, MEA plates were grown at 25 °C for 3–7 days and CFUs were counted after incubation. Concentration of live phage particles was determined by a double-agar-layer plaque technique [
29]. A hard agar medium was prepared from 2YT medium supplemented with 2% agar and a soft agar medium with 0.5% agar. The results were expressed in Plaque-Forming Units (PFU) per millilitre of suspension.
2.4. Inactivation of Microorganisms on Nonporous Stainless Steel Surface
Sterile 301 stainless steel discs of 4 cm in diameter and surface-finished on both sides were placed in sterile Petri dishes and inoculated with 50 μL of microbial suspension. The inoculum was spread evenly on the carrier, covering an area of 3.0 ± 0.5 cm2. The carriers were allowed to dry in an incubator at 37 °C for 20 min. For each test organism, two identical carriers were contaminated for survival control. Control carriers were prepared in the same way but not exposed to the aerosolised oil mixture and kept outside the test enclosures during the experimental runs.
For all the experiments, the test enclosure (0.7 m3 vapour chamber) was sealed to allow the oil mixture to be safely diffused inside and prevent dispersed oil escape. The enclosure was thermally insulated and homogeneous temperature (25 ± 2 °C) and humidity (55%) were maintained in the enclosures during all experimental runs. The oil atomisers (evaporating rate ~1.0 mL/h) were placed in the enclosure to continuously dispense the oil blend.
Contaminated stainless steel discs were then placed in the enclosure and exposed to the oil mixture dispersed via the oil atomiser for periods up to 48 h. After exposure, the discs were placed in beakers containing 5 g glass beads and 10 mL of phosphate-buffered saline (PBS) and shaken for 1 min at 150 rpm on a rotary shaker. The microbial suspensions were appropriately diluted, plated on to appropriate agar media and grown as described above. Reduction in viability (%) was calculated based on the reduction in colony (plaque) count in the dispersed oil mixture exposed discs with respect to the control (unexposed) discs.
where
is the microbial survival at time
t,
is the number of live microorganisms recovered from the disc exposed to the dispersed oil mixture and
is the number of live microorganisms recovered from the control discs.
2.5. Inactivation of Microorganisms on Agar Surface
It is also important to assess any potential impact of the presence of nutrients on surfaces that may affect the survival of microorganisms. Several experiments were undertaken to evaluate this scenario. They were conducted in the enclosure for selected microorganisms, including E. coli, B. subtilis and A. niger. Appropriate serial dilution of the bacterial and fungal cultures (to obtain 100–300 CFU) was plated on NA/MEA plates. Inoculated plates were opened inside the enclosures and placed on the bottom part of the enclosures for exposure to the essential oil mixture. To examine the long-term growth inhibitory effect of the oil mixture supplied by the oil atomiser, one set of plates with each type of microorganisms was removed from the enclosure in 0, 15, 24 and 48 h. The plates were incubated at 37 °C for 24 h for bacteria and 25 °C for 3–7 days for fungi, and CFUs were counted after incubation. Control plates were stored at room temperature and then collected, incubated and counted in the same way. Reduction in viability (%) was calculated based on the reduction in colony count on the oil vapour exposed plates with respect to the control plates (Equation (1)).
2.6. Antimicrobial Activity against Biological Aerosols
These experiments were focused on evaluation of activity of the oil mixture dispersed via oil atomiser against airborne microbes in the ambient air. The laboratory setup used for this series of experiments is shown in
Figure 2. A rotational toroid shaped 200 litres aluminium aerosol chamber (650 mm diameter and 600 mm long) was designed to keep materials in airborne form over extended periods of time [
13]. The optimal rotation speed was found to be around 7 rpm. The axis was designed to be stationary, enabling to host pipelines used for aerosol charging and monitoring.
The oil blend was dispersed via the oil atomiser strategically placed on a stationary shelf installed inside a rotating aerosol chamber (
Figure 3). Bacterial and phage aerosols were generated by a Collison nebuliser from a liquid suspension, prepared according to the procedures described above. The microbial loading time for all experiments was 2 min. The nebuliser operated at a flow rate of 6 L/min of dry and filtered compressed air.
Fungal aerosols were generated by our earlier developed device (
Figure 4) based on aerosolisation of fungal spores from cultures grown in standard 9-centimetre Petri dishes by vibration [
30]. In brief, a 10-centimetre diameter, 2 Watts, 4 Ohm speaker was hermetically sealed using soft rubber gasket to the bottom of cylindrical casing of the device. A Petri dish, containing mature fungal spores, was placed directly on the speaker’s surface and secured with thin flexible rubber strips. The speaker size was chosen to exactly accommodate a standard 9-centimetre diameter Petri dish that is placed on it so that the Petri dish vibrated simultaneously with the speaker’s membrane at different frequencies when running the experiment. A Hewlett-Packard 3312A Function Generator was used to set the amplitude and frequency of the vibrating speaker membrane to achieve the maximum possible particle concentration. A fresh dish with the fungal culture was used for each experimental run.
To trace the microbial concentration in the chamber throughout the entire experiment, a fluorescent dye (fluorescein sodium salt, C20H10Na2O2, Fluka AG, Buchs, Switzerland) at concentration of 0.5 mg/mL was added into microbial suspension (see details of the procedure in Agranovski et al. [
31] and Pyankov et al. [
32]. Experimental conditions such as the air temperature and humidity were continuously monitored and controlled throughout the entire procedure (T = 25 °C and RH = 55%), and uniformity of particle concentration and size distribution in the aerosol chamber were continuously monitored by a real-time Laser Aerosol Spectrometer (LAS, Model 4705, Aeronanotech, Moscow, Russia) to trace any unfavourable variations in aerosol supply.
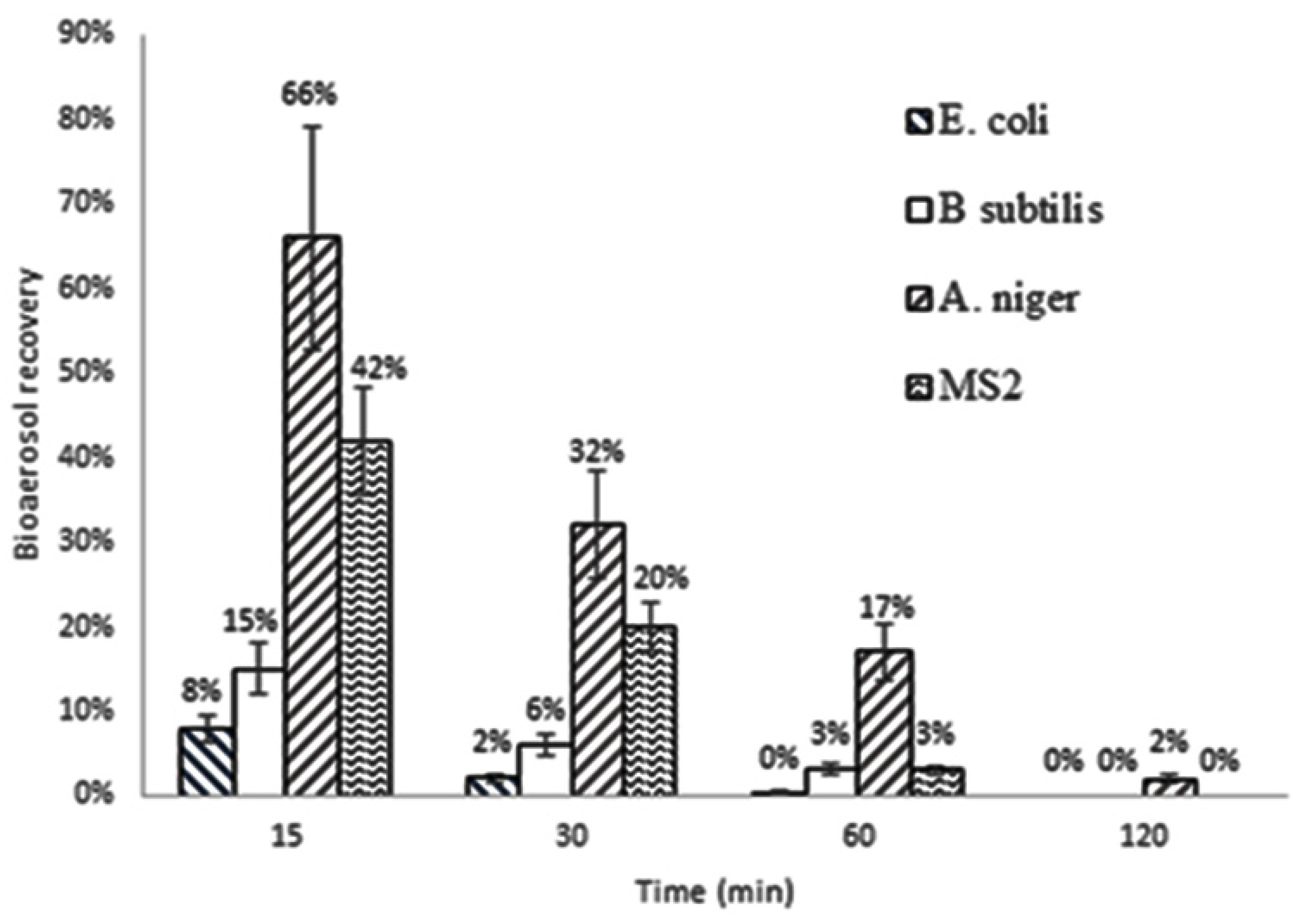
The aerosol samples were collected after 0, 10, 30, 60 and 120 min for E. coli, B. subtilis and 0, 30, 60, 120 and 200 min for A. niger at the sample flow rate of 28.3 L/min by a Single-Stage Viable Bioaerosol Impactor (Cat. No. 225-9611, SKC, Eighty Four, PA, USA) over 12 s of sampling time. Upon completion of sampling, plates were removed from the impactor and placed in an incubator set at 37 °C for 24 h for bacteria, whilst A. niger was grown at 25 °C for 3–7 days. On completion, the CFUs were counted and the results were calculated accordingly.
The
MS2 aerosol samples were collected by “bubbler” type personal bioaerosol samplers [
31,
33] over 1.5 min of sampling time. Sampler’s flow rate was 4 L of air/min. Sampling was undertaken at 15-, 30-, 60- and 120-minute time periods. Each sampler was filled with 40 mL of sampling liquid. Aliquots of collecting liquid (100 μL) from the samplers were taken for plaque assay and fluorescence measurements, enabling the identification of microbial decay as a function of time of essential oils exposure. All results were corrected to account for the sampling-related aerosol dilution, as previously described [
13].
Considering that at each sampling point, approximately 6 L of air (5.7 L by the impactor and 6 L by the sampler) were removed from the chamber (200 L) and replaced with HEPA-filtered air of the same quantity, the following equation was used to correct each particular result to account for such dilution:
where
Ct is corrected microbial concentration at time
t,
CMt is the actual measured microbial concentration at time t and
n is the time-related point number (at 5 min,
n = 0; at 30 min,
n = 1; etc.).
4. Discussion and Conclusions
Essential oils added to the ambient air as vapour or aerosol are effective antimicrobial systems and their advantages over the use of EOs in their liquid phase include an increase in activity, use at lower concentrations and ability to be used in various environments. However, the spectrum of activities of EOs in the air against particular types of micro-organism remains mainly undetermined [
34]. This study assessed the efficacy of the essential oil blend
Melaleuca alternifolia,
Eucalyptus polybractea and
Backhousia citriodora in respective ratios of 4.5:4.5:1.0, dispersed via the VaxiPod oil atomiser on two bacterial (
E. coli and
B. subtilis), one fungal (
A. niger) and one viral (
MS2 bacteriophage) strain commonly found in the atmospheric environment.
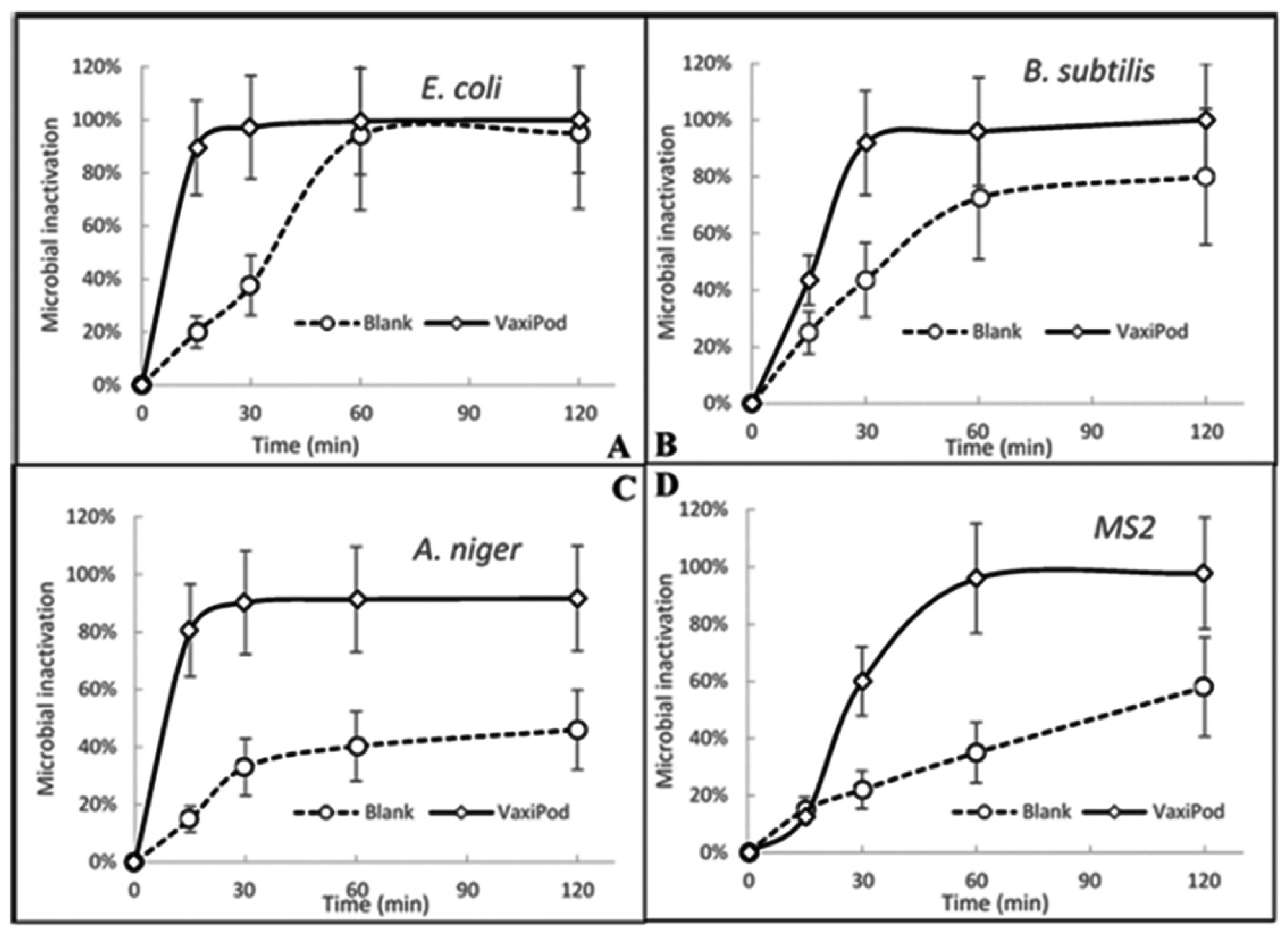
Inactivation of microbial aerosols settled on various surfaces is becoming exceptionally important research and technological question, considering that this is one of the most efficient transmission routes of a range of microbes, including COVID-19. Two surfaces used in this research demonstrated relatively similar results with slightly higher inactivation rates achieved on the stainless steel discs. It was expected that inactivation on the agar plates would be slower; however, the difference between the results is rather minor, dictating that the influence of the air environment dominates in the process, leaving the nature of the surface in second place. Of course, proper wiping of the surface with strong disinfectants would probably be more efficient; however, the proposed way of using VaxiPod would be much lower labour-consuming and could be a preferred option in many situations. The reported results look relatively similar to ones discussed in the Introduction section; however, a very important feature of the VaxiPod, related to its capability of 24 h of non-stop battery operation, enables much more flexible procedures of microbial inactivation to be used.
The results were also very encouraging when the contaminated filters were exposed to the oil blend vapour. As is seen, substantial rates of bacterial and viral inactivation eliminating 99.5% of E. coli, 95.0% of B. subtilis and 96% of MS2 on the filter surface were demonstrated within 60 min of the process run. Even for the very stress-resistant fungal strain of A. niger, the resulting decay of 91.7% was achieved within 2 h of operation. Such outcomes could be directly used in various scenarios. For example, the placement of the Vaxipod in the close vicinity to the air conditioning/ventilation air filters could firstly inactivate microorganisms on their surfaces, and then the vapour and uncaptured fine droplets delivered to the ambient air space can be used to disinfect microbial aerosols in their airborne form. Such placement would also be beneficial in achieving more even distribution of the oil vapour/droplets across substantially sized rooms, as powerful airflows created by ventilation/air conditioning systems would be used as compared to the low flowrate VaxiPod internal pump with much weaker capabilities in mixing with large-scale air flows.
In addition, a very useful feature of the VaxiPod, related to the capability of generating a second mode of aerosol particles with the diameter of a few hundred micrometres, would enable these particles to be effectively removed by even less efficient air filters used in residential and office air conditioning systems, ensuring the presence of a sufficient amount of the oil to be deposited on the filter to undertake the task.
The oil blend vapour dispersed by the Vaxipod demonstrated high efficiency in reducing airborne bacteria, fungi and viruses. The rapid inactivation of E. coli and B. subtilis with 92% and 85%, respectively, was achieved within the first 15 min, with only 0.4% and 3% remaining viable after two hours. Slightly lower inactivation of viral MS2 particle was observed over the first 30 min of the process run; however, the results became very comparable, reaching 97% of inactivation after 60 min. To inactivate 98% of robust fungal aerosols, 120 min of the process run was required.
Of course, it is obvious that these results are rather indicative for common office/residential environments as these facilities are usually not isolated from the outdoor environment and fresh microbial particles will be continuously entering the space under disinfection. However, using more efficient air filters might significantly reduce such streams. Also, a very important issue could be related to the capability of inactivation of aerosols potentially released as a short-term burst, for example, from infected individual temporary attending a place (customer of the small boutique, visitor of a sales office, coffee shop client, etc.). In this case, a limited number of contagious bioaerosols could be quickly and effectively inactivated, minimising the possibility of long-term exposure of permanent staff.
Finally, it must also be noticed that essential oils emit many volatile organic compounds (VOCs), with some considered potentially hazardous [
35]. As a result, using essential oils might be restricted for some groups of individuals.