1. Introduction
The impact of aerosols on the fog removal process occurring in lab environments can be applicable to real environmental conditions. Physical and chemical properties of aerosols affect droplet formation and size spectra, which are dependent on thermodynamical and dynamical conditions. Fog can be a natural or artificial phenomenon that has been studied extensively in the past [
1]. It affects visibility [
2,
3], air quality, climate, agriculture [
4], human health [
5], firefighting, and transportation [
2,
6,
7]. Microphysical parameters of fog can be highly variable and need to be further studied using observations and prediction models [
1,
8]. Furthermore, artificially produced fog types can also be studied for similar applications [
6,
7], plus applications to industrial processes. Fog with suitable additives has been also used as a cleaning agent of chemical species generated by the military or terrorist actions, aviation accidents, or natural disasters.
In order to obtain a better understanding of fog prediction, earlier works have extensively studied natural fog with respect to its formation and dissipation processes. Both fog and rain can act as natural “cleaners” of the lower atmosphere, because they wash out various aerosol particles as they fall into the Earth’s surface, and that includes nuclei [
9]. Due to its nature, the present work is closely connected to fog formation, dissipation, and nucleation issues occurring in the lab and in the real atmosphere.
The idea of using fog generation as an air-cleaning agent is important to develop new technologies; therefore, a project called COUNTERFOG was initiated. The main purpose of this project is to develop a fog dissipation system to reduce adverse effects of social unrest dealing with CBRN (chemical, biological, radiological, and nuclear) agents; industrial accidents; and disasters. This system uses specially developed devices and parts with novel nozzles for the generation of fog with additives, which neutralizes harmful substances within the air and deposits them on the ground [
10]. An important step for control of the presence and the concentration of chemical compounds (considered impurities) in artificially generated fog is to develop a sensor system. The current sensor [
11] used in this work is developed based on results grouped under the working name “surface photo-charge effect” (SPCE). This effect is quantified when there is an interaction between matter (gas, liquid, or solid) and an electromagnetic field. This interaction induces an electrical alternating potential difference over the frequency field [
12]. Ivanov [
13] observed the effect and suggested the name of the effect to be changed to “electromagnetic echo effect” (EMEE), since this result better reflects the nature of the effect. This way, it is not being confused with the photoelectric effect. The current work uses an EMEE sensor in a development of the cleaning properties of fog.
Many experimental investigations were performed in the past to better evaluate how various types of aerosol impurities influence the physical parameters of artificially generated fog. An automated fog generating system (AFGS) suitable for performing such tests has been developed [
14,
15]. This AFGS is used to focus on how added chemicals affect the microphysical parameters of artificially generated fog. To do this, varying concentrations of harmless chemical compounds were dissolved in distilled water for simulation of real CBRN agents. These agents, replaced with harmless chemicals, were then pulverized using a newly developed automated system in which fog droplets were generated and measured by a laser particle size analyzer. The results were analyzed to identify the influence of chemical compounds on fog parameters, such as particle spectra, leading to changes in droplet number concentration (N
d) and liquid water content (LWC), as well as mean volume diameter (MVD).
The goal of this work is to study fog microphysical parameters, such as droplet volume concentration (V
d) and MVD, as a function of chemical compounds dissolved in distilled water, and for this purpose, the EMEE system was used for investigations. Then, the chemical processes taking place for the alteration of fog spectral characteristics are reviewed. This work is designed as follows:
Section 2 focuses on experimental setup, and
Section 3 and
Section 4 are for the Results and Discussion, respectively. The last section (
Section 5) is given for the Conclusions.
2. Experiments
In this section, first, an automated fog generator is described, and then, experiments performed are provided.
2.1. Automated Fog Generator (AFG)
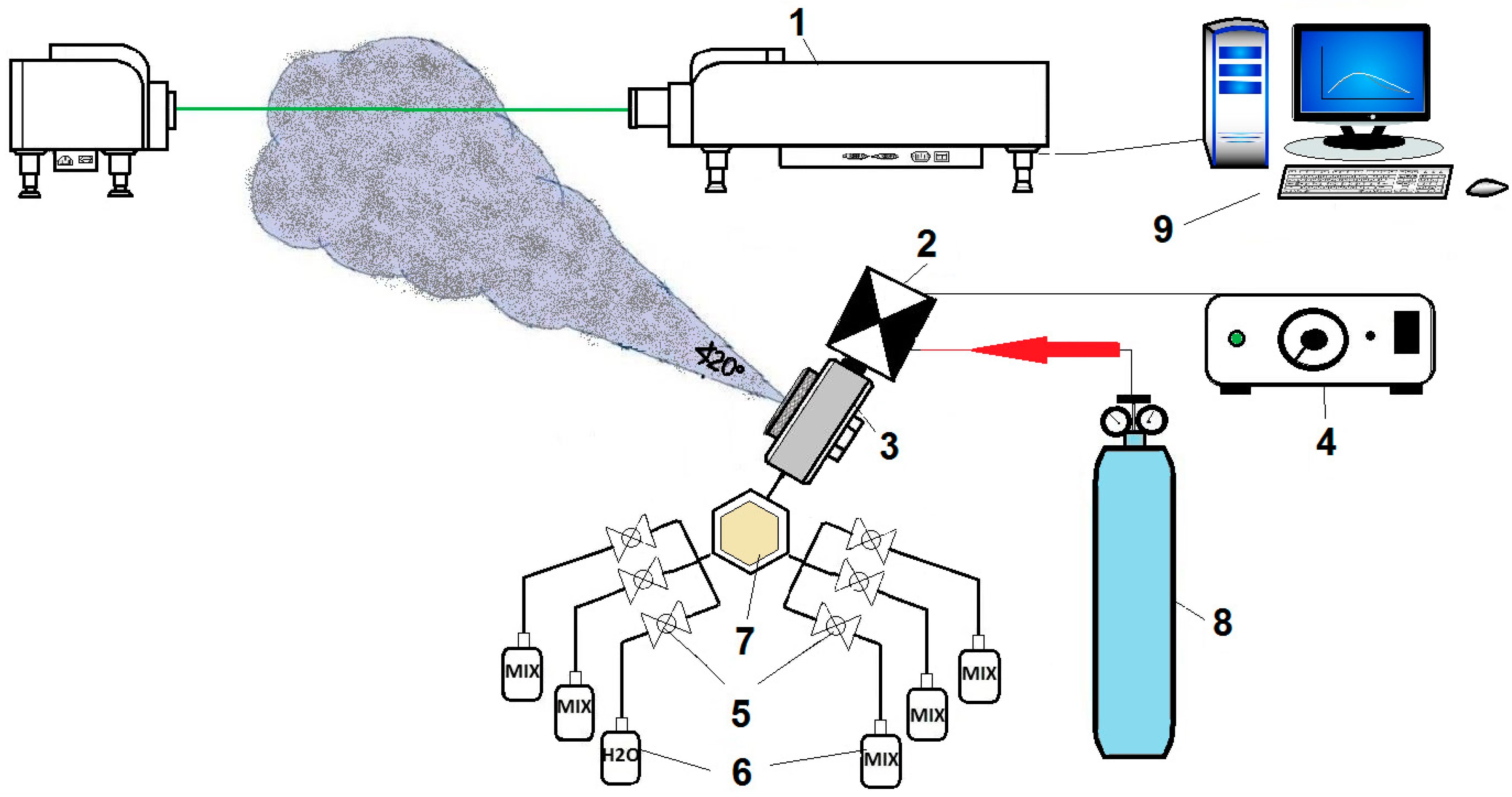
An experimental setup in the lab includes an automated fog generator (AFG), which sprays controlled fog amounts into the sampling volume of the laser particle size analyzer (LPSA). The l × w × h of the lab were 8 m × 6 m × 3 m, respectively, which was selected to reduce dynamical effects, e.g., stability of air. The relative humidity with respect to water (RH) and air temperature (Ta) inside the lab were set up at about 63% and 25 °C, respectively. This will create a near saturation environment when vapor flux into the lab increases. The AFG is able to generate fog with different chemical compositions, leading to variations in droplet sizes (d), number concentrations (Nd), and liquid water content (LWC). It is designed to work with up to six different fluids with adjustable spraying amounts over durations of 0.2–5.5 s. Switching between containers of agents is done via small valves. An important advantage of AFG is that it offers a fast and easy way to pulverize different chemical solutions and also allows a precise control of the amounts of aerosol impurities released into the fluids.
Figure 1 shows the block diagram of the experimental setup. Important components of the system are shown with numbers overlaid on the figure. Nitrogen under pressure is fed from the gas pressure tank (8) to an atomizing nozzle (3), thus drawing liquid from one of the containers (6). Depending on the impurity (composition and amount of aerosols), the corresponding container is used for fog generation. The prototype has six branches with six controlling valves (5), so that switching between different containers is performed quickly and easily. A pulse generator (4) that is specially designed for the system controls the electromagnetic valve (2). Different settings can be selected to achieve specific durations of fog spraying. The control unit generates uniform impulses with a time step in the range of 0.2 to 5.5 s. When the desired values for the impulse duration are set, the generation of an impulse is initiated mechanically. This impulse is transferred to the electromagnetic valve (2), and thus, a spraying through the nozzle with the desired duration is achieved. Fog produced by the nozzle is directed towards a laser particle size analyzer’s sampling area to measure the artificially generated fogs’ physical properties. The laser particle size analyzer (LPSA) is the Winner 319A model manufactured by Winner Particle Instrument Stock Co. Ltd., Jinan, China measuring particles between 1 and 500 μm size range, with a distance of 2 m between the LPSA transmitter and receiver.
2.2. Experiments
In the experiments, the four conditions are tested, and they are explained below.
Three kinds of chemicals were dissolved in distilled water, which are potassium dihydrogen phosphate-KH2PO4, urea-CO(NH2)2, and potassium hexacyanoferrate trihydrate-K3(Fe(CN)6). These chemicals were chosen because they have characteristics similar to CBRN agents, but they are harmless. In such a way, the presence of dangerous pollutants in fog is simulated. For example, KH2PO4 has been selected as a harmless simulant of the CRBN agents used in chemical warfare with highly lethal G- and V-series of the nerve gas class. In a water solution, KH2PO4 dissociates to give phosphate ions that resemble the CRBN molecular structure. The K3(Fe(CN)6) is chosen as a chemical warfare agent simulant due to the presence of cyanide ions in its structure. They bond each other in a stable complex state with iron, which makes the compound safe to work with. The cyanide functional group is a constituent of blood agents such as cyanogen chloride and hydrogen cyanide. The prepared solutions were then pulverized using the automated system for fog modification. Pure distilled fog water is also measured and used for comparisons.
The experiments are designed to study how different impurities (chemical compounds) described above alter the spectral distribution of artificially generated fog particles that are composed of water vapor and chemical compounds at gas pressure (P) over 100 s (T) equals to 2 bars with pulse duration of 5.5 s. Four concentrations of each of the above mentioned compounds were dissolved in distilled water at a volume mixing level of 2.5%, 5%, 7.5%, and 10%. For all of the conducted experiments, measurements were taken at each second in a distance of 70 cm from the nozzle. The 100 measurements in each set are then averaged for each solution. The Ta and RH were kept at 25 °C and 63%, respectively. These environmental conditions were monitored with thermometers and hygrometers that were controlled by air conditioners and moisture absorbers. The diameter of the nozzle is 0.4 mm with spraying angle of 20° (suggested by the manufacturer company, Lechler GmbH, Metzingen, Germany). The nozzle (model #136.316.35.A2) is a pneumatic brass-plated atomizing nozzle, which works on the siphon/gravity principle. At P = 2 bar, the water volume flux of fog droplets emitted from the nozzle was 0.13 L s−1 cm−2.
Aerosols act as condensation nuclei for the formation of fog droplets. Some chemical components, such as the ones studied in this work, can lead to the modification of the fog droplet count and their growth rates through the effects of solute on vapor pressure when RH nears 100%. Although a smaller mass of solutes acts as nuclei, the larger their size, the faster droplets will grow. A mixture of vapor and solute can also modify the vapor pressure over the solution, which can result in the growing of droplets slower or faster. This growth will strongly depend on RH, which is a function of es (saturated vapor pressure of a solution) and ea (environmental air vapor pressure). The molecular structure of the solution and its hygroscopicity also affects droplet growth.
2.3. Method
There are various aerosols in the atmosphere that act like hydrophilic (like water, i.e., solvent) and water-soluble ones (dissolved in water). The effect of soluble CCN can be important for the water evaporation (as solvent); therefore, curvature and solute effects can be considered for droplet growth. However, for small droplets, solute effects can be more critical for droplet growth or evaporation. With solute effects, the evaporation rate is lowered, resulting in condensation of the solvent. Then, the equilibrium water pressure (
ee) will be less than the saturated one (
es) over flat water surfaces. When the amount of solute is increased,
ee will decrease more, resulting in larger droplets. The evaporation rate of water molecules can be written as
where
nw,
ns, and
nt are the number of moles of water in a volume, number of moles of solute in the same volume, and the number of moles in the same volume for pure water, respectively. As a result, the evaporation rate from the water surface decreases, and this can be expressed as
where
em is the vapor pressure of the mixing (solution).
Then, the solute effect on vapor pressure of the solution (Raoult’s Law) can be written as:
where
Ns is the total number of moles of the solute,
Vd is the droplet volume,
rd is the droplet radius, and
i is the Van ’t Hoff factor accounted for the splitting of solutes into components when they dissolve. Based on Equation (3), experimental results followed up a theory that increasing droplet size results in less
s but increasing
em near to
es. On the other hand, increasing
Ns reduces vapor pressure of the mixture, which results in its faster growth. Clearly, when droplet sizes become smaller and solute mass increases, mixture-saturated vapor pressure becomes smaller, allowing droplets to grow faster and leading to an increased settling of the particles.
Large Ns values and decreasing droplet volume (smaller radius) but increasing nt (number of moles per unit volume of pure water) can result in a larger denominator of Equation (3), although the total moles of solute increases (numerator, Ns). This means that lower vapor pressure will occur in the solution but droplets will be much smaller compared to ones obtained for smaller nt values. Therefore, growth of the droplets will be different as a function of nt, and that will lead to smaller solution droplets in the air rather than ones falling to the surface. Results below provided should be considered in light of the conditions described here. In either way of the formation of smaller or larger droplets, increasing solutes can affect the life cycle of fog droplets (growth time).
4. Discussion
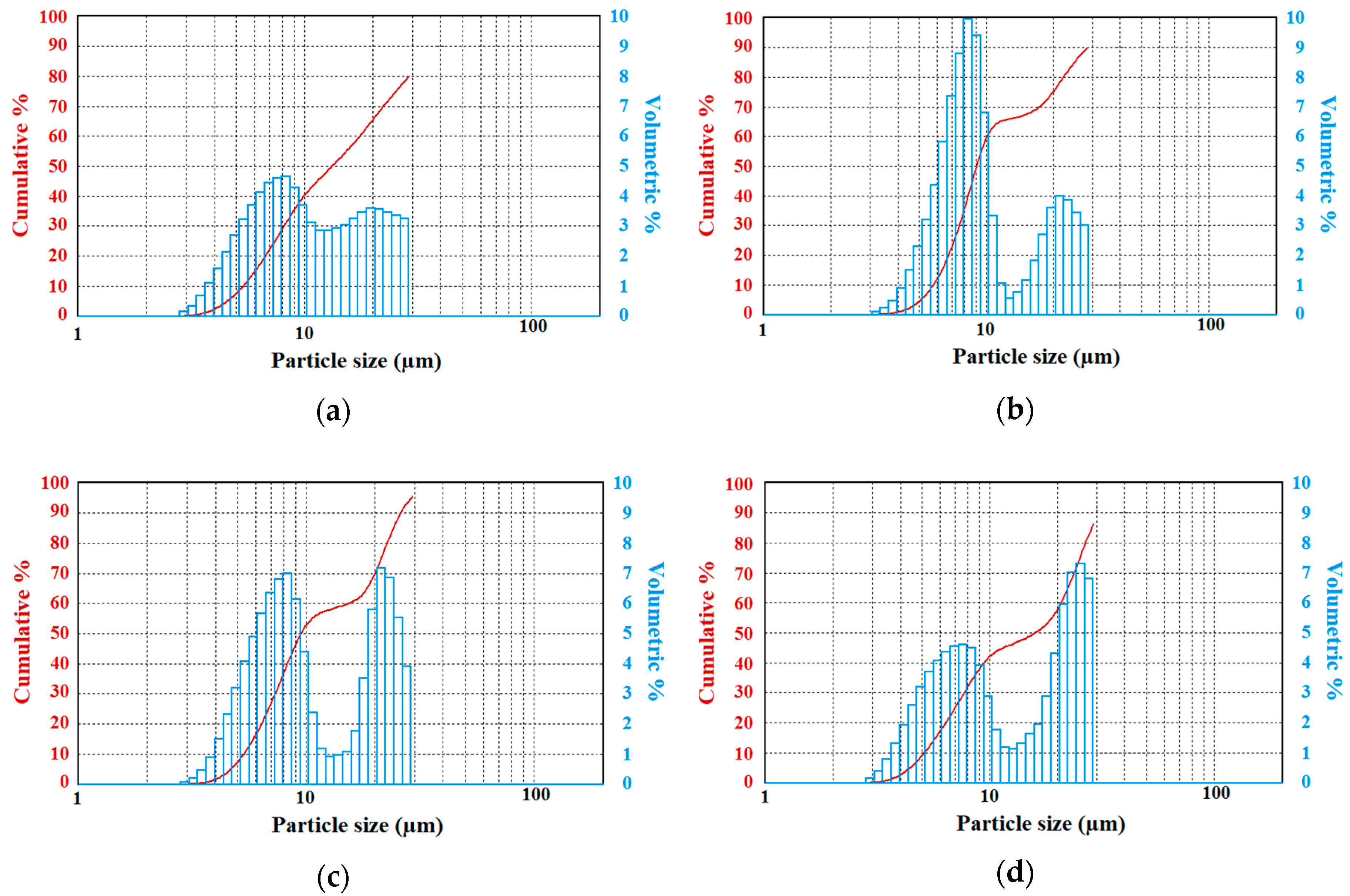
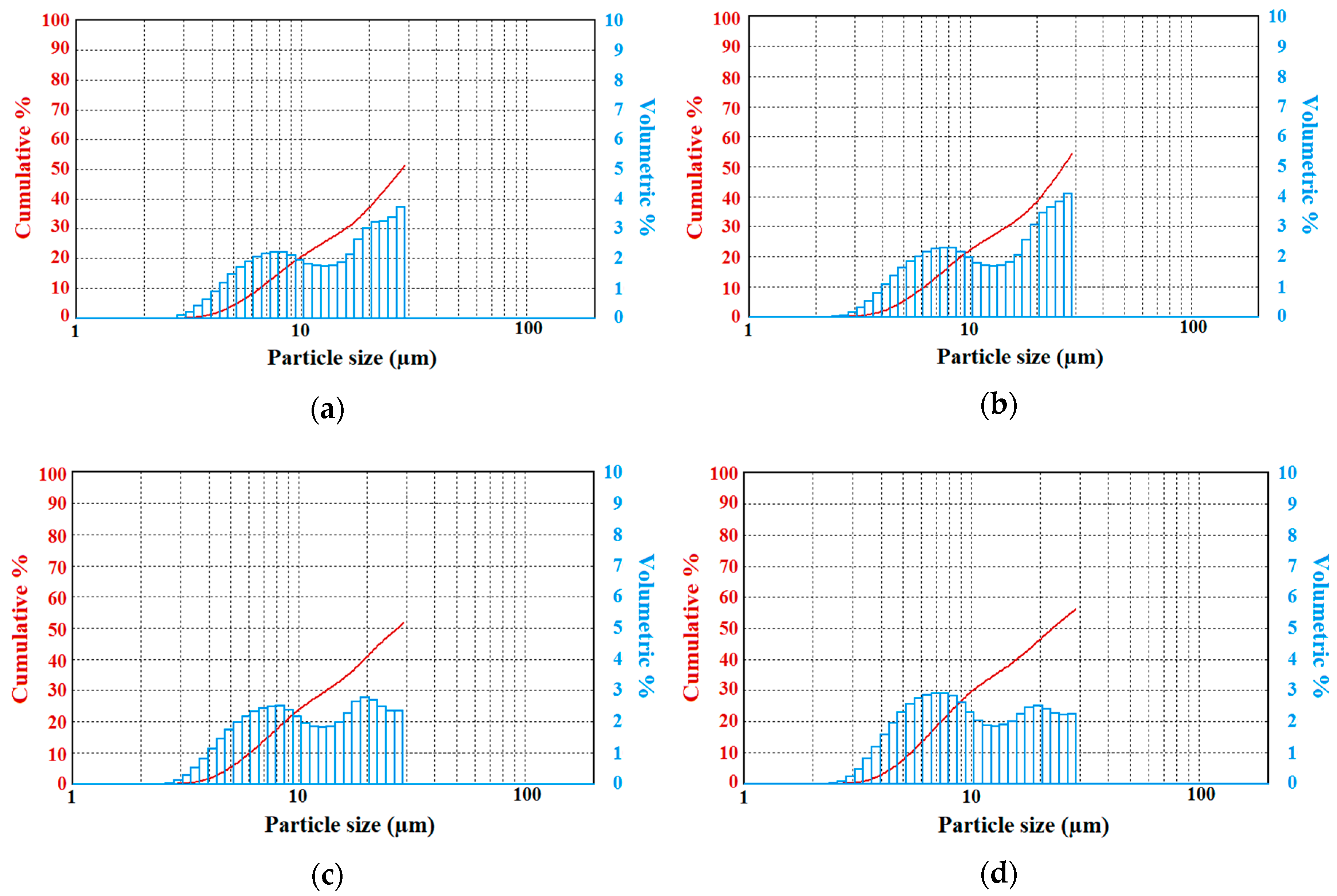
Results suggest that fog droplet spectra generated with the pure water case were found similar to real fog measurements collected in field projects [
16,
18,
19,
20]. These studies showed that fog droplet spectra had sizes from a few microns up to 30 μm, but occasionally, they reach up to 40–50 μm. When sizes get larger than 30 microns, fog droplets can settle down onto surfaces, reducing droplet counts in the air.
Nucleation characteristics of the chemicals used in solutions play an important role as cloud condensation nuclei (CCN) in the natural environments, and they can be controlled by lab set-up conditions. Then, the mixture of the vapor and CCN results in fog droplets, either by the internal mixing of vapor and solute or by direct condensation. Washing out chemical substances from the air sprayed as CBRN elements, after which particles grow faster than under regular fog conditions, results in the cleaning of atmospheric air parcels. These conditions can be modified by increasing the saturation (RH) and T, cooling rate, CCN, and dynamics of the environment.
Life cycles of fog droplets can be strongly related to lab physical set-up conditions, e.g., its volume and height, as well as to thermodynamic parameters of the environment, such as T and RH. Therefore, thermodynamic conditions similar to real fog environment conditions should be adapted for the tests. During the experiments, wetness at the surface was not observed, indicating that the physical body of the lab was not affecting our results. However, lower RH and higher T can result in much faster dissipation of the foggy conditions. Therefore, experiments in the future need to be further repeated at various thermodynamical conditions near the saturation levels rather than at low RH values. In the analysis, the generation of droplets using various chemical compounds was not easy because of the control of thermodynamic conditions in the lab, such as relative humidity RH and air temperature Ta. The use of pure distilled water in fact was important to validate the changes of chemical compounds and their impact on the fog microphysical structure. The most difficult issue was the measurement of droplet spectra without a fog spectral probe; therefore, we could not estimate the exact value of total droplet volume concentration (Vt) but only the % value of Vt was obtained. This was the most difficult issue; in the future, a fog spectral device is planned to be used in characterizing details of the microphysics of fog spectra with various chemical compounds.
Latest studies [
21,
22,
23,
24] suggested that chemical components of aerosols could play an important role for CCN concentration. For example, NaCl (sea salt) particles are a good source of CCN [
25]; increasing their number concentration and size as a function of turbulence can enlarge/narrow the droplet spectra, resulting in larger/smaller fog droplet sizes (see last paragraph in
Section 3). The work of Woodcock (1978) showed that salt particles, similar to the ones studied here, can significantly change droplet spectra and N
t. Therefore, the results of this work can be used to reduce CRBN concentrations that impact on the environment and human life during peace and war times. However, the results are obtained at lower RH values, which needs to be researched further near saturation levels.
Table 1 shows the mean diameters of fog droplets for the entire size range, as well as for sizes < 30 μm and > 30 μm of the pulverized solutions at 2 bar of gas pressure, which were measured by the LPSA. The results suggest that the solutions resulted in various mean values of droplet diameters depending on the solution type and volume ratio, and that may be used for the cleaning of CRBN agents from the environment.
Table 1 shows that the addition of up to 10% of potassium dihydrogen phosphate to distilled water leads to an increase of the average diameter of the droplets, compared to those of pure distilled water. The lower concentrations of solution (2.5% and 5%) led to a significant increase (~8%) in the average diameter of droplets. A concentration of 7.5% (10%) of this impurity led to a decrease of about 2% (9%) of the average diameter of the generated fog droplets.
The addition of urea to distilled water resulted in a decrease of the average diameter of the droplets compared to the case when the solution is pulverized from pure distilled water. The presence of 2.5% of urea in the water volume resulted in a decrease in the average diameter of droplets by 6%. The concentration of urea at 5% and 7.5% resulted in a decrease in the average diameter of droplets by 13% and 2%, respectively, and with the highest concentration of solution used as 10%, led to an increase in the average diameter of fog droplets by 7%. Note that the number of moles given in Equation (3) can play an important role on the final results related to droplet sizes and number concentrations, which depend on the mixing characteristics of solutes with pure water.
When introducing various concentrations of potassium hexacyanoferrate trihydrate to distilled water, the average diameter of droplets decreased significantly. As explained previously, this can be due to increasing the total number of moles (with smaller droplets). At the lowest concentration of 2.5%, the diameter of fog droplets decreased by 25%. For the solutions containing 5% and 7.5% of this chemical, the droplets are about 23% smaller, and for the highest concentration of 10%, about 21% smaller. The higher the concentration of potassium hexacyanoferrate trihydrate, the weaker the decrease of the average diameter of fog droplets.
The results of this work need to be evaluated further in more controlled environments and test the microphysics of fog in polluted environments with various chemical components. Additional information for thermodynamics and dynamics of the environment can also have an impact on fog droplet spectra [
26,
27,
28,
29,
30,
31,
32]; therefore, results need to be also tested during field projects under various meteorological conditions.
As pointed out, this work was an experimental work performed in a lab; therefore, the experiments should be further performed during field projects. In fact, the analysis performed in this work is similar to many wind tunnel and cloud chamber studies and faces similar concerns for the results.
5. Conclusions
This work uses a fog generating system and various chemical components in a solution to study the size distribution of artificially generated fog droplets. These solutions included changing volume concentrations and types of chemical impurities, including PDH, PDP, and carbamide. These chemicals resemble CRBN agents that are extensively used in warfare.
This work helps to develop new methods on how fog microphysical parameters change with the addition of chemical compounds in the pulverized solutions.
The main conclusions can be summarized as:
Artificially generated fog droplet spectra, leading to higher settling rates, can be used to reduce the amount of dangerous chemical compounds found in the air, such as CBRNs.
Increasing sizes of droplets can depend on both thermodynamic and dynamic conditions, as well as on the chemical structures of the compounds; these include solute amount and RH, as well as T.
Increasing the volume of chemical compounds usually resulted in increasing droplet mean size and multiple peaks, indicating the settling of chemical compounds through fog development into larger sizes, but evaporation was also possible.
The artificial fog generating system used can also be further developed to test the impact of various aerosol chemical properties on fog formation and modified into a compact prototype system.
In the future, the results obtained from this study will be used further to develop a better fog generating system that can also be used in the field. Field tests would be very important when fog can be used as a cleaning agent of contaminations of public sites and environments, and this may occur due to various reasons, such as terrorist attacks with CBRN agents, military actions, industrial accidents, and environmental disasters.