Characteristics and Sources of Black Carbon Aerosol in a Mega-City in the Western Yangtze River Delta, China
Abstract
1. Introduction
2. Experiment
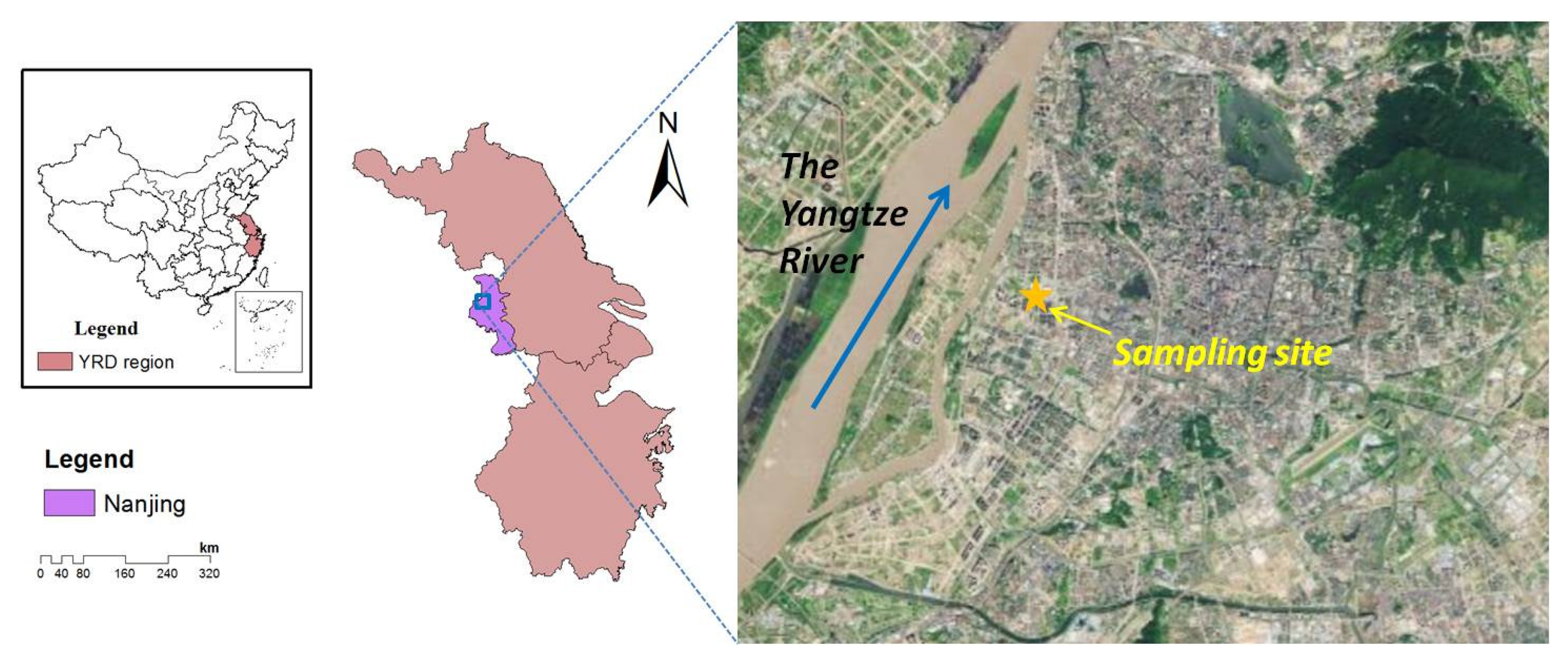
2.1. Sampling Site
2.2. Instrument Description
2.2.1. The Single Particle Soot Photometer
2.2.2. Other Instrument and Data
3. Results and Discussions
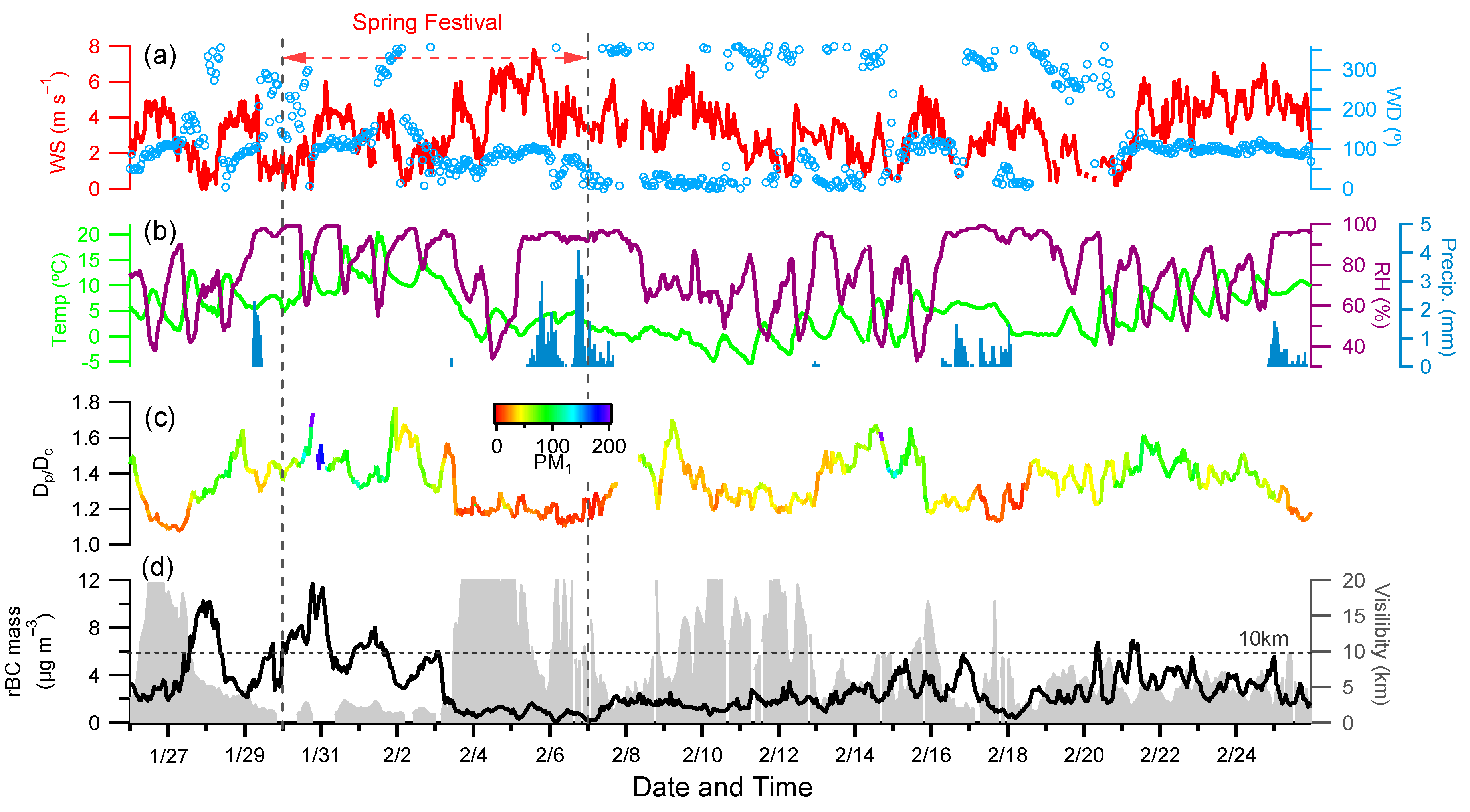
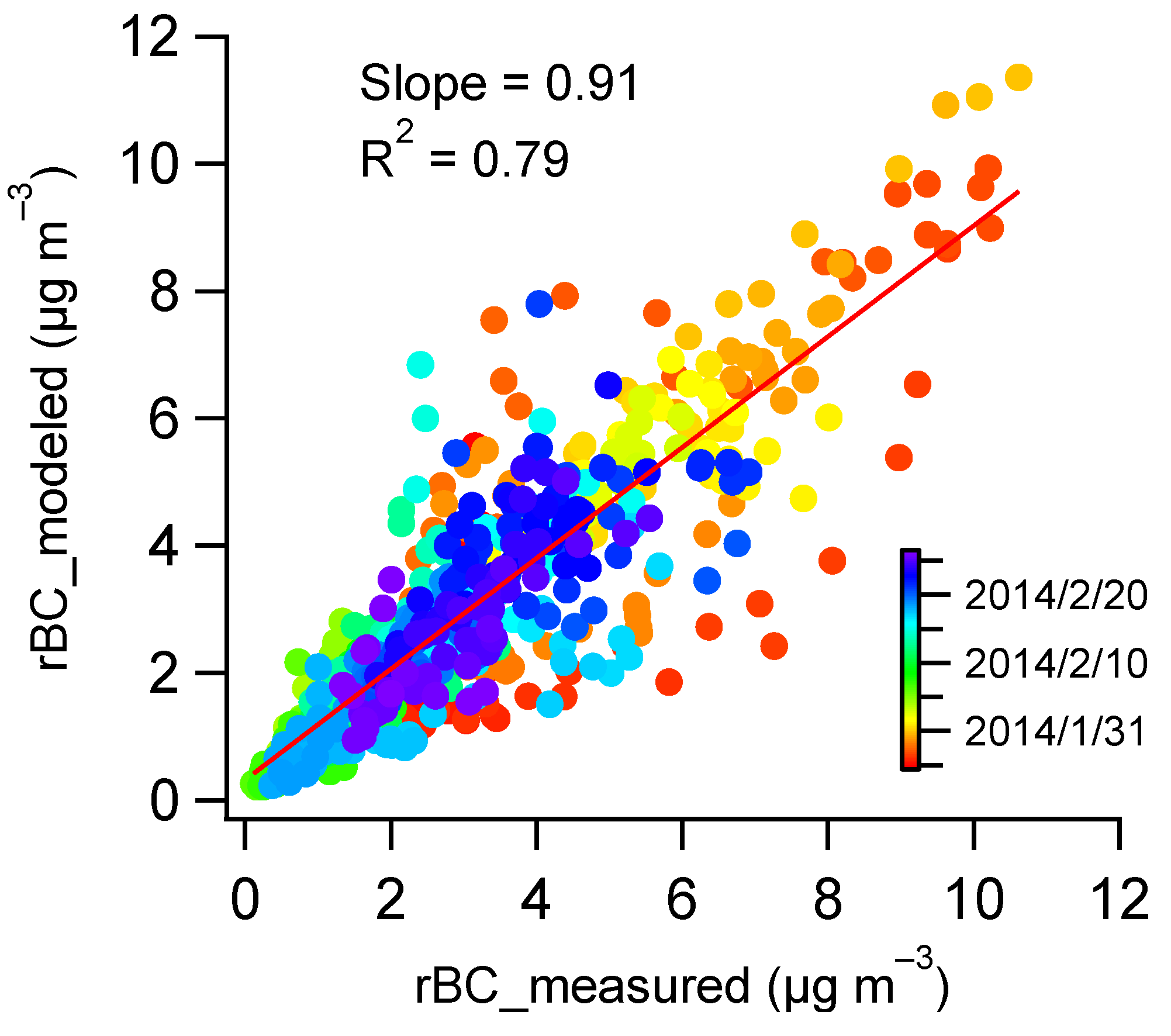
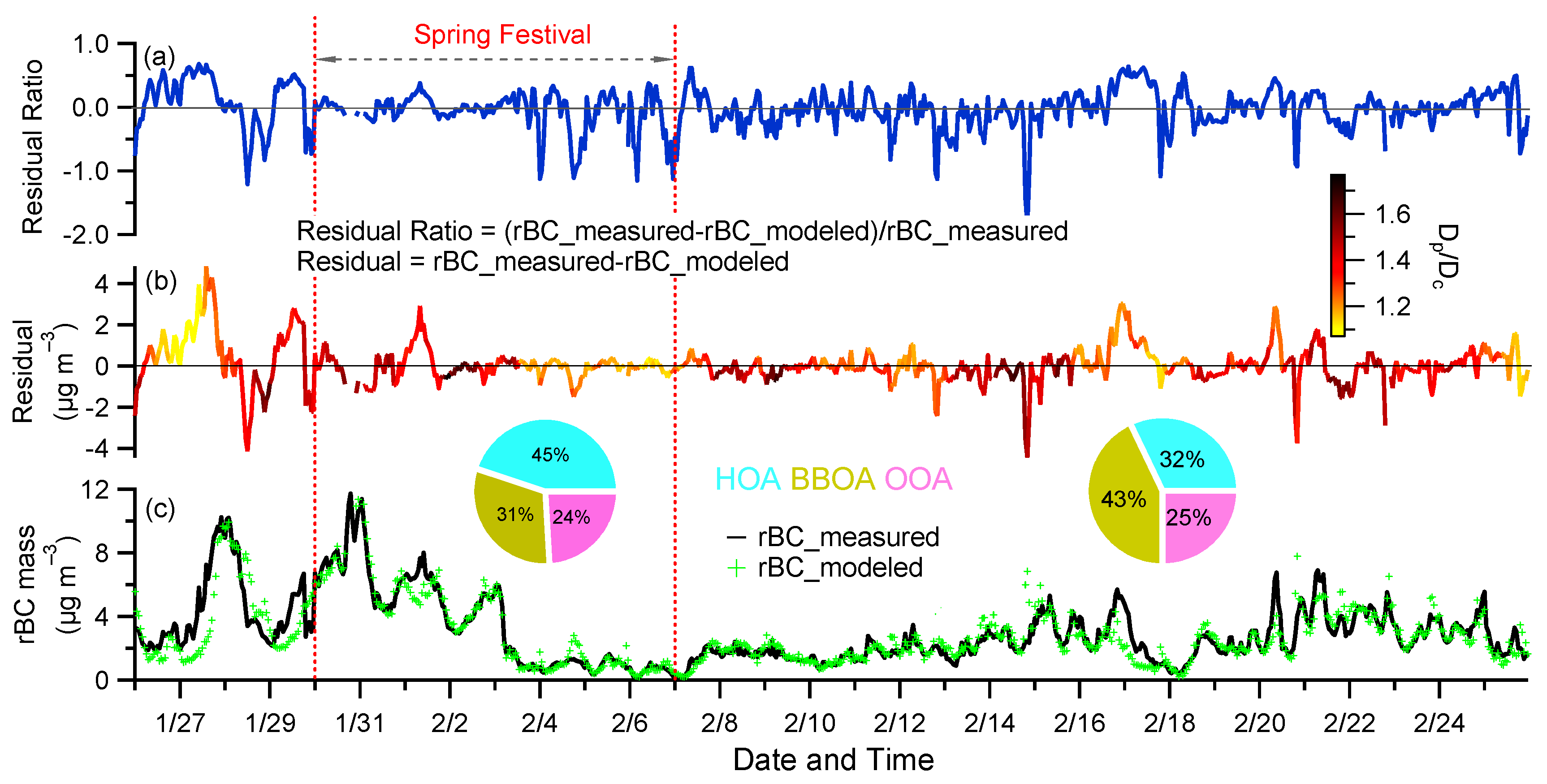
3.1. rBC Mass Loadings
3.1.1. Time Series of rBC Mass Concentration
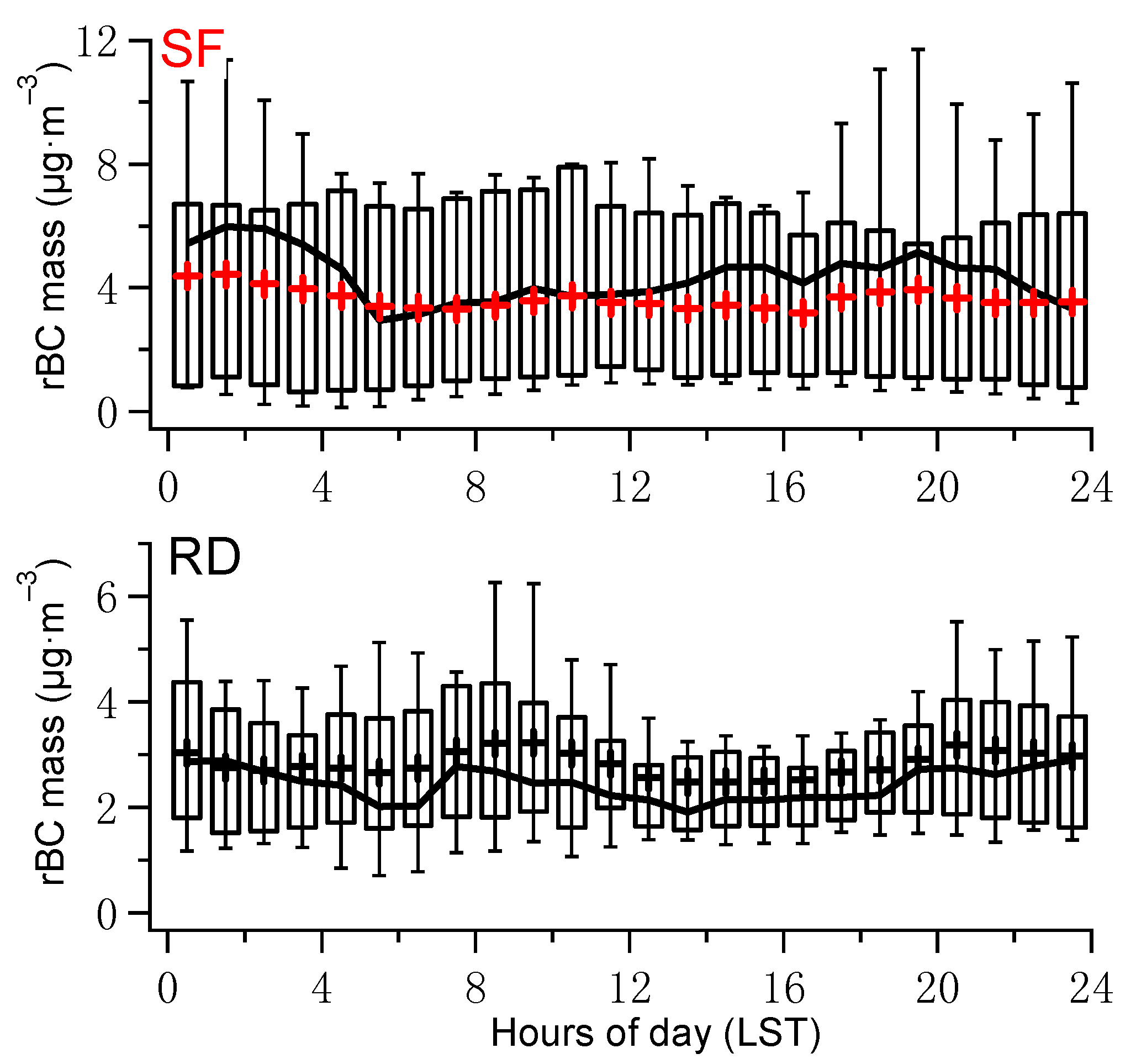
3.1.2. Diurnal Variation of rBC Mass Concentration
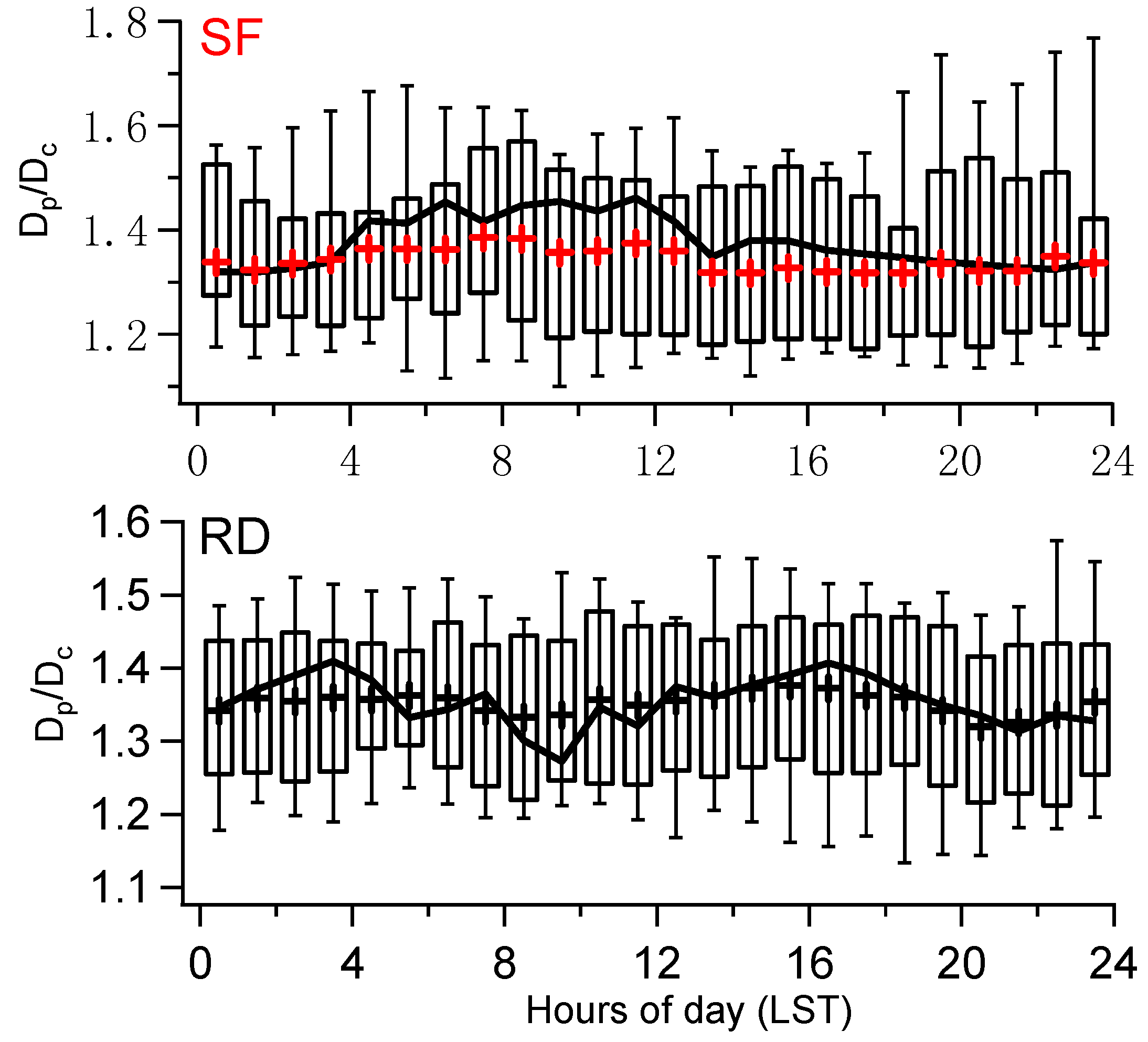
3.2. rBC Mixing State
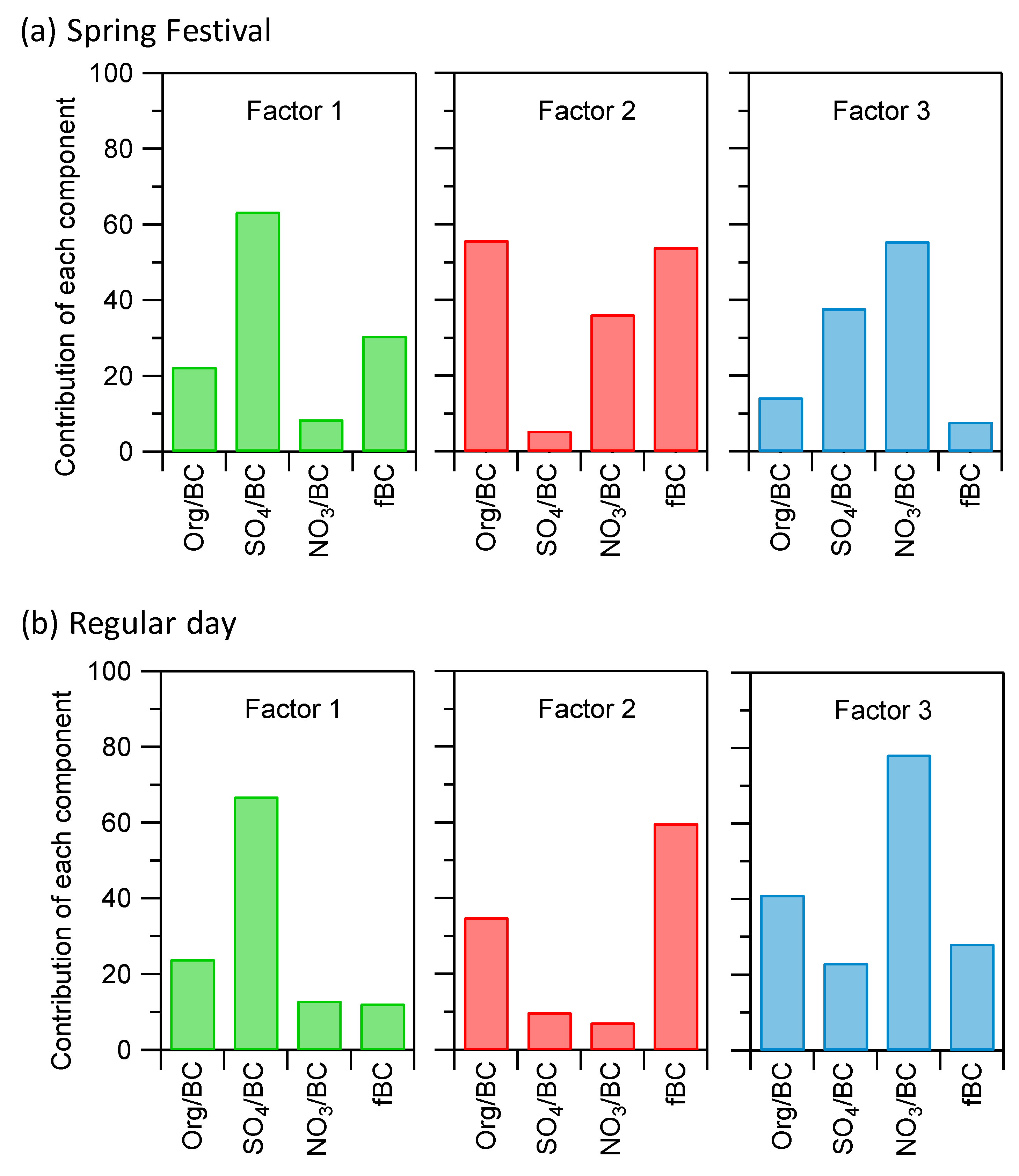
3.2.1. Diurnal Variation and Quantification of Each Components
3.2.2. Quantification of Firework Event
3.3. rBC Source Attribution by SP2 and ACSM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreae, M.O. The dark side of aerosols. Nature 2001, 409, 671–672. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Raatikainen, T.; Brus, D.; Hyvärinen, A.P.; Svensson, J.; Asmi, E.; Lihavainen, H. Black carbon concentrations and mixing state in the Finnish Arctic. Atmos. Chem. Phys. 2015, 15, 10057–10070. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Short-term effects of controlling fossil-fuel soot, biofuel soot and gases, and methane on climate, Arctic ice, and air pollution health. J. Geophys. Res. 2010, 115, D14209. [Google Scholar] [CrossRef]
- Ding, A.J.; Huang, X.; Nie, N.; Sun, J.; Kerminen, V.-M.; Petaja, T.; Su, H.; Cheng, Y.F.; Yang, X.-Q.; Wang, M.H.; et al. Enhanced haze pollution by black carbon in megacities in China. Geophys. Res. Lett. 2016, 43, 2873–2879. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.F.; Pagels, J.; Zhang, D.; Xue, H.; McMurry, P.H. Variability in morphology, hygroscopicity, and optical properties of soot aerosols during atmospheric processing. Proc. Natl. Acad. Sci. USA 2008, 105, 10291–10296. [Google Scholar] [CrossRef]
- Cappa, C.D.; Onasch, T.B.; Massoli, P.; Worsnop, D.R.; Bates, T.S.; Cross, E.S.; Davidovits, P.; Hakala, J.; Hayden, K.L.; Jobson, B.T.; et al. Radiative absorption enhancements due to the mixing state of atmospheric black carbon. Science 2012, 337, 1078–1081. [Google Scholar] [CrossRef]
- Lan, Z.J.; Huang, X.F.; Yu, K.Y.; Sun, T.L.; Zeng, L.W.; Hu, M. Light absorption of black carbon aerosol and its enhancement by mixing state in an urban atmosphere in South China. Atmos. Environ. 2013, 69, 118–123. [Google Scholar] [CrossRef]
- Lee, W.L.; Liou, K.N.; He, C.; Liang, H.-C.; Wang, T.-C.; Li, Q.; Liu, A.; Yue, Q. Impact of absorbing aerosol deposition on snow albedo reduction over the southern Tibetan plateau based on satellite observations. Theor. Appl. Climatol. 2016, 129, 1373–1382. [Google Scholar] [CrossRef]
- Schwarz, J.P.; Gao, R.S.; Spackman, J.R.; Watts, L.A.; Thomson, D.S.; Fahey, D.W.; Ryerson, T.B.; Peischl, J.; Holloway, J.S.; Trainer, M.; et al. Measurement of the mixing state, mass, and optical size of individual black carbon particles in urban and biomass burning emissions. Geophys. Res. Lett. 2008, 35, L13810. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.; Shang, D.; Zheng, J.; Du, Z.; Wu, Z.; Shao, M.; Zeng, L.; et al. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar]
- Peng, J.F.; Hu, M.; Guo, S.; Du, Z.F.; Zheng, J.; Shang, D.J.; Zamora, M.L.; Zeng, L.; Shao, M.; Wu, Y.-S.; et al. Markedly enhanced absorption and direct radiative forcing of black carbon under polluted urban environments. Proc. Natl. Acad. Sci. USA 2016, 113, 4266–4271. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Su, H.; Rose, D.; Gunthe, S.S.; Berghof, M.; Wehner, B.; Achtert, P.; Nowak, A.; Takegawa, N.; Kondo, Y.; et al. Size-resolved measurement of the mixing state of soot in the megacity Beijing, China: Diurnal cycle, aging and parameterization. Atmos. Chem. Phys. 2012, 12, 4477–4491. [Google Scholar] [CrossRef]
- Liu, D.; Allan, J.; Whitehead, J.; Young, D.; Flynn, M.; Coe, H.; McFiggans, G.; Fleming, Z.L.; Bandy, B. Ambient black carbon particle hygroscopic properties controlled by mixing state and composition. Atmos. Chem. Phys. 2013, 13, 2015–2029. [Google Scholar] [CrossRef]
- Stephens, M.; Turner, N.; Sandberg, J. Particle identification by laser-induced incandescence in a solid-state laser cavity. Appl. Opt. 2003, 42, 3726–3736. [Google Scholar] [CrossRef]
- Schwarz, J.P.; Gao, R.S.; Fahey, D.W.; Thomson, D.S.; Watts, L.A.; Wilson, J.C.; Reeves, J.M.; Darbeheshti, M.; Baumgardner, D.G.; Kok, G.L.; et al. Single-particle measurements of mid-latitude black carbon and light-scattering aerosols from the boundary layer to the lower stratosphere. J. Geophys. Res. Atmos. 2006, 111, D16207. [Google Scholar] [CrossRef]
- Taylor, J.W.; Allan, J.D.; Liu, D.; Flynn, M.; Weber, R.; Zhang, X. Assessment of the sensitivity of core/shell parameters derived using the single-particle soot photometer to density and refractive index. Atmos. Meas. Tech. 2015, 8, 1701–1718. [Google Scholar] [CrossRef]
- Moteki, N.; Kondo, Y. Effects of mixing state on black carbon measurements by laser-induced incandescence. Aerosol Sci. Technol. 2007, 41, 398–417. [Google Scholar] [CrossRef]
- Liu, D.; Flynn, M.; Gysel, M.; Targino, A.; Crawford, I.; Bower, K.; Choularton, T.; Juranyi, Z.; Steinbacher, M.; Hüglin, C.; et al. Single particle characterization of black carbon aerosols at a tropospheric alpine site in Switzerland. Atmos. Chem. Phys. 2010, 10, 7389–7407. [Google Scholar] [CrossRef]
- Slowik, J.G.; Cross, E.S.; Han, J.H.; Davidovits, P.; Onasch, T.B.; Jayne, J.T.; Williams, L.R.; Canagaratna, M.R.; Worsnop, D.R.; Chakrabarty, R.-K.; et al. An inter-comparison of instruments measuring black carbon content of soot particles. Aerosol Sci. Technol. 2007, 41, 295–314. [Google Scholar] [CrossRef]
- Schwarz, J.P.; Spackman, J.R.; Gao, R.S.; Perring, A.E.; Cross, E.; Onasch, T.B.; Ahern, A.; Wrobel, W.; Davidovits, P.; Olfert, J.; et al. The detection efficiency of the single particle soot photometer. Aerosol Sci. Technol. 2010, 44, 612–628. [Google Scholar] [CrossRef]
- Moteki, N.; Kondo, Y.; Nakamura, S. Method to measure refractive indices of small nonspherical particles: Application to black carbon particles. J. Aerosol Sci. 2010, 41, 513–521. [Google Scholar] [CrossRef]
- Liu, D.; Allan, J.D.; Young, D.E.; Coe, H.; Beddows, D.; Fleming, Z.L.; Flynn, M.J.; Gallagher, M.W.; Harrison, R.M.; James, L.; et al. Size distribution, mixing state and source apportionment of black carbon aerosol in London during wintertime. Atmos. Chem. Phys. 2014, 14, 10061–10084. [Google Scholar] [CrossRef]
- Gong, X.D.; Zhang, C.; Chen, H.; Nizkorodov, S.A.; Chen, J.M.; Yang, X. Size distribution and mixing state of black carbon particles during a heavy air pollution episode in Shanghai. Atmos. Chem. Phys. 2016, 16, 5399–5411. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Tang, L.L.; Wang, Z.; Yu, H.X.; Sun, Y.L.; Liu, D.; Qin, W.; Canonaco, F.; Prévôt, A.S.H.; Zhang, H.L.; et al. Insights into characteristics, sources, and evolution of submicron aerosols during harvest seasons in the Yangtze River delta region. China. Atmos. Chem. Phys. 2015, 15, 1331–1349. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.L.; Yu, H.; Wang, Z.; Sun, Y.; Qin, W.; Chen, W.; Chen, C.; Ding, A.; Wu, J.; et al. Chemical composition, sources and evolution processes of aerosol at an urban site in Yangtze River Delta, China during wintertime. Atmos. Environ. 2015, 123, 339–349. [Google Scholar] [CrossRef]
- Ng, N.L.; Herndon, S.C.; Trimborn, A.; Canagaratna, M.R.; Croteau, P.L.; Onasch, T.B.; Sueper, D.; Worsnop, D.R.; Zhang, Q.; Sun, Y.L.; et al. An aerosol chemical speciation monitor (ACSM) for routine monitoring of the composition and mass concentrations of ambient aerosol. Aerosol Sci. Technol. 2011, 45, 770–784. [Google Scholar] [CrossRef]
- Sun, Y.L.; Wang, Z.F.; Dong, H.B.; Yang, T.; Li, J.; Pan, X.L.; Chen, P.; Jayne, J.T. Characterization of summer organic and inorganic aerosols in Beijing, China with an aerosol chemical speciation monitor. Atmos. Environ. 2012, 51, 250–259. [Google Scholar] [CrossRef]
- Jayne, J.T.; Leard, D.C.; Zhang, X.; Davidovits, P.; Smith, K.A.; Kolb, C.E.; Worsnop, D.R. Development of an aerosol mass spectrometer for size and composition analysis of submicron particles. Aerosol Sci. Technol. 2000, 33, 49–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Jimenez, J.L.; Canagaratna, M.R.; Ulbrich, I.M.; Ng, N.L.; Worsnop, D.R.; Sun, Y. Understanding atmosphericorganic aerosols via factor analysis of aerosol massspectrometry: A review. Anal. Bioanal. Chem. 2011, 401, 3045–3067. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Huang, X.F.; Sun, T.L.; Zeng, L.W.; Guang, H.Y.; Sheng, J.L. Black carbon aerosol characterization in a coastal city in South China using a single particle soot photometer. Atmos. Environ. 2012, 51, 21–28. [Google Scholar] [CrossRef]
- Laborde, M.; Crippa, M.; Tritscher, T.; Jurányi, Z.; Decarlo, P.F.; Temime-Roussel, B.; Marchand, N.; Eckhardt, S.; Stohl, A.; Baltensperger, U.; et al. Black carbon physical properties and mixing state in the European megacity Paris. Atmos. Chem. Phys. 2013, 13, 5831–5856. [Google Scholar] [CrossRef]
- Crippa, M.; Canonaco, F.; Lanz, V.A.; Äijälä, M.; Allan, J.D.; Carbone, S.; Capes, G.; Ceburnis, D.; Dall’Osto, M.; Day, D.A.; et al. Organic aerosol components derived from 25 AMS data sets across Europe using a consistent ME-2 based source apportionment approach. Atmos. Chem. Phys. 2014, 14, 6159–6176. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Kondo, Y.; Moteki, N.; Takegawa, N.; Miyazaki, Y.; Blake, D.R. Evolution of mixing state of black carbon in polluted air from Tokyo. Geophys. Res. Lett. 2007, 34, L16803. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Kondo, Y.; Moteki, N.; Takegawa, N.; Sahu, L.; Takami, A.; Hatakeyama, S.; Yonemura, S.; Blake, D.R. Radiative impact of mixing state of black carbon aerosol in Asian outflow. J. Geophys. Res. 2008, 113, D24210. [Google Scholar] [CrossRef]
- Metcalf, A.R.; Craven, J.S.; Ensberg, J.J.; Brioude, J.; Angevine, W.; Sorooshian, A.; Hatakeyama, S.; Yonemura, S.; Blake, D.R. Black carbon aerosol over the Los Angeles basin during CalNex. J. Geophys. Res. 2012, 117, D00V13. [Google Scholar] [CrossRef]
- Zhang, G.; Bi, X.; He, J.; Chen, D.; Chan, L.Y.; Xie, G.; Wang, X.; Sheng, G.; Fu, J.; Zhou, Z. Variation of secondary coatings associated with elemental carbon by single particle analysis. Atmos. Environ. 2014, 92, 162–170. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Huang, R.J.; Zhao, Z.Z.; Zhang, N.N.; Wang, Y.C.; Ni, H.Y.; Tie, X.; Han, Y.; Zhuang, M.; Wang, M.; et al. Size distribution and mixing state of refractory black carbon aerosol from a coastal city in South China. Atmos. Res. 2016, 181, 163–171. [Google Scholar] [CrossRef]
- Drewnick, F.; Hings, S.S.; Curtius, J.; Eerdekens, G.; Williams, J. Measurement of fine particulate and gas-phase species during the New Year’s fireworks 2005 in Mainz, Germany. Atmos. Environ. 2006, 40, 4316–4327. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, Y.L.; Wang, Z.; Yin, Y. Aerosol composition and sources during the Chinese Spring Festival: Fireworks, secondary aerosol, and holiday effects. Atmos. Chem. Phys. 2015, 15, 6023–6034. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; Sun, Y.; Favez, O.; Canonaco, F.; Albinet, A.; Couvidat, F.; Liu, D.; Jayne, J.T.; Wang, Z.; et al. Limited formation of isoprene epoxydiols-derived secondary organic aerosol under NOx-rich environments in Eastern China. Geophys. Res. Lett. 2017, 44, 2035–2043. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; Croteau, P.L.; Favez, O.; Sun, Y.; Canagaratna, M.R.; Wang, Z.; Couvidat, F.; Albinet, A.; Zhang, H.; et al. Field characterization of the PM2.5 aerosol chemical speciation monitor: Insights into the composition, sources, and processes of fine particles in eastern China. Atmos. Chem. Phys. 2017, 17, 14501–14517. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Q.; Chen, M.; Ge, X.; Ren, J.; Qin, D. Chemical composition, sources, and processes of urban aerosols during summertime in northwest China: Insights from high-resolution aerosol mass spectrometry. Atmos. Chem. Phys. 2014, 14, 12593–12611. [Google Scholar] [CrossRef]

| Period | a | b | c | r2 |
|---|---|---|---|---|
| Spring Festival (SF) | 0.949 ± 0.061 | 0.224 ± 0.027 | 0.053 ± 0.010 | 0.98 |
| Regular Days (RD) | 0.408 ± 0.036 | 0.328 ± 0.017 | 0.057 ± 0.006 | 0.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jiang, L.; Chen, C.; Liu, D.; Du, S.; Zhang, Y.; Yang, Y.; Tang, L. Characteristics and Sources of Black Carbon Aerosol in a Mega-City in the Western Yangtze River Delta, China. Atmosphere 2020, 11, 315. https://doi.org/10.3390/atmos11040315
Li J, Jiang L, Chen C, Liu D, Du S, Zhang Y, Yang Y, Tang L. Characteristics and Sources of Black Carbon Aerosol in a Mega-City in the Western Yangtze River Delta, China. Atmosphere. 2020; 11(4):315. https://doi.org/10.3390/atmos11040315
Chicago/Turabian StyleLi, Jun, Lei Jiang, Cheng Chen, Dantong Liu, Songshan Du, Yunjiang Zhang, Yifan Yang, and Lili Tang. 2020. "Characteristics and Sources of Black Carbon Aerosol in a Mega-City in the Western Yangtze River Delta, China" Atmosphere 11, no. 4: 315. https://doi.org/10.3390/atmos11040315
APA StyleLi, J., Jiang, L., Chen, C., Liu, D., Du, S., Zhang, Y., Yang, Y., & Tang, L. (2020). Characteristics and Sources of Black Carbon Aerosol in a Mega-City in the Western Yangtze River Delta, China. Atmosphere, 11(4), 315. https://doi.org/10.3390/atmos11040315





