Abstract
Temporal rainwater chemistry was used to reveal air pollution in the Maolan National Karst Forest Park (MNKFP), which is representative of the typical karst forest region of southwest China (SW China). The rainwater ions’ sources, variations, trends, and potential environmental effects were investigated from 2007 to 2010 and from 2013 to 2014. Based on the analysis of the temporal ionic concentrations of rainwater in the MNKFP, significant variations of ions were observed, including in NH4+ (9.7~266.6 μeq L−1) and SO42− (14.5~1396.4 μeq L−1), which were mainly controlled by variations in the source and rainfall amount; a decreased trend of rainwater pH was also observed. Accordingly, NH4+, Ca2+, SO42−, and Cl− were regarded as the most dominant ions. Typical ionic ratios and positive matrix factorization (PMF) model-based source apportionment suggested that anthropogenic inputs (coal combustion, industrial, traffic, and agricultural emissions) contributed 51% of F−, 93% of NO3−, 62% of SO42−, and 87% of NH4+, while the natural sources (crustal dust and sea salt) were the main sources of Cl− (74%), Na+ (82%), K+ (79%), Mg2+ (94%), and Ca2+ (93%). In combination with the reducing neutralization trend of temporal rainwater observed in the MNKFP and the potential effect of rainwater ion deposition on karst forests, more detailed monitoring of the rainfall-related deposition process is required for a better understanding of its potential environmental effects on the Earth’s surface.
1. Introduction
Rainwater is the most important sink of air pollutants, such as acid gases and particulate matter (PM) [1,2,3,4]. In-cloud and below-cloud scavenging processes are the keys to the removal of air contaminants during a whole rainfall event [5,6], and the rainwater pH and chemical species are changed concomitantly [7,8,9]. Moreover, investigating rainwater chemical composition is helpful for understanding air quality, which can be used to explore the origins of air contaminants based on the different physical and chemical processes of these contaminants [10,11,12]. Typically, previous studies have classified the sources of rainwater chemical components (major ions) into three types: sea-salt input (marine sources), terrestrial dust (crustal sources), and human-made emissions (anthropogenic sources) [13,14,15]. In addition to source variations, factors affecting the chemical composition of rainwater include geomorphic situations, meteorological conditions, and environmental policies [4,16,17].
In southwest China, an evident heterogeneity of rainwater chemistry has been observed, in particular in karst landform areas [3,6,18]; the most widely distributed karst area is in Guizhou Province [19,20,21]. Since the end of the last century, this area has been regarded as an acid rain control area with a high level of acid deposition [7], and many governmental emission reduction measures have been implemented to reduce rainwater-acidifying potential and to increase neutralizing potential [22]. As one of the most sensitive terrestrial ecosystems, the karst ecosystem has responded strongly to rainfall (especially acid rain) due to the severe karstification [23,24,25,26,27]. Acidified rainwater not only facilitates the migration of materials (from the surface to underground) but also accelerates the weathering process of carbonate rocks and causes intense soil erosion in a karst ecosystem. On the other hand, the weathering products (mainly calcium and magnesium) provide a source of alkaline matter for the neutralization process of rainwater [3,28]. Moreover, rainwater is an important source of nutrients (e.g., nitrogen, phosphorus, and potassium) for some barren karst ecosystems (e.g., a karst forest ecosystem) due to the weak capacity of the soil for nutrient retention [18,29].
Forests are important parts of terrestrial ecosystems and are regarded as the Earth’s lungs. However, the structure and function of a forest system is significantly disturbed by atmosphere-related pollution and accompanying acid deposition [8,30,31,32,33,34,35]. Chemistry-varied rainwater can further impact biogeochemical cycles via chemical elemental redistribution in forest ecosystems [2,32]. For example, rainwater-related ion deposition significantly changes a land’s carbon cycle (e.g., the soil respiration process) [36,37]. Previous studies took the Maolan National Karst Forest Park (MNKFP), the most typical karst forest in the region of southwest China (SW China), as a case to investigate rainwater chemistry based on strontium and calcium isotope constraints, and they reported on the qualitative source identification and short-term material source of rainwater [8,38]. However, only a long-term variation in the chemical components of rainwater can provide more comprehensive information on the temporal evolution of air contamination (especially local air quality), and such variation can be further applied as an indicator to assess the influences of anthropogenic and natural inputs. To our knowledge, the relatively long-term measurement-based studies on rainwater chemistry in the MNKFP have been very limited, and temporal variations in the acidifying and neutralizing potential of rainwater are also unclear in the karst forest ecosystem. Moreover, the quantitative contribution (e.g., model-based source identification) of the different sources of the rainwater components in the MNKFP has been rarely studied.
To advance the information on rainwater chemistry and its potential environmental effects in the karst forest region, the present study conducted a systematic investigation in the MNKFP based on temporal rainwater chemistry (2007–2010 and 2013–2014). The key objectives were to: (1) clarify the ionic composition of rainwater in a karst forest, (2) explore the temporal variations of rainwater ion compositions and trends in terms of acidifying and neutralizing potentials, (3) identify the source contribution of related ions, and (4) discuss the potential environmental effects of rainfall-related ion deposition on the forest ecosystem.
2. Materials and Methods
2.1. Study Area and Sampling
The MNKFP is the most representative of the karst forest landscape in the subtropical region of China. It has a landmass of 213 km2 and is located in one of three globally continuous larger karst areas (southwest China karst; Figure 1) [16]. This park is distinctly encircled by a subtropical virgin evergreen forest with a forest coverage of 88.6% (elevation is 430~1078 m). Since 1996, the MNKFP has been included in the World Biosphere Reserves Network and the World Heritage List due to the primitiveness of the forest vegetation and the richness of fauna. According to government statistics, there are 2199 species of higher plants in 241 families and 870 genera in the MNKFP, such as Taxus chinensis and Incense cedar. Karst geomorphology, including karst funnels, karst depressions, karst valleys (basin), karst trough valleys, and karst hydrological landscapes, is widespread in the MNKFP. The lithology of the MNKFP is controlled by the shallow marine carbonate with sporadically distributed sandstone (Figure 1). The MNKFP is characterized by a subtropical monsoon climate (average air temperature of 15.3 °C). The wet season (from April to September) is generally the season when major rainfall events happen (~80%) [38]; the annual rainfall amount is 1750 mm.
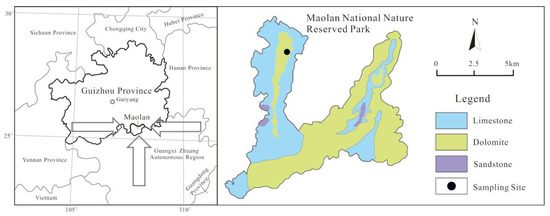
Figure 1.
Background of the sampling site in the Maolan National Karst Forest Park (MNKFP).
The rainwater sampling site was selected in the northwest of the MNKFP (Figure 1). Polyethylene (PE) samplers (diameter of approximately 65 cm and volume of 20 L) were manually installed to sample the rainwater, and they were set 12 m apart over the land’s surface. A lid was also used to avoid deposited dust on non-rainy days. The samplers were thoroughly washed with ultrapure water after each rainfall event. The filtration of the rainwater samples was completed using Millipore membrane filters (0.22 μm, acetate membrane, MILLIPORE, Billerica, MA, USA). After filtration, the samples were stored in a clean PE bottle and kept refrigerated at 4 °C. From 2007 to 2010, a total of 104 daily rainwater samples were collected [8,38]. From August 2013 to June 2014, nine monthly mixed samples were obtained, and these were proportionally mixed based on the corresponding rainfall amount of each rainfall event in different months. The analytical data of the mixed sample represent the monthly weighted-mean values [39].
2.2. Measurement and Quality Control
The pH values of daily rainwater samples were measured by portable multiple parameter meters after collection. Both the daily rainwater samples and the monthly mixed samples were separated and saved in two clean PE bottles for detecting contents of anions and cations (nitric acid acidified, pH < 2), respectively. The pre-cleaned procedure of PE bottles was taken from the literature [40]. Ion chromatography (IC, Dionex DX-120, DIONEX, Sunnyvale, CA, USA) with an anion column (IonPac AS23, DIONEX, Sunnyvale, CA, USA) was applied to detect the major anion contents, including Cl−, F−, NO3−, and SO42−. The detection limits of these anions were 0.04, 0.03, 0.06, and 0.10 mg L−1, respectively. The inductively coupled plasma atomic emission spectroscopy (ICP-AES, Thermo Scientific IRIS Intrepid-II, Thermo Fisher Scientific, Waltham, MA, USA) was applied to measure the major cation contents of K+, Na+, Ca2+, and Mg2+. The detection limits of these cations were 0.01, 0.03, 0.04, and 0.01 mg L−1, respectively. The NH4+ concentration of the daily rainwater samples was determined by the spectrophotometer (Nessler method) with a detection limit of 0.02 mg L−1. For quality control, the measurement was performed with the replicates, standard reference materials, and procedural blanks. In brief, the measured replicate samples implied an acceptable repeatability for all ions (within 5%). The standard reference material (GB W08606, National Research Center for Certified Reference Materials, China) was regularly applied to ensure quality assurance for major ion analysis. Furthermore, the procedural blanks of detected ions were below the detection limit or <5% in the rainwater samples, indicating a reliable analytical process. Moreover, the ionic balance was also applied for the daily rainwater samples. The total cations (K+, Na+, Ca2+, Mg2+, and NH4+) and total anions (Cl−, F−, NO3−, and SO42−) presented good ion balances (R2 = 0.94 and p < 0.01), suggesting the good quality of the chemical detection [41].
2.3. Data Processing Method
The equation below was used for calculating the monthly volume-weighted mean (VWM) concentrations of each ion of previous observational data [18]:
where C is the monthly VWM concentration, Ci is the ionic concentration of each sample in the same month, and Pi is the corresponding rainfall amount of each sample.
After a normal distribution test (Kolmogorov–Smirnov test) of the data of ion concentrations, the Spearman’s rank correlation coefficient and principal component analysis (PCA) were performed (via SPSS 21.0) for potential source identification. More details can be found in previous studies [40,42]. The contribution of various origins to rainwater ionic species was quantified by using the model of positive matrix factorization (PMF), which is a widely used receptor model based on the ionic concentrations and corresponding uncertainty values [43]. The modeling process was concluded via EPA (Environmental Protection Agency) PMF, version 5.0. More details on the PMF model process can be found in Supplementary Materials (Text S1).
3. Results and Discussion
3.1. Rainfall Amount and pH Distribution
The yearly total rainfall amounts of the study area were 1051, 1430, 1058, 1059, 1027, and 1288 mm from 2007 to 2010 and 2013 to 2014, respectively, with a mean value of 1152 mm. These results were relatively stable during study period but much lower than the long-term (>50 years) average value of 1750 mm in the MNKFP. As shown in Figure 2, the monthly rainfall amount of each year changed significantly, with a peak in June/July, and approximately 46%–60% of the rainfall was concentrated from June to August (summer).
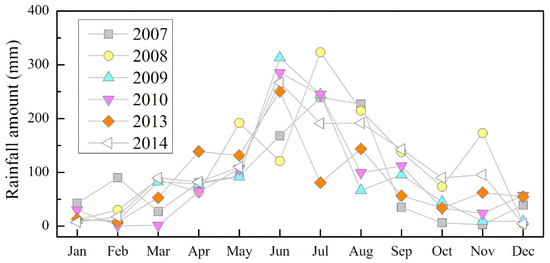
Figure 2.
Monthly rainfall amount during the study period.
As illustrated in Figure 3a, rainwater pH values (frequency distribution) presented a unimodal distribution, which was quite different from the bimodal distributions of rainwater pH observed in other areas, e.g., the Mediterranean region [44]. The pH of each rainwater sample ranged from 3.8 to 7.2, with a mean value of 5.2 and a VWM value of 5.3. An intermediate pH (4.7–5.6) in Figure 3a was recorded in about 56% of rainwater samples, and acidic pH (pH < 5.6) accounted for 75%. This pH distribution implied the acidic characteristics of rainwater in the MNKFP. According to carbonic acid equilibrium, the rainwater HCO3− concentration under this pH condition was very low, and the organic anionic species (e.g., plant-released oxalate) could have led to a slight ion imbalance [45] (Section 2.2). It is noteworthy that the reducing trend of rainwater pH in time-based linear regression (Figure 3b) was synchronously similar to that in some urban regions [46]. Overall, these results reflected that the buffer capacity of rainwater may be declining, that is, the input of acid compounds was increased and/or the input of base materials (for neutralization) was decreased. Further discussion about the neutralization process can be seen in Section 3.4.
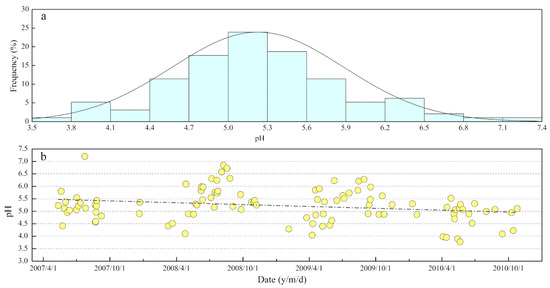
Figure 3.
The pH values of rainwater (a) frequency distribution and (b) time series; n = 104.
3.2. Ion Compositions and Temporal Variations
3.2.1. Overview of Ion Compositions
Figure 4 illustrates the statistical results of major ion concentrations on a monthly scale, including the ranges, mean values, VWM values, and relative percentages of each ion in the study period. As expected, great variations in the concentration for each ion in rainwater were observed in the MNKFP. All the ion concentration values showed a wide range, e.g., the NH4+ concentration ranged from 9.7 to 266.6 μeq L−1 and that of SO42− ranged from 14.5 to 1396.4 μeq L−1 (Figure 4a), which means that the arithmetic mean value could not reflect the overall level of rainwater ion concentration. Therefore, the VWM values were more appropriate for comparison [14]. Accordingly, the major ion concentrations in the MNKFP followed the order of SO42− (79.9 μeq L−1) > Cl− (25.7 μeq L−1) > NO3− (18.1 μeq L−1) > F− (5.0 μeq L−1) and NH4+ (56.3 μeq L−1) > Ca2+ (52.9 μeq L−1) > Mg2+ (12.3 μeq L−1) > K+ (7.2 μeq L−1) > Na+ (6.6 μeq L−1) (Figure 4a).
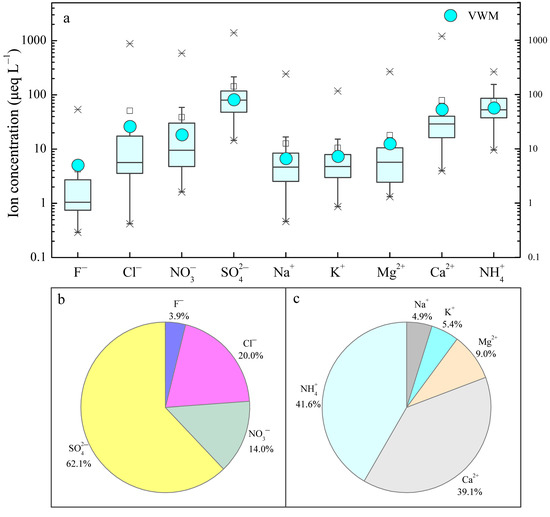
Figure 4.
(a) Overview of rainwater ion concentrations (μeq L−1), (b) rainwater anion composition percentages, and (c) rainwater cation composition percentages from 2007 to 2014 in the MNKFP. VWM: volume-weighted mean.
The composition percentages of anions and cations are also shown in Figure 4b,c, indicating that SO42−, Cl−, NH4+, and Ca2+ were the predominant rainwater species in the MNKFP. SO42− and Cl− accounted for 62.1% and 20.0%, respectively, of all measured anions, making them the most and the second richest anions (Figure 4b). The highest percentage of SO42− implied the potential impact of intense human activities [43,47]. As seen in Figure 4c, NH4+, Ca2+, Mg2+, K+, and Na+ accounted for 41.6%, 39.1%, 9.0%, 5.4%, and 4.9% of the total cations, respectively. Therefore, NH4+ was the leading cation, and Ca2+ was also a significant contributor compared to Mg2+, K+, and Na+. Both agriculture and geology are responsible for cationic composition. With the accelerated development of agricultural production, continual overfertilization discharges vast NH4-contained pollutants into the atmospheric environment, which can be supported by the increasing application rate of synthetic nitrogen fertilizer (about 10 kg N/(km2·yr) in karst agriculture over the last few decades [48]. Furthermore, the wide distribution of carbonate is the most probable source of Ca-enriched dust, which is an important origin of rainwater Ca2+ [40]. In total, the NH4+ and Ca2+ concentrations made up 80.7% of the cations, while SO42− and Cl− made up 82.1% of the anions. These results reflected that the secondary ammonium sulfate and ammonium nitrate in fine inorganic atmospheric aerosols are the most probably contributors of rainwater ions that could be washed out from the atmosphere by rainfall [49].
Here, we also summarize the ion concentrations of rainwater in different karst systems, such as karst city (Guiyang city) and karst agriculture (Puding), as well as the published data in different environmental types (Table 1). From the perspective of synchronous rainwater in different karst systems, the concentrations of SO42− and Ca2+ concentrations in the MNKFP were much lower than those observed in karst city, agricultural areas, and the Bohemian karst region [3,28,40,50,51], and the concentrations of F− and NO3− were also lower than that in karst city [40,50]. In contrast, the concentrations of Na+ and K+ in rainwater were similar in different karst systems. The concentrations of Cl−, Mg2+, and NH4+ in the MNKFP were among the corresponding ion concentrations in different karst systems (Table 1). These results can be explained by the differences of the relative intensity of human activities under various karst environmental types, such as coal combustion emissions (e.g., SO42−), traffic emissions (e.g., NO3−), industrial emissions (e.g., F−), and agricultural production intensity (e.g., NH4+ from nitrogen fertilizer and F− from phosphorus fertilizer) [18,52]. Furthermore, with the acceleration of urbanization, the construction industry and other human activities continually discharge vast Ca-containing pollutants into atmosphere [43], which are responsible for higher Ca2+ levels in karst urban rainwater relative to forest areas (MNKFP). The results in other inland/coastal megacities [13,43], deserts [53], mountain areas [54], and oceanic islands [55] further support the impact of different anthropogenic emission intensities on rainwater ion species (Table 1).

Table 1.
Comparison of ion concentration (in μeq L−1) of rainwater in the MNKFP and other published data.
3.2.2. Temporal Variations
In southwest China, in general, March to May, June to August, September to November, and December to February are defined as spring, summer, autumn, and winter, respectively [56]. Relatively higher total ion concentrations were found in spring, autumn, and winter, while summer was accompanied by lower concentrations (Figure 5a,b). Each cation and anion also showed similar seasonal variation trends. Though the seasonal pattern was not obvious, the variations of ion concentrations could still be explained by two factors. Firstly, seasonal variation in potential material sources is an important factor, including variations of atmospheric particulate matter (PM; high Ca2+ content) and atmospheric acid gases [14]. Secondly, relatively frequent precipitation in summer (rainy season) could effectively scour various species in the atmosphere and further cause a short retention time for air contaminants compared to other seasons [57], whereas more atmospheric materials can be captured by rainwater in low rain-frequency seasons [58]. Generally, in the rainfall scouring process, atmospheric materials, including sulfur and nitrogen oxides, particulate nitrate, Ca/Mg-mineral, and the new particles produced by these materials (new particle formation and gas-to-particle conversion in the process of cloud condensation nuclei, especially on the nanoscale) [59], are powerfully washed down during the early rain stage, resulting in higher ion concentrations under the condition of low rainfall amounts [39]. On the contrary, in the rainy season, without the continuing supplements of suspended materials or gaseous atmospheric pollutants, the ion concentrations of the late rain stage (prolonged and heavy rainfall event) decrease progressively and remain on a relatively low level (reflecting the in-cloud process, that is, rainwater presents a similar ion concentration to cloud-water) [5,60,61]. This decrease could also be associated with the dilution effect widely observed in rainwater research [41,62,63,64,65] that has shown a decreased ion concentration with an increased rainfall amount. Moreover, the combined influence of synoptic easterly airflow that transports a cleaner marine air mass over a region is another potential reason for lower ion concentrations during monsoons [66].

Figure 5.
Monthly variations of (a) cation concentrations, rainfall amount (RA), and pH. (b) Anion concentration in the MNKFP.
3.3. Source of the Rainwater Ions
3.3.1. Source Identification
Because of similar physicochemical features and potential co-sources of some air species (e.g., sulfur and nitrogen oxides) [67], the correlation coefficient, which was applied in this study, is an effective tool for distinguishing common sources of rainwater ions. As presented in Figure 6, the rainfall amount displayed obvious negative correlations with all ions (p < 0.01), which further supported the vital influence of rainfall amount on ion concentration variations. Nevertheless, obvious positive correlations were found among most of the ion species, such as r = 0.91 for SO42− and NH4+, r = 0.66 for Ca2+ and Mg2+, r = 0.60 for SO42− and Cl−, and r = 0.60 for SO42− and NO3− (p < 0.01; Figure 6). Thus, it was hard to gain further source information of these ions via correlation analysis.
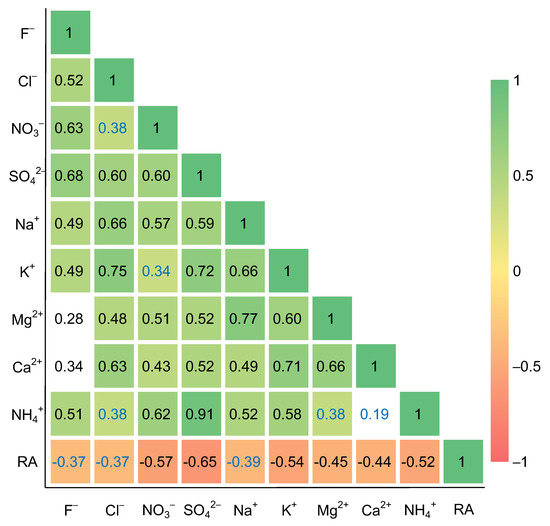
Figure 6.
Spearman’s rank correlation coefficients between monthly major ions in rainwater and RA; white cells denote statistically non-significant correlations (p > 0.05), blue font cells denote the significance level of p < 0.05, and other cells denote the significance level of p < 0.01.
To further distinguish the relationships of different rainwater ions and their sources, typical ion equivalent ratios, including SO42−/Na+, Cl−/Na+, NO3−/Na+, SO42−/NO3−, Mg2+/Ca2+, and SO42−/Ca2+ ratios, were calculated. As shown in Figure 7a, about a quarter of rainwater showed low Cl−/Na+ ratios (relative to seawater), while most of the rainwater presented a higher Cl−/Na+ ratio [68]. Overall, the Cl−/Na+ ratios of rainwater in the MNKFP are comparable to that in karst urban and rural regions [18,40,50]. Combined with the fact that the concentrations of rainwater Cl− and Na+ (typical sea-salt ions) in the MNKFP were much less than that of oceanic rainwater (Yongxing Island; Table 1) [55], we concluded that Cl− and Na+ were undergoing a strong depleted or dilution process during the transportation of atmospheric clouds [14], while the contributions of human activity to Cl− and Na+ were limited. Moreover, high SO42−/Na+ and NO3−/Na+ ratios were observed in most of the rainwater samples (Figure 7a,b), implying a distinct influence of human input, that is, the SO42− and NO3− in rainwater mainly originated from anthropogenic emissions [47]. In particular, the SO42−/NO3− ratios presented consistently high values (exceeding 1 up to 36.1; Figure 7c) in almost all samples, further revealing that fixed pollution emission sources were the primary contributors (e.g., local coal-burning emissions) [69,70], while the contribution of mobile sources (e.g., vehicle emissions) was relatively weakened [53,71]. It can be seen in Figure 7d that because of the low Mg2+/Ca2+ ratios (mean value 0.3), most of the samples are scattered between the calcite and dolomite dissolution line and the calcite dissolution line, reflecting a significant impact of atmospheric dust-calcite dissolution (originated from carbonate weathering) on rainwater Ca2+ and Mg2+ [28]. In contrast, the marine contribution to rainwater Ca2+ and Mg2+ was relatively limited compared with seawater, as seen in Figure 7d. Moreover, although the ionic ratios associated with F−, K+, and NH4+ are not plotted in Figure 7, previous studies have suggested the primary contributors on these ions. The main sources of K+ were attributed to soil dust and biomass burning (e.g., agricultural straw burning), and the higher F− concentration in rainwater was accounted by anthropogenic emissions [72,73,74]. As for rainwater NH4+, agriculture-related processes, including fertilization and biomass burning, are the primary origins [52,53], as supported by the high NH4+/NO3− ratios (up to 25.7 with a mean value of 10.0).
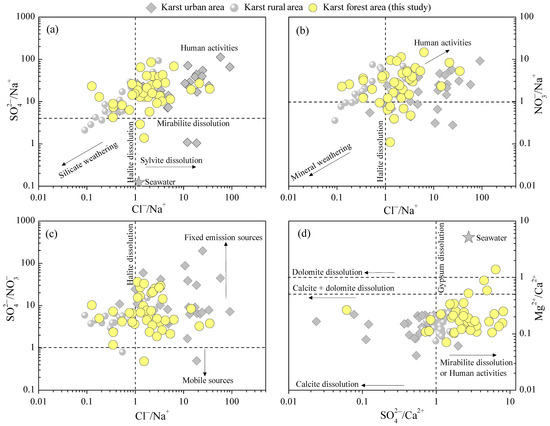
Figure 7.
The relationship between the ratios of typical ions (equivalent ratio) in karst forest, urban, and rural rainwater, including SO42−/Na+ vs. Cl−/Na+ (a), NO3−/Na+ vs. Cl−/Na+ (b), SO42−/NO3− vs. Cl−/Na+ (c), and Mg2+/Ca2+ vs. SO42−/Ca2+ (d) ratios. The calcite and dolomite dissolution line is the second horizontal dotted line in (d), and the calcite dissolution line is the x axis in (d). Data sources for karst urban and rural rainwater chemistry and other reference values are from [18,40,50,68].
3.3.2. Source Contributions
Based on the time series of rainwater chemistry data, two principal components (PCs) were recognized with a cumulative variance of 71%. Obviously, these two PCs could be easily distinguished as two contributors to rainwater ions, including anthropogenic emissions and natural sources. As shown in Figure 8a, PC1—highly loaded with F−, NO3−, SO42−, and NH4+—explained 36% of the variance. This PC has mainly displayed certain anthropogenic emission sources, such as industrial emissions, coal combustion, traffic emissions, and agricultural emissions [72,75]. PC2 had high loadings on Cl−, Na+, K+, Mg2+, and Ca2+, which was likely indicative of natural sources, such as crustal dust and sea salt [14,43]. Two contributors (factors) were further extracted from the PMF model, and the contributions of each contributor (anthropogenic and natural source) to rainwater ions are shown in Figure 8b. Anthropogenic sources contributed 51% of F−, 93% of NO3−, 62% of SO42−, and 87% of NH4+, while 74% of Cl−, 82% of Na+, 79% of K+, 94% of Mg2+, and 93% Ca2+ were attributed to natural sources. In combination with the previous discussion, the specific anthropogenic source contributions can be well-constrained, e.g., the contribution of vehicle emissions and coal combustion to rainwater NO3− were found to be no more than 93%, agriculture’s contribution to rainwater NH4+ was less than 87%, and the soil-derived dust’s contribution to Mg2+ and Ca2+ was found to be up to more than 90%.
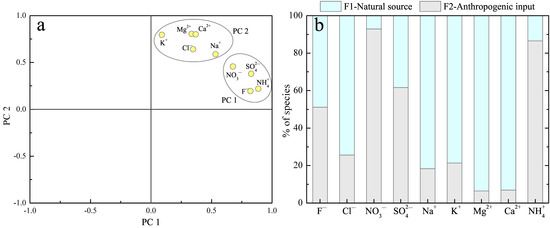
Figure 8.
(a) The principal component analysis of rainwater ions and (b) positive matrix factorization (PMF)-extracted relative contribution fractions of anthropogenic and natural sources to rainwater ions in the MNKFP.
3.4. Trends in Acidifying and Neutralizing Potential
Generally, the ratio of neutralizing potential (NP) and acidifying potential (AP) is applied to reflect the rainwater neutralizing process, which is defined as the equivalent concentration ratio of (ammonia and calcium) and (nitrate and sulfate), as follows [14,76,77]:
where nss denotes the non-sea salt part of corresponding ions—the detailed calculation can be found in previous study [53]. The time series of NP/AP are shown in Figure 9a. The high value of NP/AP (1.13 on average) implied that rainwater in the MNKFP had a significant neutralization potential to adjust the high loading sulfur/nitrate-caused acidity. It is noteworthy that the NP/AP ratio presented an obvious declining trend, with the regression intercept decreasing from 1.4 to 0.9 within four years (Figure 9a), indicating that the reducing neutralization trend was probably the temporal trend for MNKFP rainwater. A similar trend was also observed in the rainwater of some urban regions [46]. Furthermore, the time series of neutralization factors (NFs; NF of X = [X]/[NO3− + nss-SO42−], X = NH4+, nss-Ca2+, and nss-Mg2+) of NH4+, Ca2+, and Mg2+ were also calculated and are plotted in Figure 9b–d. The NFs of these three ions showed large variations with ranges from 0.06 to 1.82 (0.76 on average), from 0.02 to 1.41 (0.38 on average), and from 0.004 to 0.39 (0.07 on average) of the NFs of NH4+, Ca2+, and Mg2+, respectively, suggesting the leading role of NH4+ and Ca2+ neutralization over Mg2+. Both the NFs of NH4+ and Ca2+ showed an evident decreasing trend, while the NF of Mg2+ presented an increasing trend over the period (Figure 9b–d). Due to the confirmation of magnesium as a representative marker of cement dust [78], the increasing trend in the NF of Mg2+ (although the NF was small) likely reflected the growing potential influence of construction activities on rainwater. Similar trends of NFs were also observed in urban rainwater [46]. The temporal trends of rainwater acidification and neutralization in the MNKFP (karst forest area) were different to that in the rainwater of karst urban and agricultural regions (revealing an alkaline rain trend) [18,40], which can be explained by relatively fewer alkaline substance sources, such as NH3 volatilization from agricultural production and Ca-enriched dust from the construction industry, in the forest area.
NP/AP = [NH4+ + nss-Ca2+]/[NO3− + nss-SO42−]
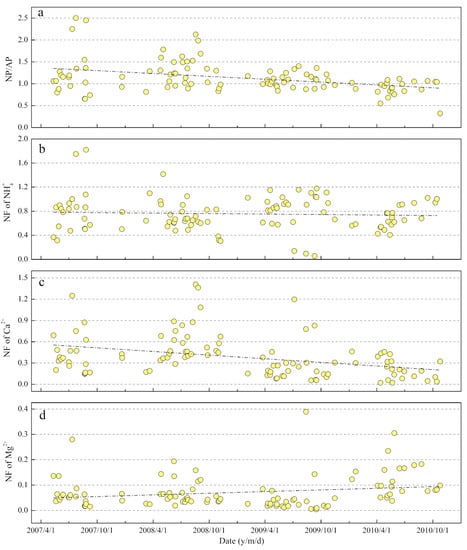
Figure 9.
Time series of (a) neutralizing potential/acidifying potential (NP/AP), (b) neutralization factor (NF) of NH4+, (c) NF of Ca2+, and (d) NF of Mg2+.
3.5. Ions Deposition Flux and Potential Environmental Effects
According to the VWM concentrations (Table 1) of each ion and the average rainfall amount (1152 mm) over the study period, the annual deposition fluxes of rainwater ions were calculated and are plotted in Figure 10. Fairly large variations in ionic deposition fluxes were observed in different ions in MNKFP rainwater with a range of 0.06 (F−) to 0.92 (SO42−) keq ha−1. The annual wet deposition flux of total ions was calculated as 3.0 keq ha−1. Though the yearly deposition flux was relatively low over China, it was equivalent to the monthly deposition flux in some heavily polluted regions (~2.5 keq ha−1) [43,46,53,54]; however, the potential environmental effects of rainwater ions on such a karst forest ecosystem are non-negligible. As the important influence factors, wet nitrogen and acid deposition significantly the change soil respiration of the forest ecosystem, which is one of the largest CO2 sources to atmosphere, on a global scale [79]. A previous study found that the interactive effects of nitrogen and acid deposition significantly reduced soil respiration via the inhibition of enzyme activity and microbial biomass in forest soil caused by soil acidification [36]. Decreased soil respiration may have similarly occurred in the MNKFP (karst forest) and further impacted the global carbon cycle based on the observed trend of rainwater pH, nitrogen, and sulfur (Section 3.1 and Section 3.4). In addition, other inorganic ions of rainwater, including K+, Na+, Ca2+, Mg2+, and Cl−, together with rainwater nitrogen and sulfur, could inhibit the decomposition rates of leaf litters and the carbon and nitrogen loss of a forest system [80]. Even more remarkable is that rainfall-actuated acid deposition (as the exogenous acid) has significantly enhanced the weathering processes of carbonate rock in karst areas and further affected global climate change [81,82,83,84]. Different from economically developed and densely populated areas (wet acid deposits dominated by nitrates) [85], wet acid deposition in the karst forest (MNKFP) was found to be controlled by sulfur with a significantly high contribution of 82% in total acid deposition (Figure 10). Therefore, more attention should be paid to rainfall-related ion deposition, particularly sulfur wet deposition in the karst forest region.
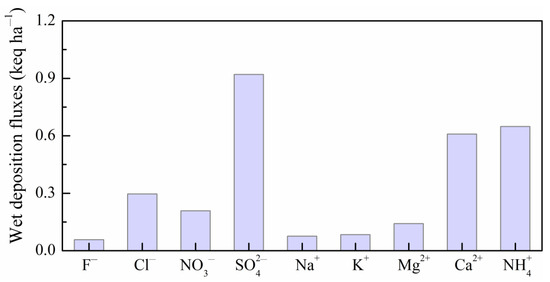
Figure 10.
The annual rainwater ions deposition fluxes in the MNKFP.
4. Conclusions
In conclusion, a temporal rainwater investigation in a typical karst forest (MNKFP) was concluded to understand the variations of rainwater chemistry, sources of rainwater ions, trends in acidifying and neutralizing potentials, and environmental effects on the karst forest system. As expected, the rainwater ion composition significantly varied in the study period based on the analysis of temporal rainwater chemistry, and a reduced trend of time-based rainwater pH was also observed. In particular, the concentrations of NH4+ and SO42− ranged from 9.7 to 266.6 μeq L−1 and from 14.5 to 1396.4 μeq L−1, respectively. NH4+, Ca2+, SO42−, and Cl− were the most dominant ions, with obvious monthly variations. Source variations and rainfall amounts were found to be the vital factors affecting rainwater ion concentration. Source identification indicated that Mg2+, Ca2+, K+, Cl−, and Na+ were mainly controlled by natural sources, while anthropogenic inputs were the primary sources of F−, NO3−, SO42−, and NH4+. The contributions were also described by a PMF model. A reducing neutralization trend of temporal rainwater was observed in the MNKFP. Given the potential effect of rainwater ion deposition on enzyme activity and microbial biomass, the decomposition of leaf litter, carbon and nitrogen loss, and carbonate rock weathering processes in a karst forest, more focus on rainfall-related ion deposition is needed.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4433/11/12/1315/s1. [86,87,88] cited in supplementary.
Author Contributions
Conceptualization, G.H. and J.Z.; methodology, G.H. and J.Z.; software, J.Z. and G.H.; validation, G.H.; formal analysis, G.H. and J.Z.; investigation, G.H.; resources, G.H.; data curation, G.H.; writing—original draft preparation, J.Z. and G.H.; writing—review and editing, G.H. and J.Z.; supervision, G.H.; project administration, G.H.; funding acquisition, G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 41325010 and 41661144029.
Acknowledgments
The authors thank Yang Tang from Institute of Geochemistry, Chinese Academy of Sciences for sampling and laboratory work. The authors also thank Rui Qu, Shitong Zhang, Wenxiang Zhou from CUGB for language polishing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, J.; Li, Z.; Guo, J.; Sun, L.; Huang, W.; Xue, W.; Fan, T.; Cribb, M. Satellite-Derived 1-km-Resolution PM1 Concentrations from 2014 to 2018 across China. Environ. Sci. Technol. 2019, 53, 13265–13274. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.H.; Nogarotto, D.C.; Mortatti, J.; Pozza, S.A. Chemical composition of rainwater in an urban area of the southeast of Brazil. Atmos. Pollut. Res. 2019, 10, 520–530. [Google Scholar] [CrossRef]
- Wu, Q.; Han, G.; Tao, F.; Tang, Y. Chemical composition of rainwater in a karstic agricultural area, Southwest China: The impact of urbanization. Atmos. Res. 2012, 111, 71–78. [Google Scholar] [CrossRef]
- Szép, R.; Mateescu, E.; Niță, I.-A.; Birsan, M.-V.; Bodor, Z.; Keresztesi, Á. Effects of the Eastern Carpathians on atmospheric circulations and precipitation chemistry from 2006 to 2016 at four monitoring stations (Eastern Carpathians, Romania). Atmos. Res. 2018, 214, 311–328. [Google Scholar] [CrossRef]
- Gonçalves, F.; Ramos, A.; Freitas, S.; Silva Dias, M.A.; Massambani, O. In-cloud and below-cloud numerical simulation of scavenging processes at Serra Do Mar region, SE Brazil. Atmos. Environ. 2002, 36, 5245–5255. [Google Scholar] [CrossRef]
- Xiao, H.W.; Xiao, H.Y.; Long, A.M.; Wang, Y.L.; Liu, C.Q. Chemical composition and source apportionment of rainwater at Guiyang, SW China. J. Atmos. Chem. 2013, 70, 269–281. [Google Scholar] [CrossRef]
- Larssen, T.; Lydersen, E.; Tang, D.; He, Y.; Gao, J.; Liu, H.; Duan, L.; Seip, H.M.; Vogt, R.D.; Mulder, J.; et al. Acid Rain in China. Environ. Sci. Technol. 2006, 40, 418–425. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Wu, Q.; Tang, Y. Effects of agricultural alkaline substances on reducing the rainwater acidification: Insight from chemical compositions and calcium isotopes in a karst forests area. Agric. Ecosyst. Environ. 2020, 290, 106782. [Google Scholar] [CrossRef]
- Facchini Cerqueira, M.R.; Pinto, M.F.; DeRossi, I.N.; Esteves, W.T.; Rachid Santos, M.D.; Costa Matos, M.A.; Lowinsohn, D.; Matos, R.C. Chemical characteristics of rainwater at a southeastern site of Brazil. Atmos. Pollut. Res. 2014, 5, 253–261. [Google Scholar] [CrossRef]
- Keresztesi, Á.; Nita, I.-A.; Birsan, M.-V.; Bodor, Z.; Szép, R. The risk of cross-border pollution and the influence of regional climate on the rainwater chemistry in the Southern Carpathians, Romania. Environ. Sci. Pollut. Res. 2020, 27, 9382–9402. [CrossRef]
- Cereceda-Balic, F.; De La Gala-Morales, M.; Palomo-Marín, R.; Fadic, X.; Vidal, V.; Funes, M.; Rueda-Holgado, F.; Pinilla-Gil, E. Spatial distribution, sources, and risk assessment of major ions ad trace elements in rainwater at Puchuncaví Valley, Chile: The impact of industrial activities. Atmos. Pollut. Res. 2020, 11, 99–109. [Google Scholar] [CrossRef]
- Park, S.-M.; Seo, B.-K.; Lee, G.; Kahng, S.-H.; Jang, Y.W. Chemical Composition of Water Soluble Inorganic Species in Precipitation at Shihwa Basin, Korea. Atmosphere 2015, 6, 732–750. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Liu, W.; Liang, C.-S.; Ji, J.; Zhao, T.; Zhang, X. Chemical composition of rainwater and the acid neutralizing effect at Beijing and Chizhou city, China. Atmos. Res. 2015, 164, 278–285. [Google Scholar] [CrossRef]
- Jain, C.D.; Madhavan, B.L.; Ratnam, M.V. Source apportionment of rainwater chemical composition to investigate the transport of lower atmospheric pollutants to the UTLS region. Environ. Pollut. 2019, 248, 166–174. [Google Scholar] [CrossRef]
- Liu, B.; Kang, S.; Sun, J.; Zhang, Y.; Xu, R.; Wang, Y.; Liu, Y.; Cong, Z. Wet precipitation chemistry at a high-altitude site (3,326 m a.s.l.) in the southeastern Tibetan Plateau. Environ. Sci. Pollut. Res. 2013, 20, 5013–5027. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.-J.; Wang, Z.-J.; Wu, Q.; Qin, C.-Q.; Li, S.-L. Quantifying depression trapping effect on rainwater chemical composition during the rainy season in karst agricultural area, southwestern China. Atmos. Environ. 2019, 218, 116998. [Google Scholar] [CrossRef]
- Cable, E.; Deng, Y. Trace Elements in Atmospheric Wet Precipitation in the Detroit Metropolitan Area: Levels and Possible Sources. Chemosphere 2018, 210, 1091–1098. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.-J.; Li, S.-L.; Wang, Z.-J.; Wu, Q.; Qin, C.-Q.; Yan, Z.-L. Determining rainwater chemistry to reveal alkaline rain trend in Southwest China: Evidence from a frequent-rainy karst area with extensive agricultural production. Environ. Pollut. 2020, 266, 115166. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Zhang, Q. Effects of agricultural abandonment on soil aggregation, soil organic carbon storage and stabilization: Results from observation in a small karst catchment, Southwest China. Agric. Ecosyst. Environ. 2020, 288, 106719. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Liu, M.; Van Zwieten, L.; Yang, X.; Yu, C.; Wang, H.; Song, Z. Carbon-nitrogen isotope coupling of soil organic matter in a karst region under land use change, Southwest China. Agric. Ecosyst. Environ. 2020, 301, 107027. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G. Tracing zinc sources with Zn isotope of fluvial suspended particulate matter in Zhujiang River, southwest China. Ecol. Indic. 2020, 118, 106723. [Google Scholar] [CrossRef]
- Yan, Z.; Han, X.; Lang, Y.; Guo, Q.; Li, S. The abatement of acid rain in Guizhou province, southwestern China: Implication from sulfur and oxygen isotopes. Environ. Pollut. 2020, 267, 115444. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Li, S.-L.; Yue, F.-J.; Qin, C.-Q.; Buckerfield, S.; Zeng, J. Rainfall driven nitrate transport in agricultural karst surface river system: Insight from high resolution hydrochemistry and nitrate isotopes. Agric. Ecosyst. Environ. 2020, 291, 106787. [Google Scholar] [CrossRef]
- Qin, C.; Li, S.; Waldron, S.; Yue, F.-J.; Wang, Z.-J.; Zhong, J.; Ding, H.; Liu, C.-Q. High-frequency monitoring reveals how hydrochemistry and dissolved carbon respond to rainstorms at a karstic critical zone, Southwestern China. Sci. Total Environ. 2020, 714. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Zhong, J.; Li, C. Spatial scale effects of the variable relationships between landscape pattern and water quality: Example from an agricultural karst river basin, Southwestern China. Agric. Ecosyst. Environ. 2020, 300, 106999. [Google Scholar] [CrossRef]
- Yue, F.-J.; Li, S.-L.; Waldron, S.; Wang, Z.-J.; Oliver, D.M.; Chen, X.; Liu, C.-Q. Rainfall and conduit drainage combine to accelerate nitrate loss from a karst agroecosystem: Insights from stable isotope tracing and high-frequency nitrate sensing. Water Res. 2020, 186, 116388. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G. Preliminary copper isotope study on particulate matter in Zhujiang River, southwest China: Application for source identification. Ecotoxicol. Environ. Saf. 2020, 198, 110663. [Google Scholar] [CrossRef]
- Lü, P.; Han, G.; Wu, Q. Chemical characteristics of rainwater in karst rural areas, Guizhou Province, Southwest China. Acta Geochim. 2017, 36, 572–576. [Google Scholar] [CrossRef]
- Hao, Z.; Gao, Y.; Yang, T.; Tian, J. Atmospheric wet deposition of nitrogen in a subtropical watershed in China: Characteristics of and impacts on surface water quality. Environ. Sci. Pollut. Res. 2017, 24, 8489–8503. [Google Scholar] [CrossRef]
- Siudek, P.; Frankowski, M.; Siepak, J. Seasonal variations of dissolved organic carbon in precipitation over urban and forest sites in central Poland. Environ. Sci. Pollut. Res. 2015, 22, 11087–11096. [Google Scholar] [CrossRef]
- Yeon, J.; Gautam, M.K.; Kim, I.; Lee, S.; Lee, D.; Perlman, D.H.J.; Lees, K.S. Isotopic composition of throughfall nitrates in suburban forests with different vegetations. Geosci. J. 2014, 19, 167–175. [Google Scholar] [CrossRef]
- Bhattarai, H.; Zhang, Y.-L.; Pavuluri, C.M.; Wan, X.; Wu, G.; Li, P.; Cao, F.; Zhang, W.; Wang, Y.; Kang, S.; et al. Nitrogen Speciation and Isotopic Composition of Aerosols Collected at Himalayan Forest (3326 m a.s.l.): Seasonality, Sources, and Implications. Environ. Sci. Technol. 2019, 53, 12247–12256. [Google Scholar] [CrossRef] [PubMed]
- Gioda, A.; Mayol-Bracero, O.L.; Scatena, F.N.; Weathers, K.C.; Mateus, V.L.; McDowell, W.H. Chemical constituents in clouds and rainwater in the Puerto Rican rainforest: Potential sources and seasonal drivers. Atmos. Environ. 2013, 68, 208–220. [Google Scholar] [CrossRef]
- Chen, X.; Mulder, J. Atmospheric deposition of nitrogen at five subtropical forested sites in South China. Sci. Total Environ. 2007, 378, 317–330. [Google Scholar] [CrossRef]
- Durka, W.; Schulze, E.-D.; Gebauer, G.; Voerkeliust, S. Effects of forest decline on uptake and leaching of deposited nitrate determined from 15N and 18O measurements. Nat. Cell Biol. 1994, 372, 765–767. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, G.G.; Tang, C.; Fang, H.; Duan, J.; Yu, X. Effects of One-Year Simulated Nitrogen and Acid Deposition on Soil Respiration in a Subtropical Plantation in China. Forests 2020, 11, 235. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. Space Phys. 2010, 115. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Wu, Q.; Tan, Q. Chemical and strontium isotope characterization of rainwater in karst virgin forest, Southwest China. Atmos. Environ. 2010, 44, 174–181. [Google Scholar] [CrossRef]
- Jia, G.; Chen, F. Monthly variations in nitrogen isotopes of ammonium and nitrate in wet deposition at Guangzhou, south China. Atmos. Environ. 2010, 44, 2309–2315. [Google Scholar] [CrossRef]
- Han, G.; Song, Z.; Tang, Y.; Wu, Q.; Wang, Z. Ca and Sr isotope compositions of rainwater from Guiyang city, Southwest China: Implication for the sources of atmospheric aerosols and their seasonal variations. Atmos. Environ. 2019, 214, 116854. [Google Scholar] [CrossRef]
- Tripathee, L.; Guo, J.; Kang, S.; Paudyal, R.; Sharma, C.M.; Huang, J.; Chen, P.; Sharma Ghimire, P.; Sigdel, M.; Sillanpää, M. Measurement of mercury, other trace elements and major ions in wet deposition at Jomsom: The semi-arid mountain valley of the Central Himalaya. Atmos. Res. 2020, 234, 104691. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.-J.; Xiao, M.; Wang, Z.J.; Wu, Q.; Qin, C.-Q. Dissolved organic carbon in rainwater from a karst agricultural area of Southwest China: Variations, sources, and wet deposition fluxes. Atmos. Res. 2020, 245, 105140. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Z.; Liu, W.; Wu, Y.; Zhao, T.; Jiang, H.; Zhang, X.; Zhang, J.; Zhou, L.; Wang, Y. Chemical composition of precipitation in Shenzhen, a coastal mega-city in South China: Influence of urbanization and anthropogenic activities on acidity and ionic composition. Sci. Total Environ. 2019, 662, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Aiuppa, A.; Bonfanti, P.; D’Alessandro, W. Rainwater Chemistry at Mt. Etna (Italy): Natural and Anthropogenic Sources of Major Ions. J. Atmos. Chem. 2003, 46, 89–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, X.; Cao, F.; Huang, D. Seasonal variation and sources of low molecular weight organic acids in precipitation in the rural area of Anshun. Chin. Sci. Bull. 2011, 56, 1005–1010. [Google Scholar] [CrossRef]
- Yang, F.; Tan, J.; Shi, Z.; Cai, Y.; He, K.; Ma, Y.; Duan, F.; Okuda, T.; Tanaka, S.; Chen, G. Five-year record of atmospheric precipitation chemistry in urban Beijing, China. Atmos. Chem. Phys. Discuss. 2012, 12, 2025–2035. [Google Scholar] [CrossRef]
- Keresztesi, Á.; Birsan, M.-V.; Nita, I.-A.; Bodor, Z.; Szép, R. Assessing the neutralisation, wet deposition and source contributions of the precipitation chemistry over Europe during 2000–2017. Environ. Sci. Eur. 2019, 31, 50. [Google Scholar] [CrossRef]
- Yue, F.-J.; Li, S.-L.; Liu, C.-Q.; Lang, Y.; Ding, H. Sources and transport of nitrate constrained by the isotopic technique in a karst catchment: An example from Southwest China. Hydrol. Process. 2015, 29, 1883–1893. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, X.; Ma, Y.; Wang, L.; Wu, R.; Chen, B.; Wang, W. The Concentrations, Formations, Relationships and Modeling of Sulfate, Nitrate and Ammonium (SNA) Aerosols over China. Aerosol Air Qual. Res. 2017, 17, 84–97. [Google Scholar] [CrossRef]
- Han, G.; Wu, Q.; Tang, Y. Acid rain and alkalization in southwestern China: Chemical and strontium isotope evidence in rainwater from Guiyang. J. Atmos. Chem. 2011, 68, 139–155. [Google Scholar] [CrossRef]
- Špičková, J.; Dobešová, I.; Vach, M.; Skrivan, P.; Mihaljevič, M.; Burian, M. The influence of the limestone-quarry Čertovy schody (Czech Republic) on the precipitation chemistry and atmospheric deposition. Geochemistry 2008, 68, 105–115. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, D.-S.; Lim, S.-S.; Kwak, J.-H.; Jeon, B.-J.; Lee, S.-I.; Lee, S.-M.; Choi, W.-J. Nitrogen isotope ratios of dissolved organic nitrogen in wet precipitation in a metropolis surrounded by agricultural areas in southern Korea. Agric. Ecosyst. Environ. 2012, 159, 161–169. [Google Scholar] [CrossRef]
- Rao, W.; Han, G.; Tan, H.; Jin, K.; Wang, S.; Chen, T. Chemical and Sr isotopic characteristics of rainwater on the Alxa Desert Plateau, North China: Implication for air quality and ion sources. Atmos. Res. 2017, 193, 163–172. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, L.; Fischer, E.; Li, Z.; Jiao, K. Study of chemical composition of precipitation at an alpine site and a rural site in the Urumqi River Valley, Eastern Tien Shan, China. Atmos. Environ. 2008, 42, 8934–8942. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Xiao, H.-Y.; Zhang, Z.-Y.; Wang, Y.-L.; Long, A.-M.; Liu, C.Q. Chemical characteristics and source apportionment of atmospheric precipitation in Yongxing Island. China Environ. Sci. 2016, 36, 3237–3244. [Google Scholar]
- Zeng, J.; Yue, F.-J.; Li, S.; Wang, Z.-J.; Qin, C.-Q.; Wu, Q.-X.; Xu, S. Agriculture driven nitrogen wet deposition in a karst catchment in southwest China. Agric. Ecosyst. Environ. 2020, 294, 106883. [Google Scholar] [CrossRef]
- Xiao, H.-Y.; Liu, C.-Q. Sources of nitrogen and sulfur in wet deposition at Guiyang, southwest China. Atmos. Environ. 2002, 36, 5121–5130. [Google Scholar] [CrossRef]
- Szép, R.; Bodor, Z.; Miklóssy, I.; Niță, I.-A.; Oprea, O.A.; Keresztesi, Á. Influence of peat fires on the rainwater chemistry in intra-mountain basins with specific atmospheric circulations (Eastern Carpathians, Romania). Sci. Total Environ. 2019, 647, 275–289. [Google Scholar] [CrossRef]
- Kulmala, M.; Petäjä, T.; Ehn, M.; Thornton, J.; Sipilä, M.; Worsnop, D.R.; Kerminen, V.-M. Chemistry of Atmospheric Nucleation: On the Recent Advances on Precursor Characterization and Atmospheric Cluster Composition in Connection with Atmospheric New Particle Formation. Annu. Rev. Phys. Chem. 2014, 65, 21–37. [Google Scholar] [CrossRef]
- Silva, M.P.R.; Gonçalves, F.L.T.; Freitas, S.R. Two case studies of sulfate scavenging processes in the Amazon region (Rondônia). Environ. Pollut. 2009, 157, 637–645. [Google Scholar] [CrossRef]
- Castillo, S.; Alastuey, A.; Cuevas, E.; Querol, X.; Avila, A. Quantifying Dry and Wet Deposition Fluxes in Two Regions of Contrasting African Influence: The NE Iberian Peninsula and the Canary Islands. Atmosphere 2017, 8, 86. [Google Scholar] [CrossRef]
- Li, Y.-C.; Zhang, M.; Shu, M.; Ho, S.S.H.; Liu, Z.-F.; Wang, X.-X.; Zhao, X.-Q. Chemical characteristics of rainwater in Sichuan basin, a case study of Ya’an. Environ. Sci. Pollut. Res. 2016, 23, 13088–13099. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, Y.; Xu, H.; Zhang, X.; Liu, H.-L.; Wang, S.; Zhang, W. Bulk/wet deposition of trace metals to rural, industrial, and urban areas in the Yangtze River Delta, China. Ecotoxicol. Environ. Saf. 2019, 169, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, D.; Kasimov, N.; Eremina, I.; Shinkareva, G.; Chubarova, N. Partitioning and solubilities of metals and metalloids in spring rains in Moscow megacity. Atmos. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, K. Atmospheric wet and dry deposition of trace elements at 10 sites in Northern China. Atmos. Chem. Phys. Discuss. 2015, 15, 951–972. [Google Scholar] [CrossRef]
- Dhungel, S.; Kathayat, B.; Mahata, K.; Panday, A. Transport of regional pollutants through a remote trans-Himalayan valley in Nepal. Atmos. Chem. Phys. Discuss. 2018, 18, 1203–1216. [Google Scholar] [CrossRef]
- Vet, R.; Artz, R.S.; Carou, S.; Shaw, M.; Ro, C.-U.; Aas, W.; Baker, A.; Bowersox, V.C.; Dentener, F.; Galy-Lacaux, C.; et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014, 93, 3–100. [Google Scholar] [CrossRef]
- Berner, E.K.; Berner, R.A. Global Water Cycle: Geochemistry and Environment; Prentice-Hall: New York, NY, USA, 1987; p. 76. [Google Scholar]
- Arimoto, R.; Duce, R.A.; Savoie, D.L.; Prospero, J.M.; Talbot, R.; Cullen, J.D.; Tomza, U.; Lewis, N.F.; Ray, B.J. Relationships among aerosol constituents from Asia and the North Pacific during PEM-West A. J. Geophys. Res. Space Phys. 1996, 101, 2011–2024. [Google Scholar] [CrossRef]
- Li, C.; Li, S.-L.; Yue, F.-J.; He, S.-N.; Shi, Z.-B.; Di, C.-L.; Liu, C.-Q. Nitrate sources and formation of rainwater constrained by nitrate isotopes in Southeast Asia: Example from Singapore. Chemosphere 2020, 241, 125024. [Google Scholar] [CrossRef]
- Tripathee, L.; Kang, S.; Rupakheti, D.; Zhang, Q.; Huang, J.; Sillanpää, M. Water-Soluble Ionic Composition of Aerosols at Urban Location in the Foothills of Himalaya, Pokhara Valley, Nepal. Atmosphere 2016, 7, 102. [Google Scholar] [CrossRef]
- Khare, P.; Goel, A.; Patel, D.; Behari, J. Chemical characterization of rainwater at a developing urban habitat of Northern India. Atmos. Res. 2004, 69, 135–145. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Liu, W.; Moon, S.; Zhao, T.; Zhou, X.; Zhang, J.; Wu, Y.; Jiang, H.; Zhou, L. Hydro-Geochemical and Sr Isotope Characteristics of the Yalong River Basin, Eastern Tibetan Plateau: Implications for Chemical Weathering and Controlling Factors. Geochem. Geophys. Geosystems 2019, 20, 1221–1239. [Google Scholar] [CrossRef]
- Wang, H.; Han, G. Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos. Res. 2011, 99, 190–196. [Google Scholar] [CrossRef]
- Rao, P.S.P.; Tiwari, S.; Matwale, J.L.; Pervez, S.; Tunved, P.; Safai, P.D.; Srivastava, A.K.; Bisht, D.S.; Singh, S.; Hopke, P.K. Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos. Environ. 2016, 146, 90–99. [Google Scholar] [CrossRef]
- Wu, Q.; Han, G. Sulfur isotope and chemical composition of the rainwater at the Three Gorges Reservoir. Atmos. Res. 2015, 155, 130–140. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Xiao, H.-Y.; Shen, C.-Y.; Zhang, Z.-Y.; Long, A.-M. Chemical Composition and Sources of Marine Aerosol over the Western North Pacific Ocean in Winter. Atmosphere 2018, 9, 298. [Google Scholar] [CrossRef]
- Yang, F.; Ye, B.; He, K.; Ma, Y.; Cadle, S.H.; Chan, T.; Mulawa, P.A. Characterization of Atmospheric Mineral Components of PM2.5 in Beijing and Shanghai, China. Sci. Total Environ. 2005, 343, 221–230. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Ji, Y.; Li, Q.; Ye, R.; Tian, K.; Tian, X. The Impact of Water-Soluble Inorganic Ions in Particulate Matter (PM2.5) on Litter Decomposition in Chinese Subtropical Forests. Forests 2020, 11, 238. [Google Scholar] [CrossRef]
- Li, S.-L.; Calmels, D.; Han, G.; Gaillardet, J.; Liu, C.-Q. Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: Examples from Southwest China. Earth Planet. Sci. Lett. 2008, 270, 189–199. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Major ions and δ34SSO4 in Jiulongjiang River water: Investigating the relationships between natural chemical weathering and human perturbations. Sci. Total Environ. 2020, 724, 138208. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-F.; Li, S.-L.; Zhong, J.; Maberly, S.C.; Li, C.; Wang, F.-S.; Xiao, H.-Y.; Liu, C.-Q. Climatic and anthropogenic regulation of carbon transport and transformation in a karst river-reservoir system. Sci. Total Environ. 2020, 707, 135628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, T.; Huang, X.; Han, X.; Yang, H.; Cai, Z.; Yao, L.; Han, X.; Zhang, M.; Huang, C. Characteristics, sources and environmental implications of atmospheric wet nitrogen and sulfur deposition in Yangtze River Delta. Atmos. Environ. 2019, 219, 116904. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Zheng, N.; Luo, L.; Xiao, H.; Xiao, H. Chemical characterization and source analysis of water-soluble inorganic ions in PM2.5 from a plateau city of Kunming at different seasons. Atmos. Res. 2020, 234, 104687. [Google Scholar] [CrossRef]
- Hien, P.D.; Bac, V.T.; Thinh, N.T.H. PMF receptor modelling of fine and coarse PM10 in air masses governing monsoon conditions in Hanoi, northern Vietnam. Atmos. Environ. 2004, 38, 189–201. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.H.; Mendoza, J.A.; Lee, C.H.; Kang, J.-H. Characterization and source identification of pollutants in runoff from a mixed land use watershed using ordination analyses. Environ. Sci. Pollut. Res. 2016, 23, 9774–9790. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).