The Importance of Microbial Inoculants in a Climate-Changing Agriculture in Eastern Mediterranean Region
Abstract
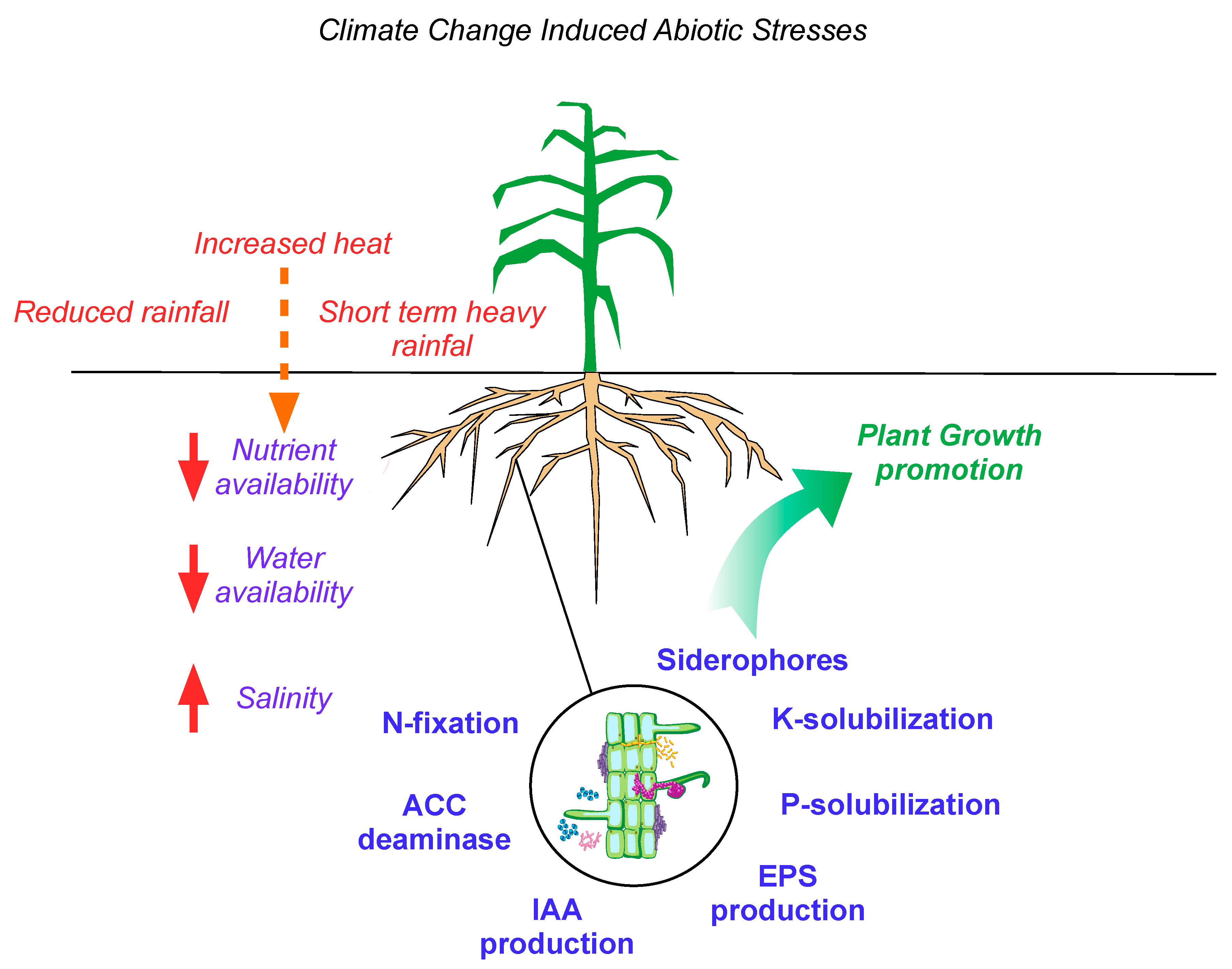
1. Introduction
2. Microbial Functions Benefit the Adaptation of Crops to Climate Change
2.1. Nitrogen Fixation Serves as a Mechanism against Nitrogen Deficiency in Soils—An Indirect Effect of Climate Change
2.2. Phytohormone Production: A Multitask Mechanism to Mitigate Drought and Salt Stress
2.3. Regulation of Inhibitory Ethylene Levels in Plant Tissues
2.4. Improved Phosphorus Availability and Acquisition Efficiency
2.5. Potassium Solubilization
2.6. Siderophore Production: Another Mechanism to Assist Plant Growth under Stress Conditions
3. Multifunctional Inoculants: The Concept of Synthetic Microbial Communities
4. Future Research Needs and Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Lelieveld, J.; Proestos, Y.; Hadjinicolaou, P.; Tanarhte, M.; Tyrlis, E.; Zittis, G. Strongly increasing heat extremes in the Middle East and North Africa (MENA) in the 21st century. Clim. Chang. 2016, 137, 245–260. [Google Scholar] [CrossRef]
- Lelieveld, J.; Hadjinicolaou, P.; Kostopoulou, E.; Chenoweth, J.; El Maayar, M.; Giannakopoulos, C.; Hannides, C.; Lange, M.A.; Tanarhte, M.; Tyrlis, E.; et al. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Chang. 2012, 114, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P. 21st century climate change in the Middle East. Clim. Chang. 2009, 92, 417–432. [Google Scholar] [CrossRef]
- Juma, C.; Asamoah, A. The New Harvest: Agricultural Innovation in Africa; Oxford University Press: Oxford, UK, 2011; Volume 38. [Google Scholar]
- Buck, S.D.; Oliveira, D.D.; Montagu, M.V. Key innovations in plant biotechnology and their applications in agriculture, industrial processes, and healthcare. Int. Plant Biotechnol. Outreach 2002, 13–33. [Google Scholar]
- Savci, S. An Agricultural Pollutant: Chemical Fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73–80. [Google Scholar] [CrossRef]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef]
- FAO. Agriculture Sourcebook Summary Climate-Smart; FAO: Rome, Italy, 2017; pp. 1–47. [Google Scholar]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Akiyama, H.; Hoshino, Y.T.; Itakura, M.; Shimomura, Y.; Wang, Y.; Yamamoto, A.; Tago, K.; Nakajima, Y.; Minamisawa, K.; Hayatsu, M. Mitigation of soil N2O emission by inoculation with a mixed culture of indigenous Bradyrhizobium diazoefficiens. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Itakura, M.; Uchida, Y.; Akiyama, H.; Hoshino, Y.T.; Shimomura, Y.; Morimoto, S.; Tago, K.; Wang, Y.; Hayakawa, C.; Uetake, Y.; et al. Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat. Clim. Chang. 2013, 3, 208–212. [Google Scholar] [CrossRef]
- Hénault, C.; Revellin, C. Inoculants of leguminous crops for mitigating soil emissions of the greenhouse gas nitrous oxide. Plant Soil 2011, 346, 289–296. [Google Scholar] [CrossRef]
- Nazaries, L.; Pan, Y.; Bodrossy, L.; Baggs, E.M.; Millard, P.; Murrell, J.C.; Singh, B.K. Evidence of Microbial Regulation of Biogeochemical Cycles from a Study on Methane Flux and Land Use Change. Appl. Environ. Microbiol. 2013, 79, 4031–4040. [Google Scholar] [CrossRef] [PubMed]
- Azcón, R. Selective interaction between free-living rhizosphere bacteria and vesicular arbuscular mycorrhizal fungi. Soil Biol. Biochem. 1989, 21, 639–644. [Google Scholar] [CrossRef]
- Singh, B.K.; Trivedi, P. Microbiome and the future for food and nutrient security. Microb. Biotechnol. 2017, 10, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Goodman, R.M. Host variation for interactions with beneficial plant-associated microbes. Annu. Rev. Phytopathol. 1999, 37, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Sammauria, R.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial inoculants: Potential tool for sustainability of agricultural production systems. Arch. Microbiol. 2020, 202, 677–693. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Porat, R.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.E.; Droby, S. Induction of resistance to Penicillium digitatum in grapefruit by β-aminobutyric acid. Eur. J. Plant Pathol. 2003, 109, 901–907. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of Drought on Nutrient Uptake and Assimilation in Vegetable Crops. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Tomer, M.D.; Schilling, K.E. A simple approach to distinguish land-use and climate-change effects on watershed hydrology. J. Hydrol. 2009, 376, 24–33. [Google Scholar] [CrossRef]
- Zittis, G.; Hadjinicolaou, P.; Lelieveld, J. Role of soil moisture in the amplification of climate warming in the eastern Mediterranean and the Middle East. Clim. Res. 2014, 59, 27–37. [Google Scholar] [CrossRef]
- Flowers, T.J.; Garcia, A.; Koyama, M.; Yeo, A.R. Breeding for salt tolerance in crop plants—The role of molecular biology. Acta Physiol. Plant. 1997, 19, 427–433. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Defez, R. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 2009, 60, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Otieno, N.; Lally, R.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.; Dowling, D. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Wu, X.; Yang, Q.; Luo, Y.; Christie, P. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ. Geochem. Health 2006, 28, 133–140. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, L. Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J. Basic Microbiol. 2013, 53. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Sutton, M.A.; Billen, G.; Bleeker, A.; Erisman, J.W.; Grennfelt, P.; Grinsven, H.V.; Grizzetti, B.; Howard, C.M.; Leip, A. Technical Summary Part I Nitrogen in Europe: The Present Position; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Myrbeck, Å.; Stenberg, M.; Rydberg, T. Establishment of winter wheat-Strategies for reducing the risk of nitrogen leaching in a cool-temperate region. Soil Tillage Res. 2012, 120, 25–31. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J.; et al. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- Omirou, M.; Anastopoulos, I.; Fasoula, D.A.; Ioannides, I.M. The effect of chemical and organic N inputs on N2O emission from rain-fed crops in Eastern Mediterranean. J. Environ. Manag. 2020, 270, 110755. [Google Scholar] [CrossRef] [PubMed]
- Anglade, J.; Billen, G.; Garnier, J. Relationships for estimating N2 fixation in legumes: Incidence for N balance of legume-based cropping systems in europe. Ecosphere 2015, 6, 1–24. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Reed, S. Functional biology of heterotrophic nitrogen-fixing bacteria. Annu. Rev. Ecol. Evol. Syst. 2011, 42. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobia from wild legumes: Diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J. Biotechnol. 2001, 91, 143–153. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Neiverth, A.; Delai, S.; Garcia, D.M.; Saatkamp, K.; de Souza, E.M.; Pedrosa, F.d.O.; Guimarães, V.F.; dos Santos, M.F.; Vendruscolo, E.C.G.; da Costa, A.C.T. Performance of different wheat genotypes inoculated with the plant growth promoting bacterium Herbaspirillum seropedicae. Eur. J. Soil Biol. 2014, 64, 1–5. [Google Scholar] [CrossRef]
- Naiman, A.D.; Latrónico, A.; García de Salamone, I.E. Inoculation of wheat with Azospirillum brasilense and Pseudomonas fluorescens: Impact on the production and culturable rhizosphere microflora. Eur. J. Soil Biol. 2009, 45, 44–51. [Google Scholar] [CrossRef]
- Herrera, J.M.; Rubio, G.; Häner, L.L.; Delgado, J.A.; Lucho-Constantino, C.A.; Islas-Valdez, S.; Pellet, D. Emerging and established technologies to increase nitrogen use efficiency of cereals. Agronomy 2016, 6, 25. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Perini, L.; Silva, M.B.; de Sousa, N.V.; Scapim, C.A.; De Oliveira, A.L.M.; Do Amaral, A.T.; Gonçalves, L.S.A. Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PLoS ONE 2019, 14, 1–19. [Google Scholar] [CrossRef]
- Zilli, J.É.; Alves, B.J.R.; Rouws, L.F.M.; Simões-Araujo, J.L.; de Barros Soares, L.H.; Cassán, F.; Castellanos, M.O.; O’Hara, G. The importance of denitrification performed by nitrogen-fixing bacteria used as inoculants in South America. Plant Soil 2020, 451, 5–24. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Philippot, L.; Peyrard, C.; Bru, D.; Breuil, M.-C.; Bizouard, F.; Justes, E.; Mary, B.; Léonard, J.; Spor, A. Peaks of in situ N2O emissions are influenced by N2O-producing and reducing microbial communities across arable soils. Glob. Chang. Biol. 2018, 24, 360–370. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, X.; Smith, D.L. Crop ecology, management and quality: Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci. 2003, 43, 1774–1781. [Google Scholar] [CrossRef]
- Rajendran, G.; Sing, F.; Desai, A.J.; Archana, G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresour. Technol. 2008, 99, 4544–4550. [Google Scholar] [CrossRef] [PubMed]
- Vicario, J.; Gallarato, L.; Paulucci, N.; Perrig, D.; Bueno, M.A.; Dardanelli, M. Co-inoculation of Legumes with Azospirillum and Symbiotic Rhizobia. In Handbook for Azospirillum; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Tilak, K.V.B.R.; Ranganayaki, N.; Manoharachari, C. Synergistic effects of plant-growth promoting rhizobacteria and Rhizobium on nodulation and nitrogen fixation by pigeonpea (Cajanus cajan). Eur. J. Soil Sci. 2006, 57, 67–71. [Google Scholar] [CrossRef]
- Baset Mia, M.A.; Shamsuddin, Z.H. Nitrogen fixation and transportation by rhizobacteria: A scenario of rice and banana. Int. J. Bot. 2010, 6, 235–242. [Google Scholar] [CrossRef][Green Version]
- Nocker, A.; Fernández, P.S.; Montijn, R.; Schuren, F. Effect of air drying on bacterial viability: A multiparameter viability assessment. J. Microbiol. Methods 2012, 90, 86–95. [Google Scholar] [CrossRef]
- Nafis, A.; Raklami, A.; Bechtaoui, N.; El Khalloufi, F.; El Alaoui, A.; Glick, B.R.; Hafidi, M.; Kouisni, L.; Ouhdouch, Y.; Hassani, L. Actinobacteria from Extreme Niches in Morocco and Their Plant Growth-Promoting Potentials. Diversity 2019, 11, 139. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Santos, S.N.; Silva, J.L.D.; Parma, M.M.; Ávila, L.A.; Visconti, A.; Zucchi, T.D.; Taketani, R.G.; Andreote, F.D.; Melo, I.S.D. Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol. Res. 2013, 168, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Lugtenberg, B.J.J.; Chin-A-Woeng, T.F.C.; Bloemberg, G.V. Microbe–plant interactions: Principles and mechanisms. Antonie Van Leeuwenhoek 2002, 81, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere Bacterial Signalling: A Love Parade Beneath Our Feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef]
- Kaur, J.; Gangwar, M.; Pandove, G. Mitigating the impact of climate change by use of microbial inoculants. Pharma Innov. J. 2018, 7, 279–288. [Google Scholar] [CrossRef]
- Shilev, S.; Azaizeh, H.; Vassilev, N.; Georgiev, D.; Babrikova, I. Interactions in Soil-Microbe-Plant System: Adaptation to Stressed Agriculture. In Microbial Interventions in Agriculture and Environment: Volume 1: Research Trends, Priorities and Prospects; Singh, D.P., Gupta, V.K., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 131–171. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Schillaci, M.; Gupta, S.; Walker, R. The Role of Plant Growth-Promoting Bacteria in the Growth of Cereals under Abiotic Stresses; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.-M.; Azcón, R. Stimulation of Plant Growth and Drought Tolerance by Native Microorganisms (AM Fungi and Bacteria) from Dry Environments: Mechanisms Related to Bacterial Effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.-L.; Luo, L. Effects of Engineered Sinorhizobium meliloti on Cytokinin Synthesis and Tolerance of Alfalfa to Extreme Drought Stress. Appl. Environ. Microbiol. 2012, 78, 8056–8061. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Czarny, J.C.; Grichko, V.P.; Glick, B.R. Genetic modulation of ethylene biosynthesis and signaling in plants. Biotechnol. Adv. 2006, 24, 410–419. [Google Scholar] [CrossRef]
- Singh, R.; Shelke, G.; Kumar, A.; Jha, P. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar] [PubMed]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability 2020, 12, 2159. [Google Scholar]
- Orozco-Mosqueda, M.d.C.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The Production of ACC Deaminase and Trehalose by the Plant Growth Promoting Bacterium Pseudomonas sp. UW4 Synergistically Protect Tomato Plants Against Salt Stress. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Tavares, M.J.; Franck, J.; Ali, S.; Glick, B.R.; Rossi, M.J. ACC deaminase plays a major role in Pseudomonas fluorescens YsS6 ability to promote the nodulation of Alpha- and Betaproteobacteria rhizobial strains. Arch. Microbiol. 2019, 201, 817–822. [Google Scholar] [CrossRef]
- Tavares, M.J.; Nascimento, F.X.; Glick, B.R.; Rossi, M.J. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Lett. Appl. Microbiol. 2018, 66, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, I.; Holloway, R.E.; Armstrong, R.D.; McLaughlin, M.J. Chemical characteristics of phosphorus in alkaline soils from southern Australia. Soil Res. 2003, 41, 61–76. [Google Scholar] [CrossRef]
- Richardson, A. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Jilani, G.; Akram, A.; Ali, R.M.; Hafeez, F.Y.; Shamsi, I.H.; Chaudhry, A.N.; Chaudhry, A.G. Enhancing crop growth, nutrients availability, economics and beneficial rhizosphere microflora through organic and biofertilizers. Ann. Microbiol. 2007, 57, 177–184. [Google Scholar] [CrossRef]
- Yazdani, M.; Bahmanyar, M.A.; Pirdashti, H.; Esmaili, M.A. Effect of phosphate solubilization microorganisms (PSM) and plant growth promoting rhizobacteria (PGPR) on yield and yield components of corn (Zea mays L.). World Acad. Sci. Eng. Technol. 2009, 37, 90–92. [Google Scholar]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Naresh Kumar, G.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Hussain, M.I.; Asghar, H.N.; Akhtar, M.J.; Arshad, M. Impact of phosphate solubilizing bacteria on growth and yield of maize. Soil Environ. 2013, 32, 71–78. [Google Scholar]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 2014, 3, 121–128. [Google Scholar] [CrossRef]
- Kumar, A. Phosphate solubilizing bacteria in agriculture biotechnology: Diversity, mechanism and their role in plant growth and crop yield. Int. J. Adv. Res. 2016, 4, 116–124. [Google Scholar] [CrossRef]
- Mirza, B.S.; Rodrigues, J.L.M. Development of a direct isolation procedure for free-living diazotrophs under controlled hypoxic conditions. Appl. Environ. Microbiol. 2012, 78, 5542–5549. [Google Scholar] [CrossRef]
- Coutinho, F.P.; Felix, W.P.; Yano-Melo, A.M. Solubilization of phosphates in vitro by Aspergillus spp. and Penicillium spp. Ecol. Eng. 2012, 42, 85–89. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Sui, J.; Liu, Z.; Li, Q.; Ji, C.; Song, X.; Hu, Y.; Wang, C.; Sa, R.; et al. Isolation and characterization of phosphofungi, and screening of their plant growth-promoting activities. AMB Express 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Omirou, M.; Ioannides, I.M.; Ehaliotis, C. Mycorrhizal inoculation affects arbuscular mycorrhizal diversity in watermelon roots, but leads to improved colonization and plant response under water stress only. Appl. Soil Ecol. 2013, 63, 112–119. [Google Scholar] [CrossRef]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, Á.; Seguel, A. Phosphorus acquisition efficiency related to root traits: Is mycorrhizal symbiosis a key factor to wheat and barley cropping? Front. Plant Sci. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Loeher, M.; Cavagnaro, T.R. Ecological intensification and arbuscular mycorrhizas: A meta-analysis of tillage and cover crop effects. J. Appl. Ecol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The Association with Two Different Arbuscular Mycorrhizal Fungi Differently Affects Water Stress Tolerance in Tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef]
- Kavadia, A.; Omirou, M.; Fasoula, D.; Trajanoski, S.; Andreou, E.; Ioannides, I.M. Genotype and soil water availability shape the composition of AMF communities at chickpea early growth stages. Appl. Soil Ecol. 2020, 150, 103443. [Google Scholar] [CrossRef]
- Toro, M.; Azcon, R.; Barea, J. Improvement of Arbuscular Mycorrhiza Development by Inoculation of Soil with Phosphate-Solubilizing Rhizobacteria To Improve Rock Phosphate Bioavailability ((sup32)P) and Nutrient Cycling. Appl. Environ. Microbiol. 1997, 63, 4408–4412. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Trotta, A.; Massa, N.; Copetta, A.; Martinotti, M.G.; Berta, G. Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 2004, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nazir, R.; Warmink, J.A.; Boersma, H.; Van Elsas, J.D. Mechanisms that promote bacterial fitness in fungal-affected soil microhabitats. FEMS Microbiol. Ecol. 2009, 71, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, J.; Ding, X.; He, X.; Zhang, F.; Feng, G. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol. Biochem. 2014, 74, 177–183. [Google Scholar] [CrossRef]
- Ordoñez, Y.M.; Fernandez, B.R.; Lara, L.S.; Rodriguez, A.; Uribe-Vélez, D.; Sanders, I.R. Bacteria with Phosphate Solubilizing Capacity Alter Mycorrhizal Fungal Growth Both Inside and Outside the Root and in the Presence of Native Microbial Communities. PLoS ONE 2016, 11, e0154438. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.K.; Mahiwal, S. Role of Potassium in Plants; Springer International Publishing: Basel, Switzerland, 2020. [Google Scholar]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Institute, I.P.; Appel, T. Principles of Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Öborn, I.; Andrist-Rangel, Y.; Askekaard, M.; Grant, C.A.; Watson, C.A.; Edwards, A.C. Critical aspects of potassium management in agricultural systems. Soil Use Manag. 2005, 21, 102–112. [Google Scholar] [CrossRef]
- Sheng, X.F.; He, L.Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef]
- Parmar, P.; Sindhu, S.S. Potassium Solubilization by Rhizosphere Bacteria: Influence of Nutritional and Environmental Conditions. J. Microbiol. Res. 2013, 3, 25–31. [Google Scholar] [CrossRef]
- Sheng, X.F. Growth promotion and increased potassium uptake of cotton and rape by a potassium releasing strain of Bacillus edaphicus. Soil Biol. Biochem. 2005, 37, 1918–1922. [Google Scholar] [CrossRef]
- Lian, B.; Wang, B.; Pan, M.; Liu, C.; Teng, H. Microbial release of potassium from K-bearing minerals by thermophilic fungus Aspergillus fumigatus. Geochim. Cosmochim. Acta 2008, 72, 87–98. [Google Scholar] [CrossRef]
- Liu, D.; Lian, B.; Dong, H. Isolation of Paenibacillus sp. and Assessment of its Potential for Enhancing Mineral Weathering. Geomicrobiol. J. 2012, 29, 413–421. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Goswami, M.P.; Bhattacharyya, L.H. Perspective of beneficial microbes in agriculture under changing climatic scenario: A review. J. Phytol. 2016. [Google Scholar] [CrossRef]
- Pindi, P.K. Liquid Microbial Consortium- A Potential Tool for Sustainable Soil Health. J. Biofertil. Biopestic. 2012, 3. [Google Scholar] [CrossRef]
- Leaungvutiviroj, C.; Ruangphisarn, P.; Hansanimitkul, P.; Shinkawa, H.; Sasaki, K. Development of a New Biofertilizer with a High Capacity for N 2 Fixation, Phosphate and Potassium Solubilization and Auxin Production. Biosci. Biotechnol. Biochem. 2010, 74, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Corneo, P.E.; Mariotte, P.; Kertesz, M.A.; Dijkstra, F.A. Effect of crop rotation on mycorrhizal colonization and wheat yield under different fertilizer treatments. Agric. Ecosyst. Environ. 2017, 247, 130–136. [Google Scholar] [CrossRef]
- Maity, A.; Sharma, J.; Pal, R.K. Novel potassium solubilizing bio-formulation improves nutrient availability, fruit yield and quality of pomegranate (Punica granatum L.) in semi-arid ecosystem. Sci. Hortic. 2019, 255, 14–20. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2014, 20. [Google Scholar] [CrossRef]
- Lucena, J.J.; Hernandez-Apaolaza, L. Iron nutrition in plants: An overview. Plant Soil 2017, 418, 1–4. [Google Scholar] [CrossRef]
- Hindt, M.; Guerinot, M. Getting a sense for signals: Regulation of the plant iron deficiency response. Biochim. Biophys. Acta 2012, 1823, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Page, M. Siderophore conjugates. Ann. N. Y. Acad. Sci. 2013, 1277, 115–126. [Google Scholar] [CrossRef]
- Boukhalfa, H.; Crumbliss, A.L. Chemical aspects of siderophore mediated iron transport. Biometals 2002, 15, 325–339. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Holmström, S.J.M. Siderophore Production by Microorganisms Isolated From a Podzol Soil Profile. Geomicrobiol. J. 2015, 32, 397–411. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Chao, Y.; Wang, S.; Tang, Y.-T.; Qiu, R.-L. Metal-tolerant Enterobacter sp. strain EG16 enhanced phytoremediation using Hibiscus cannabinus via siderophore-mediated plant growth promotion under metal contamination. Plant Soil 2017, 413, 203–216. [Google Scholar] [CrossRef]
- García, J.E.; Maroniche, G.; Creus, C.; Suárez-Rodríguez, R.; Ramirez-Trujillo, J.A.; Groppa, M.D. In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol. Res. 2017, 202, 21–29. [Google Scholar] [CrossRef]
- Cardoso, P.; Alves, A.; Silveira, P.; Sá, C.; Fidalgo, C.; Freitas, R.; Figueira, E. Bacteria from nodules of wild legume species: Phylogenetic diversity, plant growth promotion abilities and osmotolerance. Sci. Total Environ. 2018, 645, 1094–1102. [Google Scholar] [CrossRef]
- Lewis, R.W.; Islam, A.; Opdahl, L.; Davenport, J.R.; Sullivan, T.S. Comparative Genomics, Siderophore Production, and Iron Scavenging Potential of Root Zone Soil Bacteria Isolated from ‘Concord’ Grape Vineyards. Microb. Ecol. 2019, 78, 699–713. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Kong, S. Effects of Trichoderma asperellum and its siderophores on endogenous auxin in Arabidopsis thaliana under iron-deficiency stress. Int. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Roskot, N.; Steidle, A.; Eberl, L.; Zock, A.; Smalla, K. Plant-Dependent Genotypic and Phenotypic Diversity of Antagonistic Rhizobacteria Isolated from Different Verticillium Host Plants. Appl. Environ. Microbiol. 2002, 68, 3328–3338. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore—A boon to agricultural sciences. Biol. Control. 2020, 144, 104214. [Google Scholar] [CrossRef]
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Van Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ. 2013, 59, 89–94. [Google Scholar] [CrossRef]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Modified chrome azurol S method for detection and estimation of siderophores having affinity for metal ions other than iron. Environ. Sustain. 2018, 1, 81–87. [Google Scholar] [CrossRef]
- Scott, S.; Housh, A.; Powell, G.; Anstaett, A.; Gerheart, A.; Benoit, M.; Wilder, S.; Schueller, M.; Ferrieri, R. Crop Yield, Ferritin and Fe(II) boosted by Azospirillum brasilense (HM053) in Corn. Agronomy 2020, 10, 394. [Google Scholar] [CrossRef]
- Shirinbayan, S.; Khosravi, H.; Malakouti, M.J. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl. Soil Ecol. 2019, 133, 138–145. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the reward greater than the risk? Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef]
- Wallenstein, M. Managing and manipulating the rhizosphere microbiome for plant health: A systems approach. Rhizosphere 2017, 3. [Google Scholar] [CrossRef]
- Vries, F.; Wallenstein, M.D. Below-ground connections underlying above-ground food production: A framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Arcos, E.; Ferreira, E.; Videira e Castro, I. Microbial Inoculants with Autochthonous Bacteria for Biodiverse Legume Pastures in Portuguese Agro-Forestry Ecosystems BT—Biological Nitrogen Fixation and Beneficial Plant-Microbe Interaction; Springer: Cham, Switzerland, 2016; pp. 171–182. [Google Scholar]
- Pastor-Bueis, R.; Sánchez-Cañizares, C.; James, E.K.; González-Andrés, F. Formulation of a Highly Effective Inoculant for Common Bean Based on an Autochthonous Elite Strain of Rhizobium leguminosarum bv. phaseoli, and Genomic-Based Insights Into Its Agronomic Performance. Front. Microbiol. 2019, 10, 2724. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020. [Google Scholar] [CrossRef]
- Vieira, S.; Sikorski, J.; Dietz, S.; Herz, K.; Schrumpf, M.; Bruelheide, H.; Scheel, D.; Friedrich, M.W.; Overmann, J. Drivers of the composition of active rhizosphere bacterial communities in temperate grasslands. ISME J. 2020, 14, 463–475. [Google Scholar] [CrossRef]
- Montanez, A.; Rodríguez-Blanco, A.; Barlocco, C.; Beracochea, M.; Sicardi, M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Appl. Soil Ecol. 2012, 58, 21–28. [Google Scholar] [CrossRef]
- Ji, S.H.; Gururani, M.A.; Chun, S.-C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The Multifarious PGPR Serratia marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Thakur, D. Evaluation of multifarious plant growth promoting traits, antagonistic potential and phylogenetic affiliation of rhizobacteria associated with commercial tea plants grown in Darjeeling, India. PLoS ONE 2017, 12, e0182302. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Elangovan, N.; Kumar, T.S.; Govindharaju, S.; Barathi, S.; Oves, M.; Arulselvi, P.I. Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol. Res. 2017, 204, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Von Rad, U.; Mueller, M.J.; Durner, J. Evaluation of natural and synthetic stimulants of plant immunity by microarray technology. New Phytol. 2005, 165, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Kraut-Cohen, J.; Zolti, A.; Erel, R.; Dietel, K.; et al. Microbial Consortia versus Single-Strain Inoculants: An advantage in PGPM-assisted tomato production? Agronomy 2019, 9, 105. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Fernandez-Valverde, S.L.; Martinez Romero, J.C.; Martínez-Romero, E. Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Syst. Appl. Microbiol. 2019, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Shen, M.; Yang, X.; Cheng, D.; Zhao, Q. A plant growth-promoting rhizobacteria (PGPR) mixture does not display synergistic effects, likely by biofilm but not growth inhibition. Microbiology 2014, 83, 666–673. [Google Scholar] [CrossRef]
- Kong, Z.; Hart, M.; Liu, H. Paving the Way from the Lab to the Field: Using Synthetic Microbial Consortia to Produce High-Quality Crops. Front. Plant Sci. 2018, 9, 1467. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y.; et al. Core microbiomes for sustainable agroecosystems. Nat. Plants 2018, 4, 247–257. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Abbott, L.K. Unknown risks to soil biodiversity from commercial fungal inoculants. Nat. Ecol. Evol. 2017, 1, 0115. [Google Scholar] [CrossRef]
- Chenoweth, J.; Hadjinicolaou, P.; Bruggeman, A.; Lelieveld, J.; Levin, Z.; Lange, M.A.; Xoplaki, E.; Hadjikakou, M. Impact of climate change on the water resources of the eastern Mediterranean and Middle East region: Modeled 21st century changes and implications. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef]
- Sessitsch, A.; Mitter, B. 21st century agriculture: Integration of plant microbiomes for improved crop production and food security. Microb. Biotechnol. 2015, 8, 32–33. [Google Scholar] [CrossRef]
- Fasoula, D.A.; Ioannides, I.M.; Omirou, M. Phenotyping and Plant Breeding: Overcoming the Barriers. Front. Plant Sci. 2020, 10. [Google Scholar] [CrossRef]
- Omirou, M.; Ioannides, M.Ι.; Fasoula, D.A. Optimizing Resource Allocation in a Cowpea (Vigna unguiculata L. Walp.) Landrace through Whole-Plant Field Phenotyping and Non-stop Selection to Sustain Increased Genetic Gain Across a Decade. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Wei, Z.; Jousset, A. Plant Breeding Goes Microbial. Trends Plant. Sci. 2017, 22, 555–558. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavadia, A.; Omirou, M.; Fasoula, D.; Ioannides, I.M. The Importance of Microbial Inoculants in a Climate-Changing Agriculture in Eastern Mediterranean Region. Atmosphere 2020, 11, 1136. https://doi.org/10.3390/atmos11101136
Kavadia A, Omirou M, Fasoula D, Ioannides IM. The Importance of Microbial Inoculants in a Climate-Changing Agriculture in Eastern Mediterranean Region. Atmosphere. 2020; 11(10):1136. https://doi.org/10.3390/atmos11101136
Chicago/Turabian StyleKavadia, Athanasia, Michalis Omirou, Dionysia Fasoula, and Ioannis M. Ioannides. 2020. "The Importance of Microbial Inoculants in a Climate-Changing Agriculture in Eastern Mediterranean Region" Atmosphere 11, no. 10: 1136. https://doi.org/10.3390/atmos11101136
APA StyleKavadia, A., Omirou, M., Fasoula, D., & Ioannides, I. M. (2020). The Importance of Microbial Inoculants in a Climate-Changing Agriculture in Eastern Mediterranean Region. Atmosphere, 11(10), 1136. https://doi.org/10.3390/atmos11101136



