Physiological and Yield Responses of Spring Wheat Cultivars under Realistic and Acute Levels of Ozone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Experimental Plants
2.3. GH Experimental Setup
2.4. OTC Experimental Setup
2.5. O3 Fumigation and Monitoring
2.6. O3 Exposure Indices and Meteorological Parameters
2.7. Physiological Measurements
2.8. Yield Data Measurement for OTC Experiments
2.9. Statistical Analysis
3. Results
3.1. O3 Concentration and Meteorological Parameters
3.1.1. GH Experiment
3.1.2. OTC Experiment
3.2. Foliar Injury
3.3. Physiological Measurements
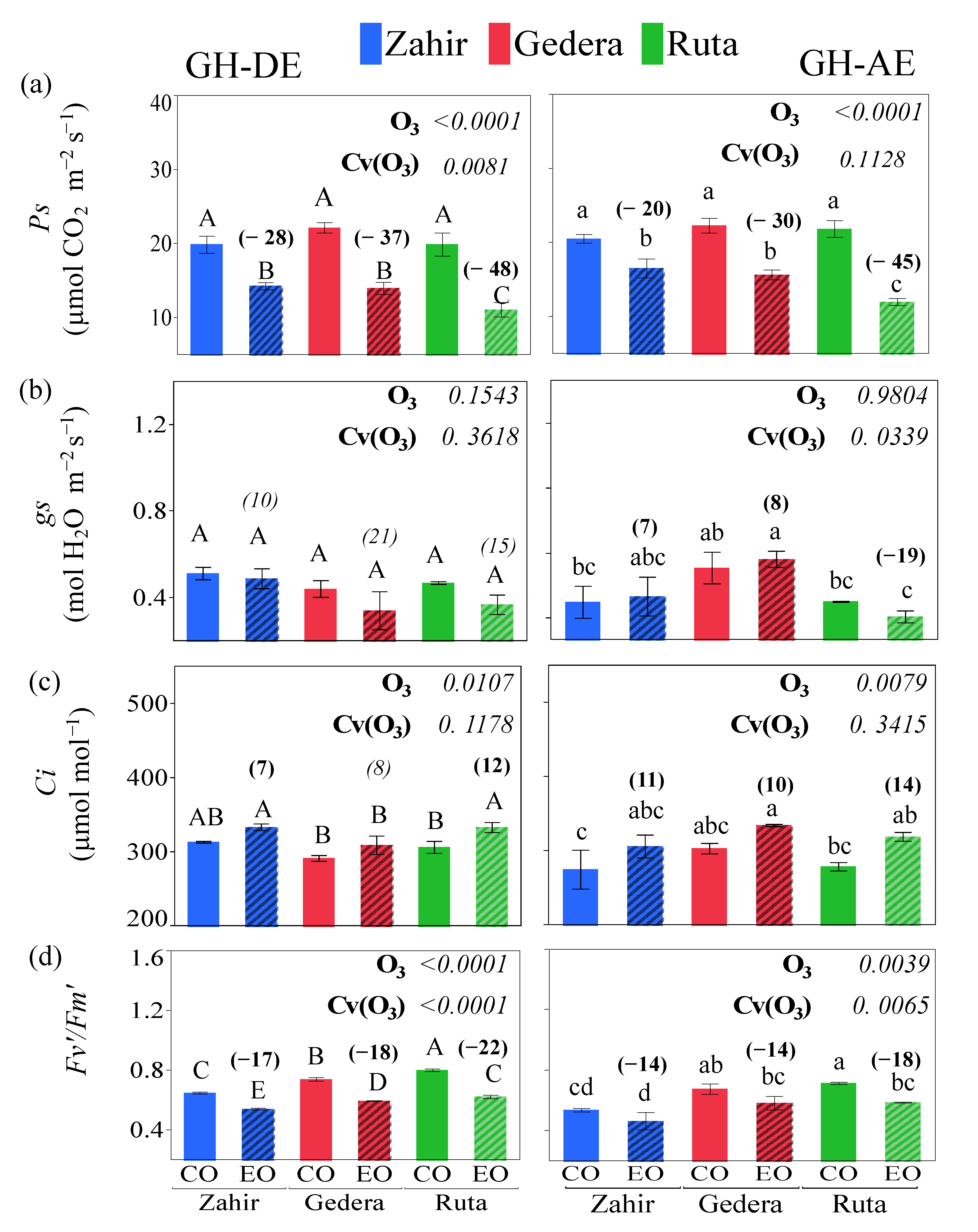
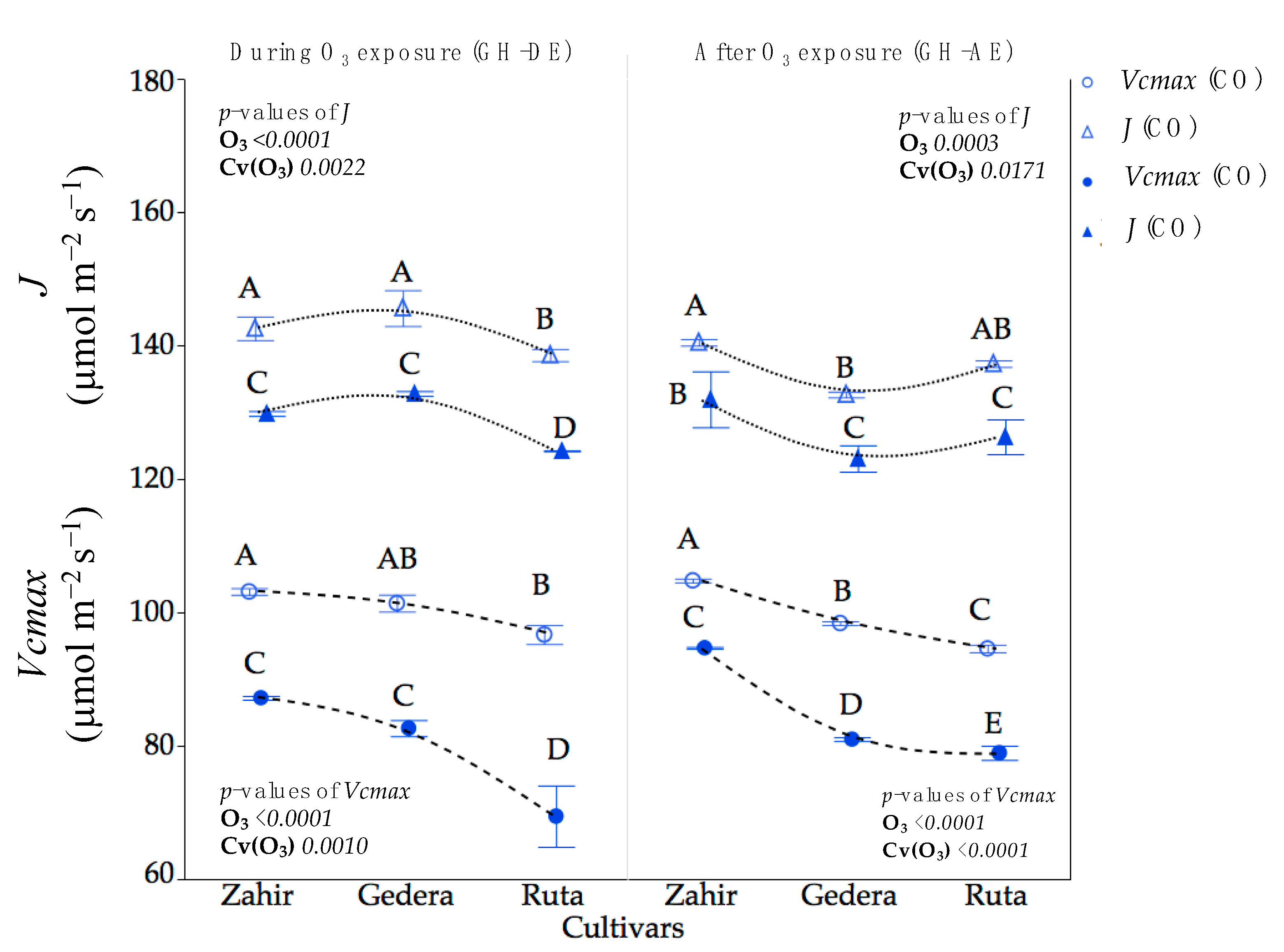
3.3.1. GH Experiment
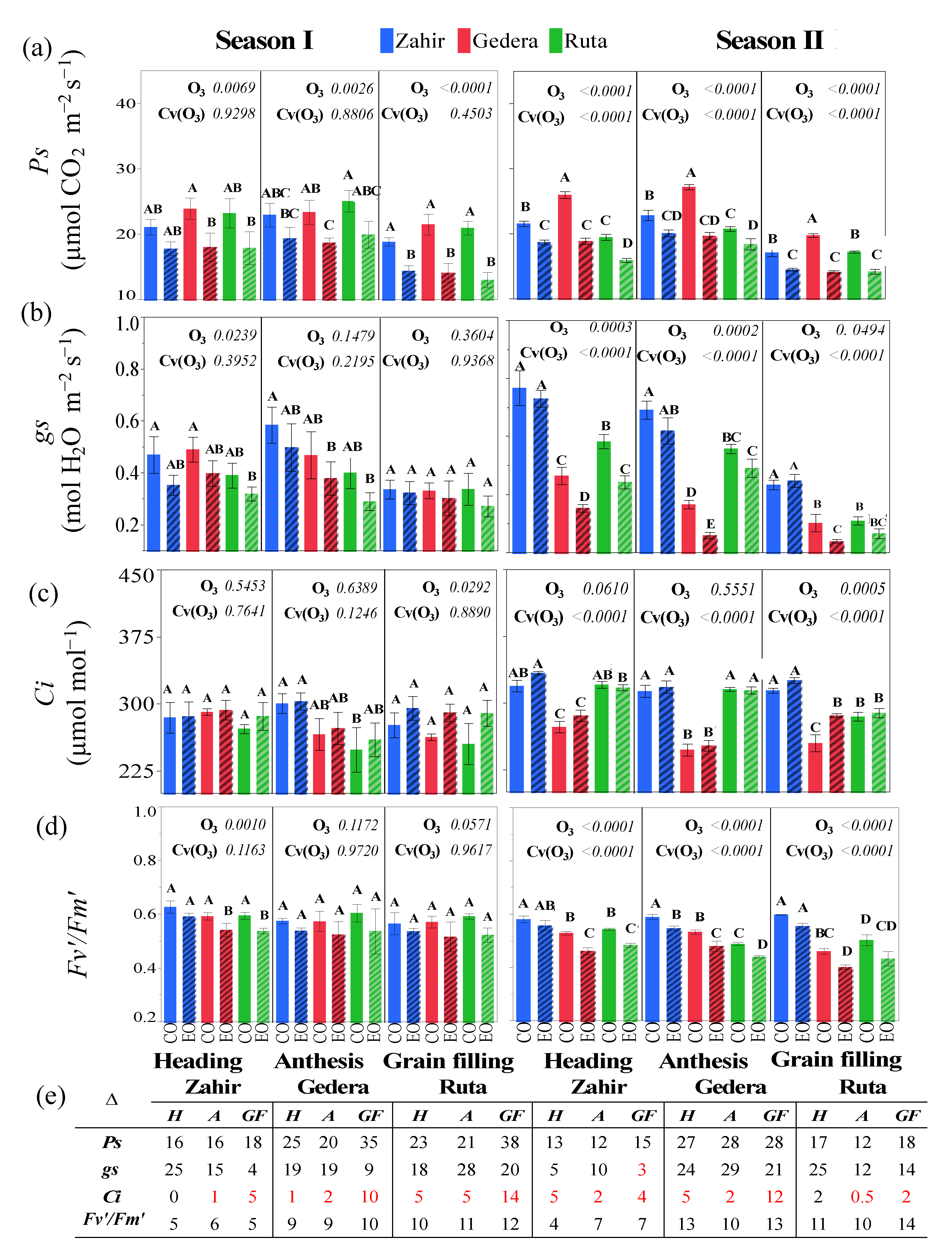
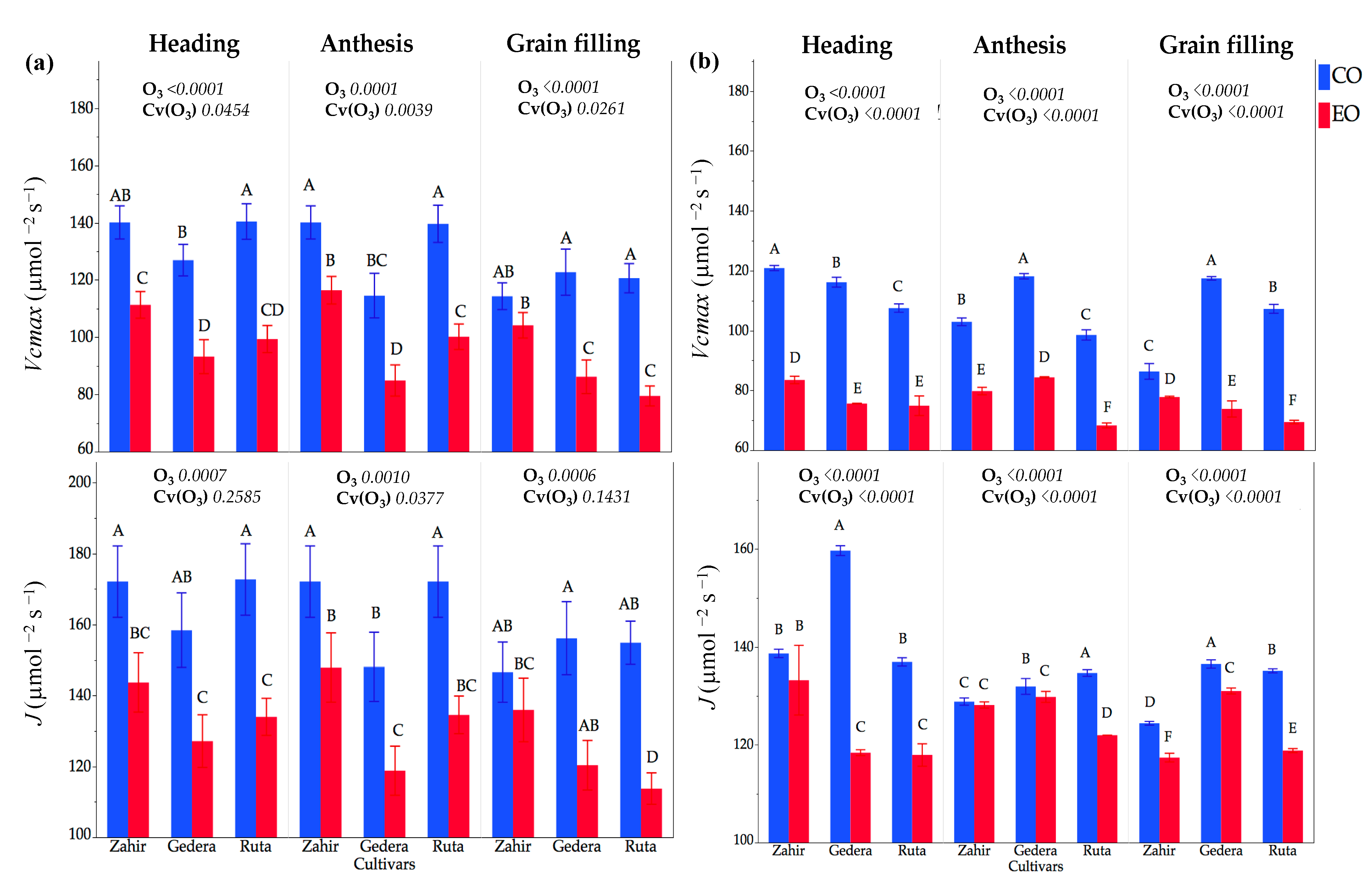
3.3.2. Physiological Measurements in OTC Experiments during Seasons I and II
3.4. Yield
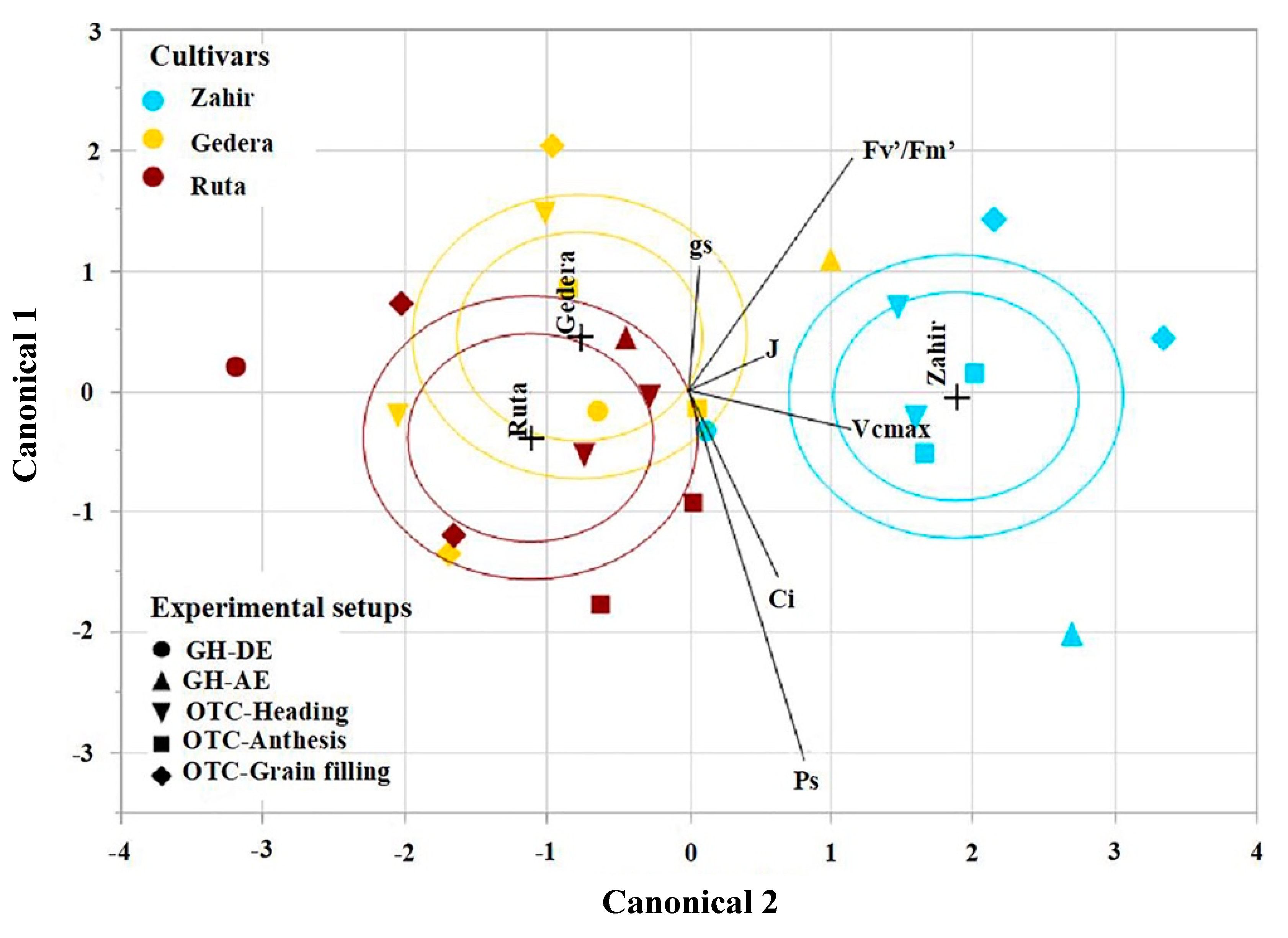
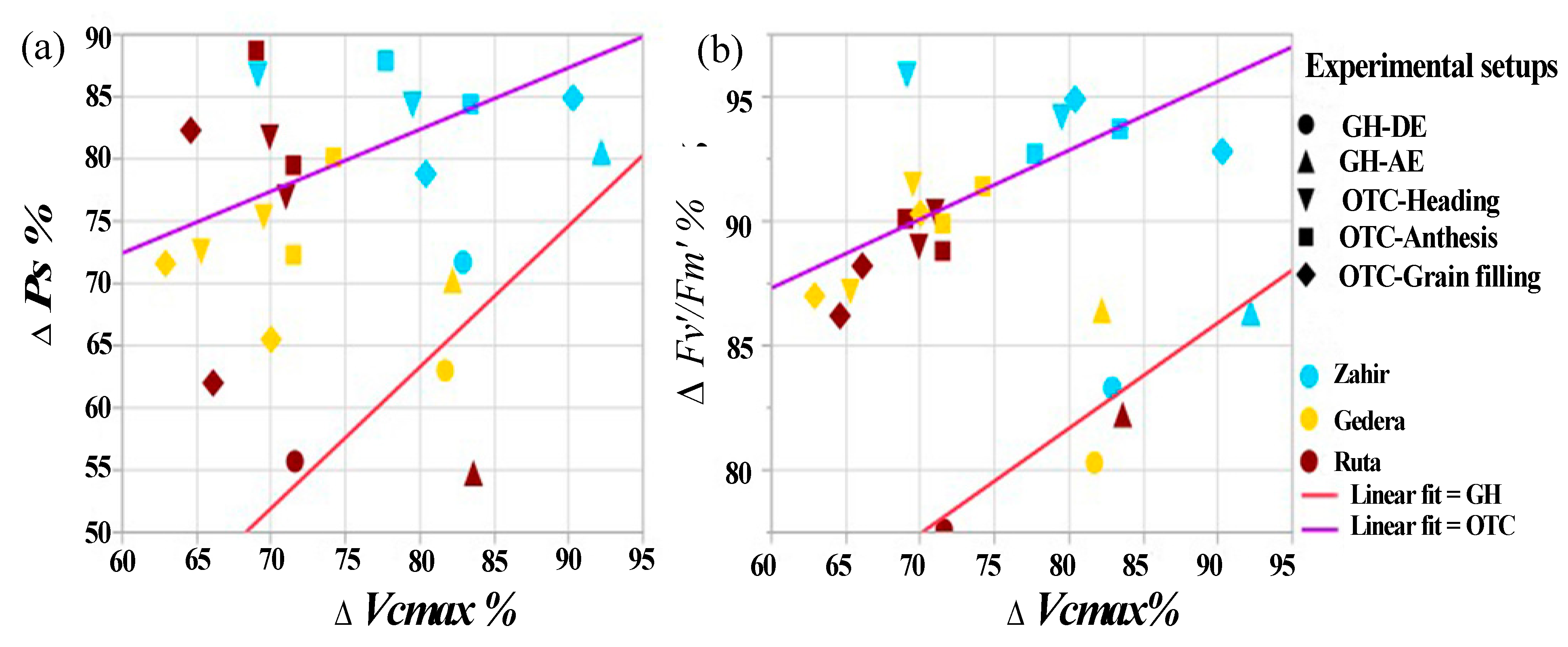
3.5. Overall Cultivar Response to O3 under All Experimental Conditions
4. Discussion
4.1. Physiological Responses under Different Levels of O3 Conditions
4.2. Cultivar-Wise Variation and Mechanism of Plant Response to O3
4.3. Physiological and Foliar Injury Responses at Different Phenological Stages
4.4. Overall Cultivar Response to O3 under All Experimental Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finlayson-Pitts, B.J.; Pitts, J.N. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Elsevier: San Diego, CA, USA, 2000; ISBN 9780080529073. [Google Scholar]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.; Mueller, N.; Burney, J.A.; Zhang, Y.; AghaKouchak, A.; Moore, F.C.; Qin, Y.; Tong, D.; Davis, S.J. Impacts of ozone and climate change on California perennial crops. In Proceedings of the AGU Fall Meeting Abstracts, San Francisco, CA, USA, 9–13 December 2019; p. GC43H-1403. [Google Scholar]
- Von Schneidemesser, E.; Driscoll, C.; Rieder, H.E.; Schiferl, L.D. How will air quality effects on human health, crops and ecosystems change in the future? Philos. Trans. R. Soc. 2020, 378, 20190330. [Google Scholar] [CrossRef]
- Fiscus, E.L.; Booker, F.L.; Burkey, K.O. Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant. Cell. Environ. 2005, 28, 997–1011. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef]
- Mills, G.; Hayes, F.; Simpson, D.; Emberson, L.; Norris, D.; Harmens, H.; Büker, P. Evidence of widespread effects of ozone on crops and (semi-)natural vegetation in Europe (1990–2006) in relation to AOT40-and flux-based risk maps. Glob. Chang. Biol. 2011, 17, 592–613. [Google Scholar] [CrossRef] [Green Version]
- Emberson, L.D.; Pleijel, H.; Ainsworth, E.A.; Van Den Berg, M.; Ren, W.; Osborne, S.; Mills, G.; Pandey, D.; Dentener, F.; Büker, P.; et al. Ozone effects on crops and consideration in crop models. Eur. J. Agron. 2018, 100, 19–34. [Google Scholar] [CrossRef]
- Feng, Z.; Kobayashi, K.; Ainsworth, E.A. Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): A meta-analysis. Glob. Chang. Biol. 2008, 14, 2696–2708. [Google Scholar] [CrossRef]
- Sarkar, A.; Agrawal, S.B. Elevated ozone and two modern wheat cultivars: An assessment of dose dependent sensitivity with respect to growth, reproductive and yield parameters. Environ. Exp. Bot. 2010, 69, 328–337. [Google Scholar] [CrossRef]
- Mills, G.; Buse, A.; Gimeno, B.; Bermejo, V.; Holland, M.; Emberson, L.; Pleijel, H. A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos. Environ. 2007, 41, 2630–2643. [Google Scholar] [CrossRef]
- Chuwah, C.; van Noije, T.; van Vuuren, D.P.; Stehfest, E.; Hazeleger, W. Global impacts of surface ozone changes on crop yields and land use. Atmos. Environ. 2015, 106, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Schauberger, B.; Rolinski, S.; Schaphoff, S.; Müller, C. Global historical soybean and wheat yield loss estimates from ozone pollution considering water and temperature as modifying effects. Agric. For. Meteorol. 2019, 265, 1–15. [Google Scholar] [CrossRef]
- Meyer, U.; Köllner, B.; Willenbrink, J.; Krause, G.H.M. Effects of different ozone exposure regimes on photosynthesis, assimilates and thousand grain weight in spring wheat. Agric. Ecosyst. Environ. 2000, 78, 49–55. [Google Scholar] [CrossRef]
- Pleijel, H.; Broberg, M.C.; Uddling, J.; Mills, G. Current surface ozone concentrations significantly decrease wheat growth, yield and quality. Sci. Total Environ. 2018, 613, 687–692. [Google Scholar] [CrossRef]
- Feng, Z.; Kobayashi, K. Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos. Environ. 2009, 43, 1510–1519. [Google Scholar] [CrossRef]
- Van Dingenen, R.; Dentener, F.J.; Raes, F.; Krol, M.C.; Emberson, L.; Cofala, J. The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmos. Environ. 2009, 43, 604–618. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Frei, M.; Burkey, K.; Emberson, L.; Uddling, J.; Broberg, M.; Feng, Z.; et al. Closing the global ozone yield gap: Quantification and cobenefits for multistress tolerance. Glob. Chang. Biol. 2018, 24, 4869–4893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, S.; Pandey, D.; Mills, G.; Hayes, F.; Harmens, H.; Gillies, D.; Büker, P.; Emberson, L. New insights into leaf physiological responses to ozone for use in crop modelling. Plants 2019, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.S.; Agrawal, S.B.; Agrawal, M. Ozone flux-effect relationship for early and late sown Indian wheat cultivars: Growth, biomass, and yield. Field Crops Res. 2021, 263, 108076. [Google Scholar] [CrossRef]
- Tai, A.P.; Sadiq, M.; Pang, J.; Yung, D.H.; Feng, Z. Impacts of Surface Ozone Pollution on Global Crop Yields: Comparing Different Ozone Exposure Metrics and Incorporating Co-effects of CO2. Front. Sustain. Food Syst. 2021, 5, 63. [Google Scholar] [CrossRef]
- Sinha, B.; Singh Sangwan, K.; Maurya, Y.; Kumar, V.; Sarkar, C.; Chandra, B.P.; Sinha, V. Assessment of crop yield losses in Punjab and Haryana using 2 years of continuous in situ ozone measurements. Atmos. Chem. Phys. 2015, 15, 9555–9576. [Google Scholar] [CrossRef] [Green Version]
- Juráň, S.; Grace, J.; Urban, O. Temporal changes in ozone concentrations and their impact on vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Biswas, D.K.; Xu, H.; Li, Y.G.; Sun, J.Z.; Wang, X.Z.; Han, X.G.; Jiang, G.M. Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Glob. Chang. Biol. 2008, 14, 46–59. [Google Scholar] [CrossRef]
- Shiff, S.; Lensky, I.M.; Bonfil, D.J. Using Satellite Data to Optimize Wheat Yield and Quality under Climate Change. Remote Sens. 2021, 13, 2049. [Google Scholar] [CrossRef]
- Fuhrer, J.; Skärby, L.; Ashmore, M.R. Critical levels for ozone effects on vegetation in Europe. Environ. Pollut. 1997, 97, 91–106. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.V.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.A.; Fatima, A.; Mishra, A.K.; Chaudhary, N.; Mukherjee, A.; Agrawal, M.; Agrawal, S.B. Assessment of ozone toxicity among 14 Indian wheat cultivars under field conditions: Growth and productivity. Environ. Monit. Assess. 2018, 190, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Grulke, N.E. Ozone exposure and stomatal sluggishness in different plant physiognomic classes. Environ. Pollut. 2010, 158, 2664–2671. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, L.; Pleijel, H.; Zhu, J.; Kobayashi, K. Differential effects of ozone on photosynthesis of winter wheat among cultivars depend on antioxidative enzymes rather than stomatal conductance. Sci. Total Environ. 2016, 572, 404–411. [Google Scholar] [CrossRef]
- Zapletal, M.; Juran, S.; Krpes, V.; Michna, K.; Cudlin, P.; Edwards, M. Effect of ozone flux on selected structural and antioxidant characteristics of a mountain norway spruce forest. Balt. For. 2018, 24, 261–267. [Google Scholar]
- Farage, P.K.; Long, S.P. The effects of O3 fumigation during leaf development on photosynthesis of wheat and pea: An in vivo analysis. Photosynth. Res. 1999, 59, 1–7. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Pang, J.; Kobayashi, K.; Zhu, J.; Ort, D.R. Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open-air field conditions. Glob. Chang. Biol. 2011, 17, 580–591. [Google Scholar] [CrossRef]
- Aidoo, M.K.; Quansah, L.; Galkin, E.; Batushansky, A.; Wallach, R.; Moshelion, M.; Bonfil, D.J.; Fait, A. A combination of stomata deregulation and a distinctive modulation of amino acid metabolism are associated with enhanced tolerance of wheat varieties to transient drought. Metabolomics 2017, 13, 138. [Google Scholar] [CrossRef]




| Experimental Setup | Phenological Stages | Season I (December 2016–April 2017) | Season II (December 2017–April 2018) | ||
|---|---|---|---|---|---|
| OTC | Heading | Control O3 (OTC-CO) | Elevated O3 (OTC-EO) | Control O3 (OTC-CO) | Elevated O3 (OTC-EO) |
| Anthesis | |||||
| Grain filling | |||||
| GH | Anthesis (August 2017) | During O3 exposure duration (GH-DE) | After O3 exposure duration (GH-AE) | ||
| Control O3 (CO) | Elevated O3 (EO) | ||||
| Seasons | Cultivars | Sowing Date | Emergence Date | Ozone Fumigation | Phenological Stage | Binning (DAE) | Heading (DAE) | Anthesis (DAE) | Grain Filling (DAE) | Harvesting Date |
|---|---|---|---|---|---|---|---|---|---|---|
| I (2016–2017) | Zahir | 1 December 2016 | 11 December 2016 | 5 February 2017 | Heading | 70–85 | 70 | 10 May 2017 | ||
| Anthesis | 85–100 | 95 | ||||||||
| Grain filling | 100–115 | 106 | ||||||||
| Gedera | 1 December 2016 | 11 December 2016 | 5 February 2017 | Heading | 75–90 | 78 | 10 May 2017 | |||
| Anthesis | 90–105 | 100 | ||||||||
| Grain filling | 105–115 | 115 | ||||||||
| Ruta | 1 December 2016 | 11 December 2016 | 5 February 2017 | Heading | 90–100 | 89 | 10 May 2017 | |||
| Anthesis | 100–110 | 102 | ||||||||
| Grain filling | 110–120 | 119 | ||||||||
| II (2017–2018) | Zahir | 5 December 2017 | 11 December 2017 | 23 December 2017 | Heading | 70–85 | 71 | 22 April 2018 | ||
| Anthesis | 80–90 | 84 | ||||||||
| Grain filling | 85–95 | 93 | ||||||||
| Gedera | 5 December 2017 | 11 December 2017 | 23 December 2017 | Heading | 75–90 | 79 | 22 April 2018 | |||
| Anthesis | 80–90 | 86 | ||||||||
| Grain filling | 90–100 | 93 | ||||||||
| Ruta | 5 December 2017 | 11 December 2017 | 23 December 2017 | Heading | 80–90 | 86 | 22 April 2018 | |||
| Anthesis | 85–95 | 90 | ||||||||
| Grain filling | 90–105 | 98 |
| Season | 12 h | 7 h | |||||
|---|---|---|---|---|---|---|---|
| OTC-CO | OTC-EO | Ambient | OTC-CO | OTC-EO | Ambient | ||
| I | Mean | 25 ± 7 | 32 ± 8 | 20 ± 4 | 27 ± 8 | 36 ± 9 | 22 ± 4 |
| Maximum | 52 | 65 | 42 | 49 | 59 | 33 | |
| Minimum | 2 | 2 | 3 | 2 | 4 | 4 | |
| AOT40 | 0.076 | 1.273 | 0.004 | 0.036 | 0.902 | 0 | |
| II | Mean | 29 ± 6 | 58 ± 12 | 37 ± 14 | 31 ± 7 | 71 ± 16 | 42 ± 14 |
| Maximum | 73 | 222 | 66 | 73 | 173 | 66 | |
| Minimum | 2 | 5 | 3 | 5 | 14 | 0 | |
| AOT40 | 0.76 | 17.43 | 3.21 | 0.54 | 14.3 | 2.59 | |
| Season | Cultivar | Treatment | Total Biomass (g m− 2) | Weight of Grains (g m−2) | Straw Biomass (g m−2) | Weight of Spikes (g m−2) | Weight of 1000 Kernels (g) |
|---|---|---|---|---|---|---|---|
| I | Zahir | OTC-CO | 1422 D | 563 AB | 696 C | 726 B | 42 A |
| OTC-EO | 1331 E | 471 BC | 690 C | 641 B | 39 AB | ||
| Gedera | OTC-CO | 1862 A | 686 A | 913 ABC | 949 A | 37 B | |
| OTC-EO | 1658 B | 532 AB | 787 BC | 871 A | 32 C | ||
| Ruta | OTC-CO | 1531 C | 433 BC | 953 AB | 578 C | 28 D | |
| OTC-EO | 1457 D | 303 C | 1067 A | 390 C | 24 D | ||
| ANOVA | Factors | O3 | <0.0001 | 0.039 | 0.919 | 0.047 | 0.003 |
| Cv(O3) | <0.0001 | 0.052 | 0.027 | 0.0001 | <0.0001 | ||
| II | Zahir | OTC-CO | 1102 D | 483 C | 460 D | 645 D | 44 B |
| OTC-EO | 886 E | 449 C | 391 E | 496 E | 41 C | ||
| Gedera | OTC-CO | 1348 C | 493 C | 544 C | 805 C | 48 A | |
| OTC-EO | 1056 D | 344 D | 448 D | 607 D | 43 BC | ||
| Ruta | OTC-CO | 2024 A | 737 A | 769 A | 1254 A | 42 C | |
| OTC-EO | 1625 B | 597 B | 642 B | 984 B | 40 D | ||
| ANOVA | Factors | O3 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Cv(O3) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, N.; Bonfil, D.J.; Tas, E. Physiological and Yield Responses of Spring Wheat Cultivars under Realistic and Acute Levels of Ozone. Atmosphere 2021, 12, 1392. https://doi.org/10.3390/atmos12111392
Chaudhary N, Bonfil DJ, Tas E. Physiological and Yield Responses of Spring Wheat Cultivars under Realistic and Acute Levels of Ozone. Atmosphere. 2021; 12(11):1392. https://doi.org/10.3390/atmos12111392
Chicago/Turabian StyleChaudhary, Nivedita, David J. Bonfil, and Eran Tas. 2021. "Physiological and Yield Responses of Spring Wheat Cultivars under Realistic and Acute Levels of Ozone" Atmosphere 12, no. 11: 1392. https://doi.org/10.3390/atmos12111392
APA StyleChaudhary, N., Bonfil, D. J., & Tas, E. (2021). Physiological and Yield Responses of Spring Wheat Cultivars under Realistic and Acute Levels of Ozone. Atmosphere, 12(11), 1392. https://doi.org/10.3390/atmos12111392






