Chemometric Study of the Correlation between Human Exposure to Benzene and PAHs and Urinary Excretion of Oxidative Stress Biomarkers
Abstract
1. Introduction
2. Experiments
2.1. Study Population
2.2. Urine Sample Collection
2.3. Chemicals and Supplies
2.4. Analysis of Urine Samples
2.5. Statistical Analysis
3. Results and Discussion
3.1. Descriptive Statistics
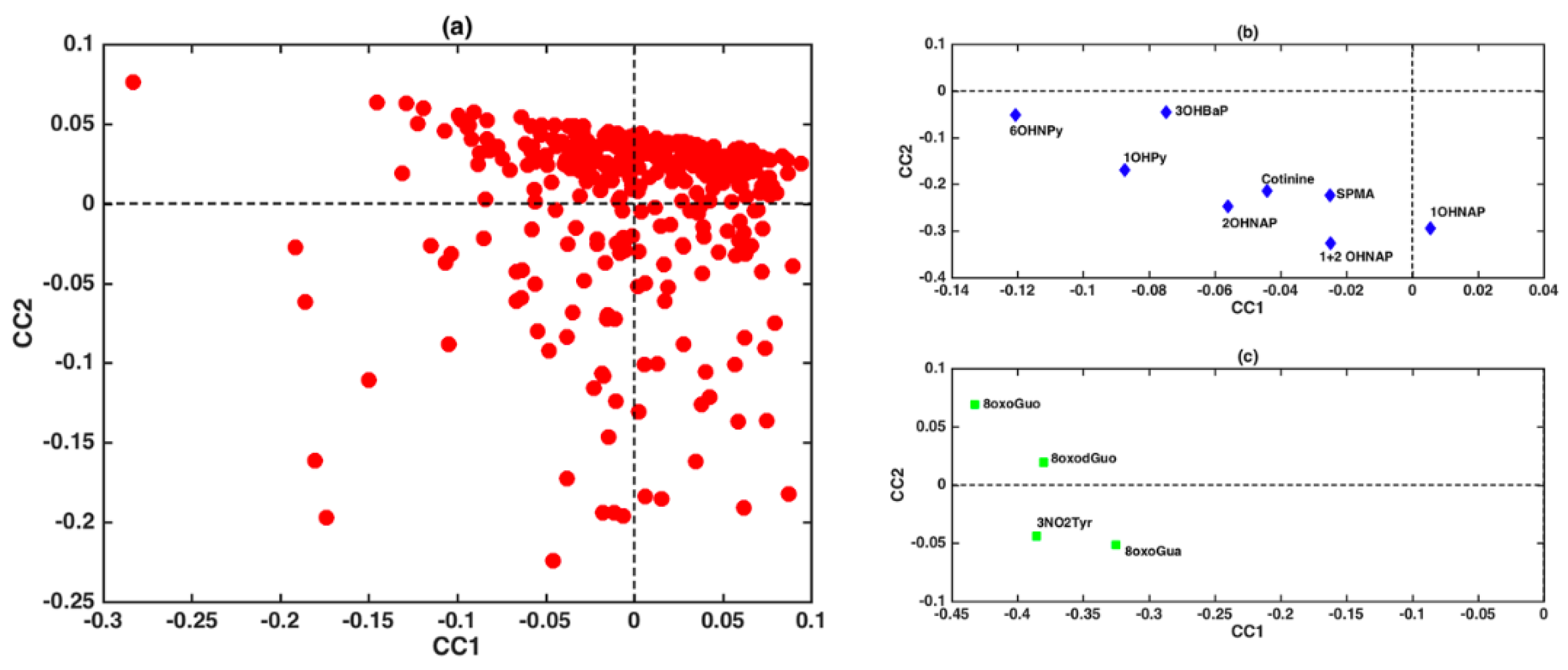
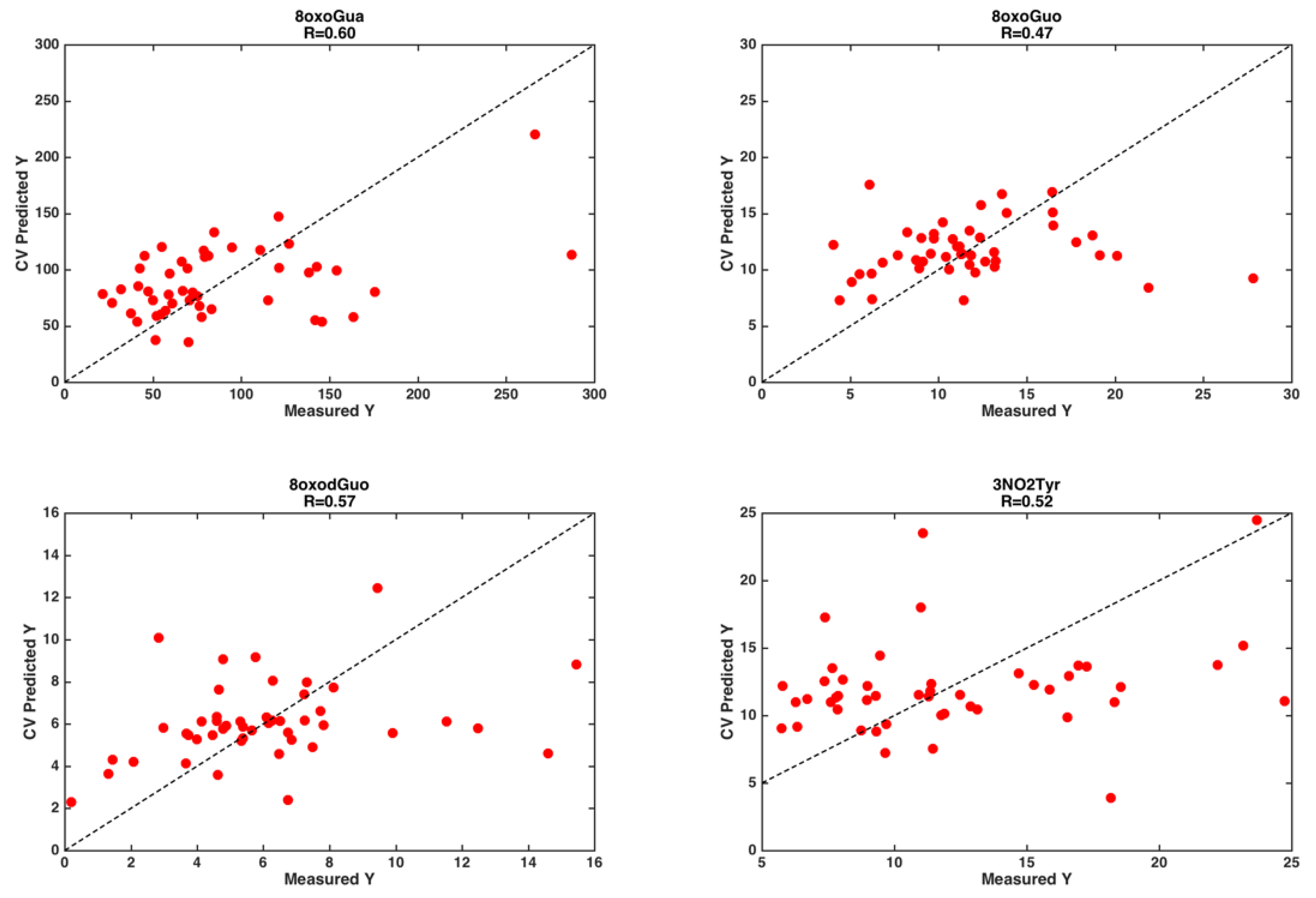
3.2. Chemometrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Air Pollution Levels Rising in Many of the World’s Poorest Cities. 2016, pp. 8–10. Available online: http://www.who.int/mediacentre/news/releases/2016/air-pollution-rising/en/ (accessed on 9 November 2020).
- International Agency for Research on Cancer. IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Outdoor air pollution. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 9–562. [Google Scholar]
- Luo, K.; Stepanov, I.; Hecht, S.S. Chemical biomarkers of exposure and early damage from potentially carcinogenic airborne pollutants. Ann. Cancer Epidemiol. 2019, 3, 5. [Google Scholar] [CrossRef]
- Ancona, C.; Bauleo, L.; Biscotti, G.; Bocca, B.; Caimi, S.; Cruciani, F.; Di Lorenzo, S.; Petrolati, M.; Pino, A.; Piras, G.; et al. A survey on lifestyle and level of biomarkers of environmental exposure in residents in Civitavecchia (Italy). Ann. Ist. Super. Sanita 2016, 52, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Chesler, R.; Hagengruber, C.; Blank, D.W.; Kestner, J.; Rawe, M. Automated determination of urinary creatinine without sample dilution: Theory and practice. Clin. Chem. 1986, 32, 446–452. [Google Scholar] [CrossRef] [PubMed]
- ACGIH. Documentation of the threshold limit values (TLVs) and biological exposure indices (BEIs). TLVs BEIs. Threshold. Limit Values Chem. Subst. Phys. Agents Biol. Expo. Indices 2014. [Google Scholar]
- Paci, E.; Pigini, D.; Bauleo, L.; Ancona, C.; Forastiere, F.; Tranfo, G. Urinary cotinine concentration and self-reported smoking status in 1075 subjects living in central Italy. Int. J. Environ. Res. Public Health 2018, 15, 804. [Google Scholar] [CrossRef] [PubMed]
- Tranfo, G.; Pigini, D.; Paci, E.; Marini, F.; Bonanni, R.C. Association of exposure to benzene and smoking with oxidative damage to nucleic acids by means of biological monitoring of general population volunteers. Environ. Sci. Pollut. Res. 2017, 24, 13885–13894. [Google Scholar] [CrossRef] [PubMed]
- Raponi, F.; Bauleo, L.; Ancona, C.; Forastiere, F.; Paci, E.; Pigini, D.; Tranfo, G. Quantification of 1-hydroxypyrene, 1- and 2-hydroxynaphthalene, 3-hydroxybenzo[a]pyrene and 6-hydroxynitropyrene by HPLC-MS/MS in human urine as exposure biomarkers for environmental and occupational surveys. Biomarkers 2017, 22, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, R.; Manini, P.; De Palma, G.; Alinovi, R.; Goldoni, M.; Niessen, W.M.A.; Mutti, A. Quantitative determination of urinary 8-oxo-7,8-dihydro-2′- deoxyguanosine, 8-oxo-7,8-dihydroguanine, 8-oxo-7,8-dihydroguanosine, and their non-oxidized forms: Daily concentration profile in healthy volunteers. Biomarkers 2010. [Google Scholar] [CrossRef] [PubMed]
- Mazerolles, G.; Hanafi, M.; Dufour, E.; Bertrand, D.; Qannari, E.M. Common components and specific weights analysis: A chemometric method for dealing with complexity of food products. Chemom. Intell. Lab. Syst. 2006. [Google Scholar] [CrossRef]
- Martens, H.; Naes, T. Multivariate Calibration; Wiley: New York, NY, USA, 1992; ISBN 978-0-471-93047-1. [Google Scholar]
- Andreoli, R.; Mutti, A.; Goldoni, M.; Manini, P.; Apostoli, P.; De Palma, G. Reference ranges of urinary biomarkers of oxidized guanine in (2′-deoxy)ribonucleotides and nucleic acids. Free Radic. Biol. Med. 2011, 50, 254–261. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC monographs on the evaluation of carcinogenic risks to humans: Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. Iarc Monogr. Eval. Carcinog. Risks Humans 2010, 92, 1. [Google Scholar]
- Tombolini, F.; Pigini, D.; Tranfo, G.; Paci, E.; Carosi, I.; Marini, F.; Bauleo, L.; Ancona, C.; Forastiere, F. Levels of urinary metabolites of four PAHs and cotinine determined in 1016 volunteers living in Central Italy. Environ. Sci. Pollut. Res. 2018, 25, 28772–28779. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Sex | Total | |
|---|---|---|---|
| Male | Female | ||
| All subjects | 188 (44.1%) | 238 (55.9%) | 426 |
| Smokers | 46 | 80 | 126 |
| Non-smokers | 142 | 158 | 300 |
| BMI (kg/m2)-median | 28.01 | 25.23 | 26.47 |
| All Subjects n. 426 | µg/g Creatinine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8-oxoGua | 8-oxoGuo | 8-oxodGuo | SPMA | Cotinine | 1-OHPy | 6-OHNPy | 3-OHBaP | 1-OHNAP | 2-OHNAP | 1 + 2 OHNAP | |
| Mean (STD) | 55.21 (38.19) | 9.70 (5.55) | 5.01 (3.99) | 0.75 (1.50) | 807.29 (2262.8) | 0.06 (0.13) | 0.06 (0.28) | 4.91 × 10−3 (0.02) | 3.44 (6.14) | 3.18 (4.93) | 6.62 (9.16) |
| 5th | 13.80 | 2.64 | 0.45 | 3.45 × 10−3 | 1.87 | 9.69 × 10−5 | 5.85 × 10−5 | 1.32 × 10−5 | 9.03 × 10−4 | 3.64 × 10−3 | 0.01 |
| 50th | 45.37 | 8.86 | 4.40 | 0.17 | 11.38 | 0.01 | 1.70 × 10−4 | 3.30 × 10−5 | 1.36 | 1.44 | 3.47 |
| 95th | 126.88 | 20.11 | 11.98 | 3.50 | 3847.49 | 0.32 | 0.42 | 0.04 | 15.25 | 11.55 | 25.41 |
| Smokers n. 126 | 8-oxoGua | 8-oxoGuo | 8-oxodGuo | SPMA | Cotinine | 1-OHPy | 6-OHNPy | 3-OHBaP | 1-OHNAP | 2-OHNAP | 1 + 2 OHNAP |
| Mean (STD) | 55.16 (39.51) | 9.21 (5.38) | 4.84 (3.54) | 2.11 (2.21) | 2702.59 (3502.4) | 0.12 (0.17) | 0.07 (0.24) | 0.01 (0.02) | 6.73 (7.36) | 5.61 (6.31) | 12.33 (11.16) |
| 5th | 16.48 | 2.39 | 0.31 | 0.13 | 287.91 | 8.05 × 10−5 | 4.99 × 10−5 | 1.16 × 10−5 | 9.83 × 10−4 | 3.25 × 10−3 | 0.01 |
| 50th | 44.67 | 8.27 | 4.60 | 1.49 | 1359.88 | 0.06 | 1.41 × 10−4 | 2.61 × 10−5 | 4.15 | 4.05 | 10.15 |
| 95th | 131.88 | 19.14 | 10.99 | 6.07 | 9529.08 | 0.44 | 0.64 | 0.03 | 22.77 | 14.24 | 32.44 |
| Non-smokers n. 300 | 8-oxoGua | 8-oxoGuo | 8-oxodGuo | SPMA | Cotinine | 1-OHPy | 6-OHNPy | 3-OHBaP | 1-OHNAP | 2-OHNAP | 1 + 2 OHNAP |
| Mean (STD) | 55.23 (37.69) | 9.91 (5.62) | 5.08 (4.17) | 0.17 (0.23) | 11.27 (13.09) | 0.04 (0.10) | 0.05 (0.29) | 4.61E-03 (0.02) | 4.94 (3.94) | 2.80 (3.80) | 4.22 (6.91) |
| 5th | 12.63 | 2.87 | 0.48 | 3.07 × 10−3 | 1.58 | 1.01 × 10−4 | 6.63 × 10−5 | 1.50 × 10−5 | 8.96 × 10−4 | 3.93 × 10−3 | 0.02 |
| 50th | 45.87 | 9.01 | 4.31 | 0.11 | 5.93 | 0.01 | 1.84 × 10−4 | 3.57 × 10−5 | 0.94 | 0.95 | 2.61 |
| 95th | 124.96 | 20.14 | 12.63 | 0.69 | 39.75 | 0.17 | 0.09 | 0.04 | 6.24 | 8.13 | 12.07 |
| All Subjects n. 322 | Smokers n. 102 | Non-Smokers n. 220 | |
|---|---|---|---|
| Mean (STD) | 8.07 (4.15) | 8.24 (4.67) | 7.98 (3.90) |
| 5th perc. | 3.01 | 3.01 | 3.02 |
| 50th perc. | 7.34 | 7.38 | 7.29 |
| 95th perc. | 15.73 | 17.24 | 14.80 |
| 8-oxoGua | 8-oxoGuo | 8-oxodGuo | SPMA | Cotinine | 1-OHPy | 6-OHNPy | 3-OHBaP | 1-OHNAP | 2-OHNAP | 1 + 2 OHNAP | 3NO2Tyr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | n. 238 | n. 181 | ||||||||||
| Mean | 62.63 ** | 10.27 * | 5.30 | 0.96 ** | 1104.28 ** | 0.08 ** | 0.07 * | 0.01 * | 4.51 ** | 3.96 * | 8.46 ** | 8.90 ** |
| STD | 42.39 | 6.05 | 4.51 | 1.73 | 2826.79 | 0.15 | 0.33 | 0.02 | 7.53 | 5.8 | 10.99 | 4.53 |
| Males | n. 188 | n. 141 | ||||||||||
| Mean | 46.00 | 8.99 | 4.65 | 0.47 | 431.27 | 0.04 | 0.04 | 0.004 | 2.09 | 2.210 | 4.29 | 6.99 |
| STD | 29.63 | 4.78 | 3.20 | 1.10 | 1120.51 | 0.09 | 0.19 | 0.02 | 3.25 | 3.32 | 5.29 | 3.32 |
| Age | BMI | 8oxoGua | 8oxoGuo | 8oxodGuo | 3NO2Tyr | Cotinine | SPMA | 1OHPy | 6OHNPy | 3OHBaP | 1OHNAP | 2OHNAP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.154 | ||||||||||||
| BMI | 0.201 | ||||||||||||
| 8oxoGua | 0.255 | −0.010 | |||||||||||
| 8oxoGuo | 0.145 | −0.003 | 0.335 | ||||||||||
| 8oxodGuo | 0.077 | −0.009 | 0.212 | 0.840 | |||||||||
| 3NO2Tyr | 0.160 | −0.019 | 0.633 | 0.623 | 0.493 | ||||||||
| Cotinine | −0.084 | −0.038 | 0.138 | −0.001 | 0.035 | 0.134 | |||||||
| SPMA | −0.049 | −0.027 | 0.060 | −0.069 | −0.076 | 0.085 | 0.638 | ||||||
| 1OHPy | 0.010 | 0.057 | 0.283 | 0.223 | 0.227 | 0.258 | 0.344 | 0.069 | |||||
| 6OHNPy | 0.122 | 0.022 | 0.306 | 0.257 | 0.258 | 0.328 | 0.117 | −0.053 | 0.441 | ||||
| 3OHBaP | 0.040 | 0.030 | −0.168 | 0.112 | 0.224 | −0.256 | 0.059 | −0.020 | 0.286 | 0.275 | |||
| 1OHNAP | −0.086 | −0.058 | 0.061 | −0.147 | −0.114 | −0.020 | 0.453 | 0.428 | 0.108 | −0.011 | −0.115 | ||
| 2OHNAP | −0.119 | 0.034 | 0.015 | 0.040 | 0.120 | 0.073 | 0.402 | 0.232 | 0.303 | 0.262 | 0.159 | 0.240 | |
| 1 + 2 OHNAP | −0.088 | 0.047 | 0.105 | −0.013 | 0.019 | 0.039 | 0.371 | 0.270 | 0.241 | 0.181 | 0.069 | 0.848 | 0.622 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonaurio, F.; Paci, E.; Pigini, D.; Marini, F.; Bauleo, L.; Ancona, C.; Tranfo, G. Chemometric Study of the Correlation between Human Exposure to Benzene and PAHs and Urinary Excretion of Oxidative Stress Biomarkers. Atmosphere 2020, 11, 1341. https://doi.org/10.3390/atmos11121341
Buonaurio F, Paci E, Pigini D, Marini F, Bauleo L, Ancona C, Tranfo G. Chemometric Study of the Correlation between Human Exposure to Benzene and PAHs and Urinary Excretion of Oxidative Stress Biomarkers. Atmosphere. 2020; 11(12):1341. https://doi.org/10.3390/atmos11121341
Chicago/Turabian StyleBuonaurio, Flavia, Enrico Paci, Daniela Pigini, Federico Marini, Lisa Bauleo, Carla Ancona, and Giovanna Tranfo. 2020. "Chemometric Study of the Correlation between Human Exposure to Benzene and PAHs and Urinary Excretion of Oxidative Stress Biomarkers" Atmosphere 11, no. 12: 1341. https://doi.org/10.3390/atmos11121341
APA StyleBuonaurio, F., Paci, E., Pigini, D., Marini, F., Bauleo, L., Ancona, C., & Tranfo, G. (2020). Chemometric Study of the Correlation between Human Exposure to Benzene and PAHs and Urinary Excretion of Oxidative Stress Biomarkers. Atmosphere, 11(12), 1341. https://doi.org/10.3390/atmos11121341









