The Effects of Ozone on Herbivore-Induced Volatile Emissions of Cultivated and Wild Brassica Rapa
Abstract
:1. Introduction
2. Materials and Methods
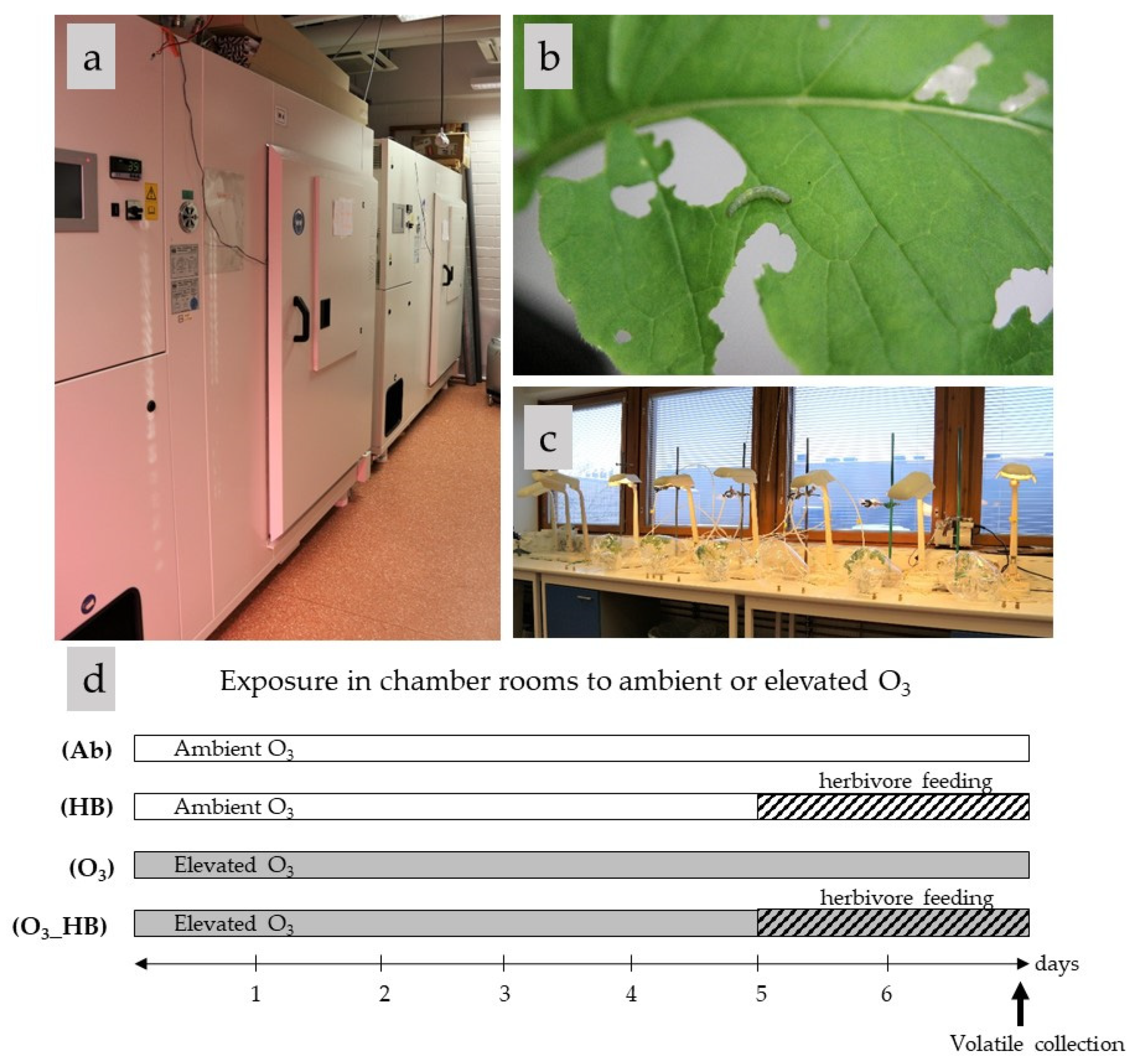
2.1. Insect and Plant Growth Conditions
2.2. Treatments
- Ab: ambient ozone (15 ppb);
- O3: elevated ozone (80 ppb);
- HB: 48 h herbivore feeding;
- O3_HB: 48 h herbivore feeding under elevated ozone.
2.3. Volatile Organic Compounds (VOCs) Collection and Analysis
2.4. Feeding Assessment
2.5. Statistical Analyses
3. Results
3.1. The Main Effect of Herbivore Feeding on Volatile Emission Rates
3.2. The Main Effect of Ozone on Volatile Emission Rates
3.3. The Interactive Effect of Ozone and Herbivore Feeding on Volatile Emissions
3.4. The Effects of Ozone Exposure on Herbivore Feeding
4. Discussion
4.1. Differences in Volatile Responses to Herbivore Feeding in Cultivated and Wild Plants
4.2. Differences in Volatile Responses to Ozone in Cultivated and Wild Plants
4.3. Differences in Volatile Responses to Interactive Ozone and Herbivory Exposure in Cultivated and Wild Plants
4.4. Effect of Elevated Ozone on the Feeding Behavior of P. xylostella Related to the Change in VOC Emissions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Compounds | Ab | O3 | HB | O3_HB | HB | O3 | HB*O3 |
|---|---|---|---|---|---|---|---|
| B. rapa (wild variety) | |||||||
| Monoterpenes | |||||||
| α-Pinene | 2.1 ± 0.4 | 3.2 ± 1.1 | 3.7 ± 1.4 | 2.9 ± 1.6 | ns | ns | ns |
| β-Pinene | 0.0 ± 0.0 | 0.3 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | ns | 0.050 | ns |
| Myrcene | 0.7 ± 0.5 | 2.8 ± 2.3 | 3.7 ± 0.6 | 4.0 ± 2.2 | ns | ns | ns |
| 3-carene | 1.2 ± 0.6 | 1.3 ± 0.9 | 4.0 ± 0.9 | 3.1 ± 1.1 | ns | ns | ns |
| Limonene | 4.3 ± 0.7 | 6.0 ± 1.6 | 5.1 ± 1.9 | 4.4 ± 1.4 | ns | ns | ns |
| 1.8 cineole | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | ns | ns | ns |
| Homoterpenes | |||||||
| (E)-DMNT 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.0 ± 3.0 | 8.8 ± 6.3 | 0.070 | ns | ns |
| Sesquiterpenoids | |||||||
| Unknown RI 1339.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 | ns | ns | ns |
| (E)-Caryophyllene | 0.7 ± 0.5 | 0.0 ± 0.0 | 8.3 ± 1.5 | 8.2 ± 5.4 | 0.002 | ns | ns |
| α-Humulene | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.9 ± 0.4 | 0.6 ± 0.4 | 0.007 | ns | ns |
| (E. E)-α-Farnesene | 0.6 ± 0.6 | 0.0 ± 0.0 | 1.2 ± 0.4 | 3.8 ± 3.0 | ns | ns | ns |
| GLVs + MeSA2 | |||||||
| (Z)-3-Hexenal | 9.6 ± 7.6 | 33.2 ± 21.6 | 30.1 ± 4.4 | 19.9 ± 6.7 | ns | ns | ns |
| Hexanal | 6.0 ± 0.3 | 13.1 ± 5.8 | 10.4 ± 2.6 | 8.6 ± 1.4 | ns | ns | ns |
| (E)-2-Hexenal | 6.7 ± 4.6 | 10.9 ± 6.1 | 8.1 ± 2.4 | 10.7 ± 3.9 | ns | ns | ns |
| (Z)-3-Hexenol | 9.9 ± 5.2 | 29.0 ± 6.8 | 20.2 ± 2.4 | 18.4 ± 7.4 | ns | ns | ns |
| (Z)-3-Hexenyl acetate | 69.0 ± 25.0 | 114.7 ± 12.8 | 101.7 ± 28.7 | 47.6 ± 9.3 | ns | ns | 0.022 |
| Hexyl acetate | 0.0 ± 0.0 | 0.0 ± 0.0 | 4.0 ± 4.0 | 0.0 ± 0.0 | ns | ns | ns |
| MeSA 2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.4 | 3.0 ± 1.9 | 0.070 | ns | ns |
| N-/or S-containing compounds | |||||||
| Dimethyl disulfide | 15.9 ± 7.1 | 1.9 ± 1.0 | 22.6 ± 21.6 | 13.9 ± 8.9 | ns | ns | ns |
| Methyl isothiocyanate | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ns | ns | ns |
| 2-methyl-5-hexenenitrile | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 3.5 ± 1.6 | 0.073 | ns | ns |
| t-butyl isothiocyanate | 0.1 ± 0.1 | 0.4 ± 0.4 | 2.8 ± 1.4 | 2.7 ± 2.6 | ns | ns | ns |
| 3-butenyl isothiocyanate | 2.2 ± 0.9 | 2.4 ± 0.8 | 3.1 ± 0.8 | 3.5 ± 2.5 | ns | ns | ns |
| Cordelia | Ab | O3 | HB | O3_HB | HB | O3 | HB*O3 |
| Monoterpenes | |||||||
| α-Pinene | 0.0 ± 0.0 | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | ns | ns | ns |
| Myrcene | 0.0 ± 0.0 | 0.9 ± 0.5 | 1.4 ± 0.5 | 2.5 ± 1.1 | ns | ns | ns |
| Limonene | 7.9 ± 1.7 | 10.2 ± 3.6 | 11.2 ± 5.2 | 5.5 ± 3.8 | ns | ns | ns |
| Linalool | 15.7 ± 15.7 | 0.0 ± 0.0 | 14.3 ± 13.9 | 1.0 ± 1.0 | ns | ns | ns |
| Homoterpenes | |||||||
| (E)-DMNT 1 | 0.5 ± 0.5 | 0.0 ± 0.0 | 8.5 ± 5.1 | 3.7 ± 2.5 | 0.1 | ns | ns |
| Sesquiterpenoids | |||||||
| Unknown RI 1423.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.2 | ns | ns | ns |
| Unknown RI 1442.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | ns | ns | ns |
| (E.E)-α-Farnesene | 0.6 ± 0.6 | 0.8 ± 0.5 | 8.8 ± 2.6 | 3.9 ± 2.0 | 0.095 | ns | ns |
| GLVs + MeSA2 | |||||||
| (Z)-3-Hexenal | 0.0 ± 0.0 | 0.0 ± 0.0 | 25.1 ± 25.1 | 74.2 ± 57.0 | ns | ns | ns |
| Hexanal | 1.7 ± 0.8 | 4.2 ± 2.4 | 4.7 ± 2.0 | 3.9 ± 1.0 | ns | ns | ns |
| (E)-2-Hexenal | 0.0 ± 0.0 | 0.0 ± 0.0 | 6.4 ± 4.6 | 14.4 ± 10.2 | 0.029 | ns | ns |
| (Z)-3-Hexenol | 4.2 ± 1.9 | 6.5 ± 3.1 | 36.1 ± 7.2 | 70.1 ± 44.7 | 0.001 | ns | ns |
| (Z)-3-Hexenyl acetate | 101.8 ± 49.2 | 103.6 ± 49.5 | 395.6 ± 80.5 | 226.8 ± 57.5 | 0.005 | ns | ns |
| Hexyl acetate | 0.7 ± 0.6 | 0.0 ± 0.0 | 4.7 ± 1.2 | 2.3 ± 0.9 | 0.049 | ns | ns |
| MeSA 2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.5 ± 1.6 | 0.2 ± 0.2 | 0.054 | ns | 0.092 |
| N-/or S-containing compounds | |||||||
| Dimethyl disulfide | 3.2 ± 1.6 | 2.9 ± 1.4 | 1.8 ± 0.7 | 4.0 ± 0.8 | ns | ns | ns |
| 2-methyl-5-hexenenitrile | 0.4 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ns | ns | ns |
| t-butyl isothiocyanate | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 | ns | ns | ns |
| Legato | Ab | O3 | HB | O3_HB | HB | O3 | HB*O3 |
| Monoterpenes + Benzyl alcohol | |||||||
| α-Pinene | 1.1 ± 0.7 | 0.6 ± 0.5 | 0.8 ± 0.4 | 0.4 ± 0.4 | ns | ns | ns |
| β-Pinene | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.5 ± 0.4 | 0.1 ± 0.1 | ns | ns | ns |
| Myrcene | 6.8 ± 6.8 | 2.2 ± 1.5 | 18.4 ± 12.6 | 6.3 ± 2.8 | ns | ns | ns |
| 3-carene | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.4 | ns | ns | ns |
| Limonene | 1.2 ± 0.2 | 1.0 ± 0.6 | 3.4 ± 1.9 | 1.5 ± 0.8 | ns | ns | ns |
| Benzyl alcohol | 0.0 ± 0.0 | 1.3 ± 0.8 | 0.1 ± 0.1 | 1.3 ± 0.4 | ns | 0.036 | ns |
| Linalool | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.4 ± 5.4 | ns | ns | ns |
| Homoterpenes | |||||||
| (E)-DMNT 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 158.8 ± 69.4 | 104.2 ± 36.3 | <0.001 | ns | ns |
| Sesquiterpenoids | |||||||
| (E)-Caryophyllene | 1.7 ± 1.0 | 0.0 ± 0.0 | 12.0 ± 8.4 | 3.5 ± 0.4 | 0.05 | ns | ns |
| Unknown RI 1459.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.4 | 0.8 ± 0.5 | 0.02 | ns | ns |
| α-Humulene | 1.9 ± 1.9 | 0.0 ± 0.0 | 1.0 ± 0.6 | 0.3 ± 0.3 | ns | ns | ns |
| (E. E)-α-Farnesene | 4.4 ± 2.3 | 6.4 ± 3.8 | 68.3 ± 30.0 | 103.7 ± 39.9 | 0.015 | ns | ns |
| GLVs + MeSA2 | |||||||
| Hexanal | 1.5 ± 1.1 | 2.6 ± 1.8 | 7.4 ± 5.0 | 4.1 ± 1.5 | ns | ns | ns |
| (E)-2-Hexenal | 5.0 ± 3.2 | 1.9 ± 1.9 | 14.7 ± 2.3 | 18.8 ± 3.1 | ns | ns | ns |
| (Z)-3-Hexenol | 23.6 ± 11.3 | 8.4 ± 4.2 | 42.1 ± 4.2 | 46.3 ± 12.6 | ns | ns | ns |
| (Z)-3-Hexenyl acetate | 116.0 ± 47.2 | 65.1 ± 30.5 | 192.8 ± 69.5 | 206.2 ± 49.1 | 0.057 | ns | ns |
| Hexyl acetate | 2.2 ± 2.2 | 8.1 ± 5.1 | 9.4 ± 4.7 | 20.0 ± 17.1 | ns | ns | ns |
| MeSA 2 | 0.0 ± 00 | 0.0 ± 0.0 | 18.6 ± 10.3 | 8.3 ± 3.8 | 0.022 | ns | ns |
| N-/or S-containing compounds | |||||||
| Dimethyl disulfide | 1.6 ± 1.1 | 0.0 ± 0.0 | 8.4 ± 6.6 | 4.7 ± 2.5 | ns | ns | ns |
| t-butyl isothiocyanate | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.5 ± 0.5 | 1.8 ± 0.8 | ns | ns | ns |
| Petita | Ab | O3 | HB | O3_HB | HB | O3 | HB*O3 |
| Monoterpenes | |||||||
| α-Pinene | 9.4 ± 3.2 | 7.7 ± 1.4 | 9.1 ± 2.0 | 7.7 ± 1.2 | ns | ns | ns |
| β-Pinene | 1.6 ± 0.8 | 0.7 ± 0.4 | 0.5 ± 0.3 | 1.0 ± 0.4 | ns | ns | ns |
| 3-carene | 5.1 ± 2.1 | 2.8 ± 1.2 | 4.1 ± 1.5 | 3.0 ± 1.6 | ns | ns | ns |
| Benzyl alcohol | 4.7 ± 2.9 | 4.1 ± 2.8 | 2.1 ± 1.2 | 2.5 ± 1.4 | ns | ns | ns |
| Limonene | 16.9 ± 10.6 | 12.0 ± 8.4 | 14.5 ± 5.9 | 7.0 ± 2.0 | ns | ns | ns |
| Linalool | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.0 ± 5.0 | 11.6 ± 11.5 | ns | ns | ns |
| Homoterpenes | |||||||
| (E)-DMNT 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 74.6 ± 22.9 | 67.0 ± 23.8 | 0.001 | ns | ns |
| Sesquiterpenoids | |||||||
| Unknown RI 1461.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.3 ± 1.4 | 4.3 ± 1.4 | 0.033 | ns | ns |
| Unknown RI 1438.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.7 ± 2.6 | 6.7 ± 2.2 | 0.015 | ns | ns |
| Unknown RI 1426.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.8 ± 1.6 | 6.6 ± 0.6 | 0.007 | ns | ns |
| (E. E)-α-Farnesene | 0.0 ± 0.0 | 0.0 ± 0.0 | 105.1 ± 31.9 | 44.3 ± 16.7 | <0.001 | ns | 0.054 |
| GLVs + MeSA2 | |||||||
| Hexanal | 34.2 ± 12.8 | 29.2 ± 11.2 | 43.7 ± 13.9 | 43.5 ± 9.3 | ns | ns | ns |
| (E)-2-Hexenal | 0.0 ± 0.0 | 8.8 ± 8.8 | 70.8 ± 15.4 | 124.5 ± 24.6 | 0.071 | ns | ns |
| (Z)-3-Hexenol | 73.4 ± 65.6 | 30.5 ± 4.0 | 86.4 ± 12.4 | 124.2 ± 32.3 | ns | ns | ns |
| (Z)-3-Hexenyl acetate | 211.7 ± 118.6 | 124.3 ± 38.3 | 198.2 ± 17.7 | 316.4 ± 66.5 | ns | ns | ns |
| Hexyl acetate | 1.9 ± 1.9 | 0.0 ± 0.0 | 4.5 ± 2.8 | 9.6 ± 5.0 | ns | ns | ns |
| MeSA 2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 73.0 ± 51.5 | 20.8 ± 20.8 | ns | ns | ns |
| N-/or S-containing compounds | |||||||
| Dimethyl disulfide | 0.0 ± 0.0 | 6.5 ± 0.6 | 7.8 ± 3.6 | 7.8 ± 3.6 | ns | ns | ns |
| Valo | Ab | O3 | HB | O3_HB | HB | O3 | HB*O3 |
| Monoterpenes | |||||||
| α-Pinene | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.2 ± 2.0 | 1.0 ± 1.0 | ns | ns | ns |
| β-Pinene | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.5 | 0.2 ± 0.2 | ns | ns | ns |
| Myrcene | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.3 | 0.5 ± 0.4 | 0.042 | ns | ns |
| 3-carene | 0.5 ± 0.2 | 0.1 ± 0.05 | 0.8 ± 0.8 | 0.7 ± 0.7 | ns | ns | ns |
| Limonene | 2.2 ± 0.3 | 2.7 ± 2.1 | 8.6 ± 2.7 | 3.7 ± 1.2 | ns | ns | ns |
| Linalool | 0.0 ± 0.0 | 11.2 ± 9.5 | 4.6 ± 4.6 | 29.6 ± 17.3 | ns | ns | ns |
| Homoterpenes | |||||||
| (E)-DMNT 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 4.6 ± 2.9 | 20.6 ± 9.2 | 0.096 | ns | ns |
| Sesquiterpenoids | |||||||
| Unknown RI 1438.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | ns | ns | ns |
| Unknown RI 1506.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.3 ± 1.2 | ns | ns | ns |
| (E. E)-α-Farnesene | 0.6 ± 0.6 | 0.3 ± 0.3 | 7.9 ± 2.3 | 19.2 ± 7.1 | ns | ns | ns |
| GLVs + MeSA2 | |||||||
| (Z)-3-Hexenal | 13.1 ± 13.1 | 0.0 ± 0.0 | 20.3 ± 12.9 | 55.70 ± 34.50 | ns | ns | ns |
| Hexanal | 6.8 ± 2.7 | 11.06 ± 3.36 | 17.0 ± 2.0 | 36.57 ± 18.33 | 0.005 | 0.061 | ns |
| (E)-2-Hexenal | 7.5 ± 7.5 | 0.0 ± 0.0 | 45.6 ± 21.8 | 111.02 ± 37.78 | ns | ns | ns |
| (Z)-3-Hexenol | 69.8 ± 62.0 | 33.20 ± 16.0 | 92.5 ± 29.8 | 190.26 ± 52.01 | ns | ns | ns |
| (Z)-3-Hexenyl acetate | 187.6 ± 145.2 | 147.1 ± 54.2 | 359.2 ± 128.5 | 719.8 ± 177.9 | ns | ns | ns |
| Hexyl acetate | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 6.7 ± 4.7 | ns | ns | ns |
| MeSA 2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 20.1 ± 8.2 | 0.017 | ns | 0.017 |
| N-/or S-containing compounds | |||||||
| Dimethyl disulfide | 4.3 ± 2.9 | 0.0 ± 0.0 | 3.5 ± 1.0 | 3.50 ± 1.24 | ns | ns | ns |
| 2-methyl-5-hexenenitrile | 0.0 ± 0.0 | 0.0 ± 0.0 | 6.3 ± 6.3 | 9.5 ± 9.5 | ns | ns | ns |
References
- Kirtman, B.; Power, S.B. Near-term climate change: Projections and predictability. In Climate Change 201 Contribution of Working Group i to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 953–1028. [Google Scholar]
- Mills, G.; Pleijel, H.; Malley, C.S.; Sinha, B.; Cooper, O.R.; Schultz, M.G. Tropospheric ozone assessment report: Present-day ozone distribution and trends relevant to vegetation. Elem. Sci. Anthr. 2018, 6, 2–46. [Google Scholar] [CrossRef]
- Sitch, S.; Cox, P.M.; Collins, W.J.C.; Huntingford, C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 2007, 448, 791–794. [Google Scholar] [CrossRef]
- Lehtomäki, H.; Geels, C.; Brandt, J.; Rao, S.; Yaramenka, K.; Åström, S.; Skou Andersen, M.; Frohn, L.M.; Im, U.; Hänninen, O. Deaths attributable to air pollution in Nordic countries: Disparities in the estimates. Atmosphere 2020, 11, 467. [Google Scholar] [CrossRef]
- Iriti, M.; Faora, F. Oxidative stress, the paradigm of ozone toxicity in plants and animals. Water Air Soil Pollut. 2008, 187, 285–301. [Google Scholar] [CrossRef]
- Viaene, P.; Deutsch, F.; Mensink, C.; Vandermeiren, K.; Vancraeynest, L.; Vanpoucke, C.; Fierens, F. Assessment of damage to vegetation in belgium based on an ozone flux model approach. Air Pollut. Model. Appl. 2016, 24, 147–153. [Google Scholar]
- Leisner, C.P.; Ainsworth, E.A. Quantifying the effects of ozone on plant reproductive growth and development. Glob. Chang. Biol. 2012, 18, 606–616. [Google Scholar] [CrossRef]
- Kangasjärvi, J.; Talvinen, J.; Utriainen, M.; Karjalainen, R. Plant defence systems induced by ozone. Plant Cell Environ. 1994, 17, 783–794. [Google Scholar] [CrossRef]
- Kangasjärvi, J.; Jaspers, P.; Kollist, H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2015, 28, 1021–1036. [Google Scholar] [CrossRef]
- Pleijel, H.; Norberg, P.A.; Selldén, G.; Skärby, L. Tropospheric ozone decreases biomass production in radish plants (Raphanus sativus) grown in rural south-west Sweden. Environ. Pollut. 1999, 106, 143–147. [Google Scholar] [CrossRef]
- Wilkinson, S.; Mills, G.; Illidge, R.; Davies, W.J. How is ozone pollution reducing our food supply? J. Exp. Bot. 2012, 63, 527–536. [Google Scholar] [CrossRef]
- Avnery, S.; Mauzerall, D.L.; Liu, J.; Horowitz, L.W. Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmos. Environ. 2011, 45, 2284–2296. [Google Scholar] [CrossRef]
- Evans, L.T. The History of Agricultural Production. Tree 1993, 8, 461. [Google Scholar]
- Chen, Y.H.; Langellotto, G.A.; Barrion, A.T. Cultivation of domesticated rice alters arthropod biodiversity and community composition. Annu. Rev. Entomol. 2013, 106, 100–110. [Google Scholar] [CrossRef]
- Chen, Y.H.; Gols, R.; Benrey, B. Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 2015, 60, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Eckardt, N.A. Evolution of domesticated bread wheat. Plant Biol. 2010, 22, 993. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Turcotte, M.M.; Poveda, K. Domestication impacts on plant-herbivore interactions: A meta-analysis. Phil. Trans. R. Soc. 2017, 372, 20160034. [Google Scholar] [CrossRef]
- Turcotte, M.M.; Turley, N.E.; Johnson, M.T.J. The impact of domestication on resistance to two generalist herbivores across 29 independent domestication events. New Phytol. 2014, 204, 671–681. [Google Scholar] [CrossRef]
- Chaudhary, B. Plant domestication and resistance to herbivory. Int. J. Plant Genom. 2013, 2013, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Chacón-Fuentes, M.; Parra, L.; Lizama, M.; Seguel, I.; Urzúa, A.; Quiroz, A. Plant flavonoid content modified by domestication. Environ. Entomol. 2017, 46, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Moreira, X.; Abdala-Roberts, L.; Gols, R.; Francisco, M. Plant domestication decreases both constitutive and induced chemical defences by direct selection against defensive traits. Sci. Rep. 2018, 8, 12678. [Google Scholar] [CrossRef]
- Mithen, R.F.; Lewis, B.G.; Heaney, R.K.; Fenwick, G.R. Glucosinolates of wild and cultivated Brassica species. Phytochemistry 1987, 26, 1969–1973. [Google Scholar]
- Moyes, C.L.; Collin, H.A.; Britton, G.; Raybould, A.F. Glucosinolates and differential herbivory in wild population of Brassica oleracea. J. Chem. Ecol. 2000, 26, 2625–2641. [Google Scholar] [CrossRef]
- Altesor, P.; Garcia, A.; Font, E.; Rodríguez-Haralambides, A.; Vilaró, F.; Oesterheld, M.; Soler, R.; González, G. Glycoalkaloids of wild and cultivated solanum: Effects on specialist and generalist insect herbivores. J. Chem. Ecol. 2014, 40, 599–608. [Google Scholar] [CrossRef]
- Maag, D.; Erb, M.; Bernal, J.S.; Wolfender, J.; Turlings, T.C.J. Maize domestication and anti-herbivore defences: Leaf-specific dynamics during early ontogeny of maize and its wild ancestors. PLoS ONE 2015, 8, 1–21. [Google Scholar] [CrossRef] [Green Version]
- De Lange, E.S.; Balmer, D.; Mauch-mani, B.; Ted, C.J. Insect and pathogen attack and resistance in maize and its wild ancestors; the teosintes. New Phytol. 2014, 204, 329–341. [Google Scholar] [CrossRef]
- Rodriguez-saona, C.; Vorsa, N.; Singh, A.P.; Johnson-Cicalese, J.; Szendrei, Z.; Mescher, M.C.; Forst, C.J. Tracing the history of plant traits under domestication in cranberries: Potential consequences on anti-herbivore defences. J. Exp. Botany 2011, 62, 2633–2644. [Google Scholar] [CrossRef]
- Gols, R.; Bukovinszky, T.; Van Dam, N.M.; Dicke, M.; Bullock, J.M.; Harvey, J.A. Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. J. Chem. Ecol. 2008, 34, 132–143. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive Function of herbivore-induced plant volatile emission in nature. Science 2001, 291, 2141. [Google Scholar] [CrossRef]
- Benrey, B.; Callejas, A.; Rios, L.; Oyama, K.; Denno, R.F. The effects of domestication of brassica and phaseolus on the interaction between phytophagous insects and parasitoids. Biol. Cont. 1998, 11, 130–140. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Cloonan, K.R.; Sanchez-Pedraza, F.; Zhou, Y.; Giusti, M.M.; Benrey, B. Differential susceptibility of wild and cultivated blueberries to an invasive frugivorous pest. J. Chem. Ecol. 2019, 45, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Rowen, E.; Kaplan, I. Eco-evolutionary factors drive induced plant volatiles: A metaanalysis. New Phytol. 2016, 210, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillard, M.D.P.; Glauser, G.; Robert, C.A.M.; Turlings, T.C.J. Fine-tuning the ‘plant domestication-reduced defense’ hypothesis: Specialist vs generalist herbivores. New Phytol. 2018, 217, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paudel, S.; Lin, P.A.; Foolad, M.R.; Ali, J.G.; Rajotte, E.G.; Felton, G.W. Induced plant defenses against herbivory in cultivated and wild tomato. J. Chem. Ecol. 2019, 45, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Heiden, A.; Hoffmann, T.; Kahl, D.; Flockow, D.; Wildt, J. Emission of volatile organic compounds from ozone-exposed plants. Ecol. Appl. 1999, 9, 1160–1167. [Google Scholar] [CrossRef]
- Fares, S.; Loreto, F.; Kleist, E.; Wildt, J. Stomatal uptake and stomatal deposition of ozone in isoprene and monoterpene emitting plants. Plant Biol. 2008, 10, 44–54. [Google Scholar] [CrossRef]
- Himanen, S.J.; Nerg, A.M.; Nissinen, A.; Pinto, D.M.; Stewart Jr, C.N.; Poppy, G.M.; Holopainen, J.K. Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytol. 2008, 181, 174–186. [Google Scholar] [CrossRef]
- Beauchamp, J.; Wisthaler, A.; Hansel, A.; Kleist, E.; Miebach, M. Ozone induced emissions of biogenic VOC from tobacco: Relationships between ozone uptake and emission of LOX products Volatile organic compound (VOC) emissions from tobacco. Cell 2005, 28, 1334–1343. [Google Scholar]
- Khaling, E.; Li, T.; Holopainen, J.K.; Blande, J.D. Elevated ozone modulates herbivore-induced volatile emissions of Brassica nigra and alters a tritrophic interaction. J. Chem. Ecol. 2016, 42, 368–381. [Google Scholar] [CrossRef]
- Brosset, A.; Saunier, A.; Kivimäenpää, M.; Blande, J.D. Does ozone exposure modify herbivore induced plant volatile emissions? Environ. Sci. Pollut. Res. 2020, 27, 30448–30459. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, S.; Li, Z.; Cowling, W.A. Center of origin and centers of diversity in an ancient crop, Brassica rapa (turnip rape). J. Hered. 2014, 105, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Campo, C.; Prakash, S. Origin and domestication. Biol. Brassica Coenospecies 1999, 4, 33–57. [Google Scholar]
- Pinto, D.M.; Blande, J.D.; Nykänen, R. Ozone degrades common herbivore-induced plant volatiles: Does this affect herbivore prey location by predators and parasitoids? J. Chem. Ecol. 2007, 1, 683–694. [Google Scholar] [CrossRef]
- Agathokleous, E.; Yu, W.L.; Ntatsi, G.; Konno, K.; Costas, J.S.; Kitaoa, M.; Koike, T. Effects of ozone and ammonium sulfate on cauliflower: Emphasis on the interaction between plants and insect herbivores. Sci. Total Environ. 2019, 659, 995–1007. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Khaling, E.; Papazian, S.; Poelman, E.H.; Holopainen, J.K.; Albrectsen, B.R.; Blande, J.D. Ozone affects growth and development of Pieris brassicae on the wild host plant Brassica nigra. Environ. Pollut. 2015, 199, 119–129. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Hermenau, U.; Baldwin, I. Instar-specific sensitivity of specialist Manduca sexta larvae to induced defences in their host plant Nicotina attenuate. Ecol. Entomol. 2001, 26, 578–586. [Google Scholar] [CrossRef]
- Do Boer, J.D.; Dicke, M. The Role of Methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem. Ecol. 2004, 30, 255–271. [Google Scholar] [CrossRef]
- Zhu, J.; Park, K. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–17460. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Gols, R.; Bullock, M.; Dicked, M.; Bukovinszky, T.; Harvey, J.A. Smelling the wood from the trees: Non-linear parasitoid responses to volatile attractants produced by wild and cultivated cabbage. J. Chem. Ecol. 2011, 37, 795–807. [Google Scholar] [CrossRef]
- Marazzi, C.; Patrian, B.; Städler, E. Secondary metabolites of the leaf surface affected by sulphur fertilisation and perceived by the diamondback moth. Chemoecoly. 2004, 14, 81–86. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Reichelt, M.; Heckel, D.G. Can sulfur fertilisation improve the effectiveness of trap crops for diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)? Pest. Manag. Sci. 2010, 66, 832–838. [Google Scholar] [CrossRef]
- Loreto, F.; Mannozzi, L.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Black, V.J.; Black, C.R.; Roberts, J.A.; Stewart, C.A. Impact of ozone on the reproductive development of plants. New Phytol. 2000, 147, 421–447. [Google Scholar] [CrossRef]
- Ashmore, M.R.; Davison, A.W. Towards a critical level of ozone for natural vegetation. In Critical Levels for Ozone in Europe: Testing and Finalising the Concepts; UNECE Workshop Report; University of Kuopio: Kuopio, Finland, 1996; pp. 58–71. [Google Scholar]
- Agathokleous, E.; Saitanis, C.J.; Koike, T. Tropospheric O3, the nightmare of wild plants: A review study. J. Agric. Meteorol. 2015, 71, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.K.; Li, Y.G.; Chen, Y.H.; Sun, J.Z.; Jiang, G.M. Assessing the genetic relatedness of higher ozone sensitivity of modern wheat to its wild and cultivated progenitors relatives. J. Exp. Bot. 2008, 59, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Morgan, P.B.; Ainsworth, E.A.; Long, S.P. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ. 2003, 26, 1317–1328. [Google Scholar] [CrossRef]
- Fiscus, E.L.; Booker, F.L.; Burkey, K.O. Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ. 2005, 28, 997–1011. [Google Scholar] [CrossRef]
- Baêsso Moura, B.; Alves, E.S.; Marabesi, M.A.; Ribeiro de Souza, S.; Schaub, M.; Vollenweider, P. Ozone affects leaf physiology and causes injury to foliage of native tree species from the tropical Atlantic Forest of southern Brazil. Sci. Total Environ. 2018, 610–611, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Vainonen, J.P.; Kangasjärvi, J. Plant signalling in acute ozone exposure. Plant Cell Environ. 2015, 38, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, T.; Nerg, A.M.; Holopainen, J.K. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but does not disturb tritrophic signalling. Environ. Pollut. 2004, 131, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E.; Gogerddan, P. The hypersensitive response, the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef] [Green Version]
- Mithöfer, A.; Wanner, G.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves. ii. continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol. 2005, 137, 1160–1168. [Google Scholar] [CrossRef] [Green Version]
- Vuorinen, T.; Nerg, A.; Ibrahim, M.A.; Reddy, G.V.P.; Holopainen, J.K. Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol. 2004, 135, 1984–1992. [Google Scholar] [CrossRef] [Green Version]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci. 2012, 196, 93–100. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsui, K.; Akakabe, Y.; Muramoto, S.; Ozawa, R.; Uefune, M. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc. Natl. Acad. Sci. USA 2014, 111, 7144–7149. [Google Scholar] [CrossRef] [Green Version]
- Heil, M.; Carlos, J.; Bueno, S. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.G.; Coleman, J.S. Plant stress and insect behavior: Cottonwood ozone and the feeding and oviposition preference of a Beetle. Oecologia 1988, 76, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bolsinger, M.; Lier, M.E.; Hughes, P.R. Influence of ozone air pollution on plant- herbivore interactions. Part 2: Effects of ozone on feeding preference, growth and consumption rates of monarch butterflies (Danaus plexippus). Environ. Pollut. 1992, 77, 31–37. [Google Scholar] [CrossRef]
- Jøndrup, P.M.; Barnes, J.D.; Port, G.R. The effect of ozone fumigation and different Brassica rapa lines on the feeding behaviour of Pieris brassicae larvae. Entomol. Exp. Appl. 2002, 104, 143–151. [Google Scholar] [CrossRef]
- Vingarzan, R. A review of surface ozone background levels and trends. Atmos. Environ. 2004, 38, 3431–3442. [Google Scholar] [CrossRef]

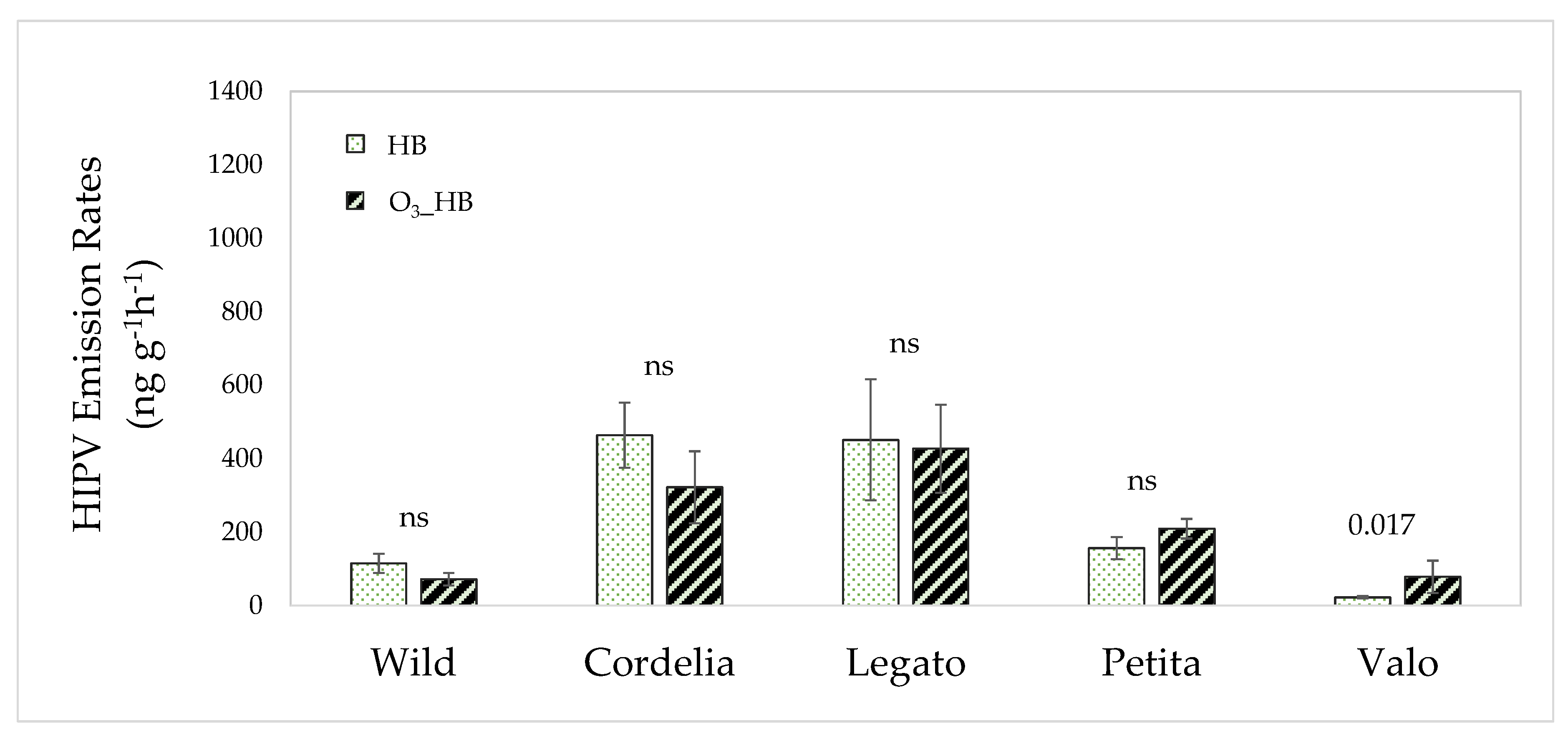

| Variety | O3 | HB | HB*O3 |
|---|---|---|---|
| Wild | ns | ns | 0.012 |
| Cordelia | ns | 0.002 | ns |
| Legato | ns | 0.001 | ns |
| Petita | ns | 0.001 | ns |
| Valo | 0.094 | 0.003 | ns |
| Variety | Herbivore-Induced Plant Volatiles | Ozone-Induced Volatiles | Interactive Effect |
|---|---|---|---|
| Wild | (E)-DMNT | β-Pinene | |
| (E)-Caryophyllene | |||
| α-Humulene | |||
| (Z)-3-Hexenyl acetate | (Z)-3-Hexenyl acetate | ||
| Methyl salicylate | |||
| 2-methyl-5-hexenenitrile | |||
| Cordelia | α-Pinene | ||
| (E)-DMNT | |||
| (E,E)-α-Farnesene | |||
| (E)-2-Hexenal | |||
| (Z)-3-Hexenol | |||
| (Z)-3-Hexenyl acetate | |||
| Hexyl acetate | |||
| Methyl salicylate | Methyl salicylate | ||
| Legato | (E)-DMNT | Benzyl alcohol | |
| (E)-Caryophyllene | |||
| Unknown RI 1459.2 | |||
| (E, E)-α-Farnesene | |||
| (Z)-3-Hexenyl acetate | |||
| Methyl salicylate | |||
| Petita | (E)-DMNT | ||
| Unknown RI 1461.5 | |||
| Unknown RI 1438.6 | |||
| Unknown RI 1426.9 | |||
| (E, E)-α-Farnesene | (E, E)-α-Farnesene | ||
| (E)-2-Hexenal | |||
| Valo | Myrcene | Hexanal | |
| (E)-DMNT | |||
| Hexanal | |||
| Methyl salicylate | Methyl salicylate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brosset, A.; Saunier, A.; Mofikoya, A.O.; Kivimäenpää, M.; Blande, J.D. The Effects of Ozone on Herbivore-Induced Volatile Emissions of Cultivated and Wild Brassica Rapa. Atmosphere 2020, 11, 1213. https://doi.org/10.3390/atmos11111213
Brosset A, Saunier A, Mofikoya AO, Kivimäenpää M, Blande JD. The Effects of Ozone on Herbivore-Induced Volatile Emissions of Cultivated and Wild Brassica Rapa. Atmosphere. 2020; 11(11):1213. https://doi.org/10.3390/atmos11111213
Chicago/Turabian StyleBrosset, Agnès, Amélie Saunier, Adedayo O. Mofikoya, Minna Kivimäenpää, and James D. Blande. 2020. "The Effects of Ozone on Herbivore-Induced Volatile Emissions of Cultivated and Wild Brassica Rapa" Atmosphere 11, no. 11: 1213. https://doi.org/10.3390/atmos11111213
APA StyleBrosset, A., Saunier, A., Mofikoya, A. O., Kivimäenpää, M., & Blande, J. D. (2020). The Effects of Ozone on Herbivore-Induced Volatile Emissions of Cultivated and Wild Brassica Rapa. Atmosphere, 11(11), 1213. https://doi.org/10.3390/atmos11111213







