Water-Soluble Anions in PM10 Samples Collected in the Metropolitan Area of Costa Rica: Temporal and Spatial Variations
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Filter Extraction
2.3. Chemical Analysis
2.4. Statistical Analysis
2.5. Time Series
3. Results and Discussion
3.1. Concentrations of Water-Soluble Ions and PM10
- (1)
- The sustained growth of the vehicular fleet, as the ratio of vehicles per 1000 inhabitants duplicated between 1994–2014, from 132 to 263 [34];
- (2)
- A policy recently implemented by the Costa Rican Oil Refinery to make two types of fuel oil available to the industrial market, one of which has a much lower sulfur content than the regular one, which contains around 2% [35].
3.2. Correlation Patterns between Variables
3.3. Principal Component Analysis (PCA)
3.4. Analysis of the Time Series
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Observatorio urbano del Gran Área Metropolitana (OUGAM). Datos Estadísticos y Geográficos del Área Metropolitana de Costa Rica; Universidad de Costa Rica: San José, Costa Rica, 2015. [Google Scholar]
- Herrera, J. Sexto Informe Anual de la Calidad del Aire del Gran Área Metropolitana de Costa Rica; Universidad Nacional: Heredia, Costa Rica, 2016; pp. 14–29. [Google Scholar]
- Li, J.J.; Wang, G.H.; Zhou, B.H.; Cheng, C.L.; Cao, J.J.; Shen, Z.X.; An, Z.S. Chemical composition and size distribution of wintertime aerosols in the atmosphere of Mt.Hua in Central China. Atmos. Environ. 2011, 45, 1251–1258. [Google Scholar] [CrossRef]
- Norouzi, S.; Khademi, H. Source identification of heavy metals in atmospheric dust using Platanus orientalis L. leaves as bioindicator. Eurasian J. Soil Sci. 2015, 4, 144–219. [Google Scholar] [CrossRef][Green Version]
- National Pollutant Inventory. Particle Matter (PM10 and PM2.5); Australian Government, Department of the Environment and Energy: San José, Costa Rica, 2019.
- United States Environmental Protection Agency. Particulate Matter (PM) Pollution. 2018. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed on 5 June 2021).
- World Health Organization. Ambient (Outdoor) Air Quality and Health. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 6 June 2021).
- Goudarzi, G.; Geravandi, S.; Mohammadi, M.J.; Vosoughi, M.; Angali, K.A.; Zallaghi, E.; Neisi, A.K.; Saeidimehr, S.; Mohammadi, B. Total number of deaths and respiratory mortality attributed to particulate matter (PM10) in Ahvaz, Iran during 2009. Int. J. Environ. Health Eng. 2015, 4, 1–7. [Google Scholar]
- Khaniabadi, Y.O.; Goudarzi, G.; Daryanoosh, S.M.; Borgini, A.; Tittarelli, A.; De Marco, A. Exposure to PM10, NO2 and O3 and impacts on human health. Environ. Sci. Pol. Res. 2017, 24, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, K.; Biswal, S.K. Effect of particulate matter (PM) on plants, climate, ecosystem and human health. Int. J. Adv. Technol. Eng. Sci. 2014, 2, 118–129. [Google Scholar]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef]
- Rahul, J.; Jain, M.K. An investigation into the impact of particulate matter on vegetation along the national highway: A review. Res. J. Environ. Sci. 2014, 7, 356–372. [Google Scholar] [CrossRef]
- Ulrichs, C.; Welke, B.; Mucha-Pelzer, T.; Goswami, A.; Mewis, I. Effect of solid particulate matter deposits on vegetation—A review. Funct. Plant Sci. Biotechnol. 2008, 2, 56–62. [Google Scholar]
- Miuc, A.; Voncina, E.; Lešnik, U. Composition of organic compounds adsorbed on PM10 in the air above Maribor. Acta Chim. Slov. 2015, 62, 834–848. [Google Scholar] [CrossRef][Green Version]
- Uchiyama, S.; Inaba, Y.; Kunugita, L. Ozone removal in the collection of carbonyl compounds in air. J. Chromatogr. A 2012, 1229, 293–297. [Google Scholar] [CrossRef]
- Satsangi, A.; Pachauri, T.; Singla, V.; Lakhani, A.; Maharaj Kumari, K. Water soluble ion species in atmospheric aerosols: Concentrations and sources at Agra in the indo-Gangetic plain (IGP). Aerosol Air Qual. Res. 2013, 13, 1877–1889. [Google Scholar] [CrossRef]
- Tan, J.; Duan, J.; Zhen, N.; He, K.; Hao, J. Chemical characteristics and source of size-fractionated atmospheric particle in haze episode in Beijing. Atmos. Res. 2016, 167, 24–33. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K.; Barrie, L.A. Photochemical and other sources of organic compounds in the Canadian high Arctic aerosol pollution during winter-spring. Environ. Sci. Technol. 2009, 43, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhuang, G.; Tang, A.; Yuan, H.; Sun, Y.; Chen, S.; Zheng, A. The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 2005, 39, 3771–3784. [Google Scholar] [CrossRef]
- Khan, M.F.; Shirasuna, Y.; Hirano, K.; Masunaga, S. Characterization of PM2.5, PM2.5−10 and PM>10 in ambient air, Yokohama, Japan. Atmos. Res. 2010, 96, 159–172. [Google Scholar] [CrossRef]
- Kawamura, K.; Bikkina, S. A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources, and transformation. Atmos. Res. 2016, 170, 140–160. [Google Scholar] [CrossRef]
- Chianese, E.; Tirimberio, G.; Riccio, A. PM2.5 and PM10 in the urban area of Naples: Chemical composition, chemical properties and influence of air masses origin. J. Atmos. Chem. 2019, 76, 151–169. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Kistler, M.; Kireeva, E.D.; Persiantseva, N.M.; Timofeev, M.A.; Shoniya, N.K.; Kopeikin, V.M. Aerosol composition and microstructure in the smoky atmosphere of Moscow during the August 2010 extreme wildfires. Izv. Atmos. Ocean. Phys. 2017, 53, 49–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.H.; Bian, Q.J.; Griffith, S.M.; Louie, P.K.; Yu, J. Sources and atmospheric processes impacting oxalate at a suburban coastal site in Hong Kong: Insights inferred from 1-year hourly measurements. J. Geophys. Res. Atmos. 2015, 120, 9772–9788. [Google Scholar] [CrossRef]
- Souza, D.Z.; Vasconcellos, P.C.; Lee, H.; Aurela, M.; Saarnio, K.; Teinilä, K.; Hillamo, R. Composition of PM2.5 and PM10 collected at urban sites in Brazil. Aerosol Air Qual. Res. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Wang, F.; Huang, J.; Xia, F.; Lou, M.; Wu, Y.; Jiang, J.H.; Xie, T.; Zhaxi, Y. Three-dimensional structure of aerosol in China: A perspective from multi-satellite observations. Atmos. Res. 2016, 178, 580–589. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, I.M.; Tsai, S.S.; Yang, C.Y. Correlation of Asian dust storm events with daily clinic visits for allergic rhinitis in Taipei, Taiwan. J. Toxicol. Environ. Health-Part A 2006, 69, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, I.; Juarros-Basterretxea, J.; Robles-Fernández, A.; Basteiro, J.; García-Cueto, E. Pruebas de bondad de ajuste en distribuciones simétricas, ¿Qué estadístico utilizar? Univ. Psychol. 2014, 14, 245–254. [Google Scholar] [CrossRef]
- Minitab. ¿Qué es el Método de Tukey para Comparaciones Múltiples? Available online: https://support.minitab.com/es-mx/minitab/18/help-and-how-to/modeling-statistics/anova/supporting-topics/multiple-comparisons/what-is-tukey-s-method/ (accessed on 7 December 2020).
- Herzog, M.H.; Francis, G.; Clarke, A. ANOVA. In Understanding Statistics and Experimental Design. Learning Materials in Biosciences; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Roy-García, I.; Rivas-Ruiz, R.; Pérez-Rodríguez, M.; Palacios-Cruz, L. Correlación: No toda correlación implica causalidad. Rev. Alerg. México 2019, 66, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kaskaoutis, G.; Singh, P.; Singh, S. Seasonal variability of atmospheric aerosol parameters over Greater Noida using ground sunphotometer observations. Aerosol Air Qual. Res. 2014, 14, 608–622. [Google Scholar] [CrossRef]
- Kuniyal, J.C.; Sharma, M.; Chand, K.; Mathela, C.S. Water soluble ionic components in particulate matter (PM10) during high pollution episode days at Mohal and Kothi in the North-Western Himalaya, India. Aerosol Air Qual. Res. 2015, 15, 529–543. [Google Scholar] [CrossRef]
- Ministerio de Ambiente y Energía. VII Plan Nacional de Energía 2015–2030; Programa de las Naciones Unidas para el Desarrollo: San José, Costa Rica, 2015.
- RECOPE. Manual de Productos; Refinadora Costarricense de Petróleo: San José, Costa Rica, 2019. [Google Scholar]
- Wang, Y.; Zhuang, G.; Chen, S.; An, Z.; Zheng, A. Characteristics and sources of formic, acetic and oxalic acids in PM2.5 and PM10 aerosols in Beijing, China. Atmos. Res. 2007, 84, 169–181. [Google Scholar] [CrossRef]
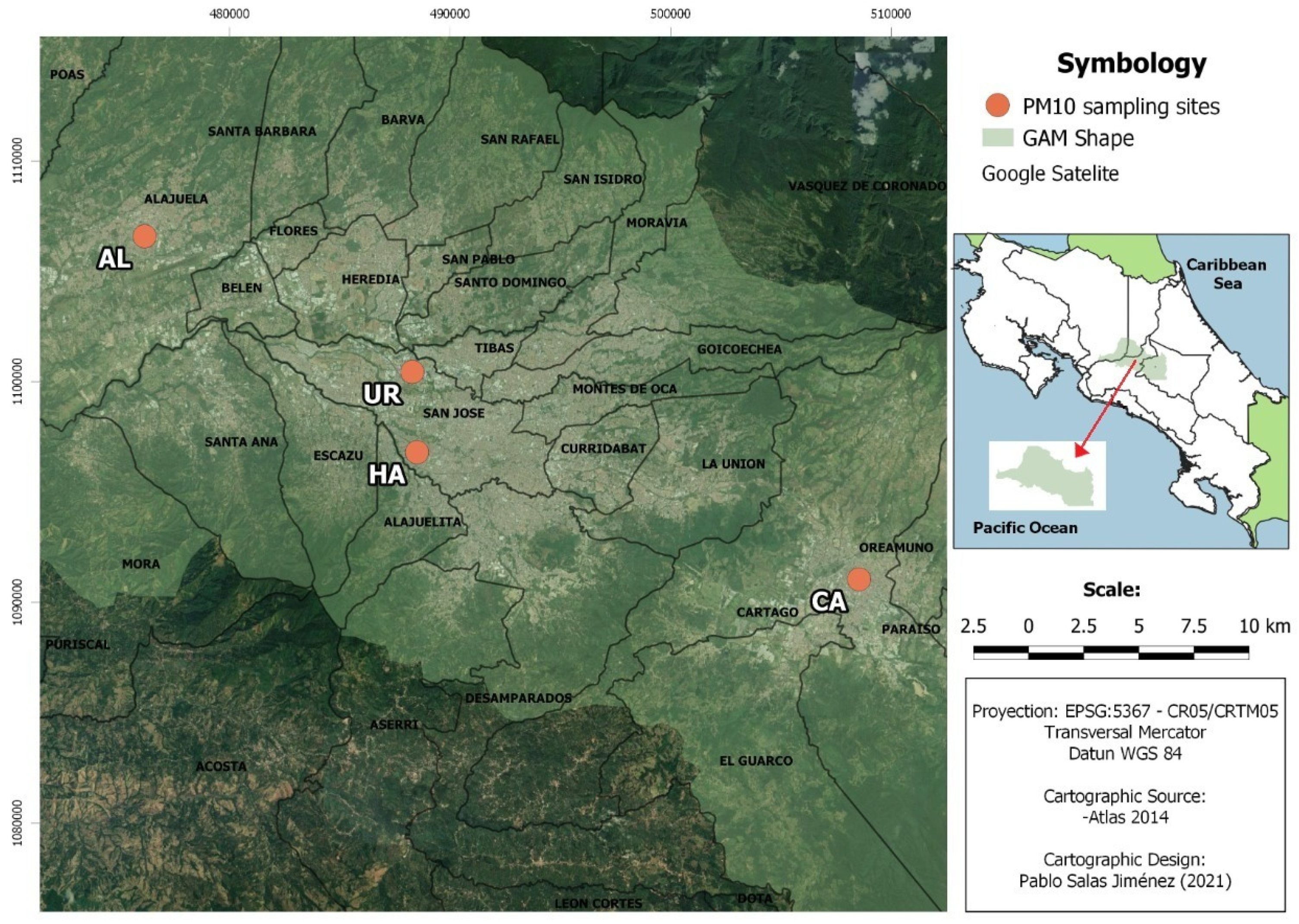






| Sites | Type | Coordinates | Height (m) | |
|---|---|---|---|---|
| Latitude | Longitude | |||
| Hatillo (HA) | Residential | 9°55′8.60″ N | 84°6′17.80″ W | 1114 |
| Uruca (UR) | Industrial−Commercial | 9°57′7.24″ N | 84°6′24.95″ W | 1098 |
| Cartago (CA) | Commercial | 9°52′1.38″ N | 83°55′19.61″ W | 1448 |
| Alajuela (AL) | Commercial−Residential | 10°0′28.12″ N | 81°13′3.88″ W | 904 |
| Sampling Sites | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|
| HA | 76 | 95 | 106 | 93 | 91 | 110 | 95 | 102 |
| UR | 100 | 87 | 101 | 98 | 94 | 114 | 101 | 99 |
| CA | 92 | 109 | 112 | 101 | 108 | 105 | 98 | 97 |
| AL | 93 | 99 | 103 | 95 | 96 | 102 | 104 | 106 |
| Ion Specie | Detection Limits (µgm−3) |
|---|---|
| Fluoride (F−) | 0.01 |
| Formate (HCOO−) | 0.01 |
| Acetate (CH3COO−) | 0.03 |
| Chloride (Cl−) | 0.02 |
| Nitrite (NO2−) | 0.02 |
| Nitrate (NO3−) | 0.02 |
| Phosphate (PO43−) | 0.03 |
| Sulfate (SO42−) | 0.03 |
| Oxalate (C2O42−) | 0.02 |
| Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM10 | F− | |||||||||||||||
| HA | 34 (19) | 27 (7) | 28 (9) | 26 (7) | 25 (6) | 26 (9) | 26 (8) | 25 (7) | 0.25 (0.11) | 0.31 (0.13) | 0.29 (0.08) | 0.18 (0.05) | 0.24 (0.07) | 0.20 (0.08) | 0.19 (0.07) | 0.21 (0.08) |
| UR | 32 (11) | 29 (8) | 31 (9) | 30 (9) | 32 (9) | 31 (8) | 32 (11) | 31 (9) | 0.18 (0.16) | 0.30 (0.11) | 0.27 (0.09) | 0.22 (0.06) | 0.21 (0.07) | 0.16 (0.09) | 0.23 (0.07) | 0.17 (0.08) |
| CA | 29 (10) | 25 (8) | 26 (9) | 26 (6) | 25 (6) | 24 (6) | 24 (7) | 26 (8) | 0.15 (0.09) | 0.16 (0.07) | 0.25 (0.14) | 0.22 (0.06) | 0.19 (0.08) | 0.21 (0.06) | 0.15 (0.07) | 0.12 (0.08) |
| AL | 27 (6) | 22 (6) | 27 (10) | 26 (6) | 28 (9) | 25 (6) | 25 (5) | 23 (4) | 0.17 (0.09) | 0.20 (0.07) | 0.22 (0.06) | 0.19 (0.06) | 0.19 (0.07) | 0.13 (0.05) | 0.11 (0.06) | 0.12 (0.08) |
| HCOO− | CH3COO− | |||||||||||||||
| HA | 0.13 (0.07) | 0.21 (0.13) | 0.26 (0.08) | 0.37 (0.11) | 0.34 (0.10) | 0.29 (0.08) | 0.32 (0.09) | 0.27 (0.06) | 0.17 (0.07) | 0.21 (0.08) | 0.24 (0.10) | 0.20 (0.08) | 0.18 (0.09) | 0.23 (0.01) | 0.25 (0.09) | 0.22 (0.07) |
| UR | 0.30 (0.07) | 0.33 (0.09) | 0.35 (0.12) | 0.42 (0.09) | 0.40 (0.08) | 0.37 (0.06) | 0.41 (0.08) | 0.34 (0.07) | 0.31 (0.08) | 0.34 (0.11) | 0.50 (0.14) | 0.43 (0.07) | 0.39 (0.09) | 0.44 (0.10) | 0.48 (0.12) | 0.42 (0.11) |
| CA | 0.65 (0.26) | 0.57 (0.32) | 0.71 (0.20) | 0.70 (0.24) | 0.66 (0.15) | 0.68 (0.23) | 0.59 (0.17) | 0.64 (0.27) | 0.24 (0.14) | 0.26 (0.10) | 0.24 (0.15) | 0.31 (0.12) | 0.28 (0.11) | 0.30 (0.16) | 0.25 (0.10) | 0.27 (0.09) |
| AL | 0.75 (0.34) | 0.55 (0.12) | 0.87 (0.25) | 0.75 (0.31) | 0.72 (0.42) | 0.66 (0.26) | 0.57 (0.31) | 0.64 (0.28) | 0.57 (0.25) | 0.62 (0.18) | 0.67 (0.22) | 0.70 (0.14) | 0.67 (0.27) | 0.63 (0.23) | 0.55 (0.18) | 0.60 (0.14) |
| Cl− | NO2− | |||||||||||||||
| HA | 1.56 (0.80) | 1.33 (0.51) | 1.41 (0.43) | 1.37 (0.37) | 1.78 (0.86) | 1.18 (0.53) | 1.05 (0.62) | 1.14 (0.71) | 0.46 (0.21) | 0.28 (0.12) | 0.42 (0.14) | 0.33 (0.17) | 0.29 (0.10) | 0.36 (0.14) | 0.32 (0.18) | 0.25 (0.09) |
| UR | 1.36 (0.60) | 1.03 (0.35) | 1.63 (0.51) | 1.46 (0.33) | 1.81 (0.81) | 1.43 (0.83) | 0.98 (0.52) | 1.06 (0.62) | 0.29 (0.10) | 0.26 (0.13) | 0.34 (0.10) | 0.30 (0.07) | 0.26 (0.09) | 0.38 (0.11) | 0.26 (0.09) | 0.38 (0.11) |
| CA | 1.29 (0.73) | 1.12 (0.45) | 1.84 (0.80) | 1.28 (0.34) | 1.74 (0.73) | 1.56 (0.81) | 1.04 (0.67) | 1.08 (0.51) | 0.19 (0.07) | 0.26 (0.10) | 0.52 (0.13) | 0.43 (0.11) | 0.37 (0.08) | 0.41 (0.02) | 0.29 (0.10) | 0.35 (0.09) |
| AL | 1.13 (0.54) | 0.94 (0.23) | 1.45 (0.40) | 1.08 (0.21) | 1.68 (0.73) | 1.29 (0.96) | 0.95 (0.56) | 1.01 (0.63) | 0.17 (0.06) | 0.21 (0.06) | 0.25 (0.10) | 0.33 (0.09) | 0.27 (0.08) | 0.34 (0.07) | 0.25 (0.07) | 0.28 (0.08) |
| NO3− | PO43− | |||||||||||||||
| HA | 1.10 (0.32) | 0.90 (0.28) | 1.20 (0.28) | 1.17 (0.24) | 1.19 (0.25) | 0.93 (0.14) | 0.87 (0.22) | 0.96 (0.19) | 1.46 (0.58) | 0.87 (0.38) | 0.77 (0.21) | 0.95 (0.27) | 1.07 (0.34) | 0.91 (0.28) | 1.14 (0.33) | 1.26 (0.35) |
| UR | 0.95 (0.41) | 0.92 (0.25) | 1.21 (0.36) | 1.07 (0.26) | 1.28 (0.35) | 1.14 (0.43) | 0.99 (0.27) | 1.07 (0.39) | 1.23 (0.37) | 1.01 (0.28) | 1.08 (0.40) | 0.84 (0.27) | 1.16 (0.41) | 1.04 (0.33) | 1.26 (0.53) | 1.34 (0.48) |
| CA | 0.66 (0.29) | 1.01 (0.40) | 1.41 (0.56) | 1.33 (0.35) | 1.06 (0.36) | 1.13 (0.27) | 0.94 (0.37) | 1.02 (0.28) | 1.03 (0.55) | 0.70 (0.41) | 0.69 (0.38) | 0.71 (0.25) | 0.88 (0.36) | 0.85 (0.32) | 0.95 (0.27) | 1.01 (0.35) |
| AL | 0.84 (0.33) | 0.79 (0.22) | 1.16 (0.58) | 1.29 (0.37) | 0.90 (0.34) | 1.06 (0.42) | 0.95 (0.38) | 0.99 (0.35) | 0.84 (0.58) | 0.68 (0.37) | 0.77 (0.34) | 0.81 (0.29) | 0.89 (0.33) | 0.85 (0.28) | 0.91 (0.24) | 0.96 (0.37) |
| SO42− | C2O42− | |||||||||||||||
| HA | 4.94 (2.16) | 4.43 (1.44) | 4.02 (1.77) | 3.84 (1.11) | 3.70 (1.06) | 3.82 (1.37) | 3.57 (1.25) | 3.89 (1.62) | 0.32 (0.07) | 0.26 (0.09) | 0.22 (0.06) | 0.28 (0.08) | 0.24 (0.05) | 0.30 (0.07) | 0.26 (0.08) | 0.29 (0.05) |
| UR | 4.51 (2.18) | 4.11 (0.92) | 4.28 (1.15) | 4.10 (1.36) | 4.17 (1.06) | 4.35 (1.27) | 4.13 (0.98) | 4.05 (1.05) | 0.52 (0.11) | 0.47 (0.12) | 0.61 (0.24) | 0.57 (0.19) | 0.64 (0.32) | 0.61 (0.26) | 0.50 (0.23) | 0.55 (0.28) |
| CA | 3.98 (1.68) | 3.71 (1.36) | 4.01 (1.80) | 3.42 (1.28) | 3.51 (0.98) | 3.45 (1.45) | 3.22 (0.75) | 3.13 (0.62) | 0.27 (0.13) | 0.35 (0.12) | 0.53 (0.16) | 0.41 (0.11) | 0.47 (0.15) | 0.44 (0.12) | 0.36 (0.18) | 0.40 (0.10) |
| AL | 4.15 (1.73) | 4.03 (1.58) | 4.53 (1.36) | 3.67 (0.88) | 3.75 (0.98) | 3.86 (0.81) | 3.67 (0.78) | 3.57 (0.69) | 0.41 (0.01) | 0.47 (0.17) | 0.52 (0.14) | 0.49 (0.21) | 0.40 (0.16) | 0.44 (0.14) | 0.37 (0.12) | 0.40 (0.10) |
| HA | CA | |||||||||||||||||
| Parameter | PM10 | F− | HCOO− | CH3COO− | Cl− | NO2− | NO3− | PO43− | SO42− | PM10 | F− | HCOO− | CH3COO− | Cl− | NO2− | NO3− | PO43− | SO42− |
| F− | 0.018 | −0.092 | ||||||||||||||||
| HCOO− | −0.017 | −0.081 | −0.028 | 0.024 | ||||||||||||||
| CH3COO− | −0.032 | 0.089 | 0.401 | 0.018 | 0.040 | 0.062 | ||||||||||||
| Cl− | −0.011 | −0.107 | −0.042 | −0.094 | 0.037 | 0.094 | 0.152 | 0.078 | ||||||||||
| NO2− | −0.033 | −0.239 | 0.247 | 0.212 | 0.107 | −0.103 | 0.278 | 0.059 | 0.121 | 0.287 | ||||||||
| NO3− | 0.497 | −0.149 | 0.386 | 0.546 | 0.208 | 0.404 | 0.430 | 0.184 | 0.138 | 0.006 | 0.273 | 0.435 | ||||||
| PO43− | 0.012 | −0.163 | −0.175 | −0.229 | −0.008 | −0.070 | −0.036 | 0.114 | −0.025 | −0.048 | 0.021 | 0.108 | −0.179 | −0.162 | ||||
| SO42− | 0.574 | 0.053 | 0.094 | −0.085 | 0.036 | 0.087 | 0.520 | 0.106 | 0.482 | 0.120 | 0.096 | 0.030 | 0.118 | 0.259 | 0.611 | −0.092 | ||
| C2O42− | 0.141 | −0.032 | 0.258 | 0.262 | 0.031 | 0.528 | 0.256 | −0.193 | −0.041 | −0.081 | 0.457 | −0.09 | 0.111 | 0.221 | 0.430 | 0.315 | −0.122 | 0.077 |
| UR | AL | |||||||||||||||||
| F− | −0.034 | 0.079 | ||||||||||||||||
| HCOO− | −0.026 | 0.150 | 0.219 | 0.115 | ||||||||||||||
| CH3COO− | 0.091 | 0.162 | 0.396 | 0.066 | −0.005 | 0.427 | ||||||||||||
| Cl− | −0.009 | 0.026 | 0.160 | 0.199 | 0.170 | −0.040 | 0.381 | 0.062 | ||||||||||
| NO2− | 0.014 | 0.347 | 0.343 | 0.422 | 0.325 | 0.095 | 0.281 | 0.383 | 0.497 | 0.106 | ||||||||
| NO3− | 0.635 | 0.169 | 0.408 | 0.435 | 0.174 | 0.432 | 0.462 | 0.246 | 0.351 | 0.451 | 0.224 | 0.492 | ||||||
| PO43− | 0.140 | −0.107 | −0.026 | 0.046 | −0.035 | −0.098 | 0.002 | 0.069 | 0.066 | 0.046 | 0.230 | 0.043 | 0.108 | −0.041 | ||||
| SO42− | 0.530 | 0.016 | 0.077 | 0.185 | 0.277 | 0.203 | 0.577 | 0.016 | 0.414 | 0.134 | 0.269 | 0.006 | 0.196 | 0.110 | 0.462 | −0.075 | ||
| C2O42− | 0.055 | 0.112 | 0.210 | 0.325 | 0.157 | 0.405 | 0.290 | 0.065 | 0.131 | 0.117 | 0.138 | 0.083 | 0.055 | 0.142 | 0.485 | 0.162 | 0.009 | 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Murillo, J.; Soto-Murillo, T.; Rojas-Marín, J.F.; Beita-Guerrero, V.H.; Hidalgo-Gutiérrez, M. Water-Soluble Anions in PM10 Samples Collected in the Metropolitan Area of Costa Rica: Temporal and Spatial Variations. Atmosphere 2021, 12, 1264. https://doi.org/10.3390/atmos12101264
Herrera-Murillo J, Soto-Murillo T, Rojas-Marín JF, Beita-Guerrero VH, Hidalgo-Gutiérrez M. Water-Soluble Anions in PM10 Samples Collected in the Metropolitan Area of Costa Rica: Temporal and Spatial Variations. Atmosphere. 2021; 12(10):1264. https://doi.org/10.3390/atmos12101264
Chicago/Turabian StyleHerrera-Murillo, Jorge, Tomas Soto-Murillo, José Félix Rojas-Marín, Victor Hugo Beita-Guerrero, and María Hidalgo-Gutiérrez. 2021. "Water-Soluble Anions in PM10 Samples Collected in the Metropolitan Area of Costa Rica: Temporal and Spatial Variations" Atmosphere 12, no. 10: 1264. https://doi.org/10.3390/atmos12101264
APA StyleHerrera-Murillo, J., Soto-Murillo, T., Rojas-Marín, J. F., Beita-Guerrero, V. H., & Hidalgo-Gutiérrez, M. (2021). Water-Soluble Anions in PM10 Samples Collected in the Metropolitan Area of Costa Rica: Temporal and Spatial Variations. Atmosphere, 12(10), 1264. https://doi.org/10.3390/atmos12101264






