Effect of a New Manure Amendment on Ammonia Emissions from Poultry Litter
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Treatments
2.2. Flux Measurements and Litter Collection
2.3. Litter Analysis
2.4. Data Analysis
3. Results and Discussion
3.1. Broiler Performance
3.2. Litter Properties
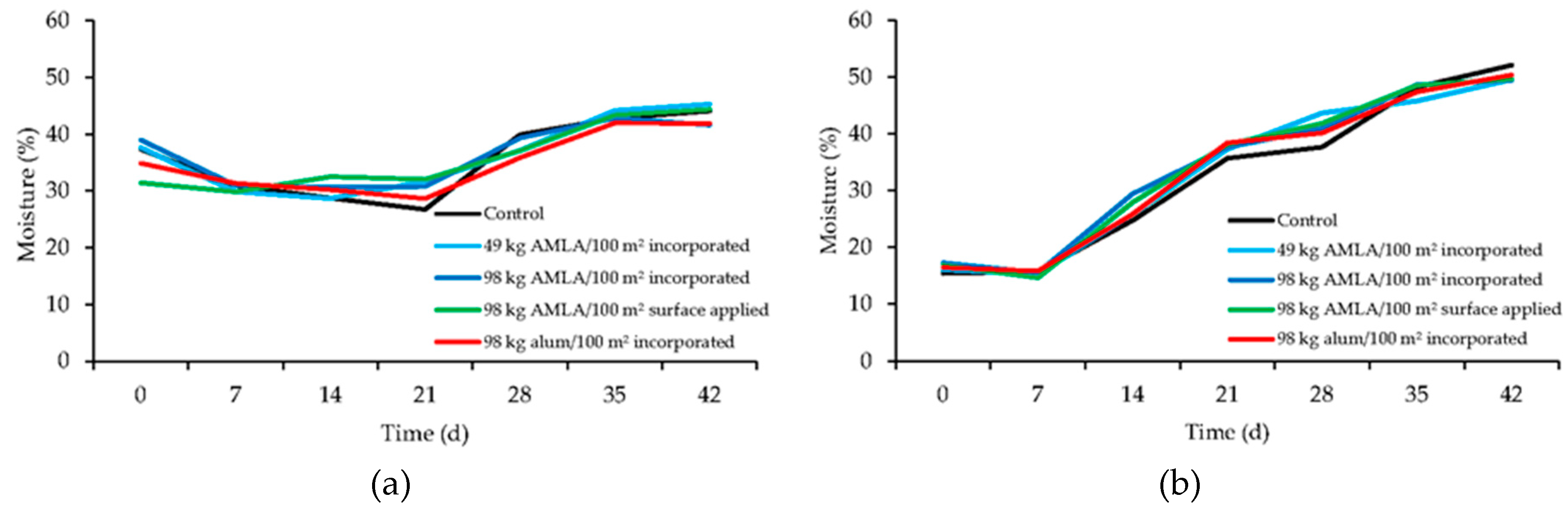
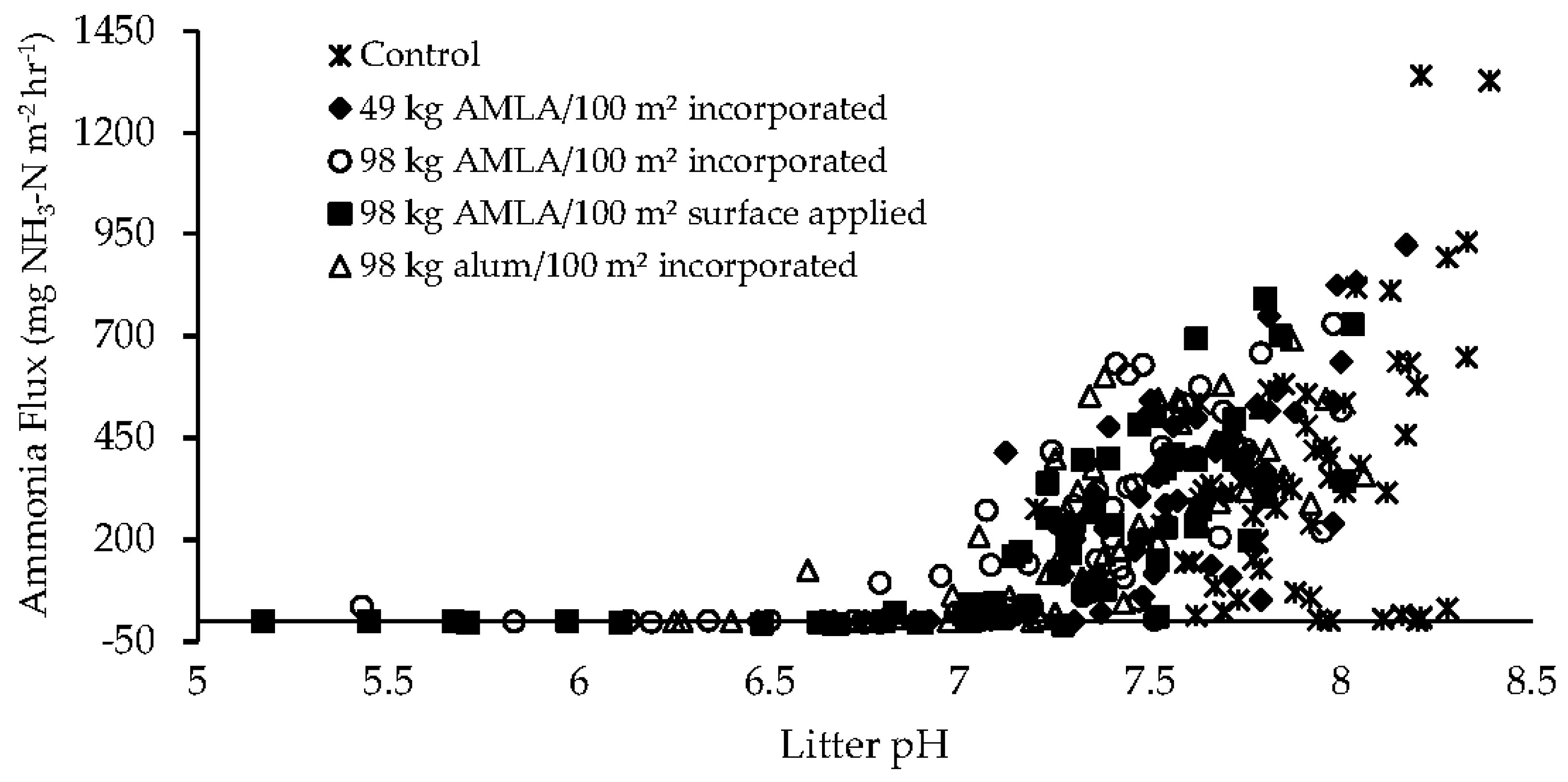
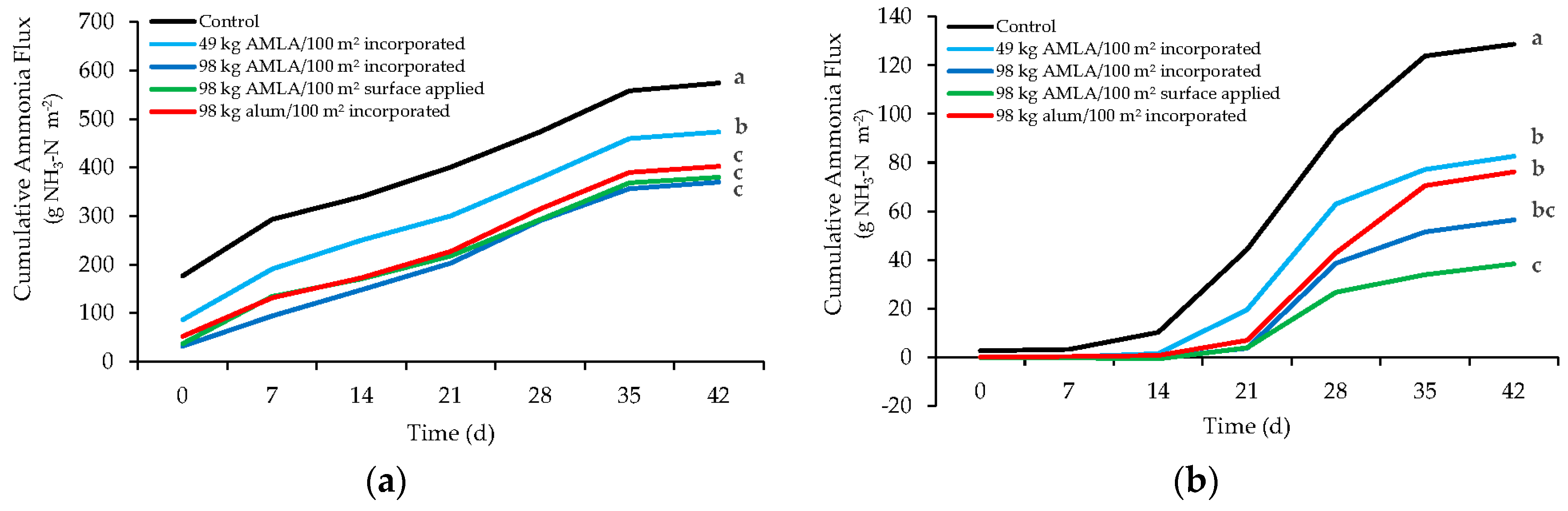
3.3. Ammonia Flux
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Battye, R.; Battye, W.; Overcash, C.; Fudge, S. Development and Selection of Ammonia Emission Factors; Final Report; EC/R Inc.: Durham, NC, USA, 1994. [Google Scholar]
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity evaluation and emission characteristics of poultry litter co-combustion process. Appl. Sci. 2019, 9, 4116. [Google Scholar] [CrossRef]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Miles, D.; Burns, R.; Pote, D.; Berg, K.; Choi, I. Ammonia emission factors from broiler litter in barns, in storage, and after land application. J. Environ. Qual. 2011, 40, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- National Chicken Council. Broiler Chicken Industry Key Facts 2019. Available online: https://www.nationalchickencouncil.org/about-the-industry/statistics/broiler-chicken-industry-key-facts/ (accessed on 10 January 2020).
- Nagaraj, M.; Wilson, C.P.; Saenmahayak, B.; Hess, J.B.; Bilgili, S.F. Efficacy of litter amendment to reduce pododermatitis in broiler chickens. J. Appl. Poult. Res. 2007, 16, 255–261. [Google Scholar] [CrossRef]
- Wheeler, E.; Casey, K.; Gates, R.; Xin, H.; Liang, Y.; Topper, P. Litter management strategies in relation to ammonia emissions from floor-raised birds. In Proceedings of the Mitigating Air Emissions from Animal Feeding Operations Conference, Iowa State University, Ames, IA, USA, 19–21 May 2008; pp. 99–103. [Google Scholar]
- Carlile, F.S. Ammonia in poultry houses: A literature review. Worlds Poult. Sci. J. 1984, 40, 99–113. [Google Scholar] [CrossRef]
- Miles, D.M.; Branton, S.L.; Lott, B.D. Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poult. Sci. 2004, 83, 1650–1654. [Google Scholar] [CrossRef]
- Miles, D.M.; Miller, W.W.; Branton, S.L.; Maslin, W.R.; Lott, B.D. Ocular responses to ammonia in broiler chickens. Avian Dis. 2006, 50, 45–49. [Google Scholar] [CrossRef]
- Donham, K.J. Air Quality Relationships to Occupational Health in the Poultry Industry. In Proceedings of the 1996 National Waste Management, Auburn, AL, USA, 21–23 October 1996; Blake, J.P., Patterson, P.H., Eds.; Auburn University Press: Auburn, AL, USA, 1996; pp. 24–28. [Google Scholar]
- Donham, K.J. Occupational Health Hazards and Recommended Exposure Limits for Workers in Poultry Buildings. In Proceedings of the 2000 National Waste Management, Auburn, AL, USA, 2000; Blake, J.P., Patterson, P.H., Eds.; Auburn University Press: Auburn, AL, USA, 2000; pp. 92–109. [Google Scholar]
- Kling, H.F.; Quarles, C.L. Effect of atmospheric ammonia and the stress of infectious bronchitis vaccination on Leghorn males. Poult. Sci. 1974, 53, 1161–1167. [Google Scholar] [CrossRef]
- Anderson, D.P.; Beard, C.W.; Hanson, R.P. The adverse effects of ammonia on chickens including resistance to infection with Newcastle Disease virus. Avian Dis. 1964, 8, 369–379. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Viets, F.G., Jr. Nitrogen enrichment of surface water by absorption of ammonia volatilized from cattle feedlots. Science 1969, 166, 514–515. [Google Scholar] [CrossRef]
- Schroder, H. Nitrogen losses from Danish agriculture-trends and consequences. Agric. Ecosyst. Environ. 1985, 17, 279–289. [Google Scholar] [CrossRef]
- ApSimon, H.M.; Kruse, M.; Bell, J.N. Ammonia emissions and their role in acid deposition. Atmos. Environ. 1987, 21, 1939–1946. [Google Scholar] [CrossRef]
- van Breemen, N.; Burrough, P.A.; Velthorst, E.J.; van Dobben, H.F.; de Wit, T.; Ridder, T.B.; Reijinders, H.F.R. Soil acidification from atmospheric ammonium sulphate in forest canopy through fall. Nature 1982, 299, 548–550. [Google Scholar] [CrossRef]
- Barthelmie, R.J.; Pryor, S.C. Implications of ammonia emissions for fine aerosol formation and visibility impairment: A case study from the Lower Fraser Valley, British Columbia. Atmos. Environ. 1998, 32, 345–352. [Google Scholar] [CrossRef]
- McCubbin, D.R.; Apelberg, B.J.; Roe, S.; Divita, F., Jr. Livestock ammonia management and particulate-related health benefits. Environ. Sci. Technol. 2002, 36, 1141–1146. [Google Scholar] [CrossRef]
- Elliot, H.A.; Collins, N.E. Factors affecting ammonia release in broiler houses. Trans. ASAE 1982, 25, 413–418, 424. [Google Scholar] [CrossRef]
- Arogo, J.; Westerman, P.W.; Herber, A.J.; Robarge, W.P.; Classen, J.J. Ammonia emissions from animal feeding operations. In Animal Agriculture and the Environment: National Center for Manure and Animal Waste Management White Papers; Rice, J.M., Caldwell, D.F., Humenik, F.J., Eds.; ASABE: St. Joseph, MI, USA, 2006; pp. 41–88. [Google Scholar]
- Sajeev, E.P.M.; Winiwarter, W.; Amon, B. Greenhouse gas and ammonia emissions from different stages of liquid manure management changes: Abatement options and emission interactions. J. Environ. Qual. 2017, 47, 30–41. [Google Scholar] [CrossRef]
- Sutton, A.L.; Applegate, T.; Hankins, S.; Hill, B.; Allee, G.; Greene, W.; Kohn, R.; Meyer, D.; Powers, W.; van Kempen, T. Manipulation of animal diets to affect manure production, composition and odors: State of the science. In Animal Agriculture and the Environment: National Center for Manure and Animal Waste Management White Papers; Rice, J.M., Caldwell, D.F., Humenik, F.J., Eds.; ASABE: St. Joseph, MI, USA, 2006; pp. 377–408. [Google Scholar] [CrossRef]
- Borges, S.A.; Fisher da Silva, A.V.; Ariki, J.; Hooge, D.A.; Cummings, K.R. Dietary electrolyte balance for broiler chickens under moderately high ambient temperatures and relative humidities. Poult. Sci. 2003, 82, 301–308. [Google Scholar] [CrossRef]
- Puumala, M. Storage of manure in heaps. In Sustainable Handling and Utilization of Livestock Manure from Animals to Plants. In Proceedings of the NJF Seminar No. 320, Horsens, Denmark, 16–19 January 2001; p. 202. [Google Scholar]
- Dalólio, F.S.; da Silva, J.N.; de Oliveira, A.C.C.; Tinôco, I.D.F.F.; Barbosa, R.C.; de Oliveira Resende, M.; Albino, L.F.T.; Coelho, S.T. Poultry litter as biomass energy: A review and future perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Zhu, S.; Lee, S.W. Co-combustion performance of poultry wastes and natural gas in the advanced Swirling Fluidized Bed Combustor (SFBC). Waste Manag. 2005, 25, 511–518. [Google Scholar] [CrossRef]
- Atimtay, A.; Yurdakul, S. Combustion and co-combustion characteristics of torrefied poultry litter with lignite. Renew. Energy 2020, 148, 1292–1301. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R.; Miller, D.M. Effect of chemical amendments on ammonia volatilization from poultry litter. J. Environ. Qual. 1995, 24, 293–300. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R.; Miller, D.M. Evaluation of chemical amendments to reduce ammonia volatilization from poultry litter. Poult. Sci. 1996, 75, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R. Reducing phosphorus runoff and improving poultry production with alum. Poult. Sci. 1999, 78, 692–698. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R. Reducing phosphorus runoff and inhibiting ammonia loss from poultry manure with aluminum sulfate. J. Environ. Qual. 2000, 29, 37–49. [Google Scholar] [CrossRef]
- Smith, D.R.; Moore, P.A., Jr.; Haggard, B.E.; Maxwell, C.V.; Daniel, T.C.; VanDevander, K.; Davis, M.E. Effect of aluminum chloride and dietary phytase on relative ammonia losses from swine manure. J. Anim. Sci. 2004, 82, 605–611. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Xin, H.; Liang, Y.; Burns, R.T. Reduction of ammonia emissions from stored laying hen manure through topical application of zeolite, Al+ Clear, Ferix-3, or poultry litter treatment. J. Appl. Poult. Res. 2008, 17, 421–431. [Google Scholar] [CrossRef]
- Terzich, M.; Quarles, C.; Brown, J.; Goodwin, M.A. Effect of Poultry Litter Treatment (PLT) on the development of respiratory tract lesions in broilers. Avian Pathol. 1998, 27, 566–569. [Google Scholar] [CrossRef]
- Shreve, B.R.; Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R. Reduction of phosphorus in run-off from field-applied poultry litter using chemical amendment. J. Environ. Qual. 1995, 24, 106–111. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Miller, D.M. Reducing phosphorus solubility in poultry litter with aluminum, calcium, and iron amendments. J. Environ. Qual. 1994, 23, 325–330. [Google Scholar] [CrossRef]
- Eugene, B.; Moore, P.A., Jr.; Li, H.; Miles, D.; Trabue, S.; Burns, R.; Buser, M. Effects of alum additions to poultry litter on in-house ammonia and greenhouse gas concentrations and emissions. J. Environ. Qual. 2015, 44, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- McWard, G.W.; Taylor, D.R. Acidified clay litter amendment. J. Appl. Poult. Res. 2000, 9, 518–529. [Google Scholar] [CrossRef]
- Moore, P.A., Jr. Improving the sustainability of animal agriculture by treating manure with alum. In Environmental Chemistry of Animal Manure; He, Z., Ed.; Nova Science: Hauppauge, NY, USA, 2011; pp. 349–381. ISBN 1-62808-641-6. [Google Scholar]
- Worley, J.W.; Risse, L.M.; Cabrera, M.L.; Nolan, M.P. Bedding for broiler chickens: Two alternative systems. Appl. Eng. Agric. 1999, 15, 687–693. [Google Scholar] [CrossRef]
- Worley, J.W.; Cabrera, M.L.; Risse, L.M. Reduced levels of alum to amend broiler litter. Appl. Eng. Agric. 2000, 16, 441–444. [Google Scholar] [CrossRef]
- Moore, P.A., Jr. Development of a new manure amendment for reducing ammonia volatilization and phosphorus runoff from poultry litter. J. Environ. Qual. 2016, 45, 1412–1429. [Google Scholar] [CrossRef]
- Moore, P.A., Jr. Composition and Methods of Treating Animal Manure. US Patent 9,301,440, 5 April 2016. [Google Scholar]
- Adak, A.; Mallik, D.; Chaudhuri, S.K. Alum mud: Phase identification and catalytic potential for aquepis-phase decomposition of hydrogen peroxide. Clays Clay Miner. 1999, 47, 234–238. [Google Scholar] [CrossRef]
- Choi, I.H.; Moore, P.A., Jr. Effects of liquid aluminum chloride additions to poultry litter on broiler performance, ammonia emissions, soluble phosphorus, total volatile fatty acids, and nitrogen contents of litter. Poult. Sci. 2008, 87, 1955–1963. [Google Scholar] [CrossRef]
- Miles, D.M.; Owens, P.R.; Rowe, D.E. Spatial variability of litter gaseous flux within a commercial broiler house: Ammonia, nitrous oxide, carbon dioxide, and methane. Poult. Sci. 2006, 85, 167–172. [Google Scholar] [CrossRef]
- Moore, P.A.; Miles, D.M.; Burns, R.; Pote, D.; Berg, K. Evaluation and Management of Ammonia Emissions from Poultry Litter. In Best Management Practices. In Proceedings of the Workshop on Agricultural Air Quality: State of Science, Potomac, MA, USA, 5–8 June 2006; pp. 304–310. [Google Scholar]
- Self-Davis, M.L.; Moore, P.A., Jr. Method of determining water soluble phosphorus in animal manure. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Water; Southern Cooperative Series Bulletin No. 396, SERA-IEG 17; Pierzynski, G.M., Ed.; North Carolina State University: Raleigh, NC, USA, 2000; pp. 74–77. ISBN 1-58161-396-2. [Google Scholar]
- Choi, I.H.; Moore, P.A., Jr. Effect of various litter amendments on ammonia volatilization and nitrogen content of poultry litter. J. Appl. Poult. Res. 2008, 17, 454–462. [Google Scholar] [CrossRef]
- USEPA. Methods for Chemical Analysis of Water and Wastes; USEPA Rep. 600/4-79-020. USEPA, Environ; Monitoring and Support Lab.: Cincinnati, OH, USA, 1983.
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 19th ed.; Eaton, A., Clesceri, L., Greenberg, A., Franson, M., Eds.; Am. Public Health Assoc.: Washington, DC, USA, 1995; ISBN 0-87553-223-3. [Google Scholar]
- Zarcinas, B.A.; Cartwright, B.; Spouncer, L.R. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Soil Sci. Plant Anal. 1987, 18, 131–146. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide: Statistics; SAS Inst.: Cary, NC, USA, 2014. [Google Scholar]
- Schabenberger, O. Introducing the GLIMMIX Procedure for Generalized Linear Mixed Models; Proceedings SUGI 30; SAS Institute Inc.: Cary, NC, USA, 2005. [Google Scholar]
- Carr, L.E.; Wheaton, F.W.; Douglass, L.W. Empirical models to determine ammonia concentrations from broiler chicken litter. Trans. ASAE 1990, 33, 1337–1342. [Google Scholar] [CrossRef]
- Reddy, K.R.; Khaleel, R.; Overcash, M.R.; Westerman, P.W. A nonpoint source model for land areas receiving animal wastes: II. Ammonia volatilization. Trans. ASAE 1979, 22, 1398–1405. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Edwards, D.R. Long-term effects of poultry litter, alum-treated litter, and ammonium nitrate on aluminum availability in soils. J. Environ. Qual. 2005, 34, 2104–2111. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Feed Intake (kg) | Weight Gain (kg) | Feed:Gain (kg:kg) | Mortality (%) |
|---|---|---|---|---|
| Flock 2 | ||||
| Control | 3.87ab † | 2.12a | 1.83a | 7.00a |
| 49 kg AMLA/100 m2 incorporated | 3.98a | 2.18a | 1.83a | 3.00a |
| 98 kg AMLA/100 m2 incorporated | 3.77b | 2.08a | 1.81a | 5.50a |
| 98 kg AMLA/100 m2 surface applied | 3.86ab | 2.15a | 1.80a | 4.00a |
| 98 kg alum/100 m2 incorporated | 3.95a | 2.21a | 1.79a | 8.00a |
| Flock 3 | ||||
| Control | 4.14a | 2.59a | 1.62a | 8.50a |
| 49 kg AMLA/100 m2 incorporated | 4.11a | 2.53a | 1.63a | 5.50a |
| 98 kg AMLA/100 m2 incorporated | 3.97a | 2.51a | 1.58a | 4.50a |
| 98 kg AMLA/100 m2 surface applied | 4.07a | 2.53a | 1.62a | 5.00a |
| 98 kg alum/100 m2 incorporated | 4.00a | 2.45a | 1.64a | 7.50a |
| Treatment | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | ||||||||
| Control | 8.30a † | 8.21a | 7.72a | 7.99a | 7.76a | 7.92a | 8.06a | 7.99a |
| 49 kg AMLA/100 m2 incorporated | 7.76ab | 7.92ab | 7.46a | 7.57b | 7.62b | 7.81ab | 7.91a | 7.72b |
| 98 kg AMLA/100 m2 incorporated | 6.83c | 7.52c | 7.53a | 7.38b | 7.42c | 7.68b | 7.80a | 7.45c |
| 98 kg AMLA/100 m2 surface applied | 7.41bc | 7.72bc | 7.52a | 7.45b | 7.48c | 7.75b | 7.77a | 7.58bc |
| 98 kg alum/100 m2 incorporated | 7.76ab | 7.66bc | 7.22a | 7.50b | 7.45c | 7.66b | 7.83a | 7.58bc |
| Flock 3 | ||||||||
| Control | 8.19a | 8.08a | 7.78a | 7.83a | 7.67a | 7.77a | 7.62a | 7.85a |
| 49 kg AMLA/100 m2 incorporated | 6.87b | 6.93b | 7.11b | 7.34b | 7.45b | 7.62ab | 7.45ab | 7.25b |
| 98 kg AMLA/100 m2 incorporated | 6.09cd | 6.54b | 6.73cd | 7.07c | 7.34bc | 7.36c | 7.26b | 6.91cd |
| 98 kg AMLA/100 m2 surface applied | 5.63d | 6.49b | 6.53d | 7.03c | 7.17d | 7.33c | 7.31b | 6.78d |
| 98 kg alum/100 m2 incorporated | 6.41bc | 6.98b | 6.81c | 7.14c | 7.22cd | 7.51bc | 7.37b | 7.06bc |
| Treatment | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | ||||||||
| Control | 2.79b † | 2.29c | 2.14d | 2.17c | 2.24c | 3.73d | 4.64b | 2.86c |
| 49 kg AMLA/100 m2 incorporated | 4.51a | 3.87b | 3.78c | 3.42b | 3.47b | 4.76c | 5.93a | 4.25b |
| 98 kg AMLA/100 m2 incorporated | 5.45a | 6.72a | 6.27a | 5.11a | 4.38a | 5.18bc | 5.81a | 5.56a |
| 98 kg AMLA/100 m2 surface applied | 5.21a | 5.79a | 4.73b | 4.70a | 4.15a | 5.34ab | 6.46a | 5.20a |
| 98 kg alum/100 m2 incorporated | 4.99a | 5.73a | 5.46ab | 4.94a | 4.37a | 5.64a | 6.38a | 5.36a |
| Flock 3 | ||||||||
| Control | 1.30c | 1.14d | 1.55c | 2.52c | 3.27c | 4.52b | 6.67b | 2.99b |
| 49 kg AMLA/100 m2 incorporated | 2.76b | 2.86c | 2.83b | 3.66b | 4.42b | 5.10b | 8.44a | 4.29a |
| 98 kg AMLA/100 m2 incorporated | 3.90a | 3.90a | 3.60a | 4.44a | 5.42a | 6.91a | 8.93a | 5.30a |
| 98 kg AMLA/100 m2 surface applied | 3.80a | 3.68ab | 3.42a | 4.04ab | 5.07a | 6.49a | 8.93a | 5.06a |
| 98 kg alum/100 m2 incorporated | 3.83a | 3.50b | 3.34a | 3.93ab | 5.12a | 6.66a | 9.23a | 5.09a |
| Treatment | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | ||||||||
| Control | 13.3c † | 15.4b | 18.4c | 18.2a | 19.6a | 19.2a | 21.3a | 17.9b |
| 49 kg AMLA/100 m2 incorporated | 17.7ab | 19.4a | 21.3ab | 21.0a | 20.5a | 20.5a | 22.1a | 20.3a |
| 98 kg AMLA/100 m2 incorporated | 16.4b | 19.4a | 22.2a | 21.3a | 20.7a | 20.9a | 22.5a | 20.5a |
| 98 kg AMLA/100 m2 surface applied | 18.8a | 19.8a | 19.4bc | 20.0a | 21.9a | 21.0a | 22.5a | 20.5a |
| 98 kg alum/100 m2 incorporated | 17.2ab | 21.1a | 21.9a | 22.2a | 22.6a | 22.3a | 23.2a | 21.5a |
| Flock 3 | ||||||||
| Control | 22.1a | 22.3a | 22.3b | 19.7b | 20.7d | 20.3b | 24.8a | 21.8c |
| 49 kg AMLA/100 m2 incorporated | 22.7a | 22.1a | 24.0b | 21.2ab | 21.9bc | 22.4ab | 25.6a | 22.9b |
| 98 kg AMLA/100 m2 incorporated | 22.8a | 21.7a | 22.7ab | 23.0a | 23.0b | 22.9a | 25.4a | 23.1b |
| 98 kg AMLA/100 m2 surface applied | 20.8a | 23.4a | 23.7ab | 23.2a | 23.2c | 23.7a | 25.1a | 23.3b |
| 98 kg alum/100 m2 incorporated | 21.8a | 25.1a | 25.0a | 23.7a | 24.5a | 24.2a | 26.3a | 24.4a |
| Treatment | Day | Avg. | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Flock 2 | ||||||||
| Control | 1052a † | 694a | 277a | 363a | 435a | 502a | 668a | 570a |
| 49 kg AMLA/100 m2 incorporated | 513b | 623a | 354a | 299a | 464a | 484a | 573a | 473b |
| 98 kg AMLA/100 m2 incorporated | 189c | 370b | 321a | 328a | 521a | 394a | 559a | 383c |
| 98 kg AMLA/100 m2 surface applied | 220c | 578ab | 218a | 282a | 446a | 452a | 469a | 381c |
| 98 kg alum/100 m2 incorporated | 310bc | 472ab | 246a | 324a | 521a | 450a | 516a | 406bc |
| Flock 3 | ||||||||
| Control | 15.7a | 3.68a | 41.9a | 202a | 286a | 187a | 200a | 134a |
| 49 kg AMLA/100 m2 incorporated | 0.13b | 1.27b | 7.60b | 107b | 258a | 84.7a | 225a | 97.9ab |
| 98 kg AMLA/100 m2 incorporated | −0.64b | 1.71b | −2.35b | 23.0c | 208a | 76.1a | 205a | 73.2b |
| 98 kg AMLA/100 m2 surface applied | −1.09b | −0.23b | −2.84b | 27.2c | 136a | 43.0a | 183a | 55.2b |
| 98 kg alum/100 m2 incorporated | 0.22b | 1.30b | 2.67b | 37.5c | 213a | 164 a | 236a | 93.8ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, K.; Moore, P.A., Jr.; Martin, J.; Ashworth, A.J. Effect of a New Manure Amendment on Ammonia Emissions from Poultry Litter. Atmosphere 2020, 11, 257. https://doi.org/10.3390/atmos11030257
Anderson K, Moore PA Jr., Martin J, Ashworth AJ. Effect of a New Manure Amendment on Ammonia Emissions from Poultry Litter. Atmosphere. 2020; 11(3):257. https://doi.org/10.3390/atmos11030257
Chicago/Turabian StyleAnderson, Kelsey, Philip A. Moore, Jr., Jerry Martin, and Amanda J. Ashworth. 2020. "Effect of a New Manure Amendment on Ammonia Emissions from Poultry Litter" Atmosphere 11, no. 3: 257. https://doi.org/10.3390/atmos11030257
APA StyleAnderson, K., Moore, P. A., Jr., Martin, J., & Ashworth, A. J. (2020). Effect of a New Manure Amendment on Ammonia Emissions from Poultry Litter. Atmosphere, 11(3), 257. https://doi.org/10.3390/atmos11030257





