Abstract
Mercury (Hg) is a global pollutant, being highly persistent in the atmosphere, in particular gaseous elemental mercury (GEM), which can easily be emitted and then transported over long distances. In the Gulf of Trieste (northern Adriatic Sea, Italy), contamination by Hg is well characterised but little is known regarding the concentrations, sources and fate of GEM in the atmosphere. In this work, discrete measurements of GEM were recorded from several sites at different times of the year. The database is consistent with temporal night-day variations monitored using a continuous real-time device. The meteorological conditions were collected as ancillary parameters. GEM levels varied from <LOD (2.0 ng m−3) to 48.5 ng m−3 (mean 2.7 ng m−3), with no significant differences found among sites. A clear daily pattern emerged, with maximum values reached just after sunset. Air temperature, relative humidity, wind speed and direction were identified as the main micrometeorological factors influencing both the spatial and temporal variation of GEM. Our results show that average atmospheric GEM values are higher than the natural background of the Northern Hemisphere and will be useful in future selection regarding the most suitable sites to monitor atmospheric Hg depositions and fluxes from soil and water.
1. Introduction
Mercury (Hg) is a well-known global pollutant which is a cause for serious concern regarding ecosystems and human health due to its persistence, toxicity and potential bioaccumulation/biomagnification [1]. The atmosphere represents the main redistribution pathway of Hg mobilised from lithospheric reservoirs and often atmospheric depositions constitute the main source of this element for terrestrial and aquatic ecosystems, even in areas far from points of emission [2]. Once released into the environment, Hg can adversely affect the physiology of many living organisms [3], particularly in its organic form, monomethylmercury (MMHg), which is more toxic and easily biomagnified within the trophic chain due to its lipophilic nature [4]. The consumption of fish contaminated by MMHg is considered the main Hg exposure route for humans [5], whereas inhalation of inorganic Hg vapours occurs mainly via dental amalgams and in certain occupations [6]. Adverse effects of Hg on human health primarily concern the nervous system, but exposure to the different forms of this element can also damage renal and cardiovascular systems and generate negative reproductive and epigenetic outcomes [7].
As a result of human activities, the amount of Hg mobilised and circulating in the environment showed a sharp increase starting from the preindustrial period. Focusing on the atmospheric compartment, recent estimates show that Hg concentrations increased by approximately 450% above levels which would have been found in the year 1450, which would have been considered pristine conditions [8,9], and also depositions to natural ecosystems have increased by a 3–5 factor compared to preindustrial levels [10]. Anthropogenic Hg emissions into the atmosphere are associated with several activities such as coal combustion, artisanal gold mining, cement and nonferrous metal production, waste incineration, the chlor-alkali industry, etc., and were estimated at 2220 Mg in 2015 [11]. These outputs only account for approximately one-third of the total global Hg released into the atmosphere (6500–8200 Mg year−1), whereas emissions from natural processes range from 4600 to 5300 Mg year−1 [12]. However, a large part of these releases are re-emissions of previously deposed Hg from different surfaces (anthropogenic activities oceans, soil, vegetation), while current primary Hg sources such as volcanoes, geothermal activity and weathering of rocks and soils naturally enriched in Hg represent approximately only 4% of total terrestrial outputs [13,14].
Mercury occurs in the atmosphere in three different forms, operationally identified as gaseous elemental Hg (GEM), reactive gaseous Hg (RGM) and particulate bound Hg (PBM) [15]. GEM usually accounts for more than 98% of the total pool [16,17], so its concentrations can be considered indicative of total gaseous Hg levels [18]. This form is very stable in the atmosphere where it can persist for 0.5–2 years [19] and undergo long-range transport before being removed through depositions, thus impacting remote areas such as the Arctic [20]. RGM and PBM, instead, are more soluble (RGM) or easily captured by water (PBM) and show shorter atmospheric lifetimes (hours to days); they are easily removed through wet and dry deposition relatively close to the emission source [21]. Moreover, deposited Hg can be re-emitted back to the atmosphere as GEM, further accentuating the global redistribution of this element [2].
The background value of GEM is currently estimated at 1.5–1.7 ng m−3 in the Northern Hemisphere [22], slightly higher than that of the Southern Hemisphere (1.1–1.3 ng m−3 [23]) due to the greater abundance of emission and re-emission sources. [24]. However, on the local scale, atmospheric GEM shows notable spatiotemporal variations among different environments owing to the large amount of release and deposition rates [25], which can be influenced by local point-sources, the presence of oxidants in the atmosphere, topography and micrometeorological conditions [26,27]. Moreover, several different diurnal and seasonal patterns of GEM are reported in the literature for marine, coastal, rural and urban environments, making the determination of the predominant controlling factors of observed GEM variation extremely complex ([28] and references therein).
Generally, atmospheric GEM concentrations and variations are often greater in areas subject to strong anthropogenic impact [24]. Spatial and temporal atmospheric GEM trends can be helpful in identifying sources and sinks of Hg in the atmosphere (e.g., [25,29]) and providing useful information for the evaluation of release and dispersion of Hg from sites heavily contaminated by human activities and which should be considered for risk assessment [30,31].
In this work, the levels and spatial distribution of GEM were investigated in the Gulf of Trieste (Northern Adriatic Sea, Italy), a coastal environment historically contaminated by Hg. In this area, the contamination of soil, water and sediments has been well characterised and is the result of secular mining activity which took place in Idrija (NW Slovenia), where high amounts of Hg-contaminated material were dispersed into the environment and then transported by the Isonzo River freshwaters in dissolved and solid forms, flowing into the Gulf [32]. There is, however, still little known about the concentrations, sources and fate of GEM in the atmosphere of this area. To fill the gap, several discrete GEM measurements were conducted at numerous sites characterised by different Hg levels in the substrate, distributed over the coastal areas in order to compare GEM levels from both contaminated and pristine environments. To better understand temporal patterns and possible dispersion of this contaminant in the atmospheric compartment, continuous night-day measurements were also conducted. Furthermore, GEM values were correlated with the main meteorological parameters in order to elucidate the factors influencing the behaviour of the element in this area.
2. Experiments
2.1. Study Area
The Gulf of Trieste extends from the Tagliamento River mouth to Savudrija/Punta Salvore (Croatia) and covers an area of approximately 550 km2 in the north easternmost part of the Adriatic Sea. This coastal ecosystem hosts a Marine Protected Area (Miramare) where, due to a no-entry and a buffer zone, conservation and refuge for overexploited species are guaranteed [33]. On the other hand, this densely populated area is host to several varieties of industry. The coastline is largely exploited for tourism (approximately 60%) with Grado and Lignano Sabbiadoro settlements which significantly increase the resident population during summer (from 8000 to 80,000 and 6000 to 250,000, respectively). The most industrialised areas are represented by the cities of Trieste and Monfalcone (210,000 and 30,000 inhabitants, respectively) with their harbours, and also by the nearby city of Koper (Slovenia); overall these industrial sites cover about 30% of the territory (i.e., iron-steel factory, coal-fired power plant, oil pipeline, ship traffic, considerable vehicular emissions and so on). Finally, mussel farming (Mytilus galloprovincialis) and aquaculture using suspended cages (Dicenthrarcus labrax, Sparus aurata and Mugil cephalus) take place in the easternmost sector of the Gulf.
Several pollutants (i.e., trace elements and POPs) represent a concern for the area as highlighted in numerous previous studies [34,35,36,37,38,39,40,41,42,43,44]. However, mercury contamination due to secular inland mining exploitation from Idrija (NW Slovenia) shows a wide diffusion in sediments, waters and soils [32,45,46,47].
2.2. Gaseous Elemental Mercury (GEM) Measurements
GEM measurements were conducted by means of a Lumex RA-915M Portable Mercury Analyzer in selected sites representative of nonurban areas contaminated by Hg (Fossalon-FOS, Grado-GRA and Val Noghera-VN), urban areas (Monfalcone-MON, Villaggio del Pescatore-PES and Trieste-TS) and pristine areas (Basovizza-BAS and Piran-PIR) in the Gulf of Trieste (Northern Adriatic Sea; Figure 1). The Lumex Ra-915M relies on atomic absorption spectrometry (AAS): the instrument has a multipath analytical cell and Zeeman background correction provides both high sensitivity and minimal interference: the accuracy of the method is 20% [48]. The dynamic range covers four orders of magnitude (2–25,000 ng m−3), and the detection limit is governed by shot noise and equals 2.0 ng m−3 (average measuring time 5 s) and 0.3 ng m−3 (average measuring time 30 s). A complete calibration is done by a Lumex technician each year, while a calibration check of the instrument is performed prior to taking measurements by means of an internal accessory cell containing a known amount of Hg. During field work, real-time measurements were visualised on a digital display and stored in an internal data logger. Subsequently, the data were recovered by RAPID 1.00.442 software.
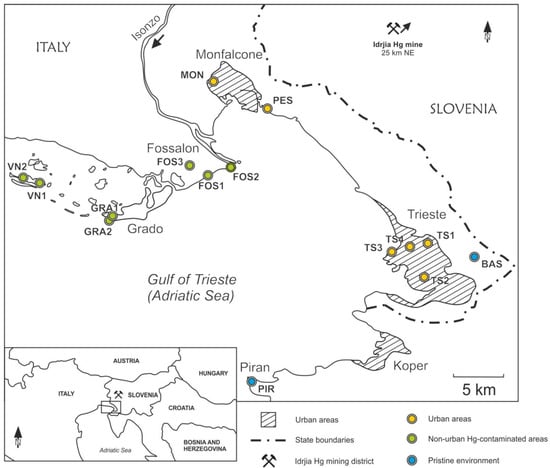
Figure 1.
Study area and selected sampling sites: on the lower left the symbol shows the location of the Idrija Hg mine responsible for the contamination of the Isonzo River Plain and the Gulf of Trieste.
The data were acquired over a variable time range, from a few hours to a maximum of 14 days, depending on sites and local conditions, and sampling rate (from 1 to 10 s). Moreover, values below 2 ng m−3 were treated with the medium bound approach, thus set to ½ LOD (1 ng m−3). All GEM time-series were resampled to a fixed sampling frequency of 1 h in order to easily process the dataset and check the relationships with some meteorological parameters provided by the “OMNIA” database from the meteorological observatory of the regional environmental protection agency (OSMER–ARPA FVG, Visco, Italy).
Univariate statistics were computed hourly with Microsoft Excel spreadsheets and then processed in Python. The Pandas library [49] was employed for time-series downsampling and re-sampling and for calculating matrices of Spearman nonparametric correlation coefficient. The wind rose chart (or polar bar chart) was obtained using the Plotly library [50] graphic tool. All time data are expressed in UTC+0 (Greenwich Mean Time; GMT).
3. Results and Discussion
3.1. GEM Level and Distribution
The univariate descriptive statistic of the surveyed sites is reported in Table 1. As previously mentioned, the pristine environment was chosen for its suitability for comparison with other impacted sites at a considerable distance from Hg-bearing sediments and soils affected by the mining activity of the Idrija Hg mine and from other potential emissions. These sites (BAS and PIR) were previously investigated and showed GEM contents that were on average lower than those found in the other sites (1.02 ± 0.32 and 1.88 ± 1.07 ng m−3, respectively) [51,52]. It is notable that MON, which is located in an urban area, showed lower values (1.19 ± 0.40 ng m−3) considering the hourly average, but the maximum reached up to 17.28 ng m−3. Overall, mean hourly values ranged between 1.20 and 3.57 ng m−3, comparable or slightly higher than the natural background levels estimated for the Northern Hemisphere (1.5–1.7 ng m−3) [22] and for the Mediterranean area (1.75–1.80 ng m−3) [53]. The highest concentrations were found at the Hg-contaminated FOS site (48.5 ng m−3). This area was originally part of the Isonzo River delta and has been affected by human activity since 1800, in particular by land reclamation (several dewatering plants) for intensive agriculture which took place after 1920: the soils are heavily contaminated (up to 40 mg kg−1) [54].

Table 1.
Basic statistics of gaseous elemental mercury (GEM) dataset calculated on raw data (not resampled) and grouped by location. (*) Data published by [52] and (§) [51].
These results are comparable to those found in other Hg-contaminated sites. Taking as an example, Muramoto et al. [55] recorded GEM ranging from 1.89 to 2.23 ng m−3 (max = 6.11 ng m−3) in the Minamata Bay (Japan). In the Mediterranean area Bagnato et al. 2013 [56] and Gibicar et al. 2009 [57] found from 1.5 ± 0.4 to 2.1 ± 0.98 and from 2.8 to 8.7 ng m−3 in the Augusta Bay (Sicily, Italy) and Rosignano (Tuscany, Italy), respectively, and the GEM values were higher during the summer period.
Due to the large amount of the dataset, the comparison of GEM concentrations was depicted by means of a box and whisker plot representation. Briefly, the median is represented by the horizontal bold line within the box, 25th and 75th percentiles are at the top and bottom. In this case, the presence of outliers is shown as circles if values are 1.5 times out of the box and as stars for values which are three times out of the box (Figure 2). Site FOS showed several outliers such as TS, thus suggesting that there are nearby sources of GEM (i.e., contaminated soils, urban activities) that, in the absence of dilution conditions, can be detected by means of continuous monitoring. On the other hand, pristine areas do not behave like active GEM sources and show data comparable to those observed in other areas (ranging from 0.3 to 10 ng m−3) with no outliers [31]. The significant difference between sample medians was confirmed by the Kruskal–Wallis test (p = same = 2.18 × 10−71), which is a nonparametric method for testing if there are statistically significant differences between two or more groups of an independent variable. The calculated ratio of urban to pristine site concentrations for GEM, on average, usually range from 1 to 1.8 [27], and the ratio found in this study, which is 1.52, falls within the ranges from other pristine-urban site studies.
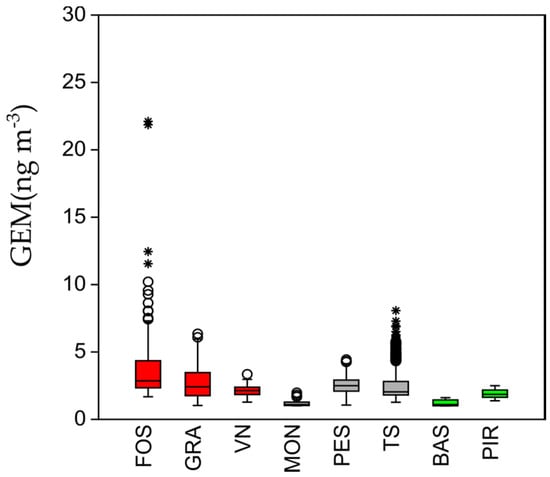
Figure 2.
Boxplots of GEM observed in the investigated sites. (red) Hg-contaminated sites; (grey) urban sites; (green) nonurban and pristine sites.
One of the main concerns arising from GEM levels is the potential risk for local inhabitants via an inhalation pathway. According to the guidelines and safety regulations reported in Oyarzun et al. [58], it can be asserted that no risk is present for local inhabitants (WHO guideline fixed at 1000 ng m−3; MRL for chronic inhalation 200 ng m−3, US OSHA and ATSDR).
3.2. GEM Time-Series
One of the goals of this study was to investigate the occurrence of daily cycles of GEM concentrations as previously reported for other contaminated sites [31]. In this context, some particular time-series, the monitoring campaigns in Fossalon (FOS3_1 and FOS1_2, 2013 and 2015), Grado (GRA2_1, 2015) and Trieste (TS2_3, 2015), are reported in Figure 3.
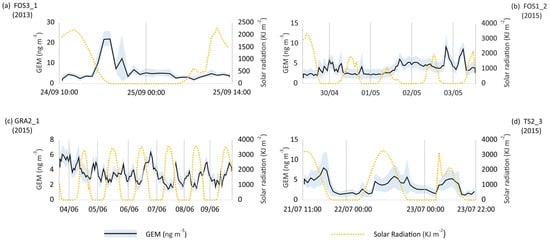
Figure 3.
Time-series of GEM (ng m−3) plotted against intensity of solar radiation (kJ m−2) at selected sites. Date is expressed as dd/mm. (a,b) Fossalon Hg-contaminated site; (c) Grado Hg-contaminated site; (d) Trieste urban area.
The time-series at FOS3_1 showed the presence of anomalous brief high-amplitude peaks that occurred at sunset and during the night (Figure 3a). As previously mentioned, values up to 48.46 ng m−3 were reached (Table 1). In other cases, the peaks were less high in amplitude but more frequent (Figure 3b,c). It can be hypothesised that the contaminated soils are characterised by an emission capacity, a continuous source of GEM that is generally higher during the day because of incident solar radiation. However, during sunrise the temperature decreases and the sea breeze drops; before the opposite land breeze occurs, a temporary atmospheric stable condition is created so that atmospheric dilution and mixing are not favoured, and GEM can concentrate in the lower layers of the atmosphere [30,31].
The occurrence of events of high atmospheric GEM levels is usually associated with conditions of stagnant air and low atmospheric mixing as shown in Figure 4 [17,59].
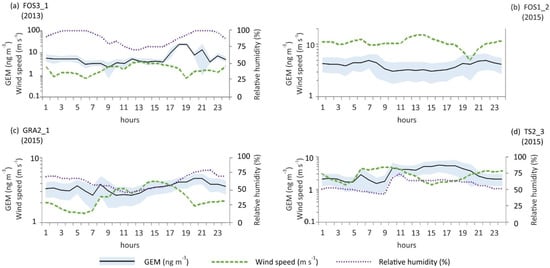
Figure 4.
Hourly average values of GEM (ng m−3) plotted against wind speed (m s−1) and relative humidity at selected sites. (a,b) Fossalon Hg-contaminated site; (c) Grado Hg-contaminated site; (d) Trieste urban area.
During diurnal hours, sea breezes can dilute atmospheric GEM, resulting in lower concentrations. However, considering that the correlation between wind speed and GEM (Table 2) is below 0.3, we hypothesise that lower levels of this contaminant during the day at the Fossalon site could also be caused by an enhanced photooxidation to RGM, which can easily be removed from the atmosphere through atmospheric depositions [60]. In coastal areas, air coming from the sea driven by breezes is usually rich in oxidants such as halogen radicals (e.g., bromine), which are thought to favour the oxidation of GEM in the presence of solar radiation [61,62] and in the marine boundary layer the reaction of GEM with bromine is considered the predominant pathway of oxidation for this species [63].

Table 2.
Spearman (r) correlation matrix between GEM concentrations and micrometeorological parameters. Dark blue and red indicate strong negative and positive significant correlations.
On the contrary, the GEM content in the urban area of Trieste appears to be influenced by solar radiation (Figure 3d).
To assess the correlation between GEM and micrometeorological parameters, Spearman’s correlation coefficients were calculated for any time-series longer than 24 h (Table 2). Generally, we found that GEM reached its highest values when air relative humidity raised, likely because they have similar behaviour with respect to wind dilution. High relative air humidity is usually associated with stagnant atmospheric conditions, which as stated above favours the accumulation of GEM in the lower atmosphere. Taking as an example the measurements conducted at Grado, a significant correlation of GEM with air relative humidity (r = 0.72; Table 2) occurred at GRA2_1 during six days of continuous monitoring. Moreover, GEM increases with temperature, and the behaviour of GEM differs between the Hg-contaminated soil of the Isonzo River Plain and lagoon, and the city of Trieste. In the first case, GEM showed negative correlations with solar radiation, whereas it showed the opposite trend in the urban area of Trieste. This difference is likely a consequence of the distribution of breeze strength between the considered sites; the Isonzo alluvial plain is characterised by a diurnal sea breeze stronger than a nocturnal land breeze, whereas the urban area of Trieste shows an opposite pattern, with higher speeds during the night. In this urban site, no relevant sources of contamination are known, thus GEM could largely be carried from contaminated areas by wind during the diurnal sea breeze. The influence of sea breezes on atmospheric GEM levels in urban areas is well documented [17,25,61,64].
The wind rose chart shows that when GEM is high (up to an hourly mean value of 8 ng m−3) with winds prevalently blowing from W and NW, in the direction of the highly contaminated Fossalon plain, it (GEM) could reach the city of Trieste via the seawater surface of the Gulf (Figure 5). It was hypothesised that a variation in wind speed is not sufficient to cause a dilution process responsible for the decrease in GEM during the night. A possible explanation could instead be that there is another significant source of GEM in the area. This could be represented by the waters of the Gulf of Trieste itself, since it is well-known that elemental Hg can be transferred not only between mining-impacted marine sediments and seawater, but also from seawater to the surrounding inhabited coastal area. In a previous study, Wänberg et al. [53] found that atmospheric GEM levels in Piran follow a similar pattern, showing higher values under conditions of low wind speed blowing from the north. The authors postulated that these air masses were enriched in GEM while passing over the contaminated gulf due to GEM emission from the surface of the sea, a process potentially relevant thanks to the abundance in the water column of this area of dissolved Hg available for reduction and subsequent volatilisation [46,65]. Unfortunately, no data regarding GEM in the Gulf of Trieste are available to more strongly support this hypothesis.
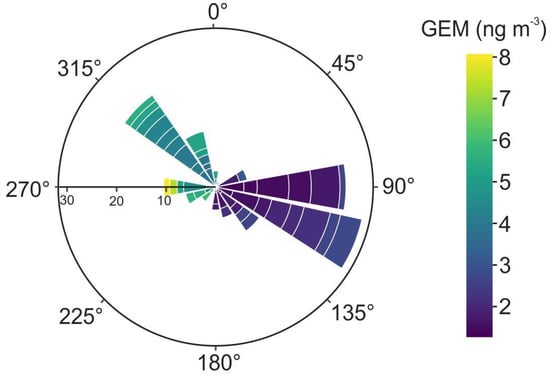
Figure 5.
Wind rose for GEM data at Trieste (time-series: TS2_3). Colours indicate GEM concentration, while the radius length is the cumulative wind speed (m s−1).
4. Conclusions
This study provides new data regarding gaseous elemental mercury (GEM) in the atmosphere around the Gulf of Trieste, which is a historically Hg-contaminated coastal area via sediment/soil-associated metal dispersion from the Isonzo River resulting from mercury mining from Idrija. The results of the study show that GEM concentrations do not reach levels of concern for the population. However, significant variations of GEM have been observed, showing that the coastal alluvial plain built up along the Isonzo River is an active source of mercury in the atmosphere. Anomalous peaks of GEM have been observed, especially at sunset or during the night, when the sea breeze turns into the land breeze, resulting in a decrease in wind speed and scarce low mixing of the low atmospheric layer in contact with the ground. These sites with high-amplitude plumes of GEM are also worth long-term monitoring for continuous data acquisition.
In the urban area of Trieste, the correlation between wind direction and GEM indicates a mass flow of GEM transported from the Isonzo coastal alluvial plain without excluding a contribution from coastal waters of the Gulf to the surrounding areas. The main difference between the nonurban contaminated land/lagoon and the urban area investigated is a different day-night pattern of GEM distribution. On the sites contaminated by Hg, GEM tends to be higher during the night, whereas urban areas show an opposite trend, with maximum values reached during the day. A possible explanation is that in the urban area, anthropogenic Hg emissions contributed significantly to the atmospheric GEM budget, whereas releases from contaminated soils at Fossalon are mainly driven by natural re-emission processes. Further research is needed to identify and quantify the emission sources in both environments, also including monitoring of other trace gases (e.g., CO2, CO, CH4, SO2) usually used as signatures of anthropogenic influence. Future study is also required to understand the contribution of atmospheric Hg deposition to the observed daily GEM patterns, to determine whether their contribution to GEM depletion is higher than that of atmospheric mixing and transport, particularly at those sites which showed a higher daily variability (e.g., over the Isonzo coastal alluvial plain). Moreover, determination of the different atmospheric Hg species, together with measurements of atmospheric oxidants such as halogen radicals, could give further information regarding GEM oxidation to RGM, which could be particularly relevant for coastal environments and contribute to the enhancement of Hg deposition on the substrate.
Author Contributions
Data curation, conceptualization, methodology, writing-original draft and writing review N.B., F.F., S.C., J.M.E., P.H. and A.A. Processing of data N.B. Data survey N.B., F.F., P.H. and S.C. Final editing A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Two anonymous reviewers are warmly acknowledged for their reviews and useful suggestions which improved the earlier version of the manuscript. Karry Close is warmly acknowledged for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Selin, N.E. Global biogeochemical cycling of mercury: A review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Fitzgerald, W.F.; Clarkson, T.W. Mercury and monomethylmercury: Present and future concerns. Environ. Health Perspect. 1991, 96, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Zhang, Y.; Jaeglé, L.; Thompson, L.; Streets, D.G. Six centuries of changing oceanic mercury. Glob. Biogeochem. Cycles 2014, 1251–1261. [Google Scholar] [CrossRef]
- Outridge, P.M.; Mason, R.P.; Wang, F.; Guerrero, S.; Heimbürger-Boavida, L.E. Updated global and oceanic mercury budgets for the United Nations global mercury assessment 2018. Environ. Sci. Technol. 2018, 52, 11466–11477. [Google Scholar] [CrossRef]
- Mason, R.P.; Sheu, G.R. Role of the ocean in the global Mercury CYCLE. Glob. Biogeochem. Cycles 2002, 16, 40–41. [Google Scholar] [CrossRef]
- UN Environment. Global Mercury Assessment 2018; UN Environment Programme: Geneva, Switzerland, 2019. [Google Scholar]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Poissant, L.; Pilote, M.; Beauvais, C.; Constant, P.; Zhang, H.H. A year of continuous measurements of three atmospheric mercury species (GEM, RGM and Hgp) in Southern Québec, Canada. Atmos. Environ. 2005, 39, 1275–1287. [Google Scholar] [CrossRef]
- Slemr, F.; Schuster, G.; Seiler, W. Distribution, speciation, and budget of atmospheric mercury. J. Atmos. Chem. 1985, 3, 407–434. [Google Scholar] [CrossRef]
- Griggs, T.; Liu, L.; Talbot, R.W.; Torres, A.; Lan, X. Comparison of atmospheric mercury speciation at a coastal and an urban site in southeastern Texas, USA. Atmosphere 2020, 11. [Google Scholar] [CrossRef]
- Mao, H.; Talbot, R.W.; Sigler, J.M.; Sive, B.C.; Hegarty, J.D. Seasonal and diurnal variations of Hg&Deg; over New England. Atmos. Chem. Phys. 2008, 8, 1403–1421. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Travnikov, O. Contribution of the intercontinental atmospheric transport to mercury pollution in the northern hemisphere. Atmos. Environ. 2005, 39, 7541–7548. [Google Scholar] [CrossRef]
- Valente, R.J.; Shea, C.; Lynn Humes, K.; Tanner, R.L. Atmospheric mercury in the great smoky mountains compared to regional and global levels. Atmos. Environ. 2007, 41, 1861–1873. [Google Scholar] [CrossRef]
- Sprovieri, F.; Pirrone, N.; Ebinghaus, R.; Kock, H.; Dommergue, A. A review of worldwide atmospheric mercury measurements. Atmos. Chem. Phys. 2010, 10, 8245–8265. [Google Scholar] [CrossRef]
- Lindberg, S.; Bullock, R.; Ebinghaus, R.; Engstrom, D.; Feng, X.; Fitzgerald, W.; Pirrone, N.; Prestbo, E.; Seigneur, C. A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 2007, 36, 19–32. [Google Scholar] [CrossRef]
- Sprovieri, F.; Pirrone, N.; Bencardino, M.; D’Amore, F.; Carbone, F.; Cinnirella, S.; Mannarino, V.; Landis, M.; Ebinghaus, R.; Weigelt, A.; et al. Atmospheric mercury concentrations observed at ground-based monitoring sites globally distributed in the framework of the GMOS network. Atmos. Chem. Phys. 2016, 16, 11915–11935. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Talbot, R.; Laine, P.; Lefer, B.; Flynn, J.; Torres, A. Seasonal and diurnal variations of total gaseous mercury in urban Houston, TX, USA. Atmosphere 2014, 5, 399–419. [Google Scholar] [CrossRef]
- Rutter, A.P.; Snyder, D.C.; Stone, E.A.; Schauer, J.J.; Gonzalez-Abraham, R.; Molina, L.T.; Ḿarquez, C.; Ćardenas, B.; De Foy, B. In situ measurements of speciated atmospheric mercury and the identification of source regions in the Mexico City metropolitan area. Atmos. Chem. Phys. 2009, 9, 207–220. [Google Scholar] [CrossRef]
- Cheng, I.; Zhang, L.; Mao, H.; Blanchard, P.; Tordon, R.; Dalziel, J. Seasonal and diurnal patterns of speciated atmospheric mercury at a coastal-rural and a coastal-urban site. Atmos. Environ. 2014, 82, 193–205. [Google Scholar] [CrossRef]
- Mao, H.; Cheng, I.; Zhang, L. Current understanding of the driving mechanisms for spatiotemporal variations of atmospheric speciated mercury: A review. Atmos. Chem. Phys. 2016, 16, 12897–12924. [Google Scholar] [CrossRef]
- Ren, X.; Luke, W.T.; Kelley, P.; Cohen, M.D.; Artz, R.; Olson, M.L.; Schmeltz, D.; Puchalski, M.; Goldberg, D.L.; Ring, A.; et al. Atmospheric mercury measurements at a suburban site in the mid-atlantic united states: Inter-annual, seasonal and diurnal variations and source-receptor relationships. Atmos. Environ. 2016, 146, 141–152. [Google Scholar] [CrossRef]
- Esbrí, J.M.; Martínez-Coronado, A.; Higueras, P.L. Temporal variations in gaseous elemental mercury concentrations at a contaminated Site: Main factors affecting nocturnal maxima in daily cycles. Atmos. Environ. 2016, 125, 8–14. [Google Scholar] [CrossRef]
- Higueras, P.; Oyarzun, R.; Kotnik, J.; Esbrí, J.M.; Martínez-Coronado, A.; Horvat, M.; López-Berdonces, M.A.; Llanos, W.; Vaselli, O.; Nisi, B.; et al. A compilation of field surveys on gaseous elemental mercury (GEM) from contrasting environmental settings in Europe, South America, South Africa and China: Separating fads from facts. Environ. Geochem. Health 2014, 36, 713–734. [Google Scholar] [CrossRef]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (gulf of Trieste, Northern Adriatic Sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Boero, F.; Brian, F.; Micheli, F. Scientific design and monitoring of mediterranean marine protected areas. In Proceedings of the CIESM Workshop Series No 8, Porto Cesareo, Italy, 21–24 October 1999; p. 64. [Google Scholar]
- Olivotti, R.; Faganeli, J.; Malej, A. Impact of “organic” pollutants on coastal waters, gulf of Trieste. Water Sci. Technol. 1986, 18, 57–68. [Google Scholar] [CrossRef]
- Adami, G.; Barbieri, P.; Piselli, S.; Predonzani, S.; Reisenhofer, E. New data on organic pollutants in surface sediments in the harbour of Trieste. Ann. Chim. 1998, 88, 745–754. [Google Scholar]
- Pozo, K.; Lazzerini, D.; Perra, G.; Volpi, V.; Corsolini, S.; Focardi, S. Levels and spatial distribution of polychlorinated biphenyls (PCBs) in superficial sediment from 15 Italian Marine Protected Areas (MPA). Mar. Pollut. Bull. 2009, 58, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Barbieri, P.; Piselli, S.; Predonzani, S.; Reisenhofer, E. Detecting and characterising sources of persistent organic pollutants (PAHs and PCBs) in surface sediments of an industrialized area (harbour of Trieste, Northern Adriatic Sea). J. Environ. Monit. 2000, 2, 261–265. [Google Scholar] [CrossRef]
- Formalewicz, M.; Rampazzo, F.; Noventa, S.; Gion, C.; Petranich, E.; Crosera, M.; Covelli, S.; Faganeli, J.; Berto, D. Organotin compounds in touristic marinas of the Northern Adriatic sea: Occurrence, speciation and potential recycling at the sediment-water interface. Environ. Sci. Pollut. Res. 2019, 26, 31142–31157. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, P.; Adami, G.; Predonzani, S.; Reisenhofer, E. Heavy metals in surface sediments near urban and industrial sewage discharges in the gulf of Trieste. Toxicol. Environ. Chem. 1999, 71, 105–114. [Google Scholar] [CrossRef]
- Cibic, T.; Acquavita, A.; Aleffi, F.; Bettoso, N.; Blasutto, O.; De Vittor, C.; Falconi, C.; Falomo, J.; Faresi, L.; Predonzani, S.; et al. Integrated approach to sediment pollution: A case study in the gulf of Trieste. Mar. Pollut. Bull. 2008, 56, 1650–1657. [Google Scholar] [CrossRef]
- Milivojevič Nemanič, T.; Leskovšek, H.; Horvat, M.; Vrišer, B.; Bolje, A. Organotin compounds in the marine environment of the bay of Piran, Northern Adriatic sea. J. Environ. Monit. 2002, 4, 426–430. [Google Scholar] [CrossRef]
- Ščančar, J.; Zuliani, T.; Turk, T.; Milačič, R. Organotin compounds and selected metals in the marine environment of Northern Adriatic sea. Environ. Monit. Assess. 2007, 127, 271–282. [Google Scholar] [CrossRef]
- Acquavita, A.; Predonzani, S.; Mattassi, G.; Rossin, P.; Tamberlich, F.; Falomo, J.; Valic, I. Heavy metal contents and distribution in coastal sediments of the gulf of Trieste (NORTHERN Adriatic sea, Italy). Water. Air. Soil Pollut. 2010, 211, 95–111. [Google Scholar] [CrossRef]
- Petranich, E.; Croce, S.; Crosera, M.; Pavoni, E.; Faganeli, J.; Adami, G.; Covelli, S. Mobility of Metal(Loid)s at the Sediment-Water Interface in Two Tourist Port Areas of the Gulf of Trieste (Northern Adriatic sea). Environ. Sci. Pollut. Res. 2018, 25, 26887–26902. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Covelli, S.; Faganeli, J.; Logar, M.; Mandić, V.; Rajar, R.; Širca, A.; Žagar, D. Mercury in contaminated coastal environments. A case study: The gulf of Trieste. Sci. Total Environ. 1999, 237–238, 43–56. [Google Scholar] [CrossRef]
- Faganeli, J.; Horvat, M.; Covelli, S.; Fajon, V.; Logar, M.; Lipej, L.; Cermelj, B. Mercury and methylmercury in the gulf of Trieste (Northern Adriatic sea). Sci. Total Environ. 2003, 304, 315–326. [Google Scholar] [CrossRef]
- Piani, A.; Acquavita, A.; Catalano, L.; Contin, M.; Mattassi, G.; De Nobili, M. Effects of long term Hg contamination on soil mercury speciation and soil biological activities. E3S Web Conf. 2013, 1, 1–4. [Google Scholar] [CrossRef]
- Sholupov, S.E.; Ganeyev, A.A. Zeeman atomic absorption spectrometry using high frequency modulated light polarization. Spectrochim. Acta Part B At. Spectrosc. 1995, 50, 1227–1236. [Google Scholar] [CrossRef]
- McKinney, W. Data structures for statistical computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; Volume 1697900, pp. 51–56. [Google Scholar]
- Sievert, C.; Parmer, C.; Hocking, T.; Chamberlain, S.; Ram, K.; Corvellec, M.; Despouy, P. Plotly: Create Interactive Web Graphics via “Plotly. Js.”. 2018. Available online: https://rdrr.io/cran/plotly/ (accessed on 28 August 2020).
- Acquavita, A.; Biasiol, S.; Lizzi, D.; Mattassi, G.; Pasquon, M.; Skert, N.; Marchiol, L. Gaseous elemental mercury level and distribution in a heavily contaminated site: The ex-chlor alkali plant in torviscosa (Northern Italy). Water. Air. Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Floreani, F.; Acquavita, A.; Petranich, E.; Covelli, S. Diurnal fluxes of Gaseous elemental mercury from the water-air interface in coastal environments of the Northern Adriatic sea. Sci. Total Environ. 2019, 668, 925–935. [Google Scholar] [CrossRef]
- Wängberg, I.; Munthe, J.; Amouroux, D.; Andersson, M.E.; Fajon, V.; Ferrara, R.; Gårdfeldt, K.; Horvat, M.; Mamane, Y.; Melamed, E.; et al. Atmospheric mercury at Mediterranean coastal stations. Environ. Fluid Mech. 2008, 8, 101–116. [Google Scholar] [CrossRef]
- Acquavita, A.; Brandolin, D.; Felluga, A.; Maddaleni, P.; Meloni, C.; Poli, L.; Skert, N.; Zanello, A. Mercury distribution and speciation in soils contaminated by historically mining activity: The Isonzo River plain. In Proceedings of the Congresso SIMP-SGI-SOGEI 2019, Parma, Italy, 16–19 September 2019. [Google Scholar]
- Marumoto, K.; Hayashi, M.; Takami, A. Atmospheric mercury concentrations at two sites in the Kyushu Islands, Japan, and evidence of long-range transport from East Asia. Atmos. Environ. 2015, 117, 147–155. [Google Scholar] [CrossRef]
- Bagnato, E.; Sproveri, M.; Barra, M.; Bitetto, M.; Bonsignore, M.; Calabrese, S.; Di Stefano, V.; Oliveri, E.; Parello, F.; Mazzola, S. The sea-air exchange of mercury (Hg) in the marine boundary layer of the Augusta Basin (Southern Italy): Concentrations and evasion flux. Chemosphere 2013, 93, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Gibičar, D.; Horvat, M.; Logar, M.; Fajon, V.; Falnoga, I.; Ferrara, R.; Lanzillotta, E.; Ceccarini, C.; Mazzolai, B.; Denby, B.; et al. Human exposure to mercury in the vicinity of chlor-alkali plant. Environ. Res. 2009, 109, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Oyarzun, R.; Higueras, P.; Esbrí, J.M.; Pizarro, J. Mercury in air and plant specimens in herbaria: A pilot study at the MAF herbarium in Madrid (Spain). Sci. Total Environ. 2007, 387, 346–352. [Google Scholar] [CrossRef]
- Nie, X.; Mao, H.; Li, P.; Li, T.; Zhou, J.; Wu, Y.; Yang, M.; Zhen, J.; Wang, X.; Wang, Y. Total gaseous mercury in a coastal city (Qingdao, China): Influence of sea-land breeze and regional transport. Atmos. Environ. 2020, 235, 1–11. [Google Scholar] [CrossRef]
- Amos, H.M.; Jacob, D.J.; Holmes, C.D.; Fisher, J.A.; Wang, Q.; Yantosca, R.M.; Corbitt, E.S.; Galarneau, E.; Rutter, A.P.; Gustin, M.S.; et al. Gas-particle partitioning of atmospheric Hg (II) and its effect on global mercury deposition. Atmos. Chem. Phys. 2012, 12, 591–603. [Google Scholar] [CrossRef]
- Malcolm, E.G.; Keeler, G.J.; Landis, M.S. The effects of the coastal environment on the atmospheric mercury cycle. J. Geophys. Res. 2003, 108, 1–10. [Google Scholar] [CrossRef]
- Lyman, S.N.; Cheng, I.; Gratz, L.E.; Weiss-Penzias, P.; Zhang, L. An updated review of atmospheric mercury. Sci. Total Environ. 2020, 707, 55–75. [Google Scholar] [CrossRef]
- Holmes, C.D.; Jacob, D.J.; Mason, R.P.; Jaffe, D.A. Sources and deposition of reactive gaseous mercury in the marine atmosphere. Atmos. Environ. 2009, 43, 2278–2285. [Google Scholar] [CrossRef]
- Bełdowska, M.; Falkowska, L.; Siudek, P.; Gajecka, A.; Lewandowska, A.; Rybka, A.; Zgrundo, A. Atmospheric mercury over the coastal zone of the gulf of Gda ń Sk oceanological and hydrobiological studies atmospheric mercury over the coastal zone of the gulf of Gda ń Sk. Int. J. Oceanogr. Hydrobiol. 2007, 36, 1–10. [Google Scholar]
- Bratkič, A.; Tinta, T.; Koron, N.; Guevara, S.R.; Begu, E.; Barkay, T.; Horvat, M.; Falnoga, I.; Faganeli, J. Mercury transformations in a coastal water column (gulf of Trieste, Northern Adriatic sea). Mar. Chem. 2018, 200, 57–67. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).