Chemical Compositions and Source Analysis of PM2.5 during Autumn and Winter in a Heavily Polluted City in China
Abstract
1. Introduction
2. Materials and Methods
2.1. PM2.5 Samples
2.2. Chemical Analysis
2.3. Date Analysis
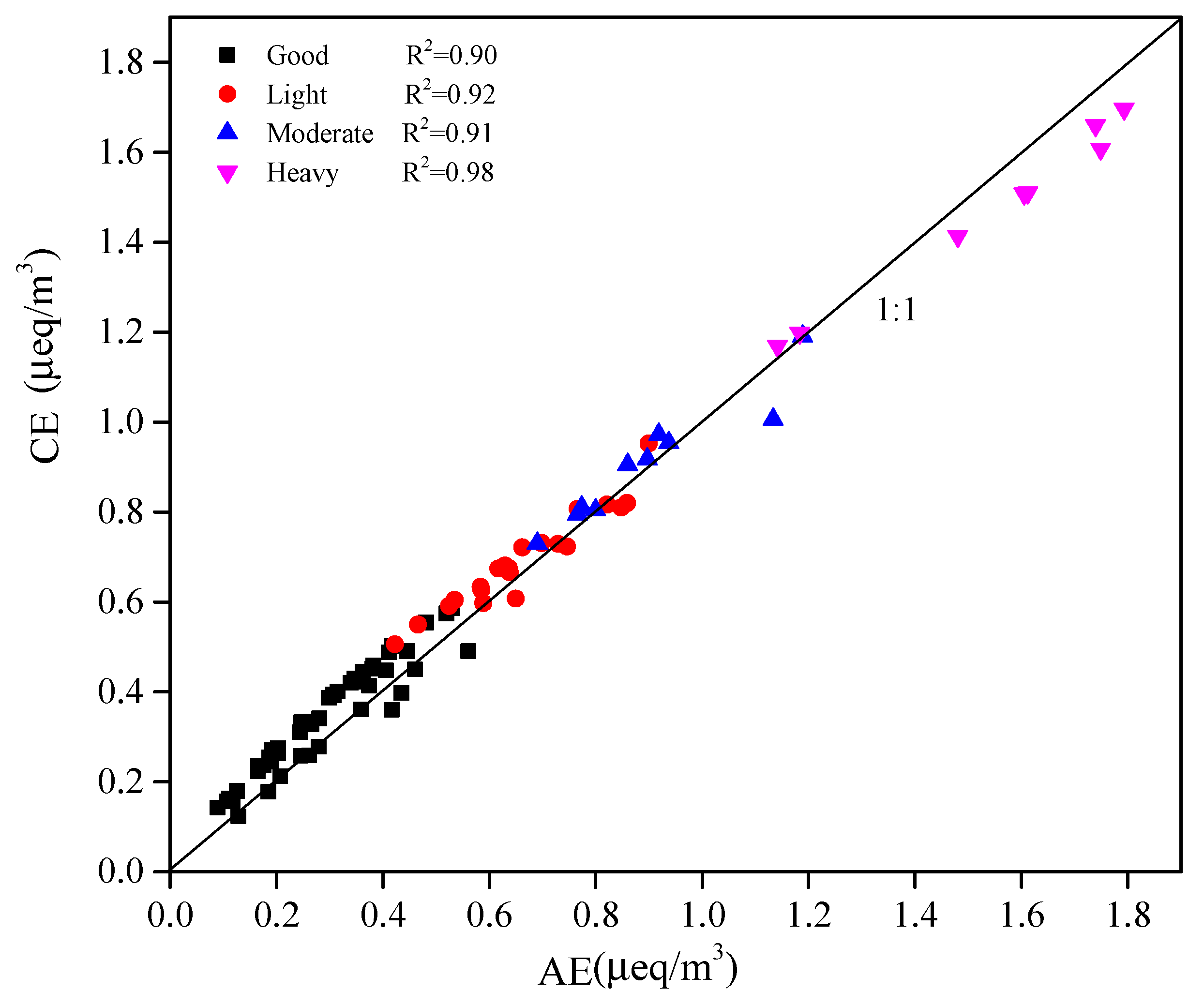
2.3.1. Ionic Balance
2.3.2. Oxidation Ratio
2.3.3. Enrichment Factor
2.3.4. Mass Reconstruction
2.3.5. Source Apportionment
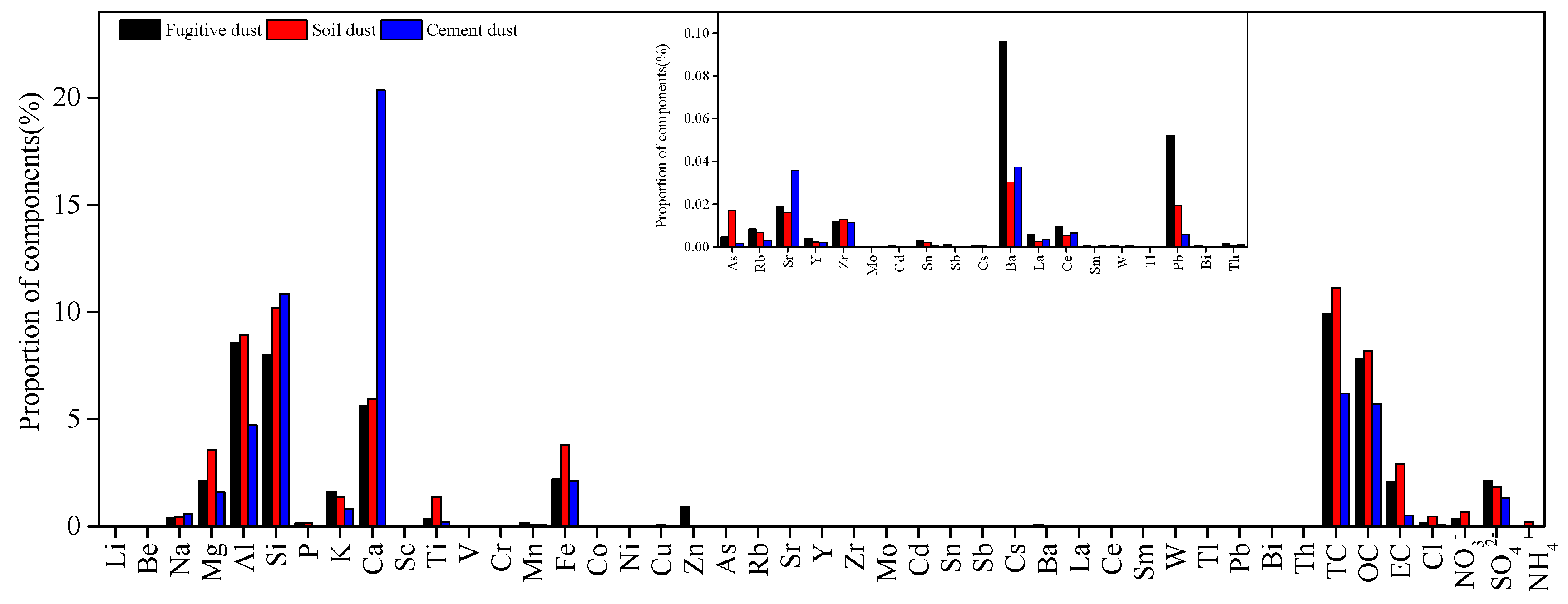
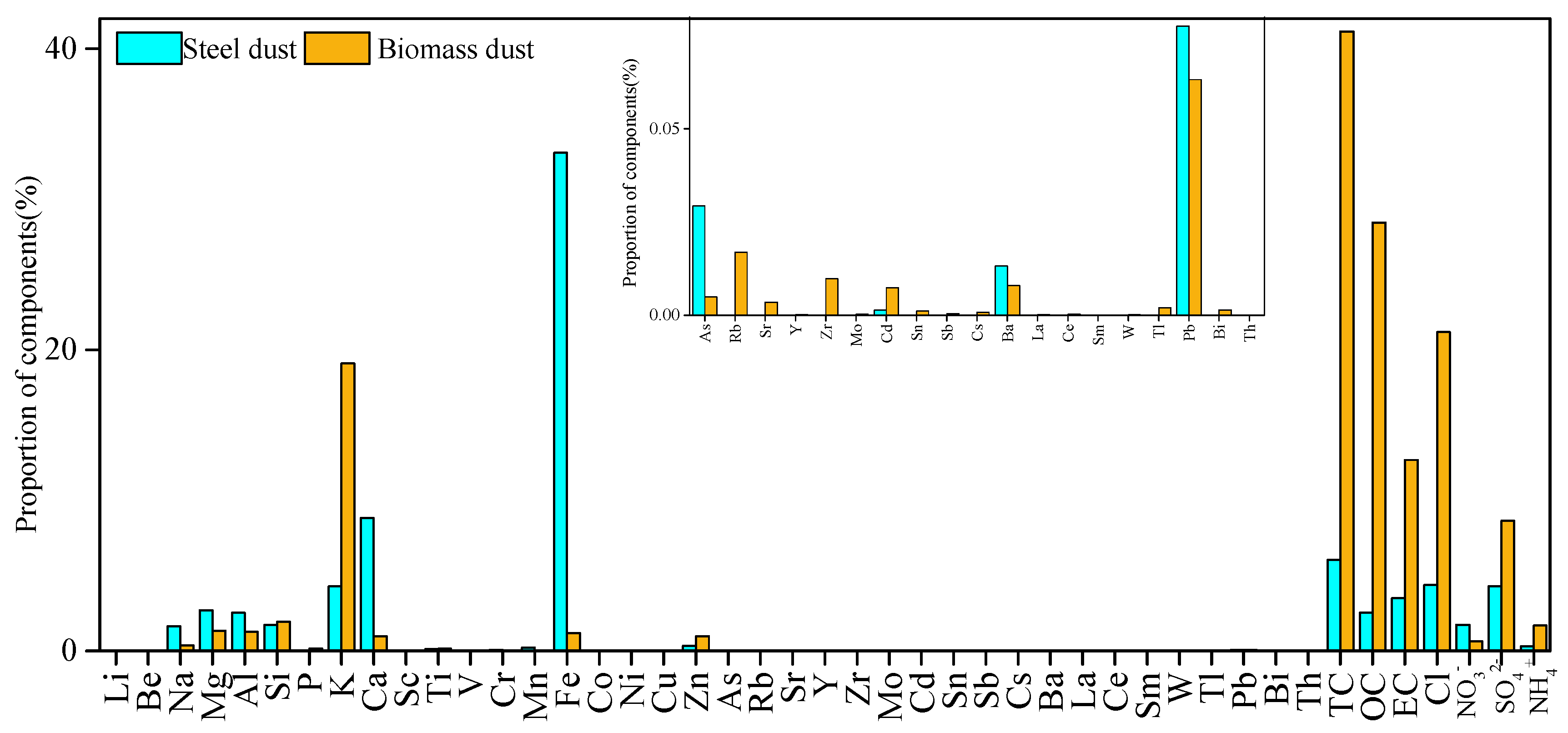
2.4. Establishment of Source Profile
3. Results and Discussions
3.1. The Characteristics of PM2.5 and Chemical Compositions
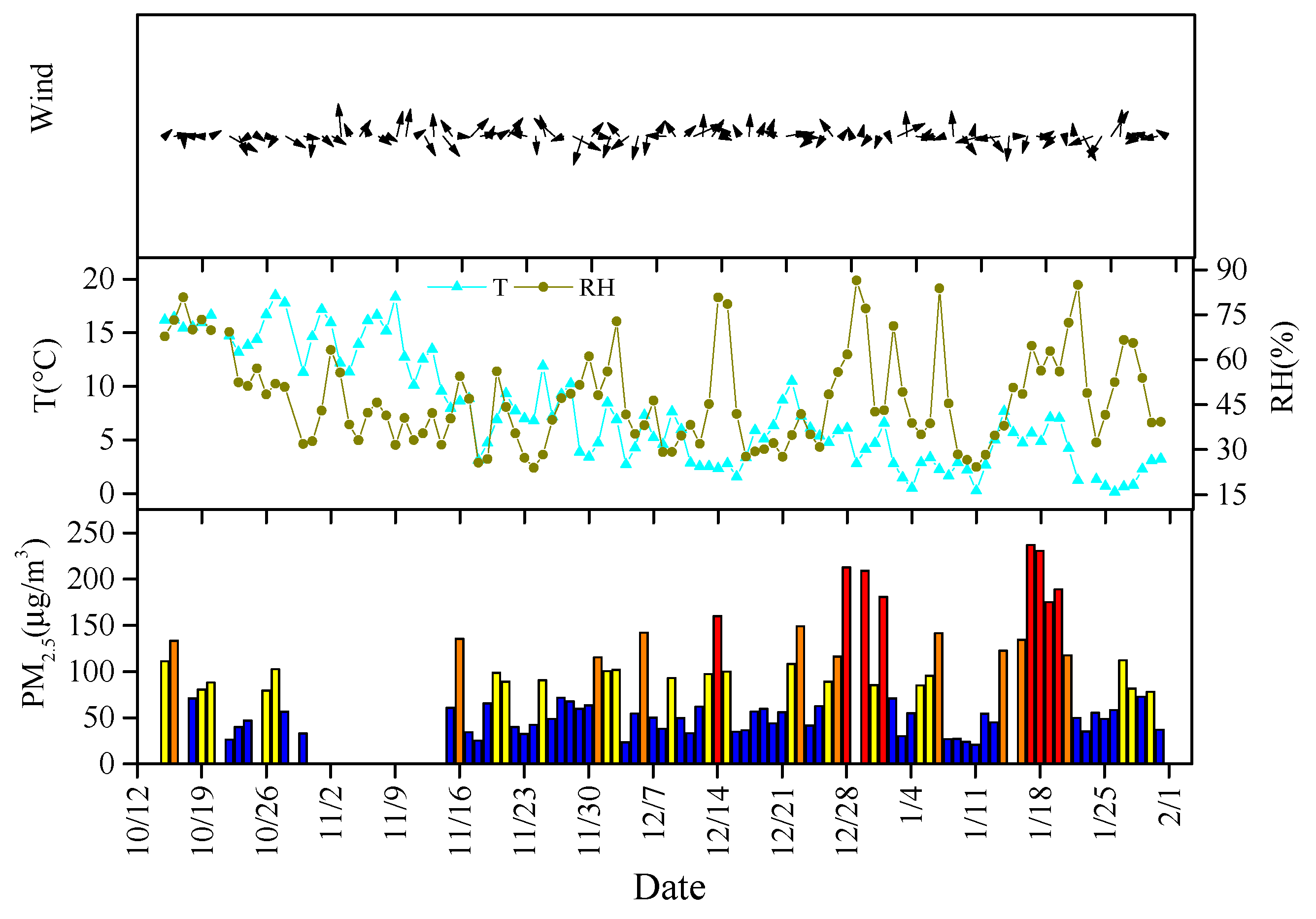
3.1.1. PM2.5 Mass Concentration
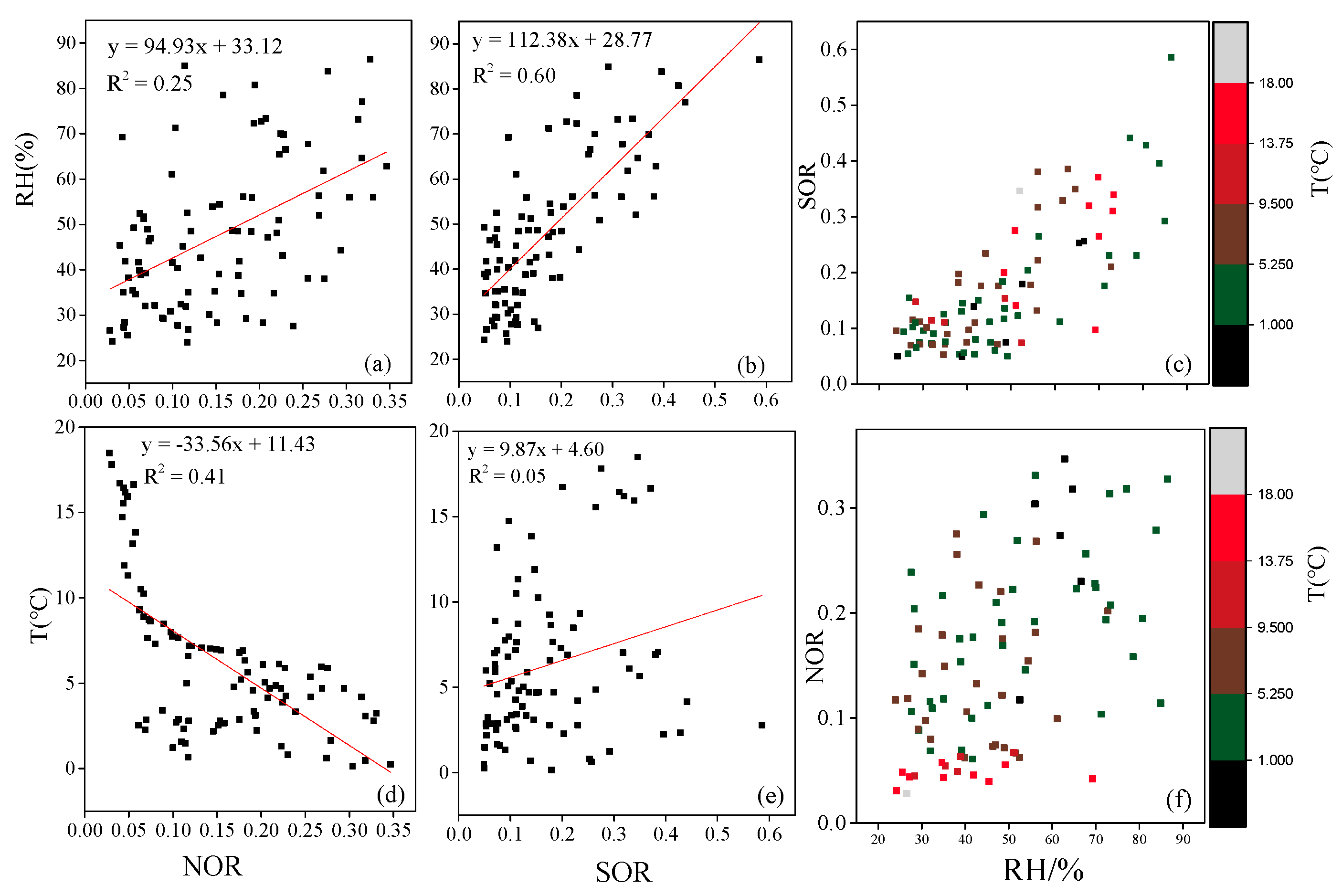
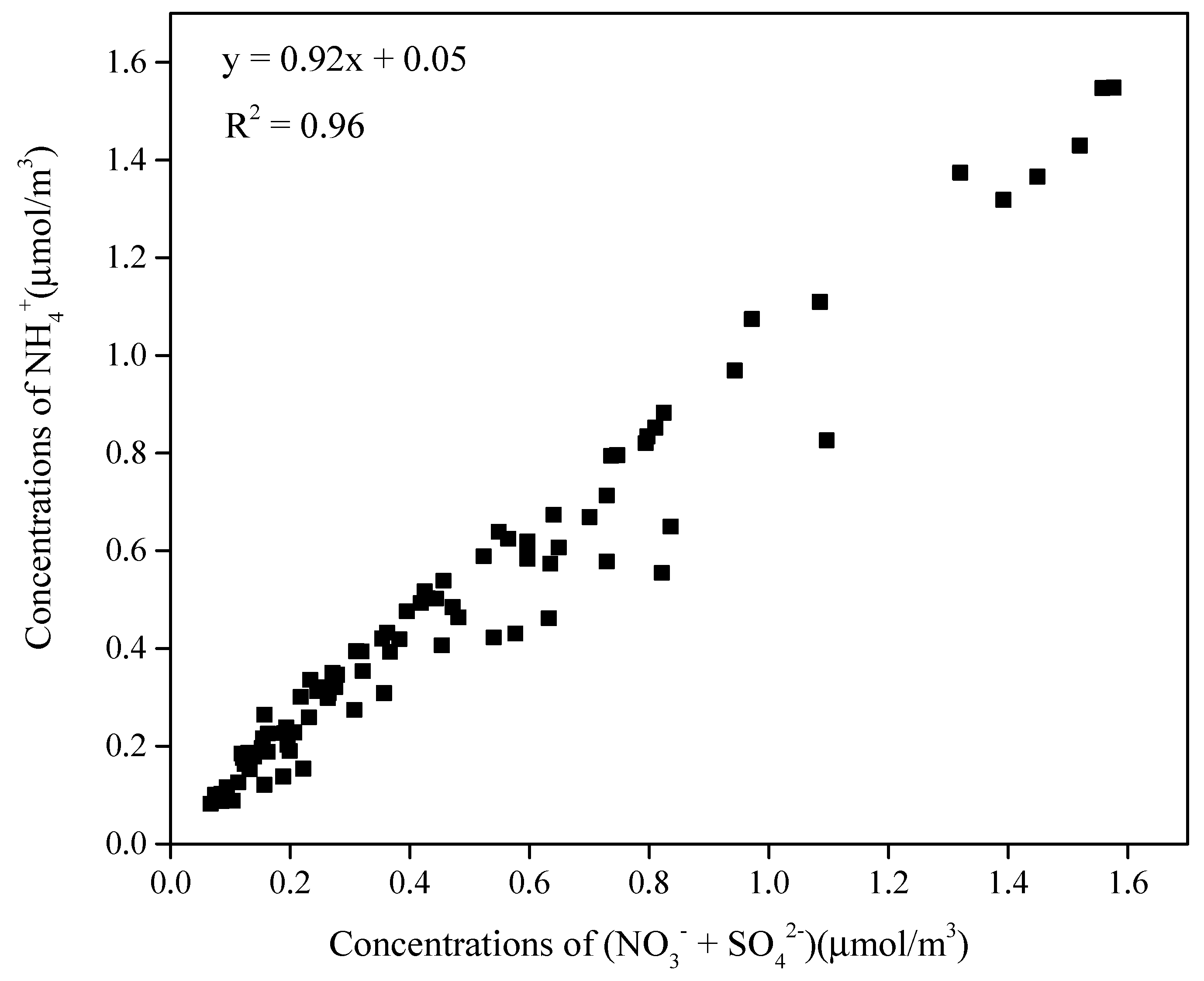
3.1.2. Water-Soluble Ions
3.1.3. Carbonaceous Aerosols
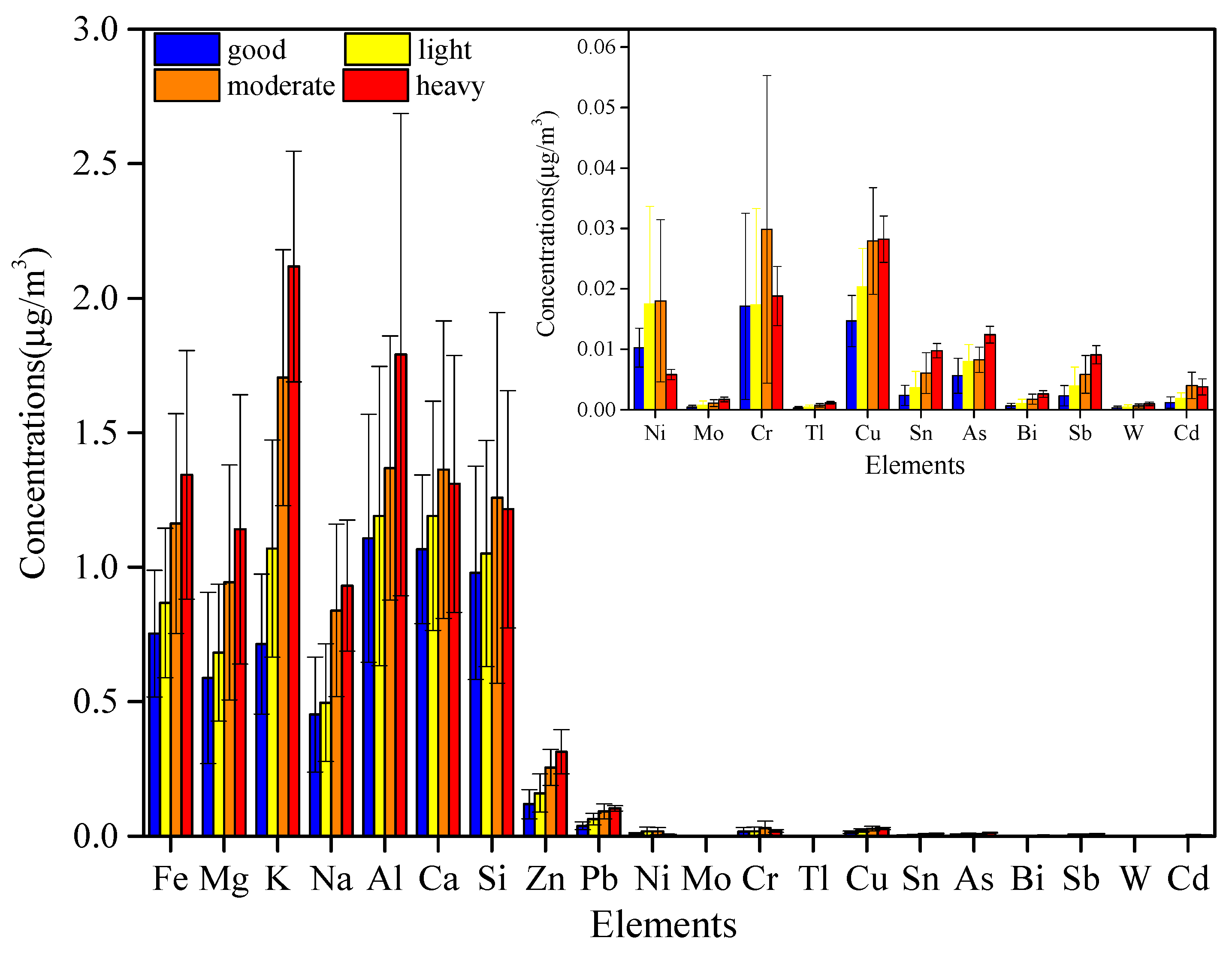
3.1.4. Inorganic Elements
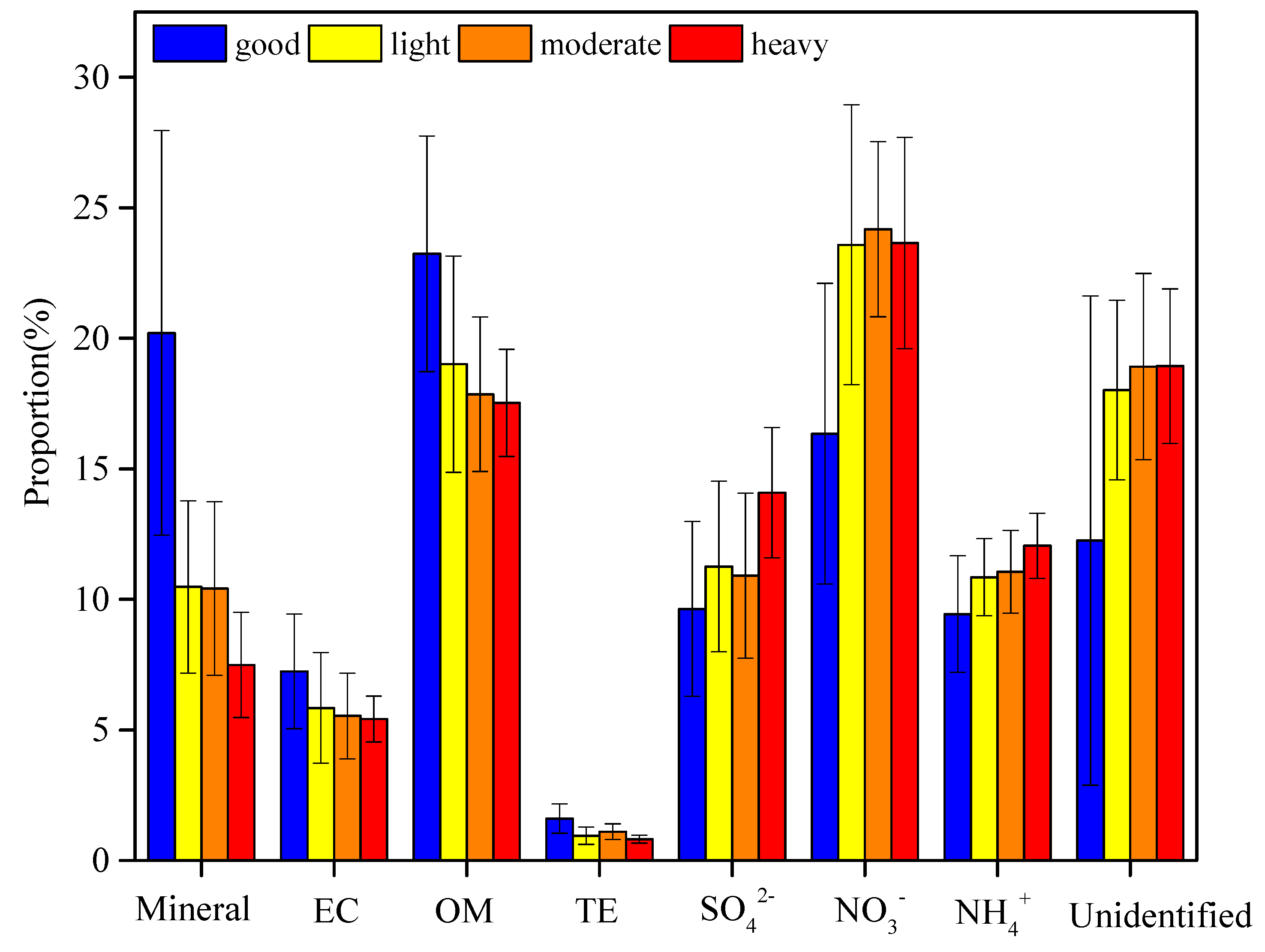
3.2. Chemical Mass Closure
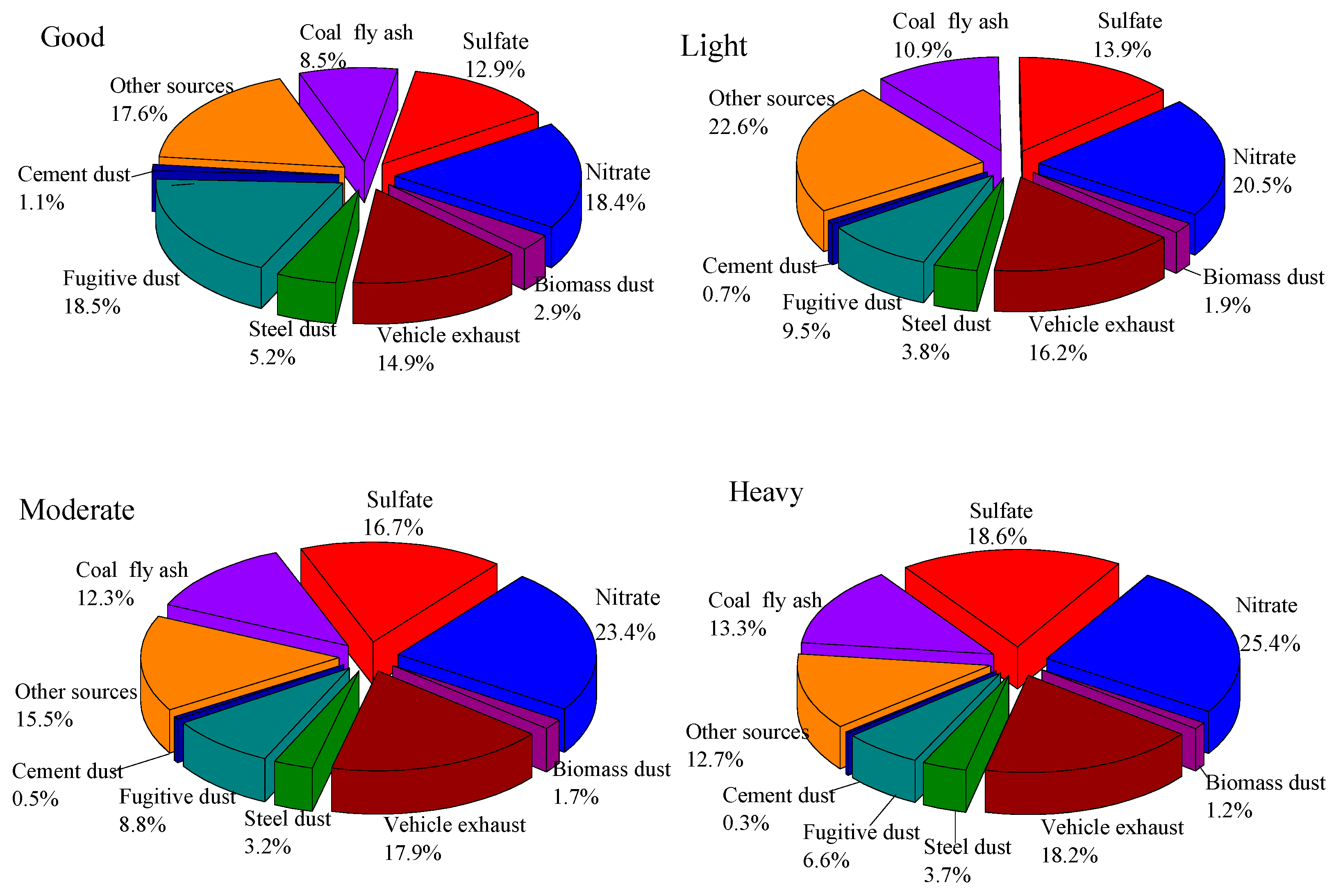
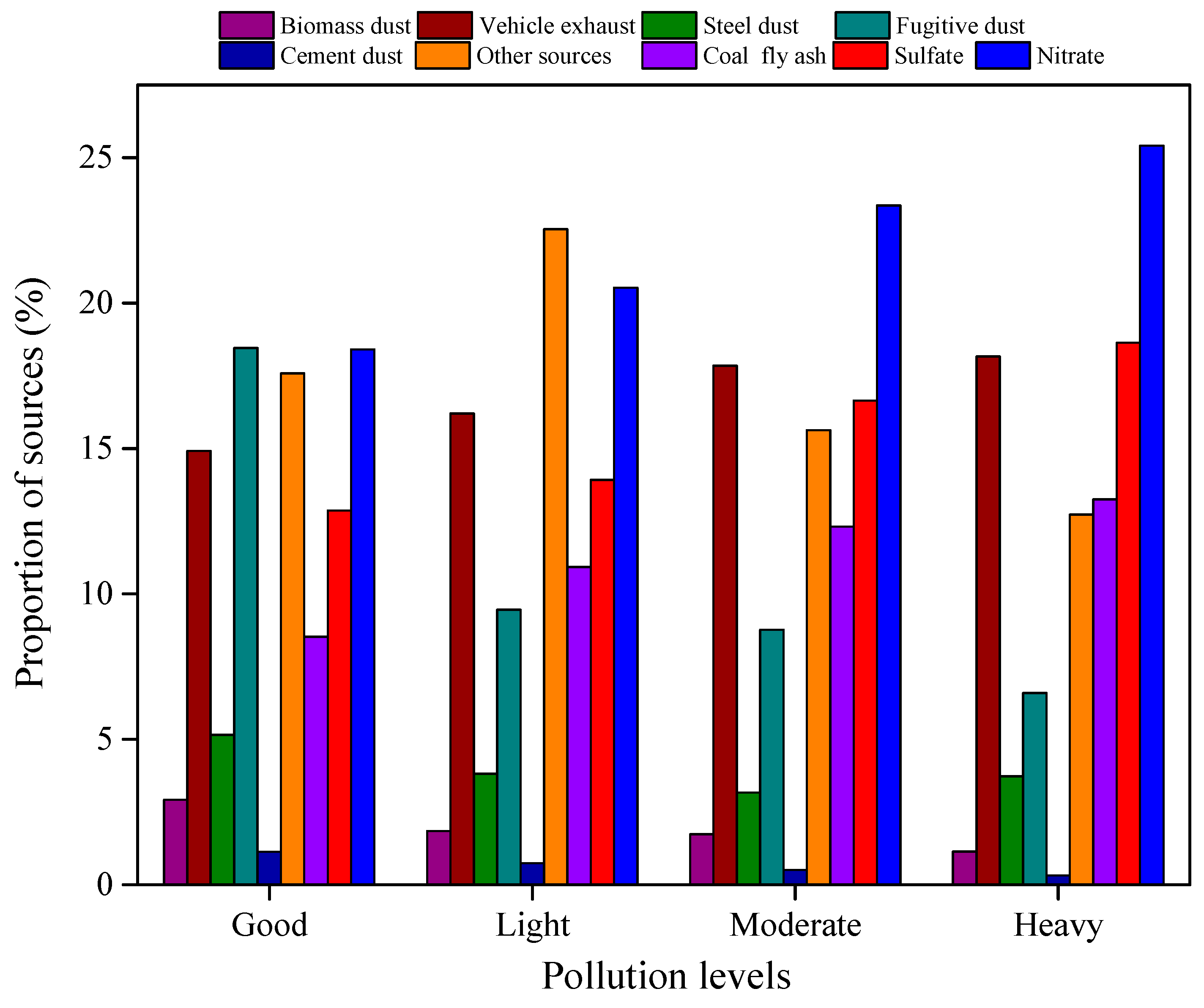
3.3. Source Apportionment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shao, M.; Tang, X.; Zhang, Y.; Li, W. City clusters in China: Air and surface water pollution. Front. Ecol. Environ. 2006, 4, 353–361. [Google Scholar] [CrossRef]
- Tang, G.; Zhu, X.; Hu, B.; Xin, J.; Wang, L.; Münkel, C.; Mao, G.; Wang, Y. Impact of emission controls on air quality in Beijing during APEC 2014: Lidar ceilometer observations. Atmos. Chem. Phys. 2015, 15, 12667–12680. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Zhang, M.; Yang, Y.; Shen, X.; Wang, Y.; Chen, D.; Guo, J. A study of the meteorological causes of a prolonged and severe haze episode in January 2013 over central-eastern China. Atmos. Environ. 2014, 98, 146–157. [Google Scholar] [CrossRef]
- Huang, M.; Deng, S.; Dong, H.; Dai, W.; Pang, J.; Wang, X. Impacts of atmospheric mercury deposition on human multimedia exposure: Projection from observations in the Pearl River Delta region, South China. Environ. Sci. Technol. 2016, 50, 10625–10634. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.J.; Molina, L.T. Megacities and atmospheric pollution. J. Air Waste Manag. Assoc. 2004, 54, 644–680. [Google Scholar] [CrossRef] [PubMed]
- Pope Iii, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, X.; Tong, D.; Davis, S.J.; Zhao, H.; Geng, G.; Feng, T.; Zheng, B.; Lu, Z.; Streets, D.G. Transboundary health impacts of transported global air pollution and international trade. Nature 2017, 543, 705–709. [Google Scholar] [CrossRef]
- Ye, W.-F.; Ma, Z.-Y.; Ha, X.-Z. Spatial-temporal patterns of PM2.5 concentrations for 338 Chinese cities. Sci. Total Environ. 2018, 631, 524–533. [Google Scholar] [CrossRef]
- Huang, G.; Cheng, T.; Zhang, R.; Tao, J.; Leng, C.; Zhang, Y.; Zha, S.; Zhang, D.; Li, X.; Xu, C. Optical properties and chemical composition of PM2.5 in Shanghai in the spring of 2012. Particuology 2014, 13, 52–59. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Zhang, Q.; Chen, C.; Wang, L.; Wei, Z.; Zhou, S.; Parworth, C.; Zheng, B.; Canonaco, F. Wintertime aerosol chemistry and haze evolution in an extremely polluted city of the North China Plain: Significant contribution from coal and biomass combustion. Atmos. Chem. Phys. 2017, 17, 4751–4768. [Google Scholar] [CrossRef]
- Ming, L.; Jin, L.; Li, J.; Fu, P.; Yang, W.; Liu, D.; Zhang, G.; Wang, Z.; Li, X. PM2.5 in the Yangtze River Delta, China: Chemical compositions, seasonal variations, and regional pollution events. Environ. Pollut. 2017, 223, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, L.; Zhou, X.; Duan, J.; Li, Y.; Hu, J.; He, K. Chemical characteristics and source apportionment of PM2.5 in Lanzhou, China. Sci. Total Environ. 2017, 601, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Cheng, L.; Chen, X.; Zhai, X.; Wang, W.; Zhang, W.; Bai, Y.; Tian, H.; Nie, L.; Zhang, S. Emission characteristics of harmful air pollutants from cremators in Beijing, China. PLoS ONE 2018, 13, e0194226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, Y.; Xin, J.; Liu, Z.; Ma, Y.; Gao, D.; Wu, J.; Zhang, W.; Wang, Y.; Shen, P. The aerosol optical properties and PM2.5 components over the world’s largest industrial zone in Tangshan, North China. Atmos. Res. 2018, 201, 226–234. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Li, Z.; Tao, Y.; Wang, F.; Zhang, X.; Xu, C.; Ma, S.; Huang, J. Particulate and gaseous pollutants in a petrochemical industrialized valley city, Western China during 2013–2016. Environ. Sci. Pollut. Res. 2018, 25, 15174–15190. [Google Scholar] [CrossRef]
- Hedley, A.J.; Wong, C.-M.; Thach, T.Q.; Ma, S.; Lam, T.-H.; Anderson, H.R. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: An intervention study. Lancet 2002, 360, 1646–1652. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, L.; Zhou, X.; Wang, Z.; Zhou, Y.; Gao, X.; Nie, W.; Wang, X.; Xu, P.; Wang, W. Evaluating PM2.5 ionic components and source apportionment in Jinan, China from 2004 to 2008 using trajectory statistical methods. J. Environ. Monit. 2011, 13, 1662–1671. [Google Scholar] [CrossRef]
- Yang, L.-X.; Wang, D.-C.; Cheng, S.-H.; Wang, Z.; Zhou, Y.; Zhou, X.-H.; Wang, W.-X. Influence of meteorological conditions and particulate matter on visual range impairment in Jinan, China. Sci. Total Environ. 2007, 383, 164–173. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.; Wang, Z.; Zhou, Y.; Cheng, S.; Xu, P.; Gao, X.; Nie, W.; Wang, X.; Wang, W. Airborne fine particulate pollution in Jinan, China: Concentrations, chemical compositions and influence on visibility impairment. Atmos. Environ. 2012, 55, 506–514. [Google Scholar] [CrossRef]
- Cao, J.; Lee, S.; Chow, J.C.; Watson, J.G.; Ho, K.; Zhang, R.; Jin, Z.; Shen, Z.; Chen, G.; Kang, Y. Spatial and seasonal distributions of carbonaceous aerosols over China. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Xu, P.; Wang, W.; Yang, L.; Zhang, Q.; Gao, R.; Wang, X.; Nie, W.; Gao, X. Aerosol size distributions in urban Jinan: Seasonal characteristics and variations between weekdays and weekends in a heavily polluted atmosphere. Environ. Monit. Assess. 2011, 179, 443–456. [Google Scholar] [CrossRef]
- Gao, X.; Yang, L.; Cheng, S.; Gao, R.; Zhou, Y.; Xue, L.; Shou, Y.; Wang, J.; Wang, X.; Nie, W. Semi-continuous measurement of water-soluble ions in PM2.5 in Jinan, China: Temporal variations and source apportionments. Atmos. Environ. 2011, 45, 6048–6056. [Google Scholar] [CrossRef]
- Gansu Province Environmental Monitoring Center. Ambient Air-Determination of the Water Soluble Cations (Li+, Na+, NH4+, K+, Ca2+, Mg2+) from Atmospheric Particles-Ion Chromatography; Minitry of Ecology and Environment, the People’s Republic of China: Beijing, China, 2016; Volume HJ800-2016.
- Gansu Province Environment Monitoring Center. Ambient Air-Determination of the Water Soluble Anions (F−, Cl−, Br−, NO2−, NO3−, PO43−, SO32−, SO42−) from Atmospheric Particles-Ion Chromatography; Minitry of Ecology and Environment, the People’s Republic of China: Beijing, China, 2016; Volume HJ799-2016.
- Liu, B.; Song, N.; Dai, Q.; Mei, R.; Sui, B.; Bi, X.; Feng, Y. Chemical composition and source apportionment of ambient PM2.5 during the non-heating period in Taian, China. Atmos. Res. 2016, 170, 23–33. [Google Scholar] [CrossRef]
- Shanghai Monitoring Center. Ambient Air and Stationary Source Emission-Determination of Metals in Ambient Particulate Matter—Inductively Coupled Plasma/mass Spectrometry (ICP-MS); Minitry of Ecology and Environment, the People’s Republic of China: Beijing, China, 2013; Volume HJ657-2013.
- Sino-Japan Friendship Center for Environmental Protection. Ambient Air and Waste Gas from Stationary Sources Emission-Determination of Metal Elements in Ambient Particle Matter-Inductively Coupled Plasma Optical Emission Spectrometry; Minitry of Ecology and Environment, the People’s Republic of China: Beijing, China, 2015; Volume HJ777-2015.
- The Weather Company LLC. Wunderground. Available online: www.wunderground.com (accessed on 1 January 2017).
- Wang, J. Oneline Monitroing and Analysis Platform on Air Quality. Available online: https://www.aqistudy.cn/ (accessed on 1 January 2017).
- Shi, G.; Xu, J.; Peng, X.; Xiao, Z.; Chen, K.; Tian, Y.; Guan, X.; Feng, Y.; Yu, H.; Nenes, A. pH of aerosols in a polluted atmosphere: Source contributions to highly acidic aerosol. Environ. Sci. Technol. 2017, 51, 4289–4296. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Q.; Canagaratna, M.R.; Zhang, Y.; Ng, N.L.; Sun, Y.; Jayne, J.T.; Zhang, X.; Zhang, X.; Worsnop, D.R. Highly time-and size-resolved characterization of submicron aerosol particles in Beijing using an Aerodyne Aerosol Mass Spectrometer. Atmos. Environ. 2010, 44, 131–140. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Engling, G.; Zhang, R.; Yang, Y.; Cao, J.; Zhu, C.; Wang, Q.; Luo, L. Chemical composition of PM2.5 in an urban environment in Chengdu, China: Importance of springtime dust storms and biomass burning. Atmos. Res. 2013, 122, 270–283. [Google Scholar] [CrossRef]
- Gao, J.; Tian, H.; Cheng, K.; Lu, L.; Zheng, M.; Wang, S.; Hao, J.; Wang, K.; Hua, S.; Zhu, C. The variation of chemical characteristics of PM2.5 and PM10 and formation causes during two haze pollution events in urban Beijing, China. Atmos. Environ. 2015, 107, 1–8. [Google Scholar] [CrossRef]
- Yao, X.; Chan, C.K.; Fang, M.; Cadle, S.; Chan, T.; Mulawa, P.; He, K.; Ye, B. The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos. Environ. 2002, 36, 4223–4234. [Google Scholar] [CrossRef]
- Du, Z.; He, K.; Cheng, Y.; Duan, F.; Ma, Y.; Liu, J.; Zhang, X.; Zheng, M.; Weber, R. A yearlong study of water-soluble organic carbon in Beijing I: Sources and its primary vs. secondary nature. Atmos. Environ. 2014, 92, 514–521. [Google Scholar] [CrossRef]
- Shao, L.; Hou, C.; Geng, C.; Liu, J.; Hu, Y.; Wang, J.; Jones, T.; Zhao, C.; BéruBé, K. The oxidative potential of PM10 from coal, briquettes and wood charcoal burnt in an experimental domestic stove. Atmos. Environ. 2016, 127, 372–381. [Google Scholar] [CrossRef]
- Tan, J.-H.; Duan, J.-C.; Ma, Y.-L.; Yang, F.-M.; Cheng, Y.; He, K.-B.; Yu, Y.-C.; Wang, J.-W. Source of atmospheric heavy metals in winter in Foshan, China. Sci. Total Environ. 2014, 493, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Reimann, C.; Caritat, P.D. Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ. Sci. Technol. 2000, 34, 5084–5091. [Google Scholar] [CrossRef]
- Vecchi, R.; Chiari, M.; D’Alessandro, A.; Fermo, P.; Lucarelli, F.; Mazzei, F.; Nava, S.; Piazzalunga, A.; Prati, P.; Silvani, F. A mass closure and PMF source apportionment study on the sub-micron sized aerosol fraction at urban sites in Italy. Atmos. Environ. 2008, 42, 2240–2253. [Google Scholar] [CrossRef]
- Tian, S.; Pan, Y.; Liu, Z.; Wen, T.; Wang, Y. Size-resolved aerosol chemical analysis of extreme haze pollution events during early 2013 in urban Beijing, China. J. Hazard. Mater. 2014, 279, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment. Technical Guideline of Source Apportionment for Particulate Matter. Beijing. 2013. Available online: http://www.mee.gov.cn/gkml/hbb/bwj/201308/W020130820340683623095.pdf (accessed on 16 February 2020).
- Zhang, T.; Cao, J.; Tie, X.; Shen, Z.; Liu, S.; Ding, H.; Han, Y.; Wang, G.; Ho, K.; Qiang, J. Water-soluble ions in atmospheric aerosols measured in Xi’an, China: Seasonal variations and sources. Atmos. Res. 2011, 102, 110–119. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, C.; Jia, X.; Xu, H.; Pan, M.; Xu, D.; Shen, X.; Zhang, J.; Tan, J.; Qian, H. Personal exposure measurements of school-children to fine particulate matter (PM2.5) in winter of 2013, Shanghai, China. PLoS ONE 2018, 13, e0193586. [Google Scholar] [CrossRef]
- Wang, H.; An, J.; Cheng, M.; Shen, L.; Zhu, B.; Li, Y.; Wang, Y.; Duan, Q.; Sullivan, A.; Xia, L. One year online measurements of water-soluble ions at the industrially polluted town of Nanjing, China: Sources, seasonal and diurnal variations. Chemosphere 2016, 148, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Turap, Y.; Talifu, D.; Wang, X.; Abulizi, A.; Maihemuti, M.; Tursun, Y.; Ding, X.; Aierken, T.; Rekefu, S. Temporal distribution and source apportionment of PM2.5 chemical composition in Xinjiang, NW-China. Atmos. Res. 2019, 218, 257–268. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.-D.; Luo, B.; Zhai, C.-Z. Particulate pollution in urban Chongqing of southwest China: Historical trends of variation, chemical characteristics and source apportionment. Sci. Total Environ. 2017, 584, 523–534. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Yang, L.-X.; Zhou, X.-H.; Xue, L.-K.; Gao, X.-M.; Zhou, Y.; Wang, W.-X. Size-fractionated water-soluble ions, situ pH and water content in aerosol on hazy days and the influences on visibility impairment in Jinan, China. Atmos. Environ. 2011, 45, 4631–4640. [Google Scholar] [CrossRef]
- Gu, J.; Du, S.; Han, D.; Hou, L.; Yi, J.; Xu, J.; Liu, G.; Han, B.; Yang, G.; Bai, Z.-P. Major chemical compositions, possible sources, and mass closure analysis of PM2.5 in Jinan, China. Air Qual. Atmos. Health 2014, 7, 251–262. [Google Scholar] [CrossRef]
- Cheng, Y.; He, K.-B.; Du, Z.-Y.; Zheng, M.; Duan, F.-K.; Ma, Y.-L. Humidity plays an important role in the PM2.5 pollution in Beijing. Environ. Pollut. 2015, 197, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, J.; Zhang, J.; Wang, L.; Yang, J.; Liang, D.; Dai, Q.; Bi, X.; Feng, Y.; Zhang, Y. Characterization and source apportionment of PM2.5 based on error estimation from EPA PMF 5.0 model at a medium city in China. Environ. Pollut. 2017, 222, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Zeng, J.; Li, Y.; Bao, L.; Cao, L.; Liu, K.; Xu, L.; Lin, J.; Liu, W.; Wang, G. Characteristics of secondary inorganic aerosol and sulfate species in size-fractionated aerosol particles in Shanghai. J. Environ. Sci. 2014, 26, 1040–1051. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, G.; Tang, A.; Wang, Y.; An, Z. Chemical characteristics of PM2.5 and PM10 in haze—Fog episodes in Beijing. Environ. Sci. Technol. 2006, 40, 3148–3155. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Sun, Y.; An, Z. The variation of characteristics and formation mechanisms of aerosols in dust, haze, and clear days in Beijing. Atmos. Environ. 2006, 40, 6579–6591. [Google Scholar] [CrossRef]
- Quan, J.; Tie, X.; Zhang, Q.; Liu, Q.; Li, X.; Gao, Y.; Zhao, D. Characteristics of heavy aerosol pollution during the 2012–2013 winter in Beijing, China. Atmos. Environ. 2014, 88, 83–89. [Google Scholar] [CrossRef]
- Zheng, G.; Duan, F.; Su, H.; Ma, Y.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Kimoto, T.; Chang, D. Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969. [Google Scholar] [CrossRef]
- Ji, D.; Zhang, J.; He, J.; Wang, X.; Pang, B.; Liu, Z.; Wang, L.; Wang, Y. Characteristics of atmospheric organic and elemental carbon aerosols in urban Beijing, China. Atmos. Environ. 2016, 125, 293–306. [Google Scholar] [CrossRef]
- Tao, J.; Gao, J.; Zhang, L.; Zhang, R.; Che, H.; Zhang, Z.; Lin, Z.; Jing, J.; Cao, J.; Hsu, S.-C. PM2.5 pollution in a megacity of southwest China: Source apportionment and implication. Atmos. Chem. Phys. 2014, 14, 5147–5196. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, C.; Tao, J.; Zhang, L.; Liang, X.; Ma, J.; Gao, H.; Huang, T.; Zhang, K. Chemical characterization and source apportionment of PM2.5 in a semi-arid and petrochemical-industrialized city, Northwest China. Sci. Total Environ. 2016, 573, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, X.; Zhang, J.; Xiao, Y.; Wang, Z.; Zhou, Y.; Wang, W. PM2.5 pollution in a petrochemical industry city of northern China: Seasonal variation and source apportionment. Atmos. Res. 2018, 212, 285–295. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Tan, J.; Duan, J.; Ma, X.; Zhang, C.; Ji, S.; Qi, M.; Lu, X.; Wang, Y. Changes of chemical composition and source apportionment of PM2.5 during 2013–2017 in urban Handan, China. Atmos. Environ. 2019, 206, 119–131. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chiang, H.-C.; Lin, S.-L.; Chen, M.-J.; Lin, T.-Y.; Chen, Y.-C. Elemental characterization and source apportionment of PM10 and PM2.5 in the western coastal area of central Taiwan. Sci. Total Environ. 2016, 541, 1139–1150. [Google Scholar] [CrossRef]
- Kara, M.; Dumanoglu, Y.; Altiok, H.; Elbir, T.; Odabasi, M.; Bayram, A. Seasonal and spatial variations of atmospheric trace elemental deposition in the Aliaga industrial region, Turkey. Atmos. Res. 2014, 149, 204–216. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, X.; Chen, H.; Xu, B.; Zhu, L.; Li, C.; Zeng, G. Source identification and potential ecological risk assessment of heavy metals in PM2.5 from Changsha. Sci. Total Environ. 2014, 493, 109–115. [Google Scholar] [CrossRef]




| Sites | Longitude and Latitude | Functional Areas |
|---|---|---|
| Environmental Monitoring Station | 116°57′ E, 36°39′ N | city downtown area |
| Architectural University | 11710′ E, 36°40′ N | New urban area |
| Lanxiang Technical School | 116°56′ E, 36°42′ N | Suburban junction area |
| Jigang | 117°10′ E, 36°43′ N | Industrial area |
| Compositions | Good | Light | Moderate | Heavy |
|---|---|---|---|---|
| F− (μg/m3) | 0.1 ± 0.06 | 0.1 ± 0.07 | 0.1 ± 0.16 | 0.1 ± 0.10 |
| Cl− (μg/m3) | 2.0 ± 1.04 | 3.6 ± 1.78 | 4.1 ± 2.34 | 6.7 ± 1.97 |
| NO3− (μg/m3) | 8.2 ± 4.47 | 22.2 ± 6.21 | 31.2 ± 5.01 | 47.2 ± 10.44 |
| SO42− (μg/m3) | 4.7 ± 2.56 | 10.6 ± 3.27 | 14.3 ± 4.47 | 27.8 ± 4.77 |
| NH4+ (μg/m3) | 4.7 ± 2.06 | 10.2 ± 1.95 | 14.2 ± 2.39 | 23.9 ± 3.51 |
| Na+ (μg/m3) | 0.4 ± 0.21 | 0.4 ± 0.21 | 0.5 ± 0.21 | 0.7 ± 0.20 |
| K+ (μg/m3) | 0.5 ± 0.18 | 0.9 ± 0.30 | 1.2 ± 0.38 | 1.9 ± 0.36 |
| Mg2+ (μg/m3) | 0.1 ± 0.02 | 0.1 ± 0.02 | 0.1 ± 0.03 | 0.1 ± 0.05 |
| Ca2+ (μg/m3) | 0.9 ± 0.26 | 1.0 ± 0.39 | 1.2 ± 0.46 | 1.1 ± 0.50 |
| OC (μg/m3) | 7.8 ± 2.05 | 12.7 ± 2.59 | 17.8 ± 3.05 | 25.9 ± 4.03 |
| EC (μg/m3) | 3.4 ± 1.17 | 5.5 ± 2.00 | 7.2 ± 2.11 | 10.1 ± 1.80 |
| OC/EC | 2.3 ± 0.76 | 2.3 ± 1.08 | 2.5 ± 1.04 | 2.6 ± 0.26 |
| Na (ng/m3) | 452.1 ± 213.65 | 496.0 ± 218.98 | 838.8 ± 320.93 | 931.4 ± 243.60 |
| Al (ng/m3) | 1107.4 ± 461.19 | 1189.8 ± 556.57 | 1368.3 ± 490.82 | 1790.9 ± 897.08 |
| Si (ng/m3) | 978.7 ± 396.31 | 1050.5 ± 420.50 | 1257.9 ± 688.98 | 1215.4 ± 441.30 |
| K (ng/m3) | 713.5 ± 260.28 | 1069.0 ± 403.55 | 1704.7 ± 475.89 | 2117.9 ± 429.00 |
| Cr (ng/m3) | 17.1 ± 21.41 | 17.4 ± 15.91 | 29.9 ± 25.43 | 18.8 ± 4.90 |
| Fe (ng/m3) | 752.9 ± 235.87 | 867.1 ± 287.09 | 1161.9 ± 408.66 | 1343.1 ± 462.69 |
| Ni (ng/m3) | 10.3 ± 23.22 | 7.5 ± 16.17 | 13.0 ± 18.43 | 5.8 ± 0.85 |
| Cu (ng/m3) | 14.7 ± 4.26 | 20.3 ± 6.42 | 27.9 ± 8.83 | 28.2 ± 3.83 |
| Zn (ng/m3) | 118.9 ± 54.08 | 159.8 ± 70.78 | 255.3 ± 67.33 | 313.4 ± 81.75 |
| As (ng/m3) | 5.6 ± 2.88 | 8.0 ± 2.80 | 8.3 ± 2.10 | 12.4 ± 1.37 |
| Mo (ng/m3) | 0.4 ± 0.31 | 0.8 ± 0.68 | 1.1 ± 0.56 | 1.7 ± 0.39 |
| Cd (ng/m3) | 1.2 ± 0.93 | 1.9 ± 0.90 | 4.0 ± 2.19 | 3.8 ± 1.32 |
| Sn (ng/m3) | 2.4 ± 1.68 | 3.6 ± 2.74 | 6.1 ± 3.39 | 9.8 ± 1.17 |
| Sb (ng/m3) | 2.3 ± 0.26 | 3.9 ± 3.16 | 5.9 ± 3.12 | 9.1 ± 1.53 |
| W (ng/m3) | 0.3 ± 0.26 | 0.5 ± 0.37 | 0.6 ± 0.33 | 2.0 ± 0.31 |
| Tl (ng/m3) | 0.3 ± 0.22 | 0.5 ± 0.35 | 0.8 ± 0.32 | 1.1 ± 0.21 |
| Pb (ng/m3) | 38.1 ± 14.73 | 63.4 ± 21.60 | 91.7 ± 27.09 | 103.3 ± 10.06 |
| Bi (ng/m3) | 0.7 ± 0.42 | 1.0 ± 0.71 | 1.7 ±0.83 | 2.6 ± 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Liu, Y.; Wang, J.; Wang, J.; Hou, L.; Lv, B.; Wang, X.; Zhao, X.; Yang, W.; Geng, C.; et al. Chemical Compositions and Source Analysis of PM2.5 during Autumn and Winter in a Heavily Polluted City in China. Atmosphere 2020, 11, 336. https://doi.org/10.3390/atmos11040336
Tian S, Liu Y, Wang J, Wang J, Hou L, Lv B, Wang X, Zhao X, Yang W, Geng C, et al. Chemical Compositions and Source Analysis of PM2.5 during Autumn and Winter in a Heavily Polluted City in China. Atmosphere. 2020; 11(4):336. https://doi.org/10.3390/atmos11040336
Chicago/Turabian StyleTian, Shasha, Yingying Liu, Jing Wang, Jian Wang, Lujian Hou, Bo Lv, Xinhua Wang, Xueyan Zhao, Wen Yang, Chunmei Geng, and et al. 2020. "Chemical Compositions and Source Analysis of PM2.5 during Autumn and Winter in a Heavily Polluted City in China" Atmosphere 11, no. 4: 336. https://doi.org/10.3390/atmos11040336
APA StyleTian, S., Liu, Y., Wang, J., Wang, J., Hou, L., Lv, B., Wang, X., Zhao, X., Yang, W., Geng, C., Han, B., & Bai, Z. (2020). Chemical Compositions and Source Analysis of PM2.5 during Autumn and Winter in a Heavily Polluted City in China. Atmosphere, 11(4), 336. https://doi.org/10.3390/atmos11040336






