Abstract
In humid locations of the Eastern U.S., sulfate is a surrogate for aerosol liquid water (ALW), a poorly measured particle constituent. Regional and seasonal variation in ALW–sulfate relationships offers a potential explanation to reconcile epidemiology and toxicology studies regarding particulate sulfur and health endpoints. ALW facilitates transfer of polar species from the gas phase to the particle phase and affects particle pH and metal oxidation state. Though abundant and a potential indicator of adverse health endpoints, ALW is largely removed in most particulate matter measurement techniques, including in routine particulate matter (PM2.5) networks that use federal reference method (FRM) monitors, which are used in epidemiology studies. We find that in 2004, a typical year in the available record, ambient ALW mass is removed during sampling and filter equilibration to standard laboratory conditions at most (94%) sites, up to 85% of the ambient water mass. The removal of ALW can induce the evaporation of other semi-volatile compounds present in PM2.5, such as ammonium nitrate and numerous organics. This produces an artifact in the PM mass measurements that is, importantly, not uniform in space or time. This suggests that PM2.5 epidemiology studies that exclude ALW are biased. This work provides a plausible explanation to resolve multi-decade discrepancies regarding ambient sulfate and health impacts in some epidemiological and toxicological studies.
1. Introduction
More than one million life years are lost in the U.S. due to fine particulate matter (PM2.5) exposure during a single year. [1] Studies link PM2.5 mass with a variety of adverse health consequences [2]. Sulfate, a major PM2.5 constituent, is often positively correlated with increased risk in many epidemiology studies [3,4], including the Harvard six cities study [5], but not all [6]. Toxicology studies do not reveal mechanistic evidence for a direct association between sulfate and health endpoints at atmospherically relevant concentrations [6,7]. Inconsistent epidemiological findings and lack of a definitive toxicological explanation are a conundrum.
A plausible explanation for the discrepancies is that sulfate is not toxic by itself, but a surrogate for co-produced constituent(s) not well characterized in PM2.5 measurements [6,8]. Historically, many epidemiological studies relating PM2.5 to health endpoints rely on data from fixed location ‘central’ monitors (e.g., [9]). Sulfate is not volatile and exhibits less spatial variability than other PM2.5 constituents [10]. Correlations between indoor (where most people spend most time) and outdoor sulfate are high [11], and measurement errors and impact on risk ratios [12] are lower for sulfate than for total PM2.5 mass. Therefore, such central monitor approaches should work well for sulfate. However, in a review of time series studies investigating the impacts of PM2.5 and sulfate on various health endpoints, Reiss et al. (2008) (using re-estimated relative risk ratios to account for misleading results from S-Plus’ default convergence criteria in the Generalized Additive Model), found little to no support for a causal association of sulfate and increased health risk at ambient concentrations. In their analysis of reported mass concentrations at central monitor locations in routine air quality networks for 2004, Reiss et al. note that, for cities across the U.S., statistical correlations between sulfate and PM2.5 vary, reducing the power of epidemiologic studies to elucidate association between specific PM2.5 constituents and health risk. Such arguments could be used to advocate for the loosening of successful sulfur-related regulations. However, there is a plausible mechanism by which sulfate may impact human health through indirect ways not assessed in many current approaches [8,13], namely by increasing the amount of aerosol liquid water (ALW) and facilitating transfer to the particle phase of polar, water-soluble organic species that are potentially toxic and induce harm.
Sulfate is a hygroscopic species that promotes water uptake. Although ALW is an abundant and ubiquitous PM2.5 chemical constituent [14], it is removed during PM2.5 mass sampling at routine air quality network sites [15], and is vaporized, and not detected by state-of-the-art particle measurement technologies such as aerosol mass spectrometers [16]. ALW facilitates transfer from the gas phase to the particle phase of polar organic species [17], which are highly soluble in a polar solvent, such as water. ALW alters the amount and chemical composition of particulate organic mass [18], potentially in ways important for human health [19]. For example, secondary organic aerosol (SOA) derived from isoprene epoxydiols is only formed when ALW is present [20]. This SOA induces expression of oxidative stress response genes in human bronchial epithelial cells, which is not seen in control experiments with sulfate aerosol [21,22,23,24]. Further, in humid locations ALW correlates with water-soluble toxic species (e.g., Fe, Cu) [25], reactive oxygen species (ROS) [25], particle oxidative potential [8], and sulfate [26]. Sulfate is the most widely reported species routinely measured and reported by air quality networks. Positive sulfate–health associations are noted in the Harvard six cities study (i.e., all humid Eastern U.S. locations, Figure 1), and generally observed in areas of the U.S. where ALW is predicted to be high [17], (e.g., Detroit [27], and New Jersey [28]) but not in areas where ALW is expected to be low [17], (e.g., Santa Clara [29], and Phoenix [30]). Associations between ALW and health end points are difficult to quantitatively assess with many current approaches.
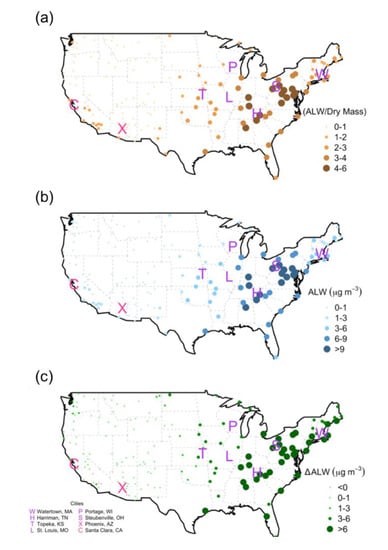
Figure 1.
Yearly average in 2004 at Interagency Monitoring of PROtected Visual Environments (IMPROVE) monitoring sites of (a) ratio of aerosol liquid water (ALW) mass per measured PM2.5 ‘dry’ mass (b) ambient ALW mass concentrations and (c) equivalent concentration loss upon filter equilibration to standard laboratory conditions. Point color and diameter are relative to ALW per dry mass ratio (orange), ALW mass (blue), and ALW mass difference (green, red). Green indicates ALW loss during equilibration and red indicates ALW gain.
Central monitors in air quality networks across the U.S. often measure PM2.5 surface mass concentrations according to the federal reference method (FRM), a filter-based mass measurement collected and averaged over 24-h (midnight to midnight). Quality assurance criteria require that after collection, FRM PM2.5 filters are equilibrated for a minimum of 24-h at 20–23 °C (±1 °C) and 30–40% (±5%) relative humidity (RH) [31]. Hourly PM2.5 mass concentration measurements using federal equivalent methods (FEM) also evaporate ALW from ambient particles prior to collection [15]. This protocol perturbs collected particles from ambient temperature and RH to standard laboratory conditions, and it is well documented that sampling artifacts arise due to the volatilization of organic material during filter sampling [32,33]. FRM sampling and equilibration procedures, in particular for water-soluble organic species, are also prone to this bias [34]. Ambient SOA equilibration experiments to laboratory conditions (RH = 35%) by El-Sayad et al. (2016) for samples collected in Baltimore, MD (i.e., humid, Eastern U.S.) find water-soluble organic matter evaporation losses are in excess of 5 μg m−3 in some cases. This is consistent with other particulate organic carbon findings by Zhang et al., (2012) for samples collected in and near Atlanta, GA (Eastern U.S.) [35]. Loss of water-soluble organic species may obscure interpretation of the health impacts of inhaled ambient PM2.5. Organic species are documented to exhibit redox activity and catalyze ROS generation upon deposition in lung fluid to induce adverse health responses, including oxidative stress, cell death, and disease [36]. Standard equilibration procedures in the FRM ensure consistency among reported mass concentrations used in PM2.5 National Ambient Air Quality Standard (NAAQS) attainment decisions for the States. For example, humid locations will have more ALW mass than arid regions for the same amount of dry PM2.5 of a given chemical composition. ALW mass concentrations per unit of measured and reported PM2.5 ‘dry’ mass are highest in the Eastern U.S. [37]. Equilibration to standard conditions normalizes the ALW content in reported PM2.5 mass across the U.S. for a strict set of laboratory conditions, asserting fairness to the mass-based NAAQS attainment process. However, data from FRM measurements are used in epidemiology studies [38], suggesting that the measurement bias associated with ALW evaporation may influence the analysis of statistical links to health endpoints.
This work explores the relationships among sulfate, ALW, and changes to PM2.5 mass upon filter equilibration across the U.S. with a focus on 2004, the year in the sulfate-epidemiology review by Reiss [6]. We characterize ALW and changes to PM2.5 mass and dry versus liquid fractionation through estimates at ambient and laboratory conditions. Precise chemical characterization of compounds associated with or formed in ALW, and then lost during filter sampling and/or equilibration remains a critical open question. The lack of understanding in ALW and the extent to which this biases PM2.5 risk estimates as they relate human exposure to water-soluble air pollution are key knowledge gaps.
2. Methods
We estimate ALW across the contiguous U.S. using surface mass concentrations of SO42− and NO3− at PM2.5 monitoring locations in the Interagency Monitoring of PROtected Visual Environments (IMPROVE) network [39]. The estimation technique has been described in detail elsewhere [40]. Briefly, IMPROVE collects 24-h PM filter measurements every three days. Teflon filters are analyzed for gravimetric fine mass, elemental concentration, and light absorption, while nylon filters are analyzed for the anions sulfate, nitrate, and chloride using ion chromatography. Data was downloaded from the IMPROVE public archive [41] on 13 July 2015 for 203 unique locations (Figure S1) from 1988 to 2014, and the database includes additional measurement details. There is some variability in the number and location of IMRPOVE sites year to year. In 2004, 158 IMPROVE sites with a minimum of 2 reported measurements per month are used for this analysis. Meteorology measurements are not available at IMPROVE sites, therefore, we employ the North American Regional Reanalysis (NARR) model [42], paired in space and time with monitoring sites, to obtain relative humidity (RH) and temperature data. We calculate ALW using NARR meteorology, IMRPOVE particle chemical composition, and the thermodynamic equilibrium model ISORROPIAv2.1 [43] that describes gas-to-particle partitioning of inorganic species, including water (Figure S2). We apply ISORROPIA as a box model where IMPROVE chemically characterized PM2.5 and NARR meteorology are inputs. We assume metastable particles, and that measured sulfate is in the form of ammonium sulfate, and nitrate is in the form of ammonium nitrate. Ammonia (NH3), dust, and organic compounds are not considered in the ALW estimates due to inconsistencies in measurements among the sites, an inability to accurately predict concentrations, and/or large uncertainties in intrinsic properties such as water uptake relationships. The IMPROVE monitoring network routinely measures ammonium (NH4+) mass, but not gas-phase ammonia. Gas-phase predictions of ammonia by atmospheric models are not accurate [44], and therefore inappropriate to use as input to the thermodynamic equilibrium is pH-dependent for which accurate constraints are elusive in routine monitoring data [45] Neglect of ammonia in our ALW mass estimates likely underestimates ALW content. Dust contains crustal species (e.g., Ca2+, K+, Mg2+) which can form insoluble species (e.g., CaSO4). Neglect of dust likely overestimates ALW mass. Organic species vary greatly in their effects on ALW [46,47], and detailed chemical characterization of the organic fraction of PM2.5 is not available. Neglect of these species introduces uncertainty, and while absolute mass concentrations are uncertain, we are confident that overall trends are qualitatively robust in space and time [37,40] Laboratory conditions for estimated ALW are 21.5 °C and 35% RH for all sites on all measurement days. These are the central values established for equilibration of FRM PM2.5 filters, and we calculate laboratory ALW estimates for the central values, as an idealized case [31].
3. Results
In 2004, the ratio of ALW mass per measured PM2.5 ‘dry’ mass, average ALW mass concentrations, and water loss upon filter equilibration to standard laboratory conditions are greatest in the Eastern U.S. (Figure 1), where both sulfate mass and RH are relatively high (Figure S3). This year is highlighted in a review of sulfate–health endpoint associations and represents typical conditions that may partly explain discrepancies in the literature among epidemiological and toxicological studies. The pattern observed for 2004 persists throughout the entire IMPROVE record analyzed in this work (Video S1, Video S2), though sulfate concentrations and absolute values of ALW mass concentrations are decreasing over past decades, consistent with findings in the Southeast U.S. [40]. The patterns in ALW and ALW:PM2.5 ratios estimated here are independently consistent with remote optical measurements that demonstrate higher RH-dependent extinction per unit of PM2.5 dry mass in the Eastern U.S. [37]. Note the spatial similarity of the humid Eastern U.S. where these ALW patterns are observed with epidemiology studies that find statistically discernible relationships among sulfate mass concentrations and health endpoints. The locations of all the Harvard six cities are in areas with relatively high ALW mass concentrations, as are other cities such as Detroit [27], New Jersey [28], and Toronto [48]. Locations where statistically robust associations among sulfate and health endpoints are not found, for example in Santa Clara, California and Phoenix, Arizona, are arid and estimated ALW is low (Figure 1). It is noted that nitrate is more hygroscopic than sulfate, but nitrate mass concentrations are generally small (<1 μg m−3 in most locations) and where they are highest, it is in the areas of the U.S. where RH is low (Figure S3). Nitrate impacts on ALW are therefore generally less than for sulfate.
While organic material is lost during FRM sampling and analysis approaches [32,33], loss of water mass is greater. ALW mass is lost during equilibration from ambient to laboratory conditions at nearly all monitoring locations (94%), with the exception of nine sites in the Southwestern U.S. where RH is low (Figure 1b, Figure S3). At all Eastern U.S. sites, throughout the entire analyzed time period, ALW mass is abundant (Figure S4, Video S1), and there is a positive difference between estimated ALW for ambient and lab conditions, (Video S2) indicating systematic ALW evaporation from filters occurs broadly for this region. Average ALW mass concentrations at most IMPROVE monitoring locations in the Eastern U.S. are >9 μg m−3 and lose the equivalent ALW mass concentration of more than 6 μg m−3 upon filter equilibration (Figure 1). This finding suggests that on average for the Eastern U.S. in 2004, ALW contributed 3 μg m−3 to reported PM2.5 ‘dry’ mass. The site with the largest ambient ALW mass concentrations and greatest amount of water loss in 2004 is Martha’s Vineyard in Massachusetts (Table 1, Figure 2). Average ALW loss at Martha’s Vineyard in 2004 was 17.5 μg m−3 or 85% of ambient water mass. ALW mass concentrations in the Eastern U.S. are substantially more abundant than the Western U.S. The majority of western sites exhibit annual average ALW mass concentrations < 3 μg m−3 in 2004. The site with the least amount of water that year is Lava Beds National Monument in Northern California, 0.6 μg m−3 (Table 1, Figure 2). The site with the largest increase in ALW mass during filter equilibration in 2004 is Death Valley National Park in Central California, where it is estimated that 0.2 μg m−3 of ALW was gained during the laboratory filter equilibration (Table 1, Figure 2). The average ambient RH at Death Valley was 28%, which is less than the 35% used for ALW estimates at laboratory conditions, explaining the estimated ALW enhancement.

Table 1.
The 2004 annual average at IMPROVE locations discussed here. Ambient and lab particles are idealized representations to indicate change due to filter equilibrations. Pie chart size is proportional by mass between ambient and lab conditions for an individual location.
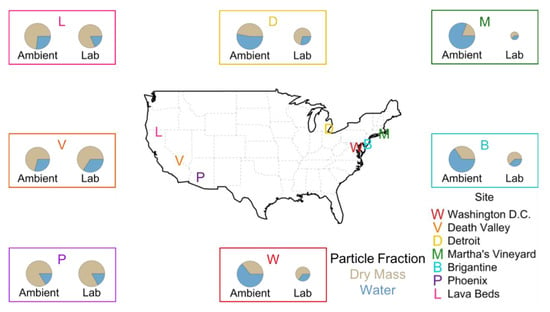
Figure 2.
Differences between ambient and laboratory-equilibrated PM2.5 absolute mass and constituent fractional contributions. Idealized particles are drawn to scale by mass for ambient and laboratory conditions for each city, but not between cities.
Fractionation of total particle mass that is ‘dry’ versus water changes dramatically upon filter equilibration in most locations. For all IMPROVE sites in the Eastern U.S. highlighted in Figure 2, Detroit, Martha’s Vineyard, Brigantine, and Washington D.C., ambient particulate matter mass is predominantly (>50%) liquid water. After equilibration, the dry fraction dominates total mass. Notable exceptions occur in arid regions of the Western U.S. with low RH, such as Death Valley, and Phoenix, AZ (Figure 2) which experienced ALW gain from ambient to filter equilibration conditions. It should be noted that, in part, the intention of standardized laboratory conditions for equilibration of PM2.5 filter samples is to remove liquid water from reported mass values used in attainment considerations. In that respect, standardized mass concentrations normalized to prescribed temperature and RH, works as intended.
In locations where RH and sulfate mass concentrations are abundant, ambient ALW mass concentrations are highest and exhibit the largest temporal variability (Figure 3, Videos S3 and S4). In 2004 in Washington D.C. median ALW mass concentrations vary from < 10 μg m−3 in the winter months to >25 μg m−3 in summer, consistent with decadal seasonal ALW patterns in this region [49] Annual average RH for the region was nearly 80% year round and exhibited little temporal variability relative to sulfate mass. Sulfate mass concentrations increase by a factor of four from summer to winter. Summertime maxima in sulfate mass concentrations in the Eastern U.S. are driven largely by changing SO2 emissions related to electricity demand, which peaks in summer on High Electricity Demand Days (HEDD) [50,51]. Temporal variance (σ = 7.4 μg m−3) in ALW for Washington D.C. in 2004 is relatively large and driven primarily by the strong seasonal trend of sulfate (Figure 3) and not RH. Sufficiently high year-round RH suggests water vapor mixing ratios do not limit ALW formation in that location and that sulfate has the determining effect on ALW mass concentrations. In contrast, in 2004 for Phoenix, AZ, RH and sulfate mass concentrations are lower (e.g., <50% RH and <2 μg m−3). Temporal variance over the year for ALW mass is small (σ = 0.4 μg m−3), in part, because sulfate mass concentrations and RH are out of phase. In Phoenix, RH is greatest in winter when ambient temperatures are low, and sulfate mass concentrations are highest in summer, same as for D.C. This suggests ambient water mixing ratios can sometimes be limiting and exert a determining impact on ALW mass concentrations, in sharp contrast to humid locations such as D.C. where RH is sufficiently high year round to facilitate ALW formation. Note that statistically robust associations between sulfate and health endpoints have been found in Eastern U.S. locations (e.g., Detroit [27], New Jersey [28]) and not in Phoenix, AZ, where sulfate mass concentrations are also substantially lower [6].
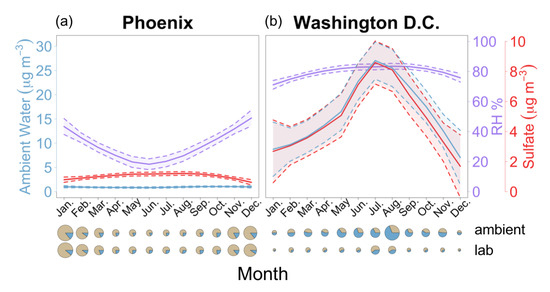
Figure 3.
Mass concentrations of aerosol liquid water and particulate sulfate are abundant and positively associated in Washington D.C., and not Phoenix, AZ in 2004. 2004 monthly mean values of ambient water content, relative humidity, and sulfate (a) (left) Phoenix, Arizona, and (b) (right) Washington D.C. Each variable is presented as the mean (solid line) and 95% confidence interval (shaded area and dotted lines). Pie charts illustrate the monthly average dry mass (brown) and water fractions (blue) for each month in each city at ambient and lab conditions. Pie chart diameter is relative to the largest monthly average mass concentration of each city.
4. Discussion
FRM-collected PM2.5 mass is perturbed from ambient conditions upon filter equilibration. Reported mass concentrations are biased low for most locations, including the 25 largest metropolitan areas in the United States (Table S1). Further, collected PM composition is chemically different from the ambient particulate matter to which individuals are exposed, potentially imparting bias in estimated risk ratios calculated in epidemiology studies that use PM2.5 FRM measurements. Discrepancies in epidemiology studies linking sulfate to health endpoints may be partly explained by mass and chemical differences in ambient vs. laboratory PM2.5 that are induced by evaporation of ALW and organic material. Further, in the absence of plausible toxicological mechanistic explanations for sulfate-induced injury it is important to note that adverse health endpoints associated with ambient sulfate may arise from other particle characteristics not explored here, such as pH or toxic metal oxidation state [8]. In the IMPROVE data set analyzed here, sulfate is correlated with water-soluble transition metals Al, Fe, Pb and Mn (R2 > 0.5) at the IMPROVE site in Lava Beds, CA for 2004 (Table S2). However, for cities in this general location (Santa Clara and Phoenix), where sulfate concentrations are less than 2 μg m−3 year round, statistically robust sulfate–health relationships are not observed in epidemiological studies [29,30]. Another complication is that partitioning of semi-volatile organic material to ALW exhibits a seasonal trend in humid areas [52], indicating that loss of organic material during equilibration is not uniform or easily parameterized from ALW data. Particulate matter in arid Southwestern U.S. locations also contains WSOC that varies by season and is associated with enhanced moisture, temperature, and ozone that may work synergistically to cause health impacts [53].
The absolute and relative amount of ALW loss and bias in FRM-reported PM2.5 changes spatially across the U.S., and for a given location throughout the year (Figure 3). There is not a single bias correction that works for all times and locations. Further, decadal decreases in sulfate and ALW mass concentrations in humid and dry locations, alike, are documented in the literature and traceable to environmental regulations, increasing efficiencies and economic factors (Figures S5 and S6) [54]. Observational evidence in visibility measurements is supportive of sulfate-driven ALW decadal trends [55], in particular in the absence of similar RH trends, suggesting that repeating epidemiology studies in certain locations may change interpretation of sulfate–health endpoint relationships if ALW is included in the analysis. These findings qualitatively translate more broadly to other locations outside of the U.S.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4433/11/2/194/s1, Figure S1: IMPROVE network monitoring locations, Figure S2: Flow chart summarizing the method for calculating aerosol liquid water, Figure S3: 2004 annual average of sulfate mass, nitrate mass, and RH% at all IMMPROVE network monitoring locations, Video S1: GIF of 1988 to 2013 yearly average ALW for IMPROVE monitoring sites, Video S2: GIF of 1988 to 2013 yearly average difference between ambient and lab ALW for IMPROVE monitoring sites, Figure S4: Defined Eastern and Western U.S., Video S3: GIF of 2004 ALW monthly average for IMPROVE monitoring sites, Video S4: GIF of 2004 monthly average difference between ambient and lab ALW for IMPROVE monitoring sites, Table S1: Chemical speciation data and calculations of aerosol liquid water content at ambient and lab conditions for the 25 largest U.S. cities discussed in Reiss et al., Table S2: 2004 annual average correlations of particulate sulfate and transition metals for a subset of cities, Figure S5: U.S. average ambient aerosol liquid water for 2001 to 2013, Figure S6: Yearly average comparing ambient water content to relative humidity, and ambient water to sulfate mass for Phoenix, AZ, and Washington D.C.
Author Contributions
Data curation, V.P.G.; Formal analysis, J.E.B.; Methodology, V.P.G.; Supervision, A.G.C.; Visualization, J.E.B.; Writing—original draft, A.G.C.; Writing—review & editing, A.G.C. and C.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded, in part by NSF grant numbers #1719252 and #1719245, NASA grant number #80NSSC19K0987.
Acknowledgments
Publicly available data from IMPROVE and NARR were necessary for the ALW calculations. Khoi Nguyen and Amy Christiansen contributed to build the ALW dataset.
Conflicts of Interest
The authors declare no conflicts.
References
- Fann, N.; Lamson, A.D.; Anenberg, S.C.; Wesson, K.; Risley, D.; Hubbell, B.J. Estimating the National Public Health Burden Associated with Exposure to Ambient PM2.5 and Ozone. Risk Anal. 2012, 32, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Cunningham, J.; Damokosh, A.I.; Neas, L.M.; Spengler, J.D.; Koutrakis, P.; Ware, J.H.; Raizenne, M.; Speizer, F.E. Health effects of acid aerosols on North American children: Respiratory symptoms. Environ. Health Perspect. 1996, 104, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Raizenne, M.; Neas, L.M.; Damokosh, A.I.; Dockery, D.W.; Spengler, J.D.; Koutrakis, P.; Ware, J.H.; Speizer, F.E. Health effects of acid aerosols on North American children: Pulmonary function. Environ. Health Perspect. 1996, 104, 506–514. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E.N. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef]
- Reiss, R.; Anderson, E.L.; Cross, C.E.; Hidy, G.; Hoel, D.; McClellan, R.; Moolgavkar, S. Evidence of health impacts of sulfate- and nitrate-containing particles in ambient air. Inhal. Toxicol. 2007, 19, 419–449. [Google Scholar] [CrossRef]
- Schlesinger, R.B. The health impact of common inorganic components of fine particulate matter (PM2.5) in ambient air: A critical review. Inhal. Toxicol. 2007, 19, 811–832. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.Y.; Zeng, L.H.; Verma, V.; Nenes, A.; Weber, R.J. Highly Acidic Ambient Particles, Soluble Metals, and Oxidative Potential: A Link between Sulfate and Aerosol Toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Comments on the updated Harvard Six Cities study. Am. J. Respir. Crit. Care Med. 2006, 174, 722–724. [Google Scholar] [CrossRef]
- Baker, K.; Scheff, P. Assessing meteorological variable and process relationships to modeled PM2.5 ammonium nitrate and ammonium sulfate in the central United States. J. Appl. Meteorol. Climatol. 2008, 47, 2395–2404. [Google Scholar] [CrossRef]
- Landis, M.S.; Norris, G.A.; Williams, R.W.; Weinstein, J.P. Personal exposures to PM2.5 mass and trace elements in Baltimore, MD, USA. Atmos. Environ. 2001, 35, 6511–6524. [Google Scholar] [CrossRef]
- Goldman, G.T.; Mulholland, J.A.; Russell, A.G.; Srivastava, A.; Strickland, M.J.; Klein, M.; Waller, L.A.; Tolbert, P.E.; Edgerton, E.S. Ambient Air Pollutant Measurement Error: Characterization and Impacts in a Time-Series Epidemiologic Study in Atlanta. Environ. Sci. Technol. 2010, 44, 7692–7698. [Google Scholar] [CrossRef] [PubMed]
- Carlton, A.G.; Pye, H.O.T.; Baker, K.R.; Hennigan, C.J. Additional benefits of federal air-quality rules: Model estiamtes of controllable biogenic secondary organic aerosol. Environ. Sci. Technol. 2018, 52, 9254–9265. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.V.; Zhang, Q.; Jimenez, J.L.; Pike, M.; Carlton, A.G. Liquid Water: Ubiquitous Contributor to Aerosol Mass. Environ. Sci. Technol. Lett. 2016, 3, 257–263. [Google Scholar] [CrossRef]
- Noble, C.A.; Vanderpool, R.W.; Peters, T.M.; McElroy, F.F.; Gemmill, D.B.; Wiener, R.W. Federal reference and equivalent methods for measuring fine particulate matter. Aerosol Sci. Technol. 2001, 34, 457–464. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Jayne, J.T.; Shi, Q.; Kolb, C.E.; Worsnop, D.R.; Yourshaw, I.; Seinfeld, J.H.; Flagan, R.C.; Zhang, X.F.; Smith, K.A.; et al. Ambient aerosol sampling using the Aerodyne Aerosol Mass Spectrometer. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Carlton, A.G.; Turpin, B.J. Particle partitioning potential of organic compounds is highest in the Eastern US and driven by anthropogenic water. Atmos. Chem. Phys. 2013, 13, 10203–10214. [Google Scholar] [CrossRef]
- Faust, J.A.; Wong, J.P.S.; Lee, A.K.Y.; Abbatt, J.P.D. Role of Aerosol Liquid Water in Secondary Organic Aerosol Formation from Volatile Organic Compounds. Environ. Sci. Technol. 2017, 51, 1405–1413. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Coggon, M.M.; Bates, K.H.; Zhang, X.; Schwantes, R.H.; Schilling, K.A.; Loza, C.L.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Organic aerosol formation from the reactive uptake of isoprene epoxydiols (IEPOX) onto non-acidified inorganic seeds. Atmos. Chem. Phys. 2014, 14, 3497–3510. [Google Scholar] [CrossRef]
- Lin, Y.H.; Arashiro, M.; Clapp, P.W.; Cui, T.Q.; Sexton, K.G.; Vizuete, W.; Gold, A.; Jaspers, I.; Fry, R.C.; Surratt, J.D. Gene Expression Profiling in Human Lung Cells Exposed to Isoprene-Derived Secondary Organic Aerosol. Environ. Sci. Technol. 2017, 51, 8166–8175. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, M.; Lin, Y.H.; Zhang, Z.F.; Sexton, K.G.; Gold, A.; Jaspers, I.; Fry, R.C.; Surratt, J.D. Effect of secondary organic aerosol from isoprene-derived hydroxyhydroperoxides on the expression of oxidative stress response genes in human bronchial epithelial cells. Environ. Sci. Process. Impacts 2018, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Fang, T.; Xu, L.; Peltier, R.E.; Russell, A.G.; Ng, N.L.; Weber, R.J. Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM2.5. Environ. Sci. Technol. 2015, 49, 4646–4656. [Google Scholar] [CrossRef] [PubMed]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Global Perspective on the Oxidative Potential of Airborne Particulate Matter: A Synthesis of Research Findings. Environ. Sci. Technol. 2014, 48, 7576–7583. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Guo, H.; Verma, V.; Peltier, R.E.; Weber, R.J. PM2.5 water-soluble elements in the southeastern United States: Automated analytical method development, spatiotemporal distributions, source apportionment, and implications for heath studies. Atmos. Chem. Phys. 2015, 15, 11667–11682. [Google Scholar] [CrossRef]
- Sisler, J.; Malm, W. The relative importance of soluble aerosols to spacial and seasonal trends of impaired visibility in the United States. Atmos. Environ. 1994, 28, 851–862. [Google Scholar] [CrossRef]
- Lippmann, M.; Ito, K.; Nadas, A.; Burnett, R.T. Association of particulate matter components with daily mortality and morbidity in urban populations. Res. Rep. Health Eff. Inst. 2000, 95, 5–72, discussion 73–82. [Google Scholar]
- Tsai, F.C.; Apte, M.G.; Daisey, J.M. An exploratory analysis of the relationship between mortality and the chemical composition of airborne particulate matter. Inhal. Toxicol. 2000, 12, 121–135. [Google Scholar] [CrossRef]
- Fairley, D. Daily mortality and air pollution in Santa Clara County, California: 1989–1996. Environ. Health Perspect. 1999, 107, 637–641. [Google Scholar] [CrossRef]
- Mar, T.F.; Norris, G.A.; Koenig, J.Q.; Larson, T.V. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ. Health Perspect. 2000, 108, 347–353. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Quality Assurance Guidance Document 2.12; Office of Air Quality Planning And Standards Air Quality Assessment Division: Research Triangle Park, NC, USA, 2016.
- Eatough, D.J.; Wadsworth, A.; Eatough, D.A.; Crawford, J.W.; Hansen, L.D.; Lewis, E.A. A multiple-system, multichannel diffusion denuder sampler for the determination of fine particulate organic material in the atmosphere. Atmos. Environ. 1993, 27, 1213–1219. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J.; Hering, S.V. Investigation of organic aerosol sampling artifacts in the los angeles basin. Atmos. Environ. 1994, 28, 3061–3071. [Google Scholar]
- El-Sayed, M.M.H.; Amenumey, D.; Hennigan, C.J. Drying-Induced Evaporation of Secondary Organic Aerosol during Summer. Environ. Sci. Technol. 2016, 50, 3626–3633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Hecobian, A.; Zheng, M.; Frank, N.H.; Edgerton, E.S.; Weber, R. Spatial and seasonal variations of fine particle water-soluble organic carbon (WSOC) over the southeastern United States: Implications for secondary organic aerosol formation. J. Atmos. Chem. Phys. 2012, 12, 6593–6607. [Google Scholar] [CrossRef]
- Pöschl, U.; Shiraiwa, M. Chemical Reviews; American Chemical Society: Washington, DC, USA, 2015; pp. 4440–4475. [Google Scholar]
- Christiansen, A.E.; Ghate, V.P.; Carlton, A.G. Aerosol Optical Thickness: Organic Composition, Associated Particle Water, and Aloft Extinction. ACS Earth Space Chem. 2019, 3, 403–412. [Google Scholar] [CrossRef]
- Van Loy, M.; Bahadori, T.; Wyzga, R.; Edgerton, B.H.E. The aerosol research and inhalation epidemiology study (ARIES): PM2.5 mass and aerosol component concentrations and sampler intercomparisons. J. Air Waste Manag. Assoc. 2000, 50, 1446–1458. [Google Scholar]
- National Park Service. Improve—Interagency Monitoring of Protected Visual Environments. 2000. Available online: http://vista.cira.colostate.edu/improve/ (accessed on 13 July 2015).
- Nguyen, T.K.V.; Capps, S.L.; Carlton, A.G. Decreasing Aerosol Water Is Consistent with OC Trends in the Southeast US. Environ. Sci. Technol. 2015, 49, 7843–7850. [Google Scholar] [CrossRef]
- IMPROVE Public Archive. Available online: http://views.cira.colostate.edu/fed/DataWizard/Default.aspx (accessed on 13 July 2015).
- Mesinger, F.; DiMego, G.; Kalnay, E.; Mitchell, K.; Shafran, P.C.; Ebisuzaki, W.; Jovic, D.; Woollen, J.; Rogers, E.; Berbery, E.H.; et al. North American regional reanalysis. Bull. Am. Meteorol. Soc. 2006, 87, 343. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+-Ca2+-Mg2+-Nh4+-Na+-SO42−-NO3−-Cl−-H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef]
- Moravek, A.; Murphy, J.G.; Hrdina, A.; Lin, J.C.; Pennell, C.; Franchin, A.; Middlebrook, A.M.; Fibiger, D.L.; Womack, C.C.; McDuffie, E.E.; et al. Wintertime spatial distribution of ammonia and its emission sources in the Great Salt Lake region. Atmos. Chem. Phys. 2019, 19, 15691–15709. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Nenes, A.; Alexander, B.; Ault, A.P.; Barth, M.C.; Clegg, S.L.; Collett, J.L.; Fahey, K.M.; Hennigan, C.J.; Herrmann, H.; et al. The Acidity of Atmospheric Particles and Clouds. Atmos. Chem. Phys. Discuss. 2019. [Google Scholar] [CrossRef]
- Petters, M.D.; Kreidenweis, S.M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 2007, 7, 1961–1971. [Google Scholar]
- Chan, M.N.; Choi, M.Y.; Ng, N.L.; Chan, C.K. Hygroscopicity of water-soluble organic compounds in atmospheric aerosols: Amino acids and biomass burning derived organic species. Environ. Sci. Technol. 2005, 39, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.T.; Stieb, D.; Brook, J.R.; Cakmak, S.; Dales, R.; Raizenne, M.; Vincent, R.; Dann, T. Associations between short-term changes in nitrogen dioxide and mortality in Canadian cities. Arch. Environ. Health 2004, 59, 228–236. [Google Scholar]
- Nguyen, T.K.V.; Ghate, V.P.; Carlton, A.G. Reconciling satellite aerosol optical thickness and surface fine particle mass through aerosol liquid water. Geophys. Res. Lett. 2016, 43, 11903–11912. [Google Scholar] [CrossRef]
- Farkas, C.M.; Moeller, M.D.; Felder, F.A.; Baker, K.R.; Rodgers, M.; Carlton, A.G. Temporalization of Peak Electric Generation Particulate Matter Emissions during High Energy Demand Days. Environ. Sci. Technol. 2015, 49, 4696–4704. [Google Scholar] [CrossRef]
- Farkas, C.M.; Moeller, M.D.; Felder, F.A.; Henderson, B.H.; Carlton, A.G. High Electricity Demand in the Northeast US: PJM Reliability Network and Peaking Unit Impacts on Air Quality. Environ. Sci. Technol. 2016, 50, 8375–8384. [Google Scholar] [CrossRef]
- El-Sayed, M.M.H.; Ortiz-Montalvo, D.L.; Hennigan, C.J. The effects of isoprene and NOx on secondary organic aerosols formed through reversible and irreversible uptake to aerosol water. Atmos. Chem. Phys. 2018, 18, 1171–1184. [Google Scholar] [CrossRef]
- Youn, J.-S.; Wang, Z.; Wonaschütz, A.; Arellano, A.; Betterton, E.A.; Sorooshian, A. Evidence of aqueous secondary organic aerosol formation from biogenic emissions in the North American Sonoran Desert. Geophys. Res. Lett. 2013, 40, 3468–3472. [Google Scholar] [CrossRef]
- Carlton, A.G.; Gouw, J.A.d.; Jimenez, J.L.; Ambrose, J.L.; Attwood, A.R.; Brown, S.; Baker, K.R.; Brock, C.; Cohen, R.C.; Edgerton, S.; et al. Synthesis of the Southeast Atmosphere Studies: Investigating fundamental atmospheric chemistry questions. Bull. Am. Meteorol. Soc. 2018, 99, 547–5567. [Google Scholar] [CrossRef]
- Li, C.; Martin, R.V. Decadal Changes in Seasonal Variation of Atmospheric Haze over the Eastern United States: Connections with Anthropogenic Emissions and Implications for Aerosol Composition. Environ. Sci. Technol. Lett. 2018, 5, 413–418. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).