Abstract
Culturing is still the most widely used method for determining fungal growth. Thus, is important to identify the most suitable culture media to assess Aspergillus spp. The aim of this study was to analyze data obtained from previous studies, aiming at identifying the most suitable culture media (malt extract agar (MEA) or dichloran-glycerol agar (DG18) to assess Aspergillus spp. isolation and growth. This study was conducted by using environmental samples (n = 1153). Most of the active sampling methods (air samples) were impacted directly onto both culture media. As for passive sampling methods, fungi were extracted from environmental matrices inoculated onto both media. Overall, total Aspergillus counts were higher in MEA (n = 617, 53.5%) than in DG18 (n = 536, 46.5%). Regarding Aspergillus sections, significant associations were detected with the media (χ2 (7) = 241.118, p < 0.001), the sampling approach (p < 0.001, 95% CI = (0.3 × 10−4), and the indoor environment (p < 0.001, 95% CI = (0.3 × 10−4)). As such, sampling approach and the culture media should be accurately selected when dealing with Aspergillus spp. exposure assessment.
1. Introduction
Airborne microorganisms are able to spread into most of our living environments, including indoor [1]. Indoor exposure to high concentrations of microorganisms has been recognized as one of the main transmission routes for infectious diseases [2,3], being responsible for various types of health effects through inhalation and ingestion. Additionally, and besides infections, human exposure to bioaerosols is associated with a wide range of respiratory health problems, due to exposure in different occupational environments [4,5,6]. In what concerns bioaerosols’ health effects, fungi play an important role. High levels of fungal particles are found in different occupational environments (animal production and waste industries, among others) [4,5,6], and their workers are at increased risk for respiratory diseases [7].
The genus Aspergillus is known to be one of the most frequently airborne fungi found in indoor and outdoor environments worldwide [8,9,10,11,12,13,14,15,16]. More recently, due to molecular identification, the genus has been subdivided into 22 different sections, including the clinically relevant species Aspergilli, Fumigati, Circumdati, Terrei, Nidulantes, Ornati, Warcupi, Candidi, Restricti, Usti, and Flavi [12]. Sections/species from Aspergillus genus are found in several habitats with different roles. In fact, they can act as decomposers of organic matter (for instance, Aspergillus section Nigri), mycotoxin producers (Aspergillus section Flavi, among others), and often as human pathogens (Aspergillus section Fumigati). The role as human pathogens can occur particularly in occupational environments, such as libraries and archives, bakeries, waste-sorting facilities, composting plants, sawmills, animal production, and other agricultural environments [8,9,10,11,12,13,14,15,16]. Aspergillus fumigatus is the species more frequently associated with respiratory symptoms, especially due to the small size of the conidia and other virulence factors, such as gliotoxin production, associated with this species. However, besides the infection ability, risk assessment should consider the toxigenic potential of species belonging to Aspergillus genus [17]. Thus, species or strains belonging to sections Circumdati, Flavi, Nigri, and Nidulantes must be considered in the risk assessment, since they include species that produce mycotoxins [7,18]. Another topic of concern in specific occupational environments, such as sawmills [14,19], is the exposure to high levels of azole-resistant Aspergillus isolates, which may increase the risk of infection, enhancing the difficulty of its clinical management [20,21,22].
Several sampling methods can be used to perform the Aspergillus assessment, as was already reported [12,15,17,23]. Active sampling methods (air sampling), although the most used method in sampling campaigns, can only reflect the load from a shorter period of time (mostly minutes). On the contrary, passive methods (such as surface swabs or settled dust) allow for the determination of the contamination levels from an increased period of time (weeks to several months) [13]. Thus, the sampling approach should comprise more than one sampling method, to allow a wider characterization of the fungal contamination and a more accurate exposure assessment [13,15].
Regarding the analysis of the collected samples, the culture method for the determination of fungal growth is still the most widely used. Traditional quantification of fungi is based on the determination of the number and type of colony forming units (CFU), providing quantitative and qualitative data on viable and culturable fungi [24,25]. Furthermore, counting culturable microorganisms not only allows a quantitative assessment (load or contamination), but also a qualitative analyses of the exposure by identifying the isolated fungi, since not all fungi pose the same hazard. After culture, fungi may be identified to the genus or species level by morphological criteria and microscopy identification [24,25].
Malt extract agar (MEA) is undoubtedly the culture medium most frequently recommended [26,27,28,29] and used in aerobiological studies, given the wide range of fungal genera found in the air. It is a nutrient-rich medium for the cultivation of environmental fungi [23,28]. MEA is particularly suitable for yeasts and molds, as it contains a high concentration of maltose and saccharides as energy sources. The acidic pH is optimum for fungal growth, whilst restricting bacterial growth [23,28]. Nevertheless, there are several other efficient culture media, such as DG18 (dichloran-glycerol Agar), firstly used for the enumeration of xerophilic molds and osmophilic yeasts. DG18 contains glycerol at 16% (w/w), which lowers the water activity (aW) from 0.999 to 0.95 [27]. Glycerol was chosen because of the advantages regarding culture of a wider range of xerophilic fungi, over sodium chloride and sugars, which have traditionally been used to formulate media of reduced aW [27]. Various studies have indicated this medium to be a better alternative for colony counting and to obtain higher diversity of genera, since it contains dichloran, which inhibits spreading of fungi belonging to the Mucorales order, such as Rhizopus and Mucor genera [13,14,15,16,30,31], and restricts the colony size of other genera. This restrictive characteristic makes the medium especially suitable for enumeration because it allows for the unobscured growth of organisms that ordinarily form small colonies [27,32]. In summary, MEA allows fast-growing fungal species to grow on its surface, especially due to high sugar content and water activity, while DG18 allows the identification of a more diverse fungal flora, but excludes fungi requiring high water activity to grow [27,32].
In highly contaminated environments, genera with a higher growth rate might inhibit the growth of Aspergillus, and, therefore, culture based-methods combined with molecular tools seems to be the best approach to obtain the data necessary to attain legal and scientific criteria [12,14,15,16,17,33]. Refined molecular tools allow for the rapid identification of fungi in air samples (for instance by high-throughput sequencing) and are being increasingly used to obtain information regarding the microbial biodiversity present in different indoor and occupational environments [13,33]. Nevertheless, it is also necessary to assess the viability of the microorganisms, since it affects biological mechanisms, such as the inflammatory and cytotoxic responses [17,34]. This means that the inflammatory potential of one environmental sample (air, settled dust, etc.) may differ depending on its microbial composition and the biological activity of the microorganisms in the sample [16,17,35,36]. This aspect underlines the importance of applying culture-based methods in exposure assessments studies [12,16,17,36], in parallel with molecular tools to overcome limitations of culture-based methods already reported [25].
To the authors’ knowledge, there are no studies regarding the evaluation of which is the most suitable culture media to assess Aspergillus spp. on environmental samples from different indoor environments in what concerns the fungal counts and diversity of sections. This study aimed to analyze data from previous studies, in order to identify the most suitable culture media between MEA or DG18 to assess Aspergillus spp. exposure. We aimed to design the most appropriate strategy on samples’ culture, depending on the applied sampling method and on the studied indoor environment.
2. Materials and Methods
2.1. Environmental Samples
This study was conducted by using environmental samples (n = 1153) collected from January of 2018 to March 2020 in several indoor and occupational environments located in the Lisbon district (Table 1). The environmental samples were collected in the scope of enlarged financed studies focused on the assessment of occupational exposure to microbiologic agents, with emphasis in the workplaces’ fungal contamination. Different companies were called to participate in the study, depending on each project goal. Workplaces enrolled in this study where selected in order to obtain the most critical scenario regarding occupational exposure to fungi [11,12,13,14,15,16].

Table 1.
Indoor and occupational environments assessed and applied sampling approaches.
Different sampling approaches were applied, and the ones where Aspergillus sections were detected and identified are listed in Table 1.
2.2. Sampling and Characterization of Viable Bioburden
Most of the active sampling methods (air samples) were impacted directly onto the culture media. Regarding passive sampling methods, fungal contamination was extracted from the environmental matrix with 0.1% Tween™ 80 saline solution (NaCl 0.9%) for 30 min at 250 rpm on an orbital laboratory shaker (Edmund Bühler SM-30, Hechingen, Germany), and 150 µL of each wash suspension was inoculated [13,14,15,16]. These laboratory procedures were followed since 2018, allowing the feed and update of an algorithm developed to assess occupational exposure to Aspergillus genus.
In all the collected samples, two different culture media were used in order to enhance the sensitivity for fungal growth: MEA supplemented with chloramphenicol (0.05%) and DG18. Sampling collection method and laboratory processing are further presented in more detail.
Regarding active sampling methods, indoor air samples in each assessed area/workplace, of a range of different volumes of air (50 to 250 L), were collected by impaction, with a flow rate of 140 L/min (Millipore air Tester, Millipore, Billerica, MA, USA), onto each plate, according to manufacturer’s instructions [13]. In some specific cases (bakeries), air samples of 300 L were collected by using the impinger Coriolis μ air sampler (Bertin Technologies) with a flow rate of 300 L/min, onto 10 mL sterile phosphate-buffered saline (PBS) with 0.05% Triton X-100 to be applied for culture and molecular methods [37]. In the firefighters’ ambulances project, the Andersen six-stage sampler with six stages and different impaction velocities of 24–1.1-m/s. were also applied. Sampler stages have cutoff sizes of 0.65, 1.1, 2.1, 3.3, 4.7, and 7.0 mm.
In two different occupational environments (bakeries and sawmills), samples were collected through a different active sampling method (filtration). In the bakeries, samples were collected in the worker breathing zone, using portable SKC Sidekick sampling pumps connected to an IOM sampler SKC, Ltd., Dorset, UK, containing 25 mm Whatman GF/A glass microfiber filters (pre-sterilized by autoclaving at a standard temperature and pressure, meaning 121° C for at least 30 min by using saturated steam under at least 15 psi of pressure) [14,15]. After gravimetric analysis, each filter was extracted in sterile 10 mL deionized water with 0.05% Tween80™ (Sigma-Aldrich, Dorset, UK) at 250 rpm for 1 min, and then a volume of 3.8 mL of sterile glycerol was added, and the solution was extracted again for 1 min at 250 rpm, then stored at −80 °C until the microbial analysis [14,15]. In sawmills, samples were collected with 37 mm conical inhalable sampling (CIS) cassettes with a conical inlet hole of 8 mm (Casella Solutions, Kempston, UK), loaded with a polycarbonate filter (pore size 1 µm) and using an airflow of 3.5 L/min. Exposed filters were transferred to 15 mL tubes containing 5 mL PBS with 0.1% BSA and sonicated for 5 min, followed by shaking for 25 min at 500 rpm. The suspension was poured into a new tube, and the extraction process repeated with 2 mL PBS–BSA before the suspensions were pooled together. Aliquots of 1 mL of the sample suspensions were stored at −20 °C, in a refrigerator, until analysis [14].
Concerning passive sampling methods, surface samples were collected by swabbing indoor sites with a 10 cm × 10 cm square stencil, which was disinfected with a 70% alcohol solution between samplings. Surface swab samples were extracted and plated onto both media [13].
Pieces of heating, ventilation, and air-conditioning (HVAC) filters from different indoor environments, filtering respiratory protection devices (FRPD), mechanical protection gloves (MPG), and vacuuming bags with 2 cm2 (1.4 cm × 1.4 cm) were collected and kept refrigerated at 4 °C, before analysis; then they were washed, and the extracts were seeded on both media [16].
Each electrostatic dust collector (EDC) having a surface exposure area of 0.0209 m2 (19 cm × 11 cm) was placed at a minimum 0.93 m above floor level, and dust was allowed to settle for 13 to 16 days. Each EDC cloth was washed, and the extracts were also seeded in MEA and DG18 [15].
Settled dust samples were weighted and washed in a ratio of 1 g (dust) per 9.1 mL of NaCl 0.9% with 0.05% Tween 80 (10 µL) for 60 min at 250 rpm, and 0.15 mL of this suspension was spread onto the referred culture media [15]. Concerning settled dust from bakeries (mainly flour), a solution of 0.9% NaCl with 0.05% Tween80 ™ (Merck S.A., Lisbon, Portugal) was added in a ratio of 4.4 g of settled dust for 40 mL of solution, and 0.15 mL was seeded onto MEA and DG18 [15].
After the incubation of MEA and DG18 at 27 °C, for 5 to 7 days, Aspergillus sections were identified microscopically, using tease mount or Scotch tape mount and lactophenol cotton blue mount procedures [38]. Morphological identification was achieved through macro- and microscopic characteristics, as noted by De Hoog et al. [38].
2.3. Statistical Analysis
Data were analyzed by using the statistical software SPSS V26.0 for Windows. Results were considered significant at 5% significance level. For the characterization of the sample, n and percentage were used. To study the relationship between the obtained Aspergillus sections and the two culture media (MEA and DG18), sampling method applied (active and passive), and the indoor environment assessed, the Chi-Square test was used or, alternatively, the Chi-Square test by Monte Carlo simulation when the applicability assumptions of the Chi-Square test were not verified. In addition to the Chi-Square test, Multiple Correspondence Analysis was used.
Aspergillus sections Terrei, Nidulantes, Clavati, Usti, Cremei, and Restricti presented a weak expression regarding prevalence. Thus, it was considered a group that was called “others”, to allow for the statistical analyses.
3. Results
Overall, total Aspergillus counts were higher in MEA (n = 617, 53.5%) than in DG18 (n = 536, 46.5%). The most frequently identified sections were Nigri (n = 295, 25.6%), Fumigati (n = 258, 22.3%), Circumdati (n = 157, 13.6%), Versicolores (n = 118, 10.1%), Candidi (n = 115, 10%), and Aspergilli (n = 102, 8.8%). The sampling approach and the studied indoor environments may have influenced these data, as it was observed that samples from air (n = 325, 28.2%), FRPD (n = 280, 24.3%), EDC (n = 167, 14.5%), and MPG (n = 125, 10.8%) were the ones with higher Aspergillus counts. Waste-sorting plants (n = 483, 41.9%), healthcare facilities (n = 231, 20%), and dwellings (n = 199, 17.3%) were the indoor environments with higher Aspergillus counts (Table 2).

Table 2.
Frequency distribution of the detected Aspergillus by sections, sampling method, and indoor environment.
Regarding Aspergillus sections, significant associations were detected with the media (χ2 (7) = 241.118, p < 0.001), the sampling approach (p < 0.001, 95% C.I. = (0.3 × 10−4), and the indoor environment (p = 1.5 × 10−4, 95% C.I. = (0.3 × 10−4)).
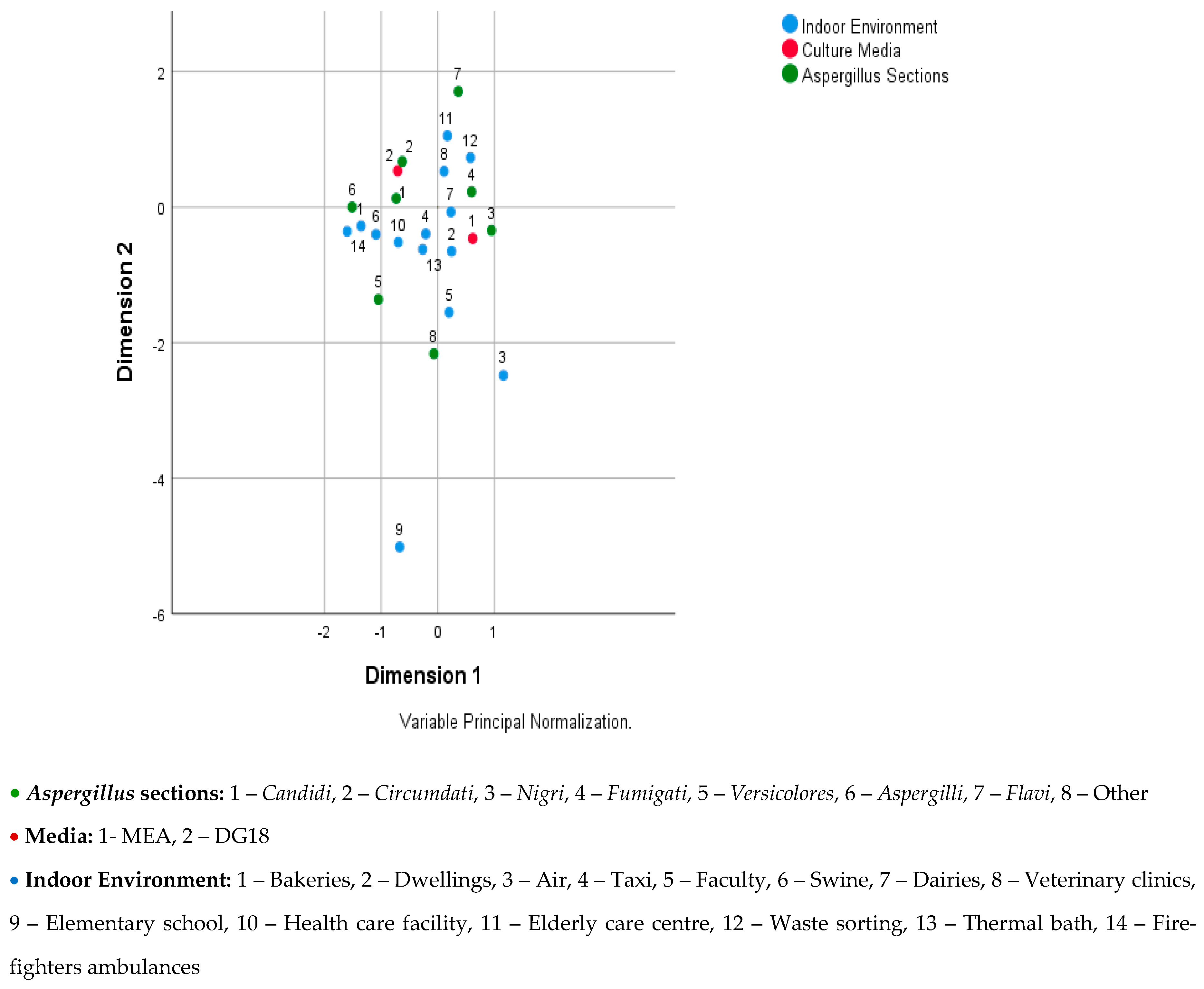
The multiple correspondences analysis was carried out in two parts, as there was a strong relationship between the sampling approach and the indoor environment, and the interpretation was more challenging to achieve. Figure 1 shows the relationship between Aspergillus sections, culture media, and indoor environment, where it can be seen that: (i) Candidi is associated to the DG18 and the indoor environments swine, healthcare facility, and taxi; (ii) Circumdati is associated to the DG18; (iii) Nigri is associated to the MEA and to the indoor environment dwellings and dairies; (iv) Fumigati is associated to the MEA and to the indoor environments waste-sorting plants, veterinary clinics, and dairies; (v) Aspergilli is associated to the indoor environment firefighters’ ambulances, bakeries, and swine and to the DG18; (vi) Flavi is associated to the indoor environment elderly care center and waste-sorting plant; (vii) others (Terrei, Nidulantes, Clavati, Usti, Cremei, and Restricti) are associated to the indoor environment college.
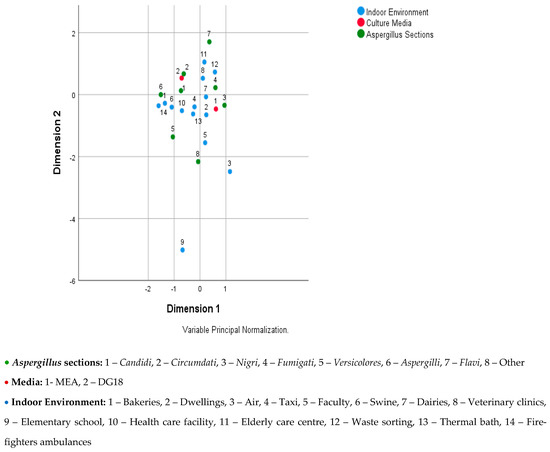
Figure 1.
Joint plot of category points for Aspergillus sections, culture media, and indoor environment.
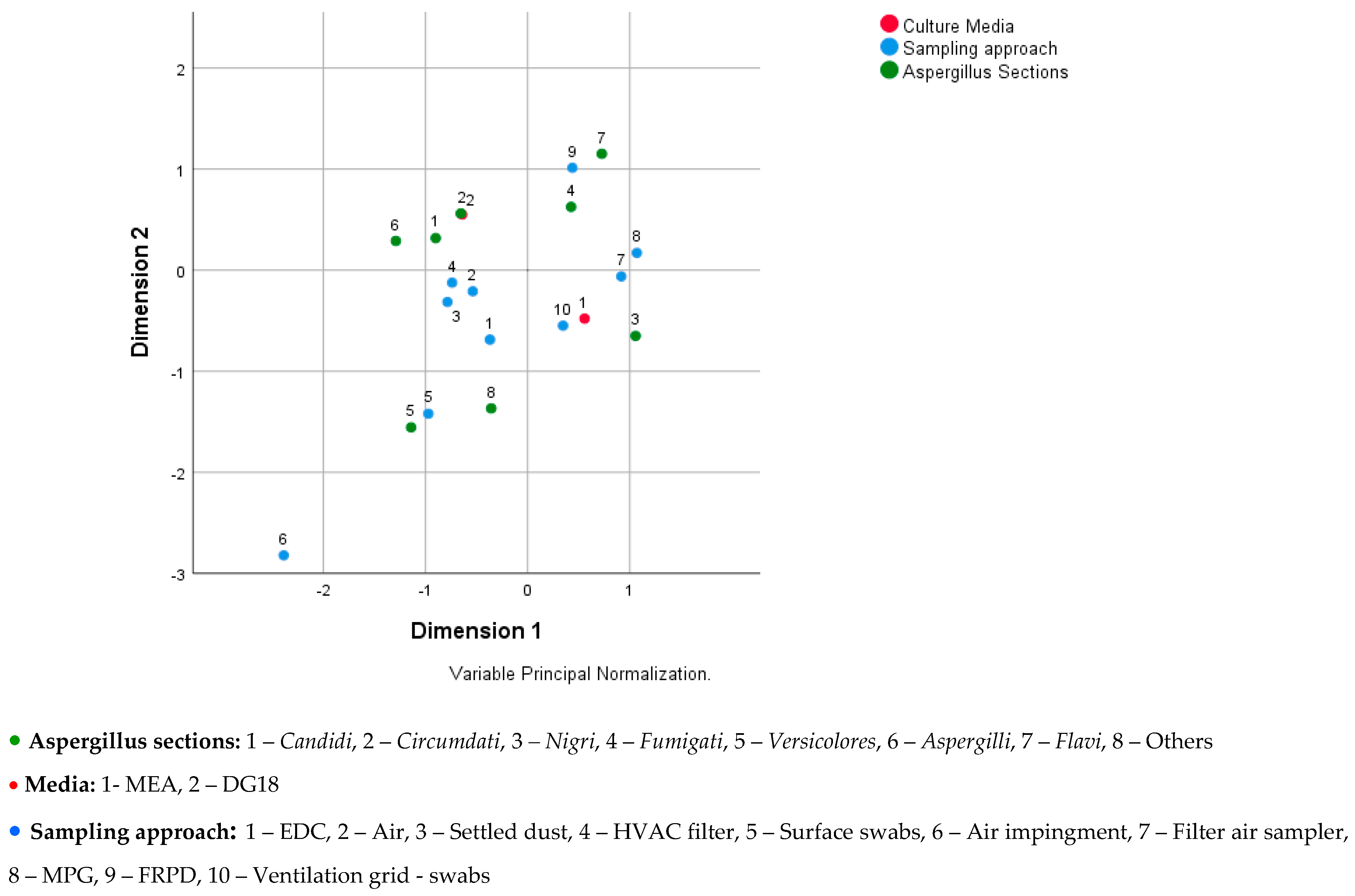
Figure 2 shows the relationship between Aspergillus sections, media, and sampling approach, where the following can be seen: (i) Candidi, Circumdati, and Aspergilli are associated to the DG18 media; (ii) Fumigati and Flavi are associated to the sampling approach FRPD; (iii) Nigri is associated to the MEA and to the sampling approach ventilation grid—swabs and filter air sampler; (iv) others (Terrei, Nidulantes, Clavati, Usti, Cremei, and Restricti) are associated to the sampling approach EDC and surface swabs; and (v) Versicolores is associated to the sampling approach surface swab.
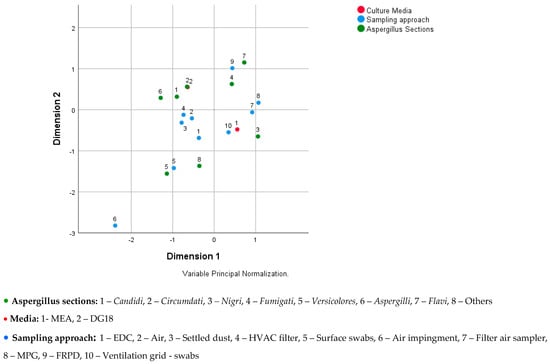
Figure 2.
Joint plot of category points for Aspergillus sections, culture media, and sampling approach.
4. Discussion
The aims of any exposure assessment on culturable fungi is to avoid overgrowth of culture plates by fast-growing species, and also to obtain as many different species as possible recovered from the assessed environment [27]. Thus, besides the field (for instance number of liters of air collected) and laboratory (serial dilutions) procedures to avoid the overgrowth, the selection of the culture media is critical to the pursuit of an accurate exposure assessment [13]. Furthermore, in different exposure assessments targeting culturable fungi, the trend was to observe higher counts on MEA and higher diversity on DG18 [13,27,30,31]. However, the studies performed until now have compared the results from both culture media, using the same sampling approach and the same indoor environment.
Aspergillus sections detected in a specific environment depend on three studied variables (culture media, sampling approach, and indoor environment assessment), reinforcing the importance of having scientific data about the background of fungal contamination to select the most suitable protocol to follow from the field to the lab bench [12]. In previous studies, protocols were suggested in order to assess fungal contamination with the following aims: (i) to understand Aspergillus occupational exposure assessment [12], (ii) to verify the compliance of a proper indoor air quality [13], and (iii) to obtain information about the Aspergillus azole resistance rates in occupational environments with expected azole pressure [14]. In all of those approaches, both culture media (MEA and DG18) were used and all different results were obtained. Taking that into account, but also understanding that a targeted approach should be defined and that resources should be saved, this study was planned to design a proper strategy on samples’ culture, depending on the used sampling method and the studied indoor environment.
In this study, the Versicolores section was still considered as an independent section. However, this section was recently integrated into the Nidulantes section. These taxonomic changes were considered after a performing polyphasic approach to these groups of fungi. In fact, they occur after deep analysis to colonies’ morphology, DNA sequencing of specific genes followed by phylogenetic analysis, and observation of different physiological features, including extrolites’ production [39,40].
The obtained data revealed that some Aspergillus sections were associated with DG18 (Candidi, Circumdati, and Aspergilli) and the indoor environment (Candidi and Aspergilli), while some were associated with MEA, besides being associated with the sampling approach as well (Nigri and Fumigati). DG18 inhibits the rapid spreading and restricts the colony size of fast growing genera [41,42], enabling fungal growth of different species [13], including fungal species with clinical and toxigenic relevance, such as specific Aspergillus sections as Fumigati.
Other sections detected in lower frequency were more frequently associated with the sampling approach (passive sampling methods) and with the indoor environment (Versicolores, Flavi, Terrei, Nidulantes, Clavati, Usti, Cremei, and Restricti). Results obtained with passive sampling methods, such as surface swabs and EDC, seem to present a wider diversity of fungal species when compared with the ones obtained by active methods [13,15,43]. Both methods are able to collect data regarding contamination from a longer period of time (from a work shift, days, weeks, or months), while active methods (air sampling) can only reflect the load of a shorter period (mostly minutes) corresponding to the sampling duration [13,14,15,44].
The EDC is a passive collection device that consists of an electrostatic polypropylene cloth [45]. This standardized method [46] is increasingly being used due to its low cost and efficacy at collecting dust [37,47], and it has already been applied for the occupational exposure assessment of fungal burden [13,15,37,46,48,49,50,51,52]. Thus, sampling with EDC allows a reliable estimation of fungal exposure, since a single EDC analysis is equal to the sum of several air-impaction measurements [53]. Additionally, we should expect the exclusive identification of some fungal species and higher fungal diversity in EDC, when compared to air samples, since the same trend was observed with surface swab samples in previous reports performed in different indoor environments [37,43,54]. Furthermore, lower contaminated environments (college) tend to have a more diversified fungal flora than the most contaminated indoor environment (waste-sorting plant), where most of the times the dominance of one fungal species indicates an increased health risk [55]. In non-problematic indoor environments, three or more genera should be found, and a dominant genus should not be observed [56].
Both MEA and DG18 can provide useful information regarding the Aspergillus genera. In the case of an exploratory exposure assessment study (to determine total Aspergillus counts), any of these can be used, or both (ideally). In fact, for the Aspergillus total counts at the genus level, results were different between the two culture media, depending on the analyzed indoor environment and on the sampling method used for collection [13,14,16]. The same tendency was observed in this study regarding Aspergillus sections. Therefore, in a specific scenario of targeted collection (towards a specific Aspergillus section, using a specific collecting approach and/or in a specific indoor environment), a decision regarding the choice of MEA or DG18 should be taken into account (Table 3). This information should be considered in a sampling strategy, to assess a particular Aspergillus section, using a defined sampling approach in a specific occupational environment.

Table 3.
Information to be considered in a sampling strategy targeting specific Aspergillus sections.
5. Conclusions
In light of our results, MEA or DG18 should be selected by taking into consideration the indoor occupational environmental to be assessed, the sampling approach applied, and also the Aspergillus section targeted. This study claims attention for the importance to accurately defining the sampling approach and the culture media applied in exposure assessment in a specific indoor environment, when targeted to Aspergillus. This information is critical when the exposure assessment needs to target specific Aspergillus sections and should be considered in field- and in bench-work protocols. As such, exposure assessors (industrial hygienists, public health officers, IAQ assessors, and laboratory technicians) should be aware of the importance of this information when planning sampling campaigns and the lab work needed.
Author Contributions
Conceptualization, C.V.; methodology, C.V., E.C., and R.S.; validation, C.V. and R.S.; formal analysis, C.V., M.D., and E.C.; investigation, C.V., M.D., and E.C.; resources, C.V.; writing—original draft preparation, C.V., M.D., and E.C.; writing—review and editing, C.V and R.S.; supervision, C.V.; project administration, C.V.; funding acquisition, C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FCT—Fundação para Ciência e Tecnologia for funding the project EXPOsE—Establishing protocols to assess occupational exposure to microbiota in clinical settings (02/SAICT/2016—Project n° 23222) and Instituto Politécnico de Lisboa, Lisbon, Portugal for funding the Project “Waste Workers’ Exposure to Bio-burden through Filtering Respiratory Protective Devices” (IPL/2018/WasteFRPD_ESTeSL) and the Project “Occupational exposure of ambulance drivers to bioburden” (IPL/2020/BIO-AmbuDrivers_ESTeSL).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
H&TRC authors gratefully acknowledge the FCT/MCTES national support through the UIDB/05608/2020 and UIDP/05608/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hernandez, H.; Martinez, L.R. Relationship of environmental disturbances and the infectious potential of fungi. Microbiology 2018, 164, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leung, G.M.; Tang, J.W.; Yang, X.; Chao, C.Y.H.; Lin, J.Z.; Lu, J.W.; Nielsen, P.V.; Niu, J.; Qian, H.; et al. Role of ventilation in airborne transmission of infectious agents in the built environment—A multidisciplinary systematic review. Indoor Air 2007, 17, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Eames, I.; Tang, J.W.; Li, Y.; Wilson, P. Airborne transmission of disease in hospitals. J. R. Soc. Interface 2009, 6, S697–S702. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J. (1–>3)-Beta-D-glucans and respiratory health: A review of the scientific evidence. Indoor Air 2005, 15, 160–169. [Google Scholar] [CrossRef]
- Eduard, W.; Heederik, D.; Duchaine, C.; Green, B.J. Bioaerosol exposure assessment in the workplace: The past, present and recent advances. J. Environ. Monit. 2012, 14, 334–339. [Google Scholar] [CrossRef]
- Heederik, D.; Von Mutius, E. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J. Allergy Clin. Immunol. 2012, 130, 44–50. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Viegas, C.; Viegas, S.; Brandão, J.; Alves-Correia, M.; Borrego, L.M.; Clemons, K.V.; Stevens, D.A.; Richardson, M. The role of occupational Aspergillus exposure in the development of diseases. Med. Mycol. 2019, 57, S196–S205. [Google Scholar] [CrossRef]
- Skórska, C.; Sitkowska, J.; Krysinska-Traczyk, E.; Cholewa, G.; Dutkiewicz, J. Exposure to airborrne microorganisms, dust and endotoxin during processing of valerian roots on farms. Ann. Agric. Environ. Med. 2005, 12, 119–126. [Google Scholar]
- Zielinska-Jankiewicz, K.; Kozajda, A.; Piotrowka, M.; Szadkowska-Stanczyk, I. Microbiological contamination with moulds in work environment in libraries and archive storage facilities. Ann. Agric. Environ. Med. 2008, 15, 71–78. [Google Scholar]
- Straumfors, A.; Corbin, M.; McLean, D.; Mannetje, A.; Olsen, R.; Afanou, A.; Daae, H.L.; Skare, Ø.; Ulvestad, B.; Johnsen, H.L.; et al. Exposure Determinants of Wood Dust, Microbial Components, Resin Acids and Terpenes in the Saw- and Planer Mill Industry. Ann. Work Expo. Health 2020, wxz096. [Google Scholar] [CrossRef]
- Viegas, C.; Carolino, E.; Sabino, R.; Viegas, S.; Veríssimo, C. Fungal Contamination in Swine: A Potential Occupational Health Threat. J. Toxicol. Environ. Health Part A 2013, 76, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. prevalence in different occupational settings. J. Occup. Environ. Hyg. 2017, 14, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Almeida, B.; Monteiro, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in healthcare centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160, 106226. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Aranha Caetano, L.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2020. [Google Scholar] [CrossRef]
- Viegas, C.; Fleming, G.; Kadir, A.; Almeida, B.; Aranha Caetano, L.; Quintal Gomes, A.; Twaruzek, M.; Kosicki, R.; Viegas, S.; Coggins, A.M. Occupational exposures to organic dust in Irish bakeries and a pizzeria restaurant. Microorganisms 2020, 8, 118. [Google Scholar] [CrossRef]
- Viegas, C.; Twarużek, M.; Dias, M.; Almeida, B.; Carolino, E.; Kosicki, R.; Soszczyńska, E.; Grajewski, J.; Aranha Caetano, L.; Viegas, S. Assessment of the microbial contamination of mechanical protection gloves used on waste sorting industry: A contribution for the risk characterization. Environ. Res. 2020, 189, 109881. [Google Scholar] [CrossRef]
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environ. Res. 2020, 183, 109177. [Google Scholar] [CrossRef]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Jeanvoine, A.; Rocchi, S.; Reboux, G.; Crini, N.; Crini, G.; Millon, L. Azole-resistant Aspergillus fumigatus in sawmills of Eastern France. J. Appl. Microbiol. 2017, 123, 172–184. [Google Scholar] [CrossRef]
- Snelders, E.; van der Lee, H.; Kuijpers, J.; Rijs, A.J.M.M.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.T.; Melchers, W.J.G.; Verweij, P.E. Emergence of azole resistance in A. fumigatus and spread of a single resistance mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013, 9, e1003633. [Google Scholar] [CrossRef]
- Verweij, P.E.; Kema, G.H.J.; Zwaan, B.; Melchers, W.J.G. Triazole fungicides and the selection of resistance to medical triazoles in the opportunistic mould Aspergillus fumigatus. Pest Manag. Sci. 2013, 69, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Dillon, H.K.; Boling, D.K.; Miller, J.D. Comparison of detection methods for Aspergillus fumigatus in environmental air samples in an occupational environment. J. Occup. Environ. Hyg. 2007, 4, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Burge, H.A.; Otten, J.A. Chapter 19 Fungi. In Bioaerosols: Assessment and Control; Macher, J., Ed.; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1999; Volume 19, pp. 1–13. [Google Scholar]
- Viegas, C.; Viegas, S.; Gomes, A.; Täubel, M.; Sabino, R. (Eds.) Exposure to Microbiological Agents in Indoor and Occupational Environments; Springer: Berlin/Heidelberg, Germany, 2017; pp. 109–128. [Google Scholar]
- Burge, H.A.; Chatigny, M.; Feeley, J.; Kreiss, K.; Morey, P.; Otten, J.; Peterson, K. Guidelines for assessment and sampling of saprophytic bioaerosols in the indoor environment. Appl. Ind. Hyg. 1987, 2, R10–R16. [Google Scholar]
- Wu, P.; Su, H.J.; Ho, H. A comparison of sampling media for environmental viable fungi collected in a hospital environment. Environ. Res. 2000, 82, 253–257. [Google Scholar] [CrossRef]
- Hung, L.L.; Miller, J.D.; Dillon, H.K. Field Guide for the Determination of Biological Contaminants in Environmental Samples, 2nd ed.; American Industrial Hygiene Association: Fairfax, VA, USA, 2005; pp. 93–100. [Google Scholar]
- Agência Portuguesa para o Ambiente. Qualidade do Ar Interior—Um Guia Técnico; Agência Portuguesa para o Ambiente: Amadora, Portugal, 2009.
- Smid, T.; Schokkin, E.; Boleij, J.S.M.; Heederik, D. Enumeration of viable fungi in occupational environments: A comparison of samplers and media. Am. Ind. Hyg. Assoc. J. 1989, 50, 235–239. [Google Scholar] [CrossRef]
- Verhoeff, A.P.; Van Wijnen, J.H.; Boleij, J.S.M.; Brunekreef, B.; Van Reenen-Hoekstra, E.S.; Samson, R.A. Enumeration and identification of airborne viable mould propagules in houses: A field comparison of selected techniques. Allergy 1990, 45, 275–284. [Google Scholar] [CrossRef]
- Chao, H.J.; Milton, D.K.; Schwartz, J.; Burge, H.A. Dustborne fungi in large office buildings. Mycopathologia 2002, 154, 93–106. [Google Scholar] [CrossRef]
- Degois, J.; Clerc, F.; Simon, X.; Bontemps, C.; Leblond, P.; Duquenne, P. First Metagenomic Survey of the Microbial Diversity in Bioaerosols Emitted in Waste Sorting Plants. Ann. Work Expo. Health 2017, 1–11. [Google Scholar] [CrossRef]
- Croston, T.L.; Nayak, A.P.; Lemons, A.R.; Goldsmith, W.; Gu, J.K.; Germolec, D.R.; Beezhold, D.H.; Green, B.J. Influence of Aspergillus fumigatus conidia viability on murine pulmonary 28 micro RNA and m RNA expression following subchronic inhalation exposure. Clin. Exp. Allergy 2016, 46, 1315–1327. [Google Scholar] [CrossRef]
- Timm, M.; Madsen, A.M.; Hansen, J.V.; Moesby, L.; Hansen, E.W. Assessment of the total inflammatory potential of bioaerosols by using a granulocyte assay. Appl. Environ. Microbiol. 2009, 75, 7655–7662. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Aranha Caetano, L.; Korkalainen, L.; Faria, T.; Pacífico, C.; Carolino, E.; Quintal Gomes, A.; Viegas, C. Cytotoxic and inflammatory potential of air samples from occupational settings with exposure to organic dust. Toxics 2017, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Monteiro, A.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Viegas, S. A Novel Multi-Approach Protocol for the Characterization of Occupational Exposure to Organic Dust—Swine Production Case Study. Toxics 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, D.; Guarro, J.; Gene, G.; Figueras, M. Atlas of Clinical Fungi—The Ultimate Bench Tool for Diagnosis, Version 4.1.4; Utr. Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2016. [Google Scholar]
- Chen, A.J.; Frisvad, J.C.; Sun, B.D.; Varga, J.; Kocsubé, S.; Dijksterhuis, J.; Kim, D.H.; Hong, S.B.; Houbraken, J.; Samson, R.A. Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology. Stud. Mycol. 2016, 84, 1–118. [Google Scholar] [CrossRef]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Bergwall, C.; Stehn, B. Comparison of selective mycological agar media for the isolation and enumeration of xerophilic moulds and osmotolerant yeasts in granulated white sugar. Zuckerindustrie 2002, 127, 259–264. [Google Scholar]
- Corry, J.E.L.; Curtis, G.D.W.; Baird, R.M. Handbook of Culture Media for Food and Water Microbiology, 3rd ed.; Royal Society of Chemistry: London, UK, 2011; ISBN 1847551459, 9781847551450. [Google Scholar]
- Viegas, C.; Faria, T.; Meneses, M.; Carolino, E.; Viegas, S.; Quintal Gomes, A.; Sabino, R. Analysis of Surfaces for Characterization of Fungal Burden—Does it Matter? Int. J. Occup. Med. Environ. Health 2016, 29, 623–632. [Google Scholar] [CrossRef]
- Badyda, A.; Gayer, A.; Czechowski, P.; Majewski, G.; Dąbrowiecki, P. Pulmonary function and incidence of selected respiratory diseases depending on the exposure to ambient PM10. Int. J. Mol. Sci. 2016, 17, 1954. [Google Scholar] [CrossRef]
- American Conference of Governmental Industrial Hygienists (ACGIH). Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2009.
- Cozen, W.; Avol, E.; Diaz-Sanchez, D.; McConnell, R.; Gauderman, W.J.; Cockburn, M.G.; Mack, T.M. Use of an electrostatic dust cloth for self-administered home allergen collection. Twin Res. Hum. Genet. 2008, 11, 150–155. [Google Scholar] [CrossRef]
- Kilburg-Basnyat, B.; Metwali, N.; Thorne, P.S. Performance of electrostatic dust collectors (EDCs) for endotoxin assessment in homes: Effect of mailing, placement, heating and electrostatic charge. J. Occup. Environ. Hyg. 2016, 13, 85–93. [Google Scholar] [CrossRef]
- Noss, I.; Wouters, I.M.; Visser, M.; Heederik, D.J.J.; Thorne, P.S.; Brunekreef, B.; Doekes, G. Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl. Environ. Microbiol. 2008, 74, 5621–5627. [Google Scholar] [CrossRef] [PubMed]
- Normand, A.C.; Vacheyrou, M.; Sudre, B.; Heederik, D.J.J.; Piarroux, R. Assessment of dust sampling methods for the study of cultivable microorganism exposure in stables. Appl. Environ. Microbiol. 2009, 75, 7617–7623. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Matthiesen, C.B.; Frederiksen, M.W.; Frederiksen, M.; Frankel, M.; Spilak, M.; Timm, M. Sampling, extraction and measurement of bacteria, endotoxin, fungi and inflammatory potential of settling indoor dust. J. Environ. Monit. 2012, 14, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- Dorado-Garcia, A.; Bos, M.E.; Graveland, H.; Van Cleef, B.A.; Verstappen, K.M.; Kluytmans, J.A.; Wagenaar, J.A.; Heederik, D.J. Risk factors for persistence of livestock-associated MRSA and environmental exposure in veal calf farmers and their family members: An observational longitudinal study. BMJ Open 2013, 3, e003272. [Google Scholar] [CrossRef] [PubMed]
- Feld, L.; Bay, H.; Angen, Ø.; Larsen, A.R.; Madsen, A.M. Survival of LA-MRSA in dust from swine farms. Ann. Work Expo. Health 2018, 62, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Damp Indoor Spaces and Health; The National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Cabo Verde, S.; Almeida, S.M.; Matos, J.; Guerreiro, D.; Meneses, M.; Faria, T.; Botelho, D.; Santos, M.; Viegas, C. Microbiological assessment of indoor air quality at diferente hospital sites. Res. Microbiol. 2015, 166, 557–563. [Google Scholar] [CrossRef]
- Hicks, J.B.; Lu, E.T.; De Guzman, R.; Weingart, M. Fungal types and concentrations from settled dust in normal residences. J. Occup. Environ. Hyg. 2005, 2, 481–492. [Google Scholar] [CrossRef]
- Hodgson, M.; Scott, R. Prevalence of fungi in carpet dust samples. In Bioaerosols, Fungi and Mycotoxins: Health Effects, Assessment, Prevention and Control; Johanning, E., Ed.; Boyd Printing Company: Albany, NY, USA, 1999; pp. 268–274. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).