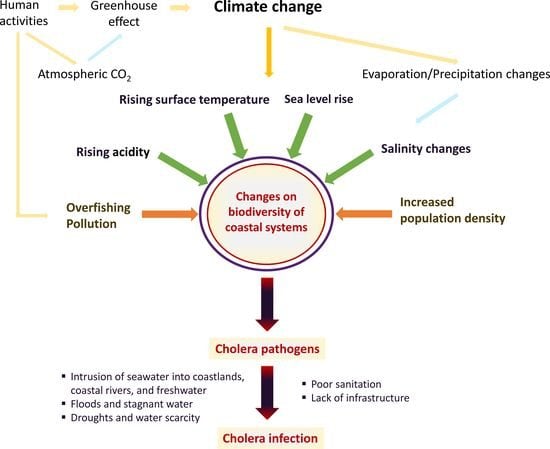
The Impact of Climate Change on Cholera: A Review on the Global Status and Future Challenges
Abstract
1. Introduction
2. Impacts of Climate Change on Aquatic Ecosystems Related to Vibrio Infections—An Overview of Evidence
3. Vibrio cholera—An Overview of Epidemiology, Transmission, and Clinical Disease
Climate-Driven Changes in the Epidemiology of Cholera and Other Vibrio Species
4. Prevention of Cholera—Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Belesova, K.; Berry, H.; Bouley, T.; Boykoff, M.; Byass, P.; Cai, W.; et al. The 2018 report of the Lancet Countdown on health and climate change: Shaping the health of nations for centuries to come. Lancet 2018, 392, 2479–2514. [Google Scholar] [CrossRef]
- Costello, A.; Abbas, M.; Allen, A.; Ball, S.; Bell, S.; Bellamy, R.; Friel, S.; Groce, N.; Johnson, A.; Kett, M.; et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet 2009, 373, 1693–1733. [Google Scholar] [CrossRef]
- Watts, N.; Amann, M.; Ayeb-Karlsson, S.; Belesova, K.; Bouley, T.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; Chambers, J.; et al. The Lancet Countdown on health and climate change: From 25 years of inaction to a global transformation for public health. Lancet 2018, 391, 581–630. [Google Scholar] [CrossRef]
- Nichols, G.; Lake, I.; Heaviside, C. Climate change and water-related infectious diseases. Atmosphere 2018, 9, 385. [Google Scholar] [CrossRef]
- ECDC. Annual Epidemiological Report on Communicable Diseases in Europe 2010; ECDC: Solna Stad, Sweden, 2010; ISBN 9789291932221. [Google Scholar]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Prim. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.D.; Nair, G.B.; Ahmed, T.; Qadri, F.; Holmgren, J. Cholera. Lancet 2017, 390, 1539–1549. [Google Scholar] [CrossRef]
- Intergovermental Panel on Climate Change. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovermental Panel on Climate Change: Geneva, Switzerland, 2014.
- Burrows, M.T.; Schoeman, D.S.; Buckley, L.B.; Moore, P.; Poloczanska, E.S.; Brander, K.M.; Brown, C.; Bruno, J.F.; Duarte, C.M.; Halpern, B.S.; et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 2011, 334, 652–655. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Walker, J.T. The influence of climate change on waterborne disease and Legionella: A review. Perspect. Public Health 2018, 138, 282–286. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The distribution and abundance of parasites in aquatic ecosystems in a changing climate: More than just temperature. Integr. Comp. Biol. 2016, 56, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Marcogliese, D.J. The impact of climate change on the parasites and infectious diseases of aquatic animals. OIE Rev. Sci. Tech. 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-cholera vibrios: The microbial barometer of climate change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Lipp, E.K.; Huq, A.; Colwell, R.R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002, 15, 757–770. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.J.; Kutz, S.; Harvell, C.D. Climate change and infectious diseases: From evidence to a predictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Singleton, F.L.; Attwell, R.W.; Jangi, M.S.; Colwell, R.R. Influence of salinity and organic nutrient concentration on survival and growth of Vibrio cholerae in aquatic microcosms. Appl. Environ. Microbiol. 1982, 43, 1080–1085. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Chang. 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Motes, M.L.; DePaola, A.; Cook, D.W.; Veazey, J.E.; Hunsucker, J.C.; Garthright, W.E.; Blodgett, R.J.; Chirtel, S.J. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 1998, 64, 1459–1465. [Google Scholar] [CrossRef]
- Dvorak, A.C.; Solo-Gabriele, H.M.; Galletti, A.; Benzecry, B.; Malone, H.; Boguszewski, V.; Bird, J. Possible impacts of sea level rise on disease transmission and potential adaptation strategies, a review. J. Environ. Manag. 2018, 217, 951–968. [Google Scholar] [CrossRef]
- Lima, F.P.; Wethey, D.S. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 2012, 3, 704. [Google Scholar] [CrossRef]
- Mora, C.; Spirandelli, D.; Franklin, E.C.; Lynham, J.; Kantar, M.B.; Miles, W.; Smith, C.Z.; Freel, K.; Moy, J.; Louis, L.V.; et al. Broad threat to humanity from cumulative climate hazards intensified by greenhouse gas emissions. Nat. Clim. Chang. 2018, 8, 1062–1071. [Google Scholar] [CrossRef]
- Soneja, S.; Jiang, C.; Romeo Upperman, C.; Murtugudde, R.; Mitchell, C.S.; Blythe, D.; Sapkota, A.R.; Sapkota, A. Extreme precipitation events and increased risk of campylobacteriosis in Maryland, U.S.A. Environ. Res. 2016, 149, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; Epstein, L.D.; Gilman, R.H.; Figueroa, D.; Cama, R.I.; Patz, J.A.; Black, R.E. Effects of El Nino and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet 2000, 355, 442–450. [Google Scholar] [CrossRef]
- Curriero, F.C.; Patz, J.A.; Rose, J.B.; Lele, S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am. J. Public Health 2001, 91, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.N.; Jagai, J.S.; Matyas, B.; DeMaria, A.; MacNeill, I.B.; Griffiths, J.K. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol. Infect. 2007, 135, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Ikeda, T.; French, N.; Baker, M.G.; Hales, S. Climate variability, weather and enteric disease incidence in New Zealand: Time series analysis. PLoS ONE 2013, 8, e83484. [Google Scholar] [CrossRef] [PubMed]
- Cann, K.F.; Thomas, D.R.; Salmon, R.L.; Wyn-Jones, A.P.; Kay, D. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 2013, 141, 671–686. [Google Scholar] [CrossRef]
- Levy, K.; Smith, S.M.; Carlton, E.J. Climate change impacts on waterborne diseases: Moving toward designing interventions. Curr. Environ. Health Rep. 2018, 5, 272–282. [Google Scholar] [CrossRef]
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef]
- Piarroux, R.; Faucher, B. Cholera epidemics in 2010: Respective roles of environment, strain changes, and human-driven dissemination. Clin. Microbiol. Infect. 2012, 18, 231–238. [Google Scholar] [CrossRef]
- Charles, R.C.; Ryan, E.T. Cholera in the 21st century. Curr. Opin. Infect. Dis. 2011, 24, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, virulence, and infective dose. PLoS Pathog. 2007, 3, e147. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cholera vaccines: WHO position paper—August 2017. Wkly Epidemiol Rec. 2017, 92, 477–498. [Google Scholar]
- Colwell, R.R.; Huq, A. Environmental reservoir of vibrio cholerae the causative agent of cholera. Ann. N. Y. Acad. Sci. 1994, 740, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Drasar, B.S.; Bradley, D.J. Long-term persistence of toxigenic Vibrio cholerae 01 in the mucilaginous sheath of a blue-green alga, Anabaena variabilis. J. Trop. Med. Hyg. 1990, 93, 133–139. [Google Scholar] [PubMed]
- Griffith, D.C.; Kelly-Hope, L.A.; Miller, M.A. Review of reported cholera outbreaks worldwide, 1995–2005. Am. J. Trop. Med. Hyg. 2006, 75, 973–977. [Google Scholar] [CrossRef]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef]
- Kovats, R.S.; Bouma, M.J.; Hajat, S.; Worrall, E.; Haines, A. El Niño and health. Lancet 2003, 362, 1481–1489. [Google Scholar] [CrossRef]
- Pascual, M.; Rodo, X.; Ellner, S.P.; Colwell, R.; Bouma, M.J. Cholera dynamics and El Nino-Southern Oscillation. Science 2000, 289, 1766–1769. [Google Scholar] [CrossRef]
- Rodó, X.; Pascual, M.; Fuchs, G.; Faruque, A.S.G. ENSO and cholera: A nonstationary link related to climate change? Proc. Natl. Acad. Sci. USA 2002, 99, 12901–12906. [Google Scholar] [CrossRef]
- Vezzulli, L.; Colwell, R.R.; Pruzzo, C. Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb. Ecol. 2013, 65, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.R.; Nur, Z.; Hassan, N.; Seidlein, L.; Dunachie, S. Pandemics, pathogenicity and changing molecular epidemiology of cholera in the era of global warming. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.K.; Basu, A.; Garg, P.; Bag, P.K.; Ghosh, A.; Bhattacharya, S.K.; Takeda, Y.; Nair, G.B. Molecular epidemiology of reemergent Vibrio cholerae O139 Bengal in India. J. Clin. Microbiol. 1998, 36, 2149–2152. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.M.; Roy, S.K.; Alim, A.R.M.A.; Siddique, A.K.; Albert, M.J. Molecular epidemiology of toxigenic Vibrio cholerae in Bangladesh studied by numerical analysis of rRNA gene restriction patterns. J. Clin. Microbiol. 1995, 33, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.M.; Ahmed, K.M.; Siddique, A.K.; Zaman, K.; Abdul Alim, A.R.M.; Albert, M.J. Molecular analysis of toxigenic Vibrio cholerae O139 Bengal strains isolated in Bangladesh between 1993 and 1996: Evidence for emergence of a new clone of the Bengal vibrios. J. Clin. Microbiol. 1997, 35, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.K.; Cash, R. Cholera outbreaks in the classical biotype era. Curr. Top. Microbiol. Immunol. 2014, 379, 1–16. [Google Scholar] [PubMed]
- Goel, A.K.; Jiang, S.C. Association of heavy rainfall on genotypic diversity in V. cholerae isolates from an outbreak in India. Int. J. Microbiol. 2011, 2011, 230597. [Google Scholar] [CrossRef]
- Pardio Sedas, V.T. Influence of environmental factors on the presence of Vibrio cholerae in the marine environment: A climate link. J. Infect. Dev. Ctries. 2007, 1, 224–241. [Google Scholar] [CrossRef]
- Lü, H.; Yuan, Y.; Sun, N.; Bi, Z.; Guan, B.; Shao, K.; Wang, T.; Bi, Z. Characterization of Vibrio cholerae isolates from 1976 to 2013 in Shandong Province, China. Braz. Braz. J. Microbiol. 2017, 48, 173–179. [Google Scholar] [CrossRef][Green Version]
- Koelle, K.; Pascual, M.; Yunus, M. Pathogen adaptation to seasonal forcing and climate change. Proc. R. Soc. B Biol. Sci. 2005, 272, 971–977. [Google Scholar] [CrossRef]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef] [PubMed]
- Jutla, A.S.; Akanda, A.S.; Griffiths, J.K.; Colwell, R.; Islam, S. Warming oceans, phytoplankton, and river discharge: Implications for cholera outbreaks. Am. J. Trop. Med. Hyg. 2011, 85, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Chatterjee, N.S. Role of vibrio cholerae exochitinase ChiA2 in horizontal gene transfer. Can. J. Microbiol. 2015, 62, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, T.; Sharma, N.C. Cholera outbreaks in India. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Asadgol, Z.; Mohammadi, H.; Kermani, M.; Badirzadeh, A.; Gholami, M. The effect of climate change on cholera disease: The road ahead using artificial neural network. PLoS ONE 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Lemaitre, J.; Pasetto, D.; Perez-Saez, J.; Sciarra, C.; Wamala, J.F.; Rinaldo, A. Rainfall as a driver of epidemic cholera: Comparative model assessments of the effect of intra-seasonal precipitation events. Acta Trop. 2019, 190, 235–243. [Google Scholar] [CrossRef]
- Chin, C.S.; Sorenson, J.; Harris, J.B.; Robins, W.P.; Charles, R.C.; Jean-Charles, R.R.; Bullard, J.; Webster, D.R.; Kasarskis, A.; Peluso, P.; et al. The origin of the Haitian cholera outbreak strain. N. Engl. J. Med. 2011, 364, 33–42. [Google Scholar] [CrossRef]
- Enserink, M. Haiti’s cholera outbreak. Cholera linked to U.N. forces, but questions remain. Science 2011, 332, 776–777. [Google Scholar] [CrossRef]
- Jutla, A.; Whitcombe, E.; Hasan, N.; Haley, B.; Akanda, A.; Huq, A.; Alam, M.; Sack, R.B.; Colwell, R. Environmental factors influencing epidemic cholera. Am. J. Trop. Med. Hyg. 2013, 89, 597–607. [Google Scholar] [CrossRef]
- WHO. Progress on Sanitation and Drinking-Water; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal contamination of drinking-water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef]
- Lee, E.C.; Azman, A.S.; Kaminsky, J.; Moore, S.M.; McKay, H.S.; Lessler, J. The projected impact of geographic targeting of oral cholera vaccination in sub-Saharan Africa: A modeling study. PLoS Med. 2019, 16, 1–17. [Google Scholar] [CrossRef]
- Escobar, L.E.; Ryan, S.J.; Stewart-Ibarra, A.M.; Finkelstein, J.L.; King, C.A.; Qiao, H.; Polhemus, M.E. A global map of suitability for coastal Vibrio cholerae under current and future climate conditions. Acta Trop. 2015, 149, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.; Smith, S.I. Recurrent cholera epidemics in Africa: Which way forward? A literature review. Infection 2018, 47, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.; Kendall, M.; Vugia, D.J.; Henao, O.L.; Mahon, B.E. Increasing rates of vibriosis in the United States, 1996–2010: Review of surveillance data from 2 systems. Clin. Infect. Dis. 2012, 54 (Suppl. 5), S391–S395. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J.; Dawson, T. Climate and cholera in KwaZulu-Natal, South Africa: The role of environmental factors and implications for epidemic preparedness. Int. J. Hyg. Environ. Health 2008, 211, 156–162. [Google Scholar] [CrossRef]
- Akanda, A.; Aziz, S.; Jutla, A.; Huq, A.; Alam, M.; Ahsan, G.; Colwell, R. Satellites and cell phones form a cholera early-warning system. Eos 2018, 99. [Google Scholar] [CrossRef]
- Kopprio, G.A.; Streitenberger, M.E.; Okuno, K.; Baldini, M.; Biancalana, F.; Fricke, A.; Martínez, A.; Neogi, S.B.; Koch, B.P.; Yamasaki, S.; et al. Biogeochemical and hydrological drivers of the dynamics of Vibrio species in two Patagonian estuaries. Sci. Total Environ. 2017, 579, 646–656. [Google Scholar] [CrossRef]
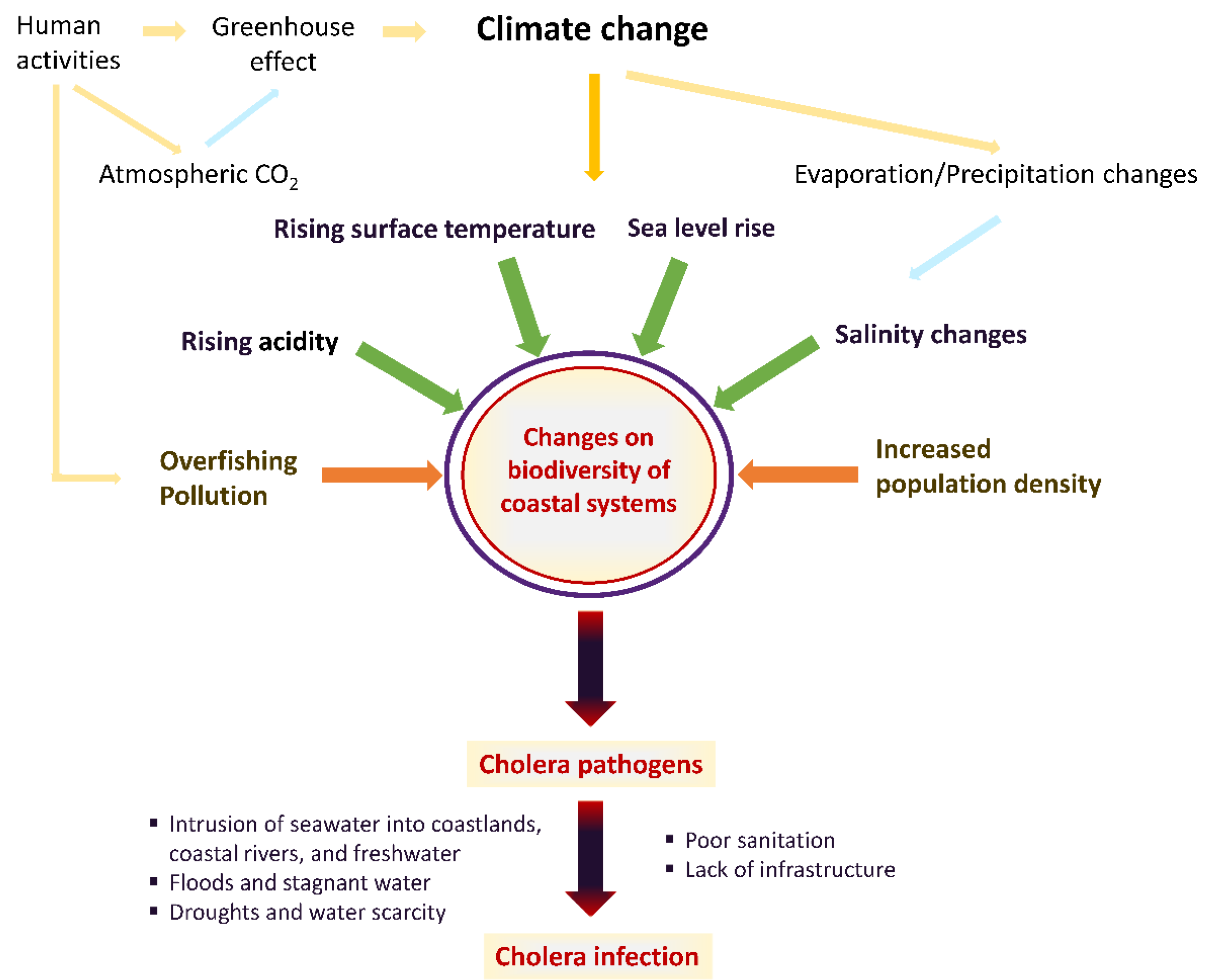
| Risk Factor | Effect of Climate Change | Vibrio cholerae Disease Potential |
|---|---|---|
| Ocean surface temperature | Increase | Bacterial replication |
| CO2 concentration | Increase | Bacterial replication |
| Oxygen levels | Decrease | Bacterial replication |
| Ocean acidification | Increase | Bacterial replication |
| Ocean pollution | Increase | Bacterial replication |
| Salinity | Increase or decrease depending on decreasing or increasing precipitation | Bacterial replication |
| Ocean level | Increase | Flooding events, disruption of water systems/Increased spread |
| Rainfall/Flood | Increase | Disruption of water systems/Increased spread |
| Drought | Increase | Increased spread |
| Period | Start from | Spread to | Cholera Strain |
|---|---|---|---|
| 1817–1823 | India (Bengal) | China, Indonesia, Europe, East Africa | V. cholerae serotype O1, classical biotype |
| 1829–1851 | India | Russia (Moscow), America (New York, Manhattan, Philadelphia, New Orleans), Hungary, Germany, London, Egypt | V. cholerae serotype O1, classical biotype |
| 1852–1859 | India | North Africa, South America (Brazil) | V. cholerae serotype O1, classical biotype |
| 1863–1879 | India (Ganges Delta) | Naples, Spain | V. cholerae serotype O1, classical biotype |
| 1881–1896 | India | Europe, Asia, South America | V. cholerae serotype O1, classical biotype |
| 1899–1923 | India | Egypt, Arabian peninsula, Persia | V. cholerae serotype O1, classical biotype |
| 1961–ongoing | Indonesia | East Pakistan, the Soviet Union, North Africa | V. cholerae serotype O1, El Tor biotype |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christaki, E.; Dimitriou, P.; Pantavou, K.; Nikolopoulos, G.K. The Impact of Climate Change on Cholera: A Review on the Global Status and Future Challenges. Atmosphere 2020, 11, 449. https://doi.org/10.3390/atmos11050449
Christaki E, Dimitriou P, Pantavou K, Nikolopoulos GK. The Impact of Climate Change on Cholera: A Review on the Global Status and Future Challenges. Atmosphere. 2020; 11(5):449. https://doi.org/10.3390/atmos11050449
Chicago/Turabian StyleChristaki, Eirini, Panagiotis Dimitriou, Katerina Pantavou, and Georgios K. Nikolopoulos. 2020. "The Impact of Climate Change on Cholera: A Review on the Global Status and Future Challenges" Atmosphere 11, no. 5: 449. https://doi.org/10.3390/atmos11050449
APA StyleChristaki, E., Dimitriou, P., Pantavou, K., & Nikolopoulos, G. K. (2020). The Impact of Climate Change on Cholera: A Review on the Global Status and Future Challenges. Atmosphere, 11(5), 449. https://doi.org/10.3390/atmos11050449