Abstract
The chemistry of the nitrate radical and its contribution to organo-nitrate formation in the troposphere has been investigated using a mesoscale 3-D chemistry and transport model, WRF-Chem-CRI. The model-measurement comparisons of NO2, ozone and night-time N2O5 mixing ratios show good agreement supporting the model’s ability to represent nitrate (NO3) chemistry reasonably. Thirty-nine organo-nitrates in the model are formed exclusively either from the reaction of RO2 with NO or by the reaction of NO3 with alkenes. Temporal analysis highlighted a significant contribution of NO3-derived organo-nitrates, even during daylight hours. Night-time NO3-derived organo-nitrates were found to be 3-fold higher than that in the daytime. The reactivity of daytime NO3 could be more competitive than previously thought, with losses due to reaction with VOCs (and subsequent organo-nitrate formation) likely to be just as important as photolysis. This has highlighted the significance of NO3 in daytime organo-nitrate formation, with potential implications for air quality, climate and human health. Estimated atmospheric lifetimes of organo-nitrates showed that the organo-nitrates act as NOx reservoirs, with particularly short-lived species impacting on air quality as contributors to downwind ozone formation.
1. Introduction
The nitrate radical (NO3) dominates night-time oxidation in urban areas in particular, where NO2 and O3 levels are elevated [1,2,3,4]. However, many studies have also highlighted the significance of NO3 in oxidation chemistry over extensive regions of the atmosphere, with high concentrations being reported over a range of atmospheric conditions [1,5,6] and a suggestion that the highest levels exist in the residual boundary layer [7].
Generated from the relatively slow oxidation of NO2 by O3 (Reaction (1)), NO3 only exists in significant concentrations during the night, reaching mixing ratios of 1 ppt or less in remote regions and 10–400 ppt in polluted urban regions [8,9,10,11,12]. Rapid photolysis rates and efficient reaction with NO result in suppressing NO3 mixing ratios with lifetimes of approximately 5 s [13] during daytime hours, but elevated levels have been predicted in winter for example [3,4].
NO3 can react with NO2 to establish a thermal equilibrium with nitrogen pentoxide (N2O5) on a timescale of minutes in the boundary layer (Reaction (2)).
The NO3 radical has a significant impact on atmospheric composition at night-time due to its reaction with numerous unsaturated hydrocarbons [14], thereby influencing the budgets of these species and their degradation products. The reactions of NO3 with unsaturated hydrocarbons, both biogenic and anthropogenic, lead to the formation of multifunctional organo-nitrates [15,16,17].
Organo-nitrates (RONO2) are mainly formed in the atmosphere through photochemical and nocturnal oxidation of anthropogenic and biogenic volatile organic compounds (VOCs); this process is initiated by the OH and NO3 radicals, respectively [18]. During the day, peroxy radicals (RO2) are mainly produced via reaction of VOCs with the OH radical, Cl radical and ozone. These radicals then go on to react with NO by two different pathways: one leads to NO-to-NO2 conversion and hence produces ozone (Reactions (3)–(5)) and the other produces organo-nitrates (Reaction (6)). The branching ratios of Reaction (6) are highly variable with the chain length of the alkyl group, e.g., ≤1% for C2H5ONO2 and ~33% for n-C8H17ONO2 [19]. During the night, the formation of organo-nitrates is dominated by NO3. It is highly reactive towards unsaturated hydrocarbons [14,20], adding to the double bond and generating organo-nitrates via this route (Reaction (7)).
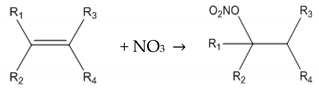
RO2 + NO → RO + NO2
NO2 + hν → NO + O(3P)
O(3P) + O2 + M → O3 + M (M=N2,O2)
RO2 + NO → RONO2

Organic nitrate destruction occurs via photolysis and reaction with OH.
RONO2 + hν → RO + NO2
RONO2 + OH → Products
The relative importance of these two destruction pathways depends on the net UV light intensity, the size of the nitrate and the substitution pattern [21]. For the alkyl nitrates with shorter chain length (≤C4), photolysis is the dominant loss process, whereas for the alkyl nitrates with longer carbon chain (≥C4), oxidation by OH is the dominant loss process [22,23,24]. The alkyl nitrates act as NOx reservoirs, releasing their sequestered NOx by these destruction processes (Reactionss (8) and (9)), which can contribute to photochemical production of O3 in areas far remote from NOx sources.
Organo-nitrates can contribute to regional ozone formation and subsequently affect air quality [25,26,27,28,29,30]. Several studies highlight the formation of organo-nitrates through the reaction of NO3 and unsaturated hydrocarbons such as isoprene and monoterpenes, which have low vapor pressures, allowing them to condense to generate a significant amount of SOA [18,31,32,33,34,35]. A study by Ng et al. [36] reported an SOA yield of between approximately 4 and 24% (in terms of organic mass) when considering the reaction between isoprene and NO3, thus highlighting the significance of these species as SOA precursors. These aerosols are known to have a major impact on the climate and human health [37,38,39,40,41,42].
The complexity of nitrate chemistry and the associated atmospheric and epidemiological implications due to the formation of organo-nitrates drive the need for continued research into the atmospheric processes, which govern NO3 and organo-nitrates in general. An important aspect of this research is the assessment of how models represent nitrate chemistry, as these are useful tools for simulating the processes governing the composition of the atmosphere during both the day- and night-time hours. We used a regional air quality model, WRF-Chem-CRI, to investigate the nitrate chemistry by comparing the model N2O5, NO2 and ozone mixing ratios with measured N2O5, NO2 and ozone mixing ratios collected during the summer months of the ClearfLo project [43], along with the processes which govern organo-nitrate formation.
2. Methodology
2.1. Measurement Site and Measurement Technique
There were no measurements of NO3 during the ClearfLo campaign; instead, we used measured N2O5, NO2 and ozone to compare modelled N2O5, NO2 and ozone, which provide an indication of the model’s ability to represent nitrate chemistry, particularly in terms of NO3. The measurements were made at an urban background site in North Kensington during the summer of the ClearfLo project using a CIMS instrument for N2O5, a chemiluminescence instrument (Air Quality Inc., Oregon City, OR, USA) for NO2 and a UV absorption TEI 49C and 49i (Thermo Scientific, Waltham, MA, USA) for ozone. The details of the instrument and the inlet configuration are described elsewhere [44,45]. The site is located within the grounds of Sion Manning School at 51.521055° N, 0.213432° W. The school is situated within a residential area approximately 7 km west of central London. The road is in close proximity to the site (10 m away) and is a minor road that only experiences high traffic volumes during school drop-off and pick-up times and rush-hour periods. There is also a major road approximately 100 m from the site, which experiences sustained high traffic volumes over the course of the day. The instrument inlet on-site was deployed at a height of ~4 m from the ground.
The N2O5 measurement was performed using a quadrupole chemical ionisation mass spectrometer (CIMS) using iodide ions (I) to detect N2O5 as NO3− (m/z = 62) at a frequency of 1 Hz. Calibration and backgrounding procedures are presented in Bannan et al. [46]. Studies such as Wang et al. [47] have noted that measurements of N2O5 at m/z 62 are not interference-free, with HNO3 and HO2NO2 being identified as key species that could cause interferences at this m/z. Measurements of N2O5 are now routinely made at m/z 235 with iodide CIMS. Nevertheless, Le Breton et al. [48] and Bannan et al. [46] measured N2O5 at m/z 62 concurrently with the Broadband Cavity Enhanced Absorption Spectrometer (BBCEAS) on both ground and airborne measurement campaigns where very good agreement was seen, albeit in more remote locations. Measurements presented here are therefore upper limits of night-time N2O5 as interferences in this measurement location are more likely in comparison to previously reported inter-comparison studies with this instrument.
2.2. WRF-Chem-CRI Model
WRF-Chem-CRI is a regional-scale, three-dimensional meteorological model with online chemistry. The model is fully coupled, whereby the chemistry and aerosol components along with the prognostic meteorological parameters are integrated over the same timestep as the transport processes, using the same advection and physical parameterisations [49]. Meteorological boundary conditions were taken from the European Centre for Medium-Range Forecasts (ECMWF) ERA-Interim reanalysis data [50]. The Model for Ozone And Related chemical Tracers (MOZART) model is an offline global transport model [51] that is used to account for the long-range transport of chemical species from outside the model domain. Biogenic emissions were estimated using an online canopy-scale model, the Model of Emissions of Gases and Aerosols from Nature (MEGAN) [52,53]. The anthropogenic emission for the UK is used in WRF-Chem-CRI from the National Atmospheric Emissions Inventory (NAEI), with a resolution of 1 km by 1 km (http://naei.beis.gov.uk; accessed on 30 September 2021). A coarser emissions database is used for Europe, e.g., TNO emission inventory [54], with a resolution of 0.125° longitude by 0.0625° latitude. The gas-phase scheme used in this study was a reduced chemical scheme, CRI-MECH, which was developed using the Master Chemical Mechanism version 3.1 (MCM 3.1), reducing the number of species and reactions by 90%, with ozone production by a given species equivalent to the number of NO to NO2 conversions that take place during its complete degradation being the primary criterion [55,56]. The CRI-MECH scheme contains 229 species, 529 gas-phase reactions and 96 photolytic reactions. The rate coefficients for the reactions in CRI-MECH specified as a function of temperature were taken from either the MCM (http://mcm.york.ac.uk/; accessed on 30 September 2021) and/or the Jet Propulsion Laboratory kinetic evaluation reports (http://jpldataeval.jpl.nasa.gov/, accessed on 30 September 2021). The model calculates photolysis using the Fast-J scheme [57] and links it to the chemical mechanism in the model. Photolysis rates in and below clouds are modulated by the Fast-J scheme using the aerosol population extinction and phase function to account for the influence of clouds and aerosols. A domain was run using a horizontal grid spacing of 15 km and a size of 134 (E-W) by 146 (N-S) grid points, covering the UK and NW Europe using a Lambert conformal map projection [58,59]. The simulation was run from 00:00 UTC on 30 July 2012 to 00:00 UTC 18 August 2012. The meteorological field was re-initialized every 3 days to ensure that the divergence of the WRF-Chem-CRI meteorology from the driving ECMWF operational/reanalysis meteorology is minimized.
3. Results and Discussion
3.1. Model Validation
The model performance of NO3 has been examined by comparing the model-measurements of NO2, O3 and N2O5 mixing ratios because of their strong dependency on the steady-state concentration of NO3. As shown in the midday and midnight ozone data, the trends in the model predictions are generally very similar to the observations (Figure 1). The modelled O3 data have strong correlations with measurements, having the coefficient of determination (r2) of 0.84 and 0.57 for midday and midnight, respectively. The difference between modelled and measured ozone data referred to as ‘bias’ are found to be −1.4 ppb for midday and −3.4 ppb for midnight. The modelled under-predictions of ozone in North Kensington are most notable at night, which could be due to the capping of the boundary layer in the model being too strong, preventing the replenishment of ground-level ozone with that from the free troposphere. The predicted midday and midnight NO2 data also show similar trends to the observations (Figure 1), with a reasonable correlation (r2 = 0.29 for midday and 0.34 for midnight) and a reasonable bias (−4.5 ppb for midday and −0.4 ppb for midnight). The model underestimation of NO2 is found to be higher during daytime compared with night-time, which can be explained by the coarse resolution of WRF-Chem-CRI, meaning that the processing of sub-grid scale emissions and fast NOx photochemistry is not captured in the simulations, thus acting as a source of model uncertainty. The measured data of N2O5 are only for night-time, which is considered as an upper-limit measurement. We compared the modelled and the measured night-time N2O5 at the surface level and found a similar trend (Figure 1) with a good correlation (r2 = 0.52), but with a sustained underestimation of model N2O5 mixing ratios (bias = −116 ppt). The model did not apply surface dynamics, assuming a uniform, flat surface, thus resulting in an underestimation of the model surface night-time N2O5 levels. The upper limits of measured night-time N2O5 because of the interferences in this urban background location are more likely responsible for the large disagreement between model-measurement of N2O5. A recent study [60] showed that HNO3 would account for >70% of the signal at m/z = 62. Considering this interference in the measurement data of N2O5 could reduce the bias to −16 ppt (~85%). Overall, the WRF-Chem-CRI model performs well in terms of predicting, NO2, O3, and night-time N2O5 levels at the North Kensington site during the ClearfLo project. Therefore, it can be assumed that the NO3 in the model provides a reasonable representation of levels present in the atmosphere.
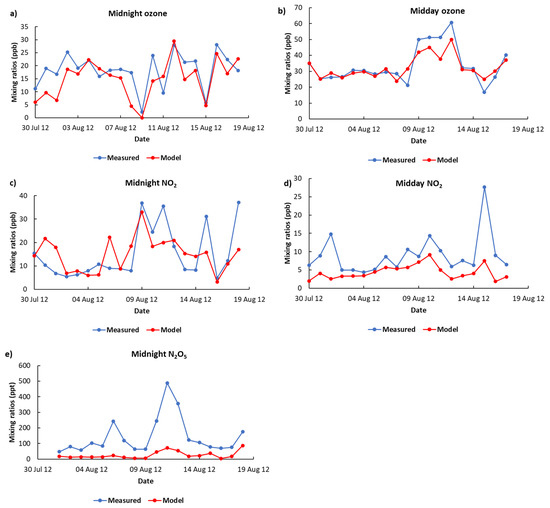
Figure 1.
Comparison of (a) midnight ozone, (b) midday ozone, (c) midnight NO2, (d) midday NO2 and (e) midnight N2O5 mixing ratios modelled by WRF-Chem-CRI with those measured during the summer months of the ClearfLo project.
3.2. Contribution of NO3 Sources Organo-Nitrates
We investigated the formation of 39 organo-nitrates in WRF-Chem-CRI from the production pathways (Table 1) and found that the organo-nitrates are formed solely from the reaction of RO2 with NO (61%) and the reaction of NO3 with alkenes (39%). Modelled mixing ratios of all 39 organo-nitrates from midnight and midday were extracted for each day for the urban background site in North Kensington, which were used for the calculation of the proportion of organo-nitrates formed from NO3. The modelled organo-nitrate mixing ratio for midday (118 ± 103 ppt) is found to be ~2-fold higher than that for midnight (71 ± 47 ppt).

Table 1.
Organo-nitrate species (shown in CRI names) and their respective production pathways and precursors, as represented in the CRI-v2 mechanism of WRF-Chem-CRI.
The contribution of organo-nitrate formation shows that a significant fraction of organo-nitrates at the urban North Kensington site is produced from NO3 in summer (see Figure 2). During summer months, the midnight NO3-sourced organo-nitrate (~62%) is found to be 3-fold higher than that at midday (~21%), which can be explained by the lower abundances of NO3 due to its strong photolysis and OH losses during midday. However, there are still substantial contributions to organo-nitrate formation through VOCs + NO3 during daytime for summer months suggesting that the reaction with VOCs in the environment with high levels of VOCs can significantly contribute to the loss of NO3. Previous studies have reported similar findings with regard to the reactivity of NO3, with losses of NO3 from reactions with organic trace gases being large enough to compete with its photolysis and reaction with NO [3,61,62]. These findings highlight the fact that the NO3 radical could be having a more dominant role in daytime oxidation than previously thought.
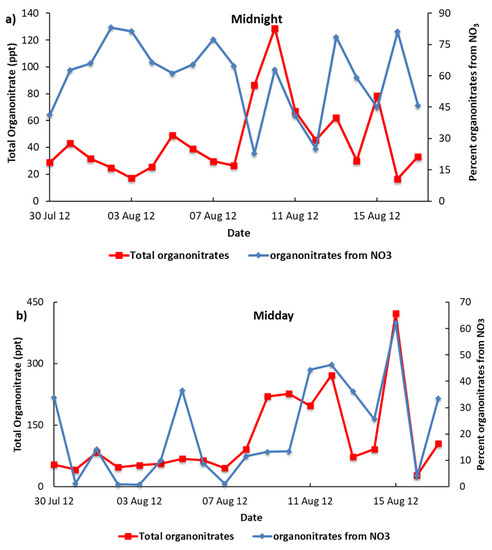
Figure 2.
Total organo-nitrate and contributions sourced from NO3 (a) midnight and (b) midday in North Kensington during summer months.
3.3. Atmospheric Implications of NO3-Sourced Organo-Nitrates
The impacts of the organo-nitrates on the atmosphere, both in terms of SOA formation and their potential contribution to O3 formation, are governed by their lifetimes, i.e., how quickly they degrade within the atmosphere. Thus, the lifetimes of these NO3-derived organo-nitrates were estimated in order to gain insight into their implications for air quality.
The eight longest-lived organo-nitrates derived from NO3 in the WRF-Chem-CRI model are: NRU12OOH (MCM analogues: C510OOH, NC4CO3H), NRN6OOH (MCM analogue: ETHO2HNO3), NRN9OOH (MCM analogues: PR1O2HNO3, PR2O2HNO3), NRN12OOH (MCM analogue: C42NO33OOH), NRTN28OOH (MCM analogues: NAPINAOOH, NAPINBOOH), NOA (MCM analogue: NOA), NRTX28OOH (MCM analogues: NBPINAOOH, NBPINBOOH) and NRU14OOH (MCM analogue: NISOPOOH).
In the model, each of these eight organo-nitrates is either lost by photolysis or by reaction with the OH radical, and the deposition loss of these organo-nitrates is not considered because of their low Henry’s law constants [63]. Model fluxes associated with these reactions, along with global modelled concentrations, were used to derive estimated lifetimes for each organo-nitrate with respect to each loss process. The lifetimes were calculated for the months of July and August. The losses of seven NO3-sourced nitrates (except NOA) in WRF-Chem-CRI are controlled by reaction with the OH radical, with shorter lifetimes often seen in July and August months, due to higher OH abundances and photolysis rates (see Table 2). For example, NRN12OOH has a shorter lifetime of around 1.0 day in July and August months (Table 2). It is likely that these seven nitrates will act as short- or long-term NOx reservoirs, thus having implications for O3 formation and air quality in and around London urban areas. However, NOA is an exception to this, with photolysis dominating its atmospheric loss.

Table 2.
The estimated atmospheric lifetimes of 8 selected organo-nitrates (derived from NO3) with respect to OH and photolysis loss.
As highlighted previously, the lifetime of these species dictates the impact that they will have on surrounding air quality. For example, in the case of NOA, its lifetime stays relatively constant at approximately 16 days. Based on the assumption that it takes approximately 1 day for an air mass to cross the U.K., organo-nitrates with these longer lifetimes will act as long-term reservoirs of NOx, persisting in the atmosphere and being essentially inert in terms of impacts on atmospheric composition or air quality in the U.K. However, the remaining seven are shorter-lived organo-nitrates, with lifetimes of less than 3 days and will start to have an impact locally, the extent of which will have a dependence on wind speed.
Two isoprene-derived organo-nitrates in WRF-Chem-CRI which are particularly short-lived are NRU12OOH and NRU14OOH. These only persist on a timescale of a few hours, thus making them short-term NOx reservoirs with definite impacts on local and regional air quality. In order to understand the atmospheric implications of these organo-nitrates, one must consider their chemistry and the impacts associated with their degradation products. NRU12OOH reacts with the OH radical to produce another organo-nitrate, NOA, which in turn degrades to produce NO2 and the acetyl peroxy radical (CH3C(O)O2). With a lifetime of between 6 and 7 h, an air mass containing NRU12OOH would have enough time to travel away from where it was generated. This means that this organo-nitrate would contribute to O3 production downwind the following day (based on the assumption that it is predominantly generated during the night), thus acting as a potential source of O3 downwind. NRU14OOH reacts with the OH radical to generate multigenerational organo-nitrates. This ultimately results in the formation of NOA, which decomposes to release NO2. As with NRU12OOH, the lifetime of NRU14OOH (approximately 9 h) allows the air to travel away from where it was generated, thus contributing to O3 formation downwind. However, its slightly longer lifetime and the less direct route of producing NOA means that NRU14OOH is able to transport the NOx further from the source, thus contributing to O3 formation and impacting air quality over a wider area.
To quantify the extent of ozone formation from organo-nitrates, another simulation was performed that involved WRF-Chem-CRI being integrated with the exclusion of the chemistry of organo-nitrates in CRI referred to as ‘WRF-Chem-CRI-WON’. Comparing the ozone midday and midnight mixing ratios from WRF-Chem-CRI to WRF-Chem-CRI-WON in North Kensington during July-August shows that up to 1.5 ppb (4%) and 0.8 ppb (2.5%) ozone can be increased during daytime and night-time, respectively, suggesting that organo-nitrates can make a non-negligible contribution to the formation of O3. During midday, the peaks of ozone increase are seen on 12 August 2012 (1.5 ppb; 4%) and 15 August 2012 (1.2 ppb; 3%) (Figure 3a) when the total organo-nitrates and NO3-derived organo-nitrates are the highest among the whole data series (see Figure 2b). The trend of increase in ozone and NO2 for midday is very similar (Pearson correlation, R = 0.7; Figure 3a,b), suggesting that the increase in ozone downwind from North Kensington is associated with maximum NO2 change (e.g., an episode of 12 August 2012; see Figure 4). The ozone increases during midnight are found to be high on 6–7 August 2012, 10–12 August and 14–15 August 2012; however, the ozone increases do not correlate very well (Pearson correlation, R = 0.3) with the increases of NO2 (see Figure 3c,d). This suggests that the ozone formed from organo-nitrates are transported from Europe to North Kensington rather than locally formed (e.g., an episode of 11 August 2012; see Figure 4).
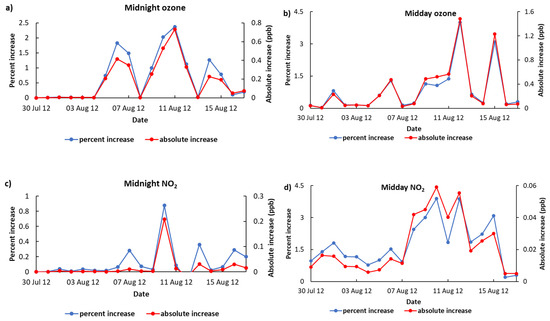
Figure 3.
The percent and absolute increase in ozone and NO2 from WRF-Chem-CRI to WRF-Chem-CRI-WON for (a) midnight ozone, (b) midday ozone, (c) midnight NO2, (d) midday NO2. Note: Absolute increase = (WRF-Chem-CRI–WRF-Chem-CRI-WON) and percent increase = (WRF-Chem-CRI–WRF-Chem-CRI-WON) × 100)/WRF-Chem-CRI-WON.
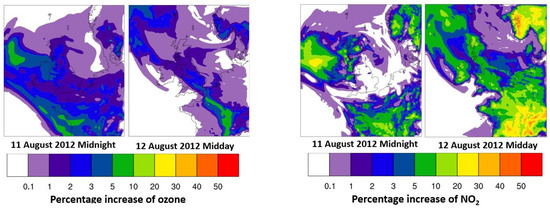
Figure 4.
Percent increases of surface ozone and NO2 mixing ratios from WRF-Chem-CRI to WRF-Chem-CRI-WON at 11 August 2012 midnight and 12 August 2012 midday. Note: Percent increase = (WRF-Chem-CRI–WRF-Chem-CRI-WON) × 100)/WRF-Chem-CRI-WON.
At present, the CRI (Common Representative Intermediates) mechanism traceable to the MCM (Master Chemical Mechanism) used in the WRF-Chem model does not contain organo-nitrates-derived SOA and is focused on optimising the representation of NO–NO2 conversions rather than SOA formation. This consequently means that the model is currently underestimating the SOA contribution from organo-nitrates. The previous study showed that the organo-nitrates (e.g., RTX28NO3, RTN28NO3) in the global model, STOCHEM-CRI, have an impact on SOA formation with a non-negligible contribution (~5%) to the total simulated global SOA [64]. The model, STOCHEM-CRI output [65] shows 0.026 µg m−3 SOA formed from the organo-nitrates, which is ~20% of the total simulated SOA (0.16 µg m−3) formed in the North Kensington in July–August. This therefore highlights the importance of organo-nitrates in the atmosphere and has scope for improving the representation of SOA in the WRF-Chem-CRI model, as the organo-nitrates represented in the chemical mechanism are likely to play an important role in this area.
4. Conclusions
NO3 has long been recognised as the dominant oxidising species in the night-time polluted troposphere, having a significant impact on the degradation chemistry and budgets of numerous VOCs. In the study, the measured night-time N2O5, NO2 and ozone during the summer were used to assess the model’s ability to represent nitrate chemistry (NO3). Extraction of midday and midnight mixing ratios of organo-nitrates gave insight into how the fraction of NO3-derived organo-nitrate varies according to time of day. The temporal and day-night analysis showed that a significant fraction of organo-nitrates are formed from NO3, even during daylight hours, attesting to a more dominant role in daytime organo-nitrate formation than previously thought. The lifetimes of selected long-lived organo-nitrates with respect to the OH radical and photolysis were then estimated, with analysis finding that most organo-nitrates were dominated by OH oxidation. Lifetimes varied on a timescale of days, highlighting their roles as short- and long-term NOx reservoirs and resultant implications for O3 production, air quality and human health. Two isoprene-derived organo-nitrates in particular, NRU12OOH and NRU14OOH were found to persist on a timescale of hours, thus making them likely to contribute to O3 formation and impact on local air quality. The analysis showed that the organo-nitrates can contribute ozone formation up to ~1.6 ppb (midday) and ~0.8 ppb (midnight) at North Kensington during the summer months. This analysis also showed that the organo-nitrates currently represented in WRF-Chem-CRI do not readily condense and therefore do not contribute to SOA. These results highlight the need to consider and improve the SOA representation in the model in the future.
Author Contributions
A.F. and M.A.H.K. analyzed the data and wrote the paper; D.E.S. conceived and designed the project; T.J.B. and C.J.P. performed the measurement of N2O5; C.J.P., M.H.L., T.J.B. and D.E.S. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
A.F. thanks EPSRC for her PhD studentship. M.A.H.K. and D.E.S. thank NERC (grant code-NE/K004905/1), Bristol ChemLabS and the Primary Science Teaching Trust under whose auspices various aspects of this work was supported. C.J.P.’s work was carried out at Jet Propulsion Laboratory, California Institute of Technology, under contract with the National Aeronautics and Space Administration (NASA), and was supported by the Upper Atmosphere Research and Tropospheric Chemistry Programs. © 2021 all rights reserved.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We thank Douglas Lowe for his continuous supports about WRF-Chem modelling throughout the project. We thank the three anonymous reviewers for their insightful reviews and suggestions which has benefitted this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wayne, R.P.; Barnes, I.; Biggs, P.; Burrows, J.P.; Canosas-Mas, C.E.; Hjorth, J.; Le Bras, G.; Moortgat, G.K.; Perner, D.; Poulet, G.; et al. The nitrate radical: Physics, chemistry, and the atmosphere. Atmos. Environ. 1991, 25, 1–203. [Google Scholar] [CrossRef]
- Platt, U.; Alicke, B.; Dubois, R.; Geyer, A.; Hofzumahaus, A.; Holland, F.; Martinez, M.; Mihelcic, D.; Klüpfel, T.; Lohrmann, B.; et al. Free radicals and fast photochemistry during BERLIOZ. J. Atmos. Chem. 2002, 42, 359–394. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Morris, W.C.; Watson, L.A.; Galloway, M.; Hamer, P.D.; Shallcross, B.M.A.; Percival, C.J.; Shallcross, D.E. Estimation of daytime NO3 radical levels in the UK urban atmosphere using the steady state approximation method. Adv. Meteorol. 2015, 2015, 294069. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.H.; Cooke, M.C.; Utembe, S.R.; Archibald, A.T.; Derwent, R.G.; Xiao, P.; Percival, C.J.; Jenkin, M.E.; Morris, W.C.; Shallcross, D.E. Global modelling of the nitrate radical (NO3) for present and pre-industrial scenarios. Atmos. Res. 2015, 164, 347–357. [Google Scholar] [CrossRef]
- Brown, S.S.; Stutz, J. Nighttime radical observations and chemistry. Chem. Soc. Rev. 2012, 41, 6405–6447. [Google Scholar] [CrossRef]
- Brown, S.S.; Ryerson, T.B.; Wollny, A.G.; Brock, C.A.; Peltier, R.; Sullivan, A.P.; Weber, R.J.; Dubé, W.P.; Trainer, M.; Meagher, J.F.; et al. Variability in nocturnal nitrogen oxide processing and its role in regional air quality. Science 2006, 311, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Fish, D.J.; Shallcross, D.E.; Jones, R.L. The vertical distribution of NO3 in the atmospheric boundary layer. Atmos. Environ. 1999, 33, 687–691. [Google Scholar] [CrossRef]
- Stone, D.; Evans, M.J.; Walker, H.; Ingham, T.; Vaughan, S.; Ouyang, B.; Kennedy, O.J.; McLeod, M.W.; Jones, R.L.; Hopkins, J.; et al. Radical chemistry at night: Comparisons between observed and modelled HOx, NO3 and N2O5 during the RONOCO project. Atmos. Chem. Phys. 2014, 14, 1299–1321. [Google Scholar] [CrossRef] [Green Version]
- Aliwell, S.R.; Jones, R.L. Measurements of tropospheric NO3 at midaltitude. J. Geophys. Res. Atmos. 1998, 103, 5719–5727. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Academic Press: Cambidge, MA, USA, 2000. [Google Scholar]
- Asaf, D.; Tas, E.; Pedersen, D.; Peleg, M.; Luria, M. Long-Term Measurements of NO3 Radical at a Semiarid Urban Site: 2. Seasonal Trends and Loss Mechanisms. Environ. Sci. Technol. 2010, 44, 5901–5907. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.L.; Brown, S.S.; Archibald, A.T.; Atlas, E.; Cohen, R.C.; Crowley, J.N.; Day, D.A.; Donahue, N.M.; Fry, J.L.; Fuchs, H.; et al. Nitrate radicals and biogenic volatile organic compounds: Oxidation, mechanisms, and organic aerosol. Atmos. Chem. Phys. 2017, 17, 2103–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics; John Wiley and Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Atkinson, R. Kinetics and mechanism of the gas-phase reactions of the NO3 radical with organic compounds. J. Phys. Chem. Ref. Data 1991, 20, 459–507. [Google Scholar] [CrossRef] [Green Version]
- Platt, U.; LeBras, G.; Poulet, G.; Burrows, J.P.; Moortgat, G. Peroxy radicals from night-time reaction of NO3 with organic compounds. Nature 1990, 348, 147–149. [Google Scholar] [CrossRef]
- Geyer, A.; Alicke, B.; Ackermann, R.; Martinez, M.; Harder, H.; BRUNE, W.; di Carlo, P.; Williams, E.; Jobson, T.; Hall, S.; et al. Direct observations of daytime NO3: Implications for urban boundary layer chemistry. J. Geophys. Res. Atmos. 2003, 108, 4368. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Saathoff, H.; Shen, X.; Ramisetty, R.; Leisner, T.; Mohr, C. Chemical Characterization of Highly Functionalized Organonitrates Contribution to Night-Time Organic Aerosol Mass Loadings and Particle Growth. Environ. Sci. Technol. 2019, 53, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Farmer, D.K.; Matsunaga, A.; Docherty, K.S.; Surratt, J.D.; Seinfeld, J.H.; Ziemann, P.J.; Jimenez, J.L. Response of an aerosol mass spectrometer to organonitrates and organosulfates and implications for atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2010, 107, 6670–6675. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.; Aschmann, S.M.; Carter, W.P.; Winer, A.M.; Pitts, J.N., Jr. Alkyl nitrate formation from the nitrogen oxide (NOx)-air photooxidations of C2-C8 n-alkanes. J. Phys. Chem. 1982, 86, 4563–4569. [Google Scholar] [CrossRef]
- Geyer, A.; Ackermann, R.; Dubois, R.; Lohrmann, B.; Müller, R.; Platt, U. Long-term observation of nitrate radicals in the continental boundary layer near Berlin. Atmos. Environ. 2001, 35, 3619–3631. [Google Scholar] [CrossRef]
- Roberts, J.M. The atmospheric chemistry of organic nitrates. Atmos. Environ. 1990, 24, 243–287. [Google Scholar] [CrossRef]
- Clemitshaw, K.C.; Williams, J.; Rattigan, O.V.; Shallcross, D.E.; Law, K.S.; Cox, R.A. Gas-phase ultraviolet absorption cross-sections and atmospheric lifetimes of several C2-C5 alkyl nitrates. J. Photoch. Photobio. A 1997, 102, 117–126. [Google Scholar] [CrossRef]
- Flocke, F.; Volz-Thomas, A.; Buers, H.J.; Patz, W.; Garthe, H.J.; Kley, D. Long-term measurements of alkyl nitrates in southern Germany: 1. General behavior and seasonal and diurnal variation. J. Geophys. Res. 1998, 103, 5729–5746. [Google Scholar] [CrossRef]
- Talukdar, R.K.; Burkholder, J.B.; Hunter, M.; Gilles, M.K.; Roberts, J.M.; Ravishankara, A.R. Atmospheric fate of several alkyl nitrates Part 2 UV absorption cross-sections and photodissociation quantum yields. J. Chem. Soc. Faraday Trans. 1997, 93, 2797–2805. [Google Scholar] [CrossRef]
- Von Kuhlmann, R.; Lawrence, M.G.; Pöschl, U.; Crutzen, P.J. Sensitivities in global scale modeling of isoprene. Atmos. Chem. Phys. 2004, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Fiore, A.M.; Horowitz, L.W.; Purves, D.W.; Levy, H.; Evans, M.J.; Wang, Y.; Li, Q.; Yantosca, R.M. Evaluating the contributing of changes in isoprene emissions to surface ozone trends over the eastern United States. J. Geophys. Res. Atmos. 2005, 110, D12303. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, L.W.; Fiore, A.M.; Milly, G.P.; Cohen, R.C.; Perring, A.; Wooldridge, P.J.; Hess, P.G.; Emmons, L.K.; Lamarque, J.-F. Observational constraints on the chemistry of isoprene nitrates over the eastern United States. J. Geophys. Res. Atmos. 2007, 112, D12S08. [Google Scholar] [CrossRef]
- Perring, A.E.; Bertram, T.H.; Farmer, D.K.; Wooldridge, P.J.; Dibb, J.; Blake, N.J.; Blake, D.R.; Singh, H.B.; Fuelberg, H.; Diskin, G.; et al. The production and persistence of ΣRONO2 in the Mexico City plume. Atmos. Chem. Phys. 2010, 10, 7215–7229. [Google Scholar] [CrossRef] [Green Version]
- Farmer, D.K.; Perring, A.E.; Wooldridge, P.J.; Blake, D.R.; Baker, A.; Meinardi, S.; Huey, L.G.; Tanner, D.; Vargas, O.; Cohen, R.C. Impact of organic nitrates on urban ozone production. Atmos. Chem. Phys. 2011, 11, 4085–4094. [Google Scholar] [CrossRef] [Green Version]
- Paulot, F.; Henze, D.K.; Wennberg, P.O. Impact of the isoprene photochemical cascade on tropical ozone. Atmos. Chem. Phys. 2012, 12, 1307–1325. [Google Scholar] [CrossRef] [Green Version]
- Griffin, R.J.; Cocker, D.R.; Flagan, R.C.; Seinfeld, J.H. Organic aerosol formation from the oxidation of biogenic hydrocarbons. J. Geophys. Res. Atmos. 1999, 104, 3555–3567. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Goldstein, A.H.; Kroll, J.H.; Ng, N.L.; Varutbangkul, V.; Flagan, R.C.; Seinfeld, J.H. Gas-phase products and secondary aerosol yields from the photooxidation of 16 different terpenes. J. Geophys. Res. Atmos. 2006, 111, D17305. [Google Scholar] [CrossRef] [Green Version]
- Rollins, A.W.; Kiendler-Scharr, A.; Fry, J.L.; Brauers, T.; Brown, S.S.; Dorn, H.-P.; Dubé, W.P.; Fuchs, H.; Mensah, A.; Mentel, T.F.; et al. Isoprene oxidation by nitrate radical: Alkyl nitrate and secondary organic aerosol yields. Atmos. Chem. Phys. 2009, 9, 6685–6703. [Google Scholar] [CrossRef] [Green Version]
- Slade, J.H.; de Perre, C.; Lee, L.; Shepson, P.B. Nitrate radical oxidation of γ -terpinene: Hydroxy nitrate, total organic nitrate, and secondary organic aerosol yields. Atmos. Chem. Phys. 2017, 17, 8635–8650. [Google Scholar] [CrossRef] [Green Version]
- Fry, J.L.; Brown, S.S.; Middlebrook, A.M.; Edwards, P.M.; Campuzano-Jost, P.; Day, D.A.; Jimenez, J.L.; Allen, H.M.; Ryerson, T.B.; Pollack, I.; et al. Secondary organic aerosol (SOA) yields from NO3 radical + isoprene based on nighttime aircraft power plant plume transects. Atmos. Chem. Phys. 2018, 18, 11663–11682. [Google Scholar] [CrossRef] [Green Version]
- Ng, N.L.; Kwan, A.J.; Surratt, J.D.; Chan, A.W.H.; Chhabra, P.S.; Sorooshian, A.; Pye, H.O.T.; Crounse, J.D.; Wennberg, P.O.; Flagan, R.C.; et al. Secondary organic aerosol (SOA) formation from reaction of isoprene with nitrate radicals (NO3). Atmos. Chem. Phys. 2008, 8, 4117–4140. [Google Scholar] [CrossRef] [Green Version]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2013: The Physical Scientific Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Nel, A. Air pollution-related illness: Effects of particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef]
- Sharaiwa, M.; Ueda, K.; Pozzer, A.; Lammel, G.; Kampf, C.J.; Fushimi, A.; Enami, S.; Arangio, A.M.; Fröhlich-Nowoisky, J.; Fujitani, Y.; et al. Aerosol Heatlth Effects from Molecular to Global Scales. Environ. Sci. Technol. 2017, 51, 13545–13567. [Google Scholar] [CrossRef]
- Rollins, A.W.; Browne, E.C.; Min, K.-E.; Pusede, S.E.; Wooldridge, P.J.; Gentner, D.R.; Goldstein, A.H.; Liu, S.; Day, D.A.; Russell, L.M.; et al. Evidence for NOx control over nighttime SOA formation. Science 2012, 337, 1210–1212. [Google Scholar] [CrossRef]
- Kiendler-Scharr, A.; Mensah, A.A.; Friese, E.; Topping, D.; Nemitz, E.; Prevot, A.S.H.; Äijälä, M.; Allan, J.; Canonaco, F.; Canagaratna, M.; et al. Ubiquity of organic nitrates from nighttime chemistry in the European submicron aerosol. Geophys. Res. Lett. 2016, 43, 7735–7744. [Google Scholar] [CrossRef] [Green Version]
- Bohnenstengel, S.I.; Belcher, S.E.; Aiken, A.; Allan, J.D.; Allen, G.; Bacak, A.; Bannan, T.J.; Barlow, J.F.; Beddows, D.C.S.; Bloss, W.J.; et al. Meteorology, Air Quality, and Health in London: The ClearfLo Project. Bull. Am. Meteorol. Soc. 2015, 96, 779–804. [Google Scholar] [CrossRef] [Green Version]
- Bannan, T.J.; Bacak, A.; Muller, J.B.; Booth, A.M.; Jones, B.; Le Breton, M.; Leather, K.E.; Ghalaieny, M.; Xiao, P.; Shallcross, D.E.; et al. Importance of direct anthropogenic emissions of formic acid measured by a chemical ionisation mass spectrometer (CIMS) during the Winter ClearfLo Campaign in London, January 2012. Atmos. Environ. 2014, 83, 301–310. [Google Scholar] [CrossRef]
- Bannan, T.J.; Booth, A.M.; Bacak, A.; Muller, J.B.A.; Leather, K.E.; Le Breton, M.; Jones, B.; Young, D.; Coe, H.; Allan, J.; et al. The first UK measurements of nitryl chloride using a chemical ionisation mass spectrometer in central London in the summer of 2012, and an investigation of the role of Cl atom oxidation. J. Geophys. Res. Atmos. 2015, 120, 5638–5657. [Google Scholar] [CrossRef]
- Bannan, T.J.; Bacak, A.; Le Breton, M.; Flynn, M.; Ouyang, B.; McLeod, M.; Jones, R.; Malkin, T.L.; Whalley, L.K.; Heard, D.E.; et al. Ground and airborne UK measurements of nitryl chloride: An investigation of the role of Cl atom oxidation at Weybourne Atmospheric Observatory. J. Geophys. Res. Atmos. 2017, 122, 11–154. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, T.; Yan, C.; Tham, Y.J.; Xue, L.; Xu, Z.; Zha, Q. Large daytime signals of N2O5 and NO3 inferred at 62 amu in a TD-CIMS: Chemical interference or a real atmospheric phenomenon? Atmos. Meas. Tech. 2014, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Le Breton, M.; Bacak, A.; Muller, J.B.A.; Bannan, T.J.; Kennedy, O.; Ouyang, B.; Xiao, P.; Ashfold, M.N.R.; Bauguitte, S.J.-B.; Shallcross, D.E.; et al. The first airborne inter-comparison of N2O5 measurements over the UK using a Chemical Ionisation Mass Spectrometer (CIMS) and Broadband Cavity Enhanced Absorption Spectrometer (BBCEAS) during the RONOCO 2010/2011 campaign. Anal. Meth. 2014, 6, 9731–9743. [Google Scholar] [CrossRef] [Green Version]
- Grell, G.A.; Peckham, S.E.; Schmitz, R.; McKeen, S.A.; Frost, G.; Skamarock, W.C.; Eder, B. Fully coupled “online” chemistry within the WRF model. Atmos. Environ. 2005, 39, 6957–6975. [Google Scholar] [CrossRef]
- Dee, D.P.; Uppala, S.M.; Simmons, A.J.; Berrisford, P.; Poli, P.; Kobayashi, S.; Andrae, U.; Balmaseda, M.A.; Balsamo, G.; Bauer, P.; et al. The ERA-interim reanalysis: Configuration and performance of the data assimilation system. Q. J. R. Meteorol. Soc. 2011, 137, 553–597. [Google Scholar] [CrossRef]
- Emmons, L.K.; Walters, S.; Hess, P.G.; Lamarque, J.-F.; Pfizer, G.G.; Fillmore, D.; Granier, C.; Guenther, A.; Kinnison, D.; Laepple, T.; et al. Description and evaluation of the Model for Ozone and Related chemical Tracers, version 4 (MOZART-4). Geosci. Model. Dev. 2010, 3, 43–67. [Google Scholar] [CrossRef] [Green Version]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, P.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef] [Green Version]
- Sakulyanontvittaya, T.; Duhl, T.; Wiedinmyer, C.; Helmig, D.; Matsunaga, S.; Potosnak, M.; Milford, J.; Guenther, A. Monoterpene and sesquiterpene emission estimates for the United States. Environ. Sci. Technol. 2008, 42, 1623–1629. [Google Scholar] [CrossRef] [Green Version]
- Kuenen, J.J.P.; Visschedijk, A.J.H.; Jozwicka, M.; Denier van der Gon, H.A.C. TNO-MACC_IIemission inventory; a multi-year (2003–2009) consistent high-resolution European emission inventory for air quality modelling. Atmos. Chem. Phys. 2014, 14, 10963–10976. [Google Scholar] [CrossRef] [Green Version]
- Jenkin, M.E.; Watson, L.A.; Utembe, S.R.; Shallcross, D.E. A Common Representative Intermediates (CRI) mechanism for VOC degradation. Part 1: Gas phase mechanism development. Atmos. Environ. 2008, 42, 7185–7195. [Google Scholar] [CrossRef]
- Watson, L.A.; Shallcross, D.E.; Utembe, S.R.; Jenkin, M.E. A Common Representative Intermediates (CRI) mechanism for VOC degradation. Part 2: Gas phase mechanism reduction. Atmos. Environ. 2008, 42, 7196–7204. [Google Scholar] [CrossRef]
- Wild, O.; Zhu, X.; Prather, M.J. Fast-J: Accurate simulation of IN- and Below-Cloud Photolysis in Tropospheric Chemical Models. J. Atmos. Chem. 2000, 37, 245–282. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Clements, J.; Lowe, D.; McFiggans, G.; Percival, C.J.; Shallcross, D.E. Investigating the behaviour of the CRI-MECH gas-phase chemistry scheme on a regional scale for different seasons using the WRF-Chem model. Atmos. Res. 2019, 229, 145–156. [Google Scholar] [CrossRef]
- Archer-Nicholls, S.; Lowe, D.; Utembe, S.; Allan, J.; Zaveri, R.A.; Fast, J.D.; Hodnebrog, Ø.; van der Gon, H.D.; McFiggans, G. Gaseous chemistry and aerosol mechanism developments for version 3.5.1 of the online regional model, WRF-Chem. Geosci. Model. Dev. 2014, 7, 2557–2579. [Google Scholar] [CrossRef] [Green Version]
- Dörich, R.; Eger, P.; Lelieveld, J.; Crowley, J.N. Iodide CIMS and m/z 62: The detection of HNO3 as NO3- in the presence of PAN, peroxyacetic acid and ozone. Atmos. Meas. Tech. 2021, 14, 5319–5332. [Google Scholar] [CrossRef]
- Liebmann, J.; Karu, E.; Sobanski, N.; Schuladen, J.; Ehn, M.; Schallhart, S.; Quéléver, L.; Hellen, H.; Hakola, H.; Hoffmann, T.; et al. Direct measurement of NO3 radical reactivity in a boreal forest. Atmos. Chem. Phys. 2018, 18, 3799–3815. [Google Scholar] [CrossRef] [Green Version]
- Liebmann, J.M.; Muller, J.B.A.; Kubistin, D.; Claude, A.; Holla, R.; Plass-Dülmer, C.; Lelieveld, J.; Crowley, J.N. Direct measurements of NO3 reactivity in and above the boundary layer of a mountaintop site: Identification of reactive trace gases and comparison with OH reactivity. Atmos. Chem. Phys. 2018, 18, 12045–12059. [Google Scholar] [CrossRef] [Green Version]
- Kames, J.; Schurath, U. Alkyl nitrates and bifunctional nitrates of atmospheric interest: Henry’s law constants and their temperature dependencies. J. Atmos. Chem. 1992, 15, 79–95. [Google Scholar] [CrossRef]
- Utembe, S.R.; Cooke, M.C.; Archibald, A.T.; Shallcross, D.E.; Derwent, R.G.; Jenkin, M.E. Simulating secondary organic aerosol in a 3-D Lagrangian chemistry transport model using the reduced Common Representative Intermediates mechanism (CRI v2-R5). Atmos. Environ. 2011, 45, 1604–1614. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Jenkin, M.E.; Foulds, A.; Derwent, R.G.; Percival, C.J.; Shallcross, D.E. A modeling study of secondary organic aerosol formation from sesquiterpenes using the STOCHEM global chemistry and transport model. J. Geophys. Res. Atmos. 2017, 122, 4426–4439. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).