Characteristics of Aerosol during a Severe Haze-Fog Episode in the Yangtze River Delta: Particle Size Distribution, Chemical Composition, and Optical Properties
Abstract
1. Introduction
2. Experimental Methods
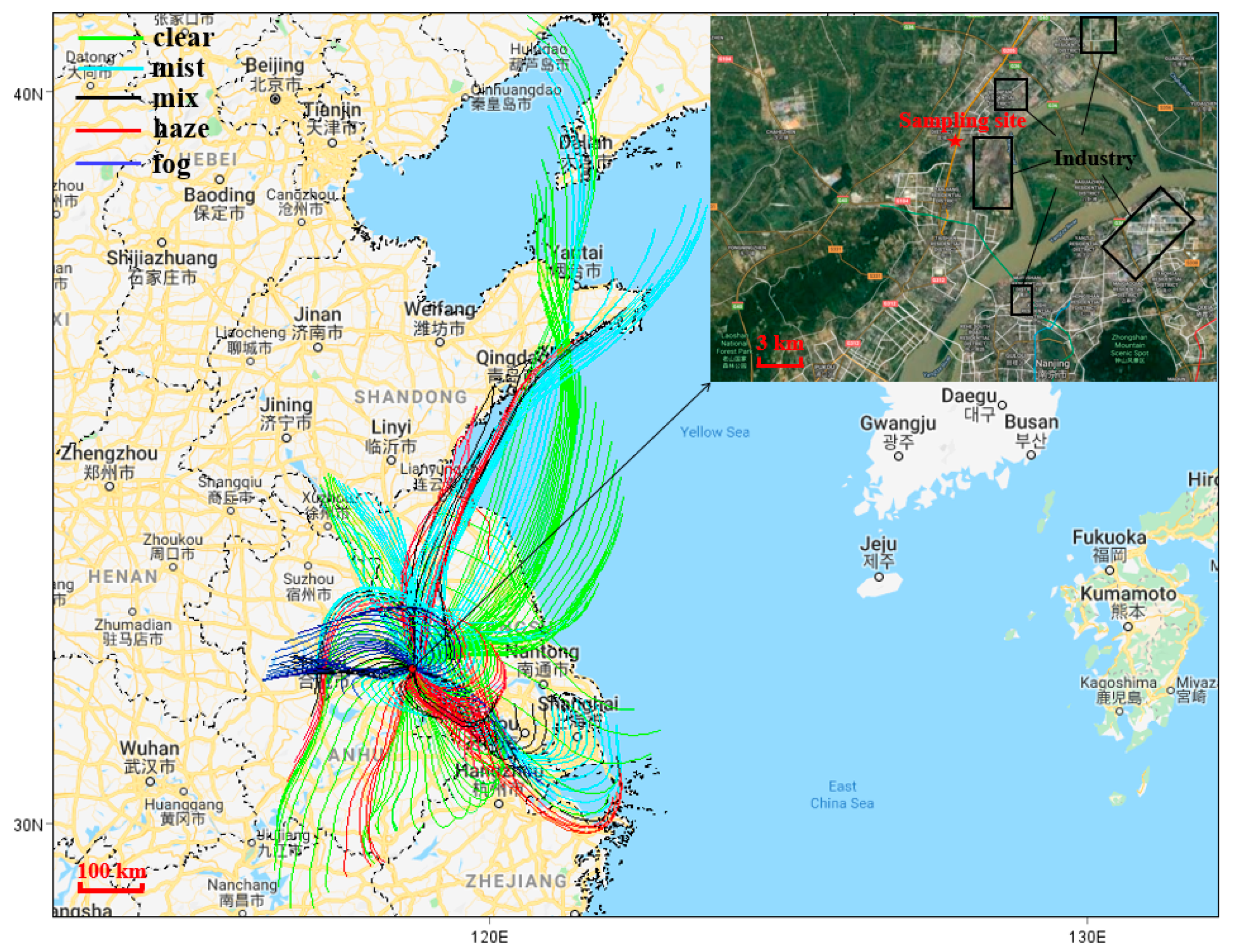
2.1. Description of the Observation Site
2.2. Description of Observational Instruments
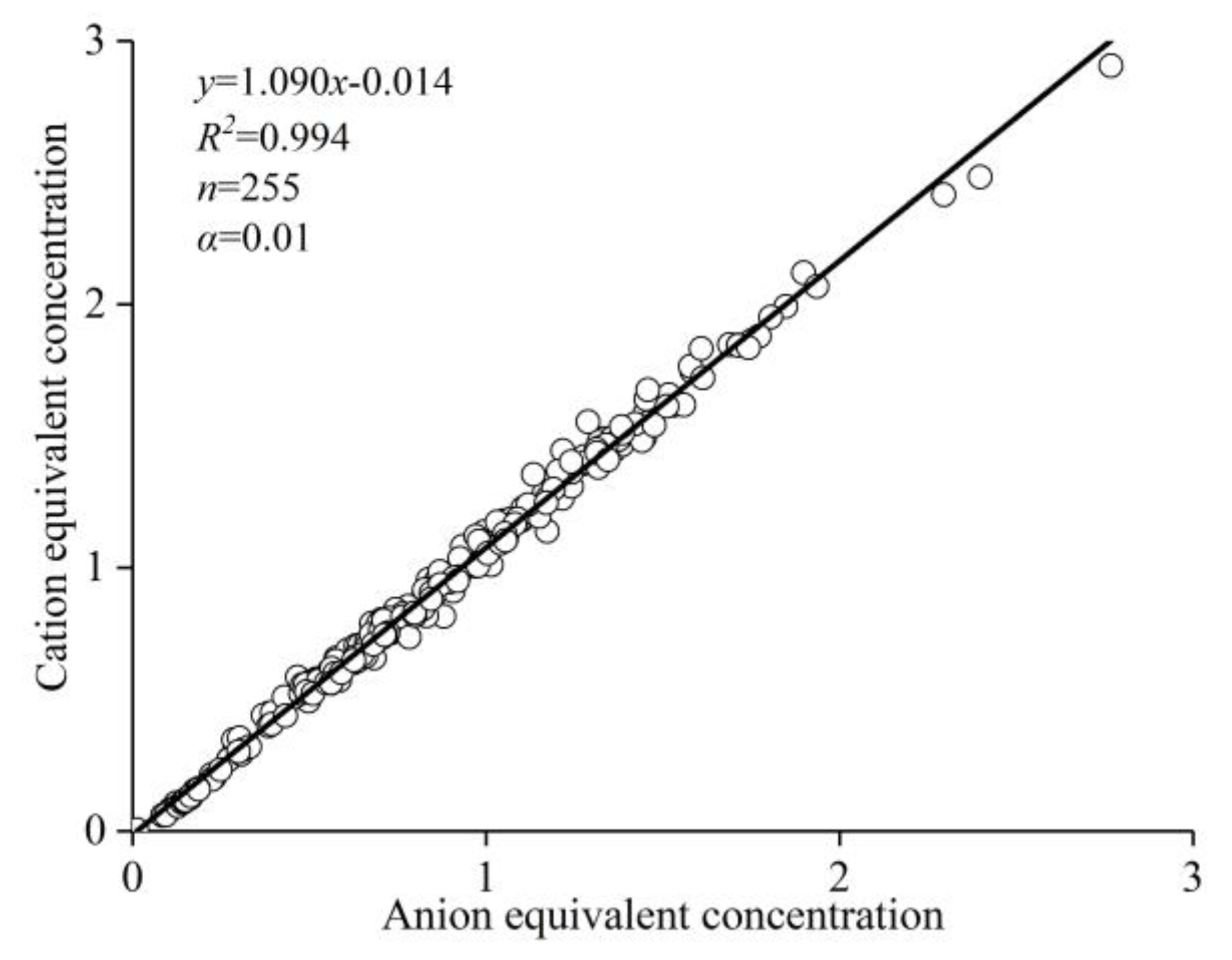
2.3. Data Validity Analysis
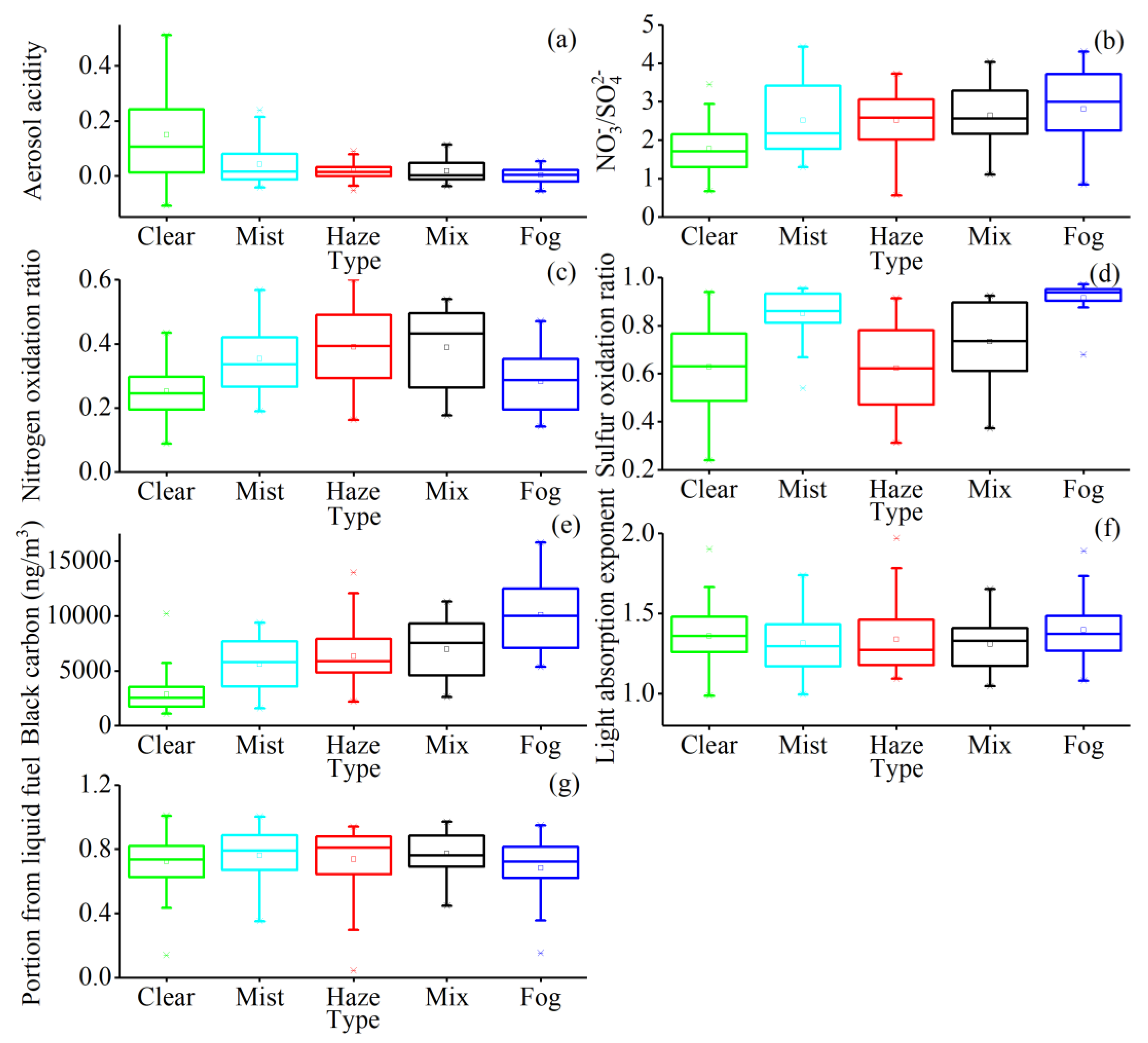
2.4. Aerosol Acidity
2.5. Nitrogen Oxidation Ratio (NOR) and Sulfur Oxidation Ratio (SOR)
2.6. Absorption Coefficients for Liquid and Solid Fuel-Sourced BC
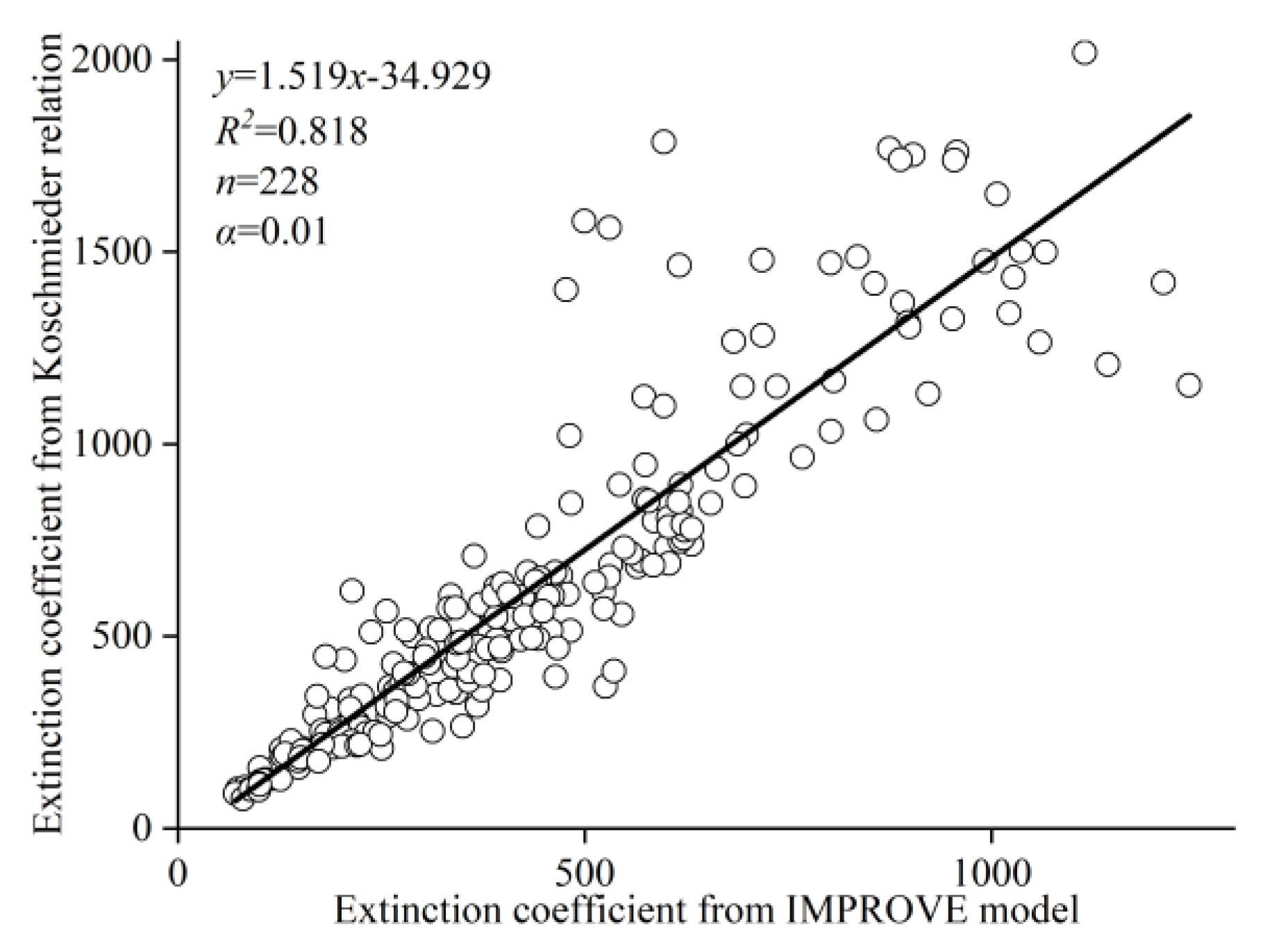
2.7. IMPROVE Model
3. Results and Discussion
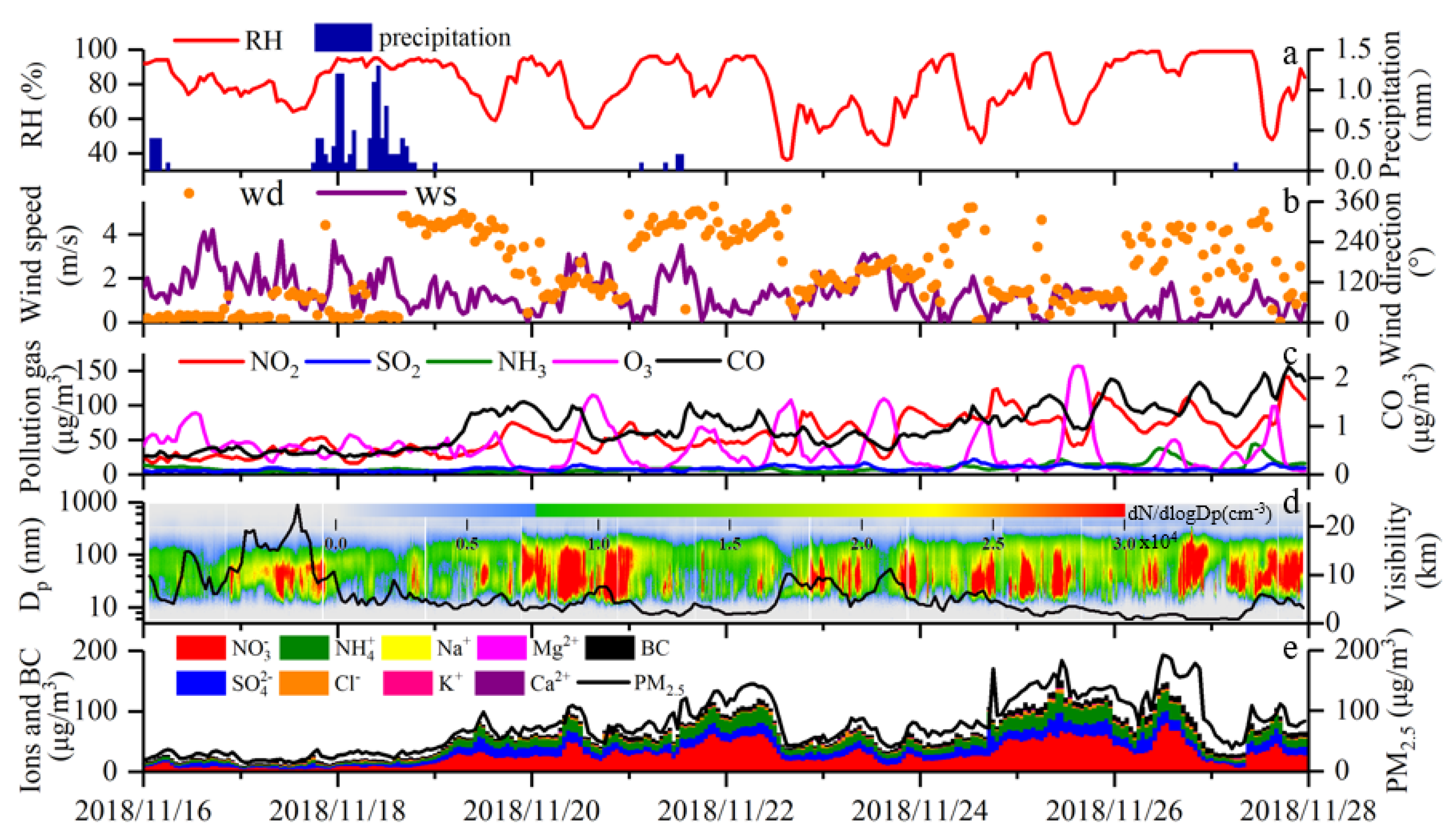
3.1. Overview of the Observations
3.2. Classification in the Haze-Fog Episode
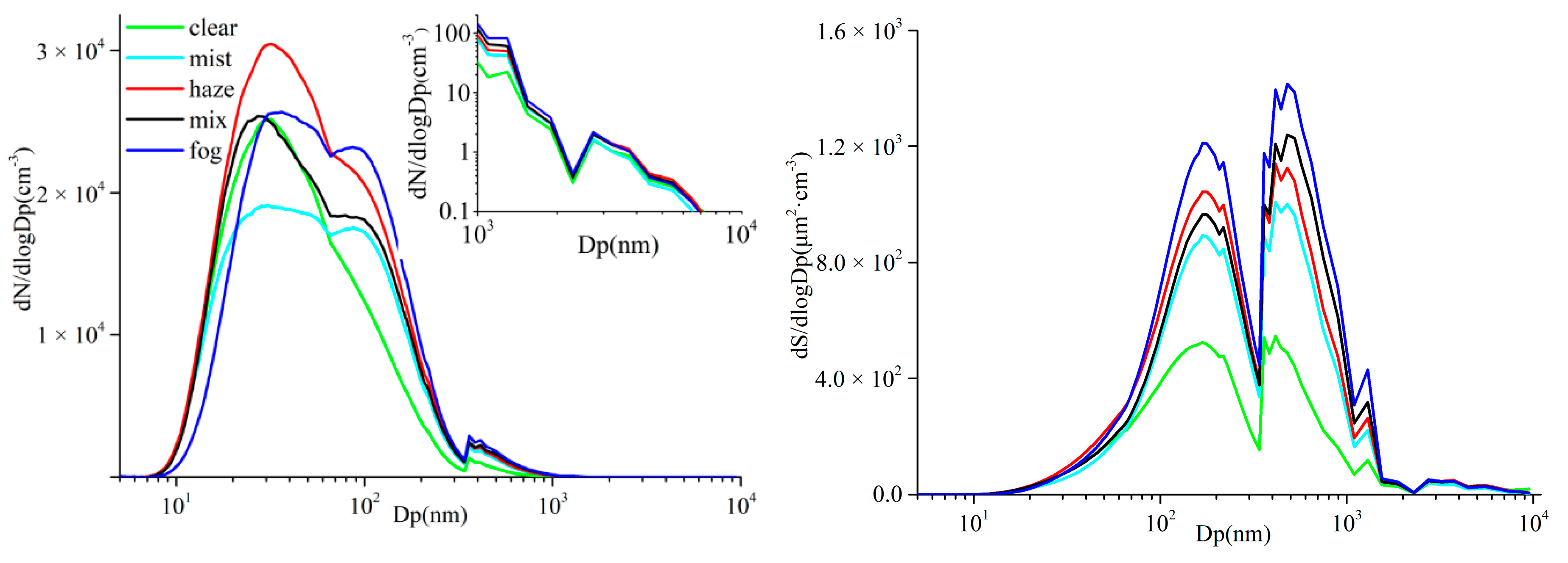
3.3. Characteristics of Aerosol Particle Size Distribution in Different Processes
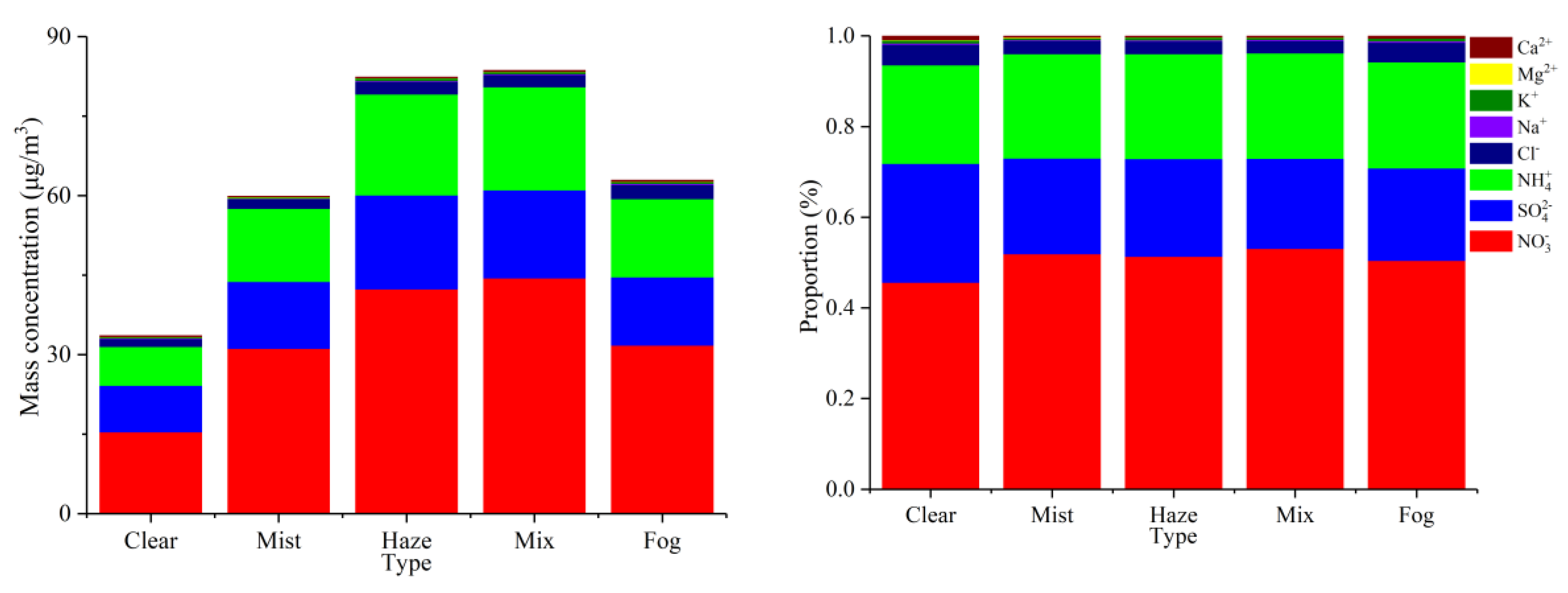
3.4. Characteristics of Aerosol Chemical Composition in Different Processes
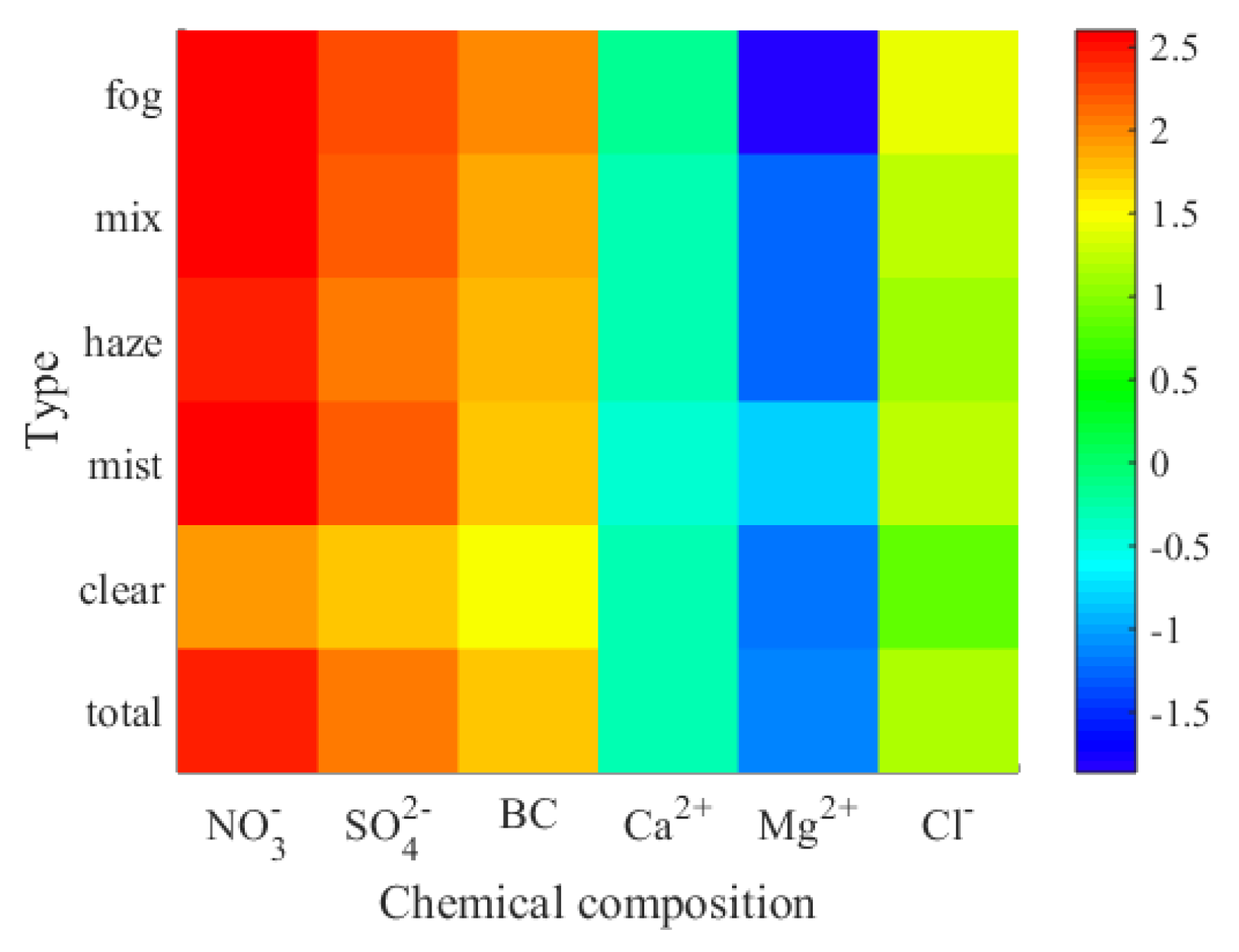
3.4.1. Distribution Characteristics of Water Soluble Ions in Different Processes
3.4.2. Distribution Characteristics of BC in Different Processes
3.5. Effect of PM2.5 Component on Extinction Coefficient in Different Processes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; An, J.; Cheng, M.; Shen, L.; Zhu, B.; Li, Y.; Wang, Y.; Duan, Q.; Sullivan, A.; Xia, L. One year online measurements of water-soluble ions at the industrially polluted town of Nanjing, China: Sources, seasonal and diurnal variations. Chemosphere 2016, 148, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kong, S.F.; Wu, F.; Cheng, Y.; Zheng, S.; Yan, Q.; Zheng, H.; Yang, G.; Zheng, M.; Liu, D.; et al. Estimating the open biomass burning emissions in central and eastern China from 2003 to 2015 based on satellite observation. Atmos. Chem. Phys. 2018, 18, 11623–11646. [Google Scholar] [CrossRef]
- Ramanathan, V.; Crutzen, P.J.; Kiehl, J.T.; Rosenfeld, D. Aerosols, climate, and the hydrological cycle. Science 2001, 294, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Andreae, M.O.; Jones, C.D.; Cox, P.M. Strong present-day aerosol cooling implies a hot future. Nature 2005, 435, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem Phys. 2005, 5, 715–737. [Google Scholar] [CrossRef]
- Chang, D.; Song, Y.; Liu, B. Visibility trends in six megacities in China 1973–2007. Atmos. Res. 2009, 94, 161–167. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y. Analysis of long-term variations of fog and haze in China in recent 50 years and their relations with atmospheric humidity. Sci. China Earth Sci. 2014, 57, 36–46. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Yan, Q.; Wu, F.; Cheng, Y.; Zheng, S.; Wu, J.; Yang, G.; Zheng, M.; Tang, L.; et al. The impacts of pollution control measures on PM2.5 reduction: Insights of chemical composition, source variation and health risk. Atmos. Environ. 2019, 197, 103–117. [Google Scholar] [CrossRef]
- Leng, C.; Zhang, Q.; Zhang, D.; Xu, C.; Cheng, T.; Zhang, R.; Tao, J.; Chen, J.; Zha, S.; Zhang, Y.; et al. Variations of cloud condensation nuclei (CCN) and aerosol activity during fog–haze episode: A case study from Shanghai. Atmos. Chem. Phys. 2014, 14, 12499–12512. [Google Scholar] [CrossRef]
- Fu, W.; Chen, Z.; Zhu, Z.; Liu, Q.; Qi, J.; Dang, E.; Wang, M.; Dong, J. Long-Term Atmospheric Visibility Trends and Characteristics of 31 Provincial Capital Cities in China during 1957–2016. Atmosphere 2018, 9, 318. [Google Scholar] [CrossRef]
- Deng, Z.; Zhao, C.; Ma, N.; Liu, P.; Ran, L.; Xu, W.; Chen, J.; Liang, Z.; Liang, S.; Huang, M.; et al. Size-resolved and bulk activation properties of aerosols in the north China plain. Atmos. Chem. Phys. 2011, 11, 3835–3846. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, J.; Quan, J.; Gao, Y.; Zhao, D.; Chen, P.; He, H. Impact of aerosol composition on cloud condensation nuclei activity. Atmos. Chem. Phys. 2012, 12, 3783–3790. [Google Scholar] [CrossRef]
- Zhang, S.T. Observation study on characteristics of haze, fog, and haze-fog transition in Nanjing. Nanjing Uni. Infor. Sci. Technol. 2015, 6, 1–126. (In Chinese) [Google Scholar]
- Husar, R.B.; Prospero, J.M.; Stowe, L.L. Characterization of tropospheric aerosols over the oceans with the NOAA advanced very high resolution radiometer optical thickness operational product. J. Geophys. Res. 1997, 102, 16889–16909. [Google Scholar] [CrossRef]
- Yao, X.; Chan, C.K.; Fang, M.; Cadle, S.; Chan, T.; Mulawa, P.; He, K.; Ye, B. The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos. Environ. 2002, 36, 4223–4234. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, B.; Wang, H.; Zhang, E. Haze variations over 1980–2009 and connecting factors over the Yangtze River Delta Region. China Environ. Sci. 2013, 33, 1929–1936. [Google Scholar]
- Wang, H.; Zhu, B.; Shen, L.; Xu, H.; An, J.; Xue, G.; Cao, J. Water-soluble ions in atmospheric aerosols measured in five sites in the Yangtze River Delta, China: Size-fractionated, seasonal variations and sources. Atmos. Environ. 2015, 123, 370–379. [Google Scholar] [CrossRef]
- Lu, W.; Wang, H.; Zhu, B.; Shi, S.; Kang, H. Distribution characteristics of PM2.5 mass concentration and their impacting factors including meteorology and transmission in North Suburb of Nanjing during 2014 to 2016. Acta Sci. Cir. 2019, 39, 1039–1048. (In Chinese) [Google Scholar]
- Zhang, X.; Wang, Y.; Niu, T.; Zhang, X.; Gong, S.; Zhang, Y.; Sun, J. Atmospheric aerosol compositions in China: Spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols. Atmos. Chem. Phys. 2012, 12, 779–799. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Wang, Y.; Li, W.; Zhang, Q.; Wang, W.; Quan, J.; Cao, G.; Wang, J.; Yang, Y.; et al. Factors contributing to haze and fog in China. Chin. Sci. Bull. 2013, 58, 1178–1187. (In Chinese) [Google Scholar]
- Fu, Q.; Zhuang, G.; Wang, J.; Xu, C.; Huang, K.; Li, J.; Hou, B.; Lu, T.; Streets, D.G. Mechanism of formation of the Heaviest pollution episode ever recorded in the Yangtze river Delta, China. Atmos. Environ. 2008, 42, 2023–2036. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Du, J.; Yang, X.; Gao, S.; Chen, J.; Geng, F. Evolution of the mixing state of fine aerosols during haze events in Shanghai. Atmos. Res. 2012, 104–105, 193–201. [Google Scholar] [CrossRef]
- Hu, R.; Wang, H.; Yin, Y.; Chen, K.; Zhu, B.; Zhang, Z.; Kang, H.; Shen, L. Mixing state of ambient aerosols during different fog-haze pollution episodes in the Yangtze River Delta, China. Atmos. Environ. 2018, 178, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, B.; Ji, D.; Cheng, M.; Gao, W.; Shi, S.; Xie, Y.; Yang, S.; Gao, M.; Fu, H.; et al. Characteristics of fine particle explosive growth events in Beijing, China: Seasonal variation, chemical evolution pattern and formation mechanism. Atmos. Environ. 2019, 687, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Duan, J.; Xu, C.; Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Kong, L.; Tao, J.; Zhang, R.; et al. Insights into a historic severe haze event in Shanghai: Synoptic situation, boundary layer and pollutants. Atmos. Chem. Phys. 2016, 16, 9221–9234. [Google Scholar] [CrossRef]
- Pan, L.; Che, H.; Geng, F.; Xia, X.; Wang, Y.; Zhu, C.; Chen, M.; Gao, W.; Guo, J. Aerosol optical properties based on ground measurements over the Chinese Yangtze Delta Region. Atmos. Environ. 2010, 44, 2587–2596. [Google Scholar] [CrossRef]
- Kang, H.; Zhu, B.; Su, J.; Wang, H.; Zhang, Q.; Wang, F. Analysis of a long-lasting haze episode in Nanjing, China. Atmos. Res. 2013, 120–121, 78–87. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J. Atmospheric extinction of a persistent fog/haze event in Nanjing during December 2007. Acta Sci. Cir. 2016, 36, 2305–2313. (In Chinese) [Google Scholar]
- Wang, L.; Ji, D.; Li, Y.; Gao, M.; Tian, S.; Wen, T.; Liu, Z.; Wang, L.; Xu, P.; Jiang, C.; et al. The impact of relative humidity on the size distribution and chemical process of major water-soluble inorganic ions in the megacity of Chongqing, China. Atmos. Res. 2017, 192, 19–29. [Google Scholar] [CrossRef]
- Xue, G.; Zhu, B.; Wang, H. Size distributions and source apportionment of soluble ions in aerosol in Nanjing. Environ. Sci. 2014, 35, 1633–1643. (In Chinese) [Google Scholar]
- Qin, Y.; Zhu, B.; Zhou, J.; Pang, B. Characteristics and source apportionment of water-soluble ions in dry deposition in the summer and autumn of Nanjing. Environ. Sci. 2016, 37, 2025–2033. (In Chinese) [Google Scholar]
- Liu, Z.; Gao, W.; Yu, Y.; Hu, B.; Xin, J.; Sun, Y.; Wang, L.; Wang, G.; Bi, X.; Zhang, G.; et al. Characteristics of PM2.5 mass concentrations and chemical species in urban and background areas of China: Emerging results from the CARE-China network. Atmos. Chem. Phys. 2018, 18, 8849–8871. [Google Scholar] [CrossRef]
- Yang, J.; Niu, Z.; Shi, C.; Liu, D.; Li, Z. Microphysics of Atmospheric Aerosols During Winter Haze/Fog Events in Nanjing. Environ. Sci. 2010, 31, 1425–1431. (In Chinese) [Google Scholar]
- Shen, X.; Sun, J.; Zhang, X.; Zhang, Y.; Zhang, L.; Che, H.; Ma, Q.; Yu, X.; Yue, Y.; Zhang, Y. Characterization of submicron aerosols and effect on visibility during a severe haze-fog episode in Yangtze River Delta, China. Atmos. Environ. 2015, 120, 307–316. [Google Scholar] [CrossRef]
- Jacob, D.J.; Waldman, J.M.; Munger, J.W.; Hoffmann, M. A field investigation of physical and chemical mechanisms affecting pollutant concentrations in fog droplets. Tellus B Chem. Phys. Meteorol. 1984, 36, 272–285. [Google Scholar] [CrossRef]
- Pandis, S.N.; Seinfeld, J.H.; Pilinis, C. Heterogeneous sulfate production in an urban fog. Atmos Environ. 1992, 26, 2509–2522. [Google Scholar] [CrossRef]
- Pandis, S.N.; Seinfeld, J.H.; Pilinis, C. The smog-fog-smog cycle and acid deposition. J. Geophys. Res. 1990, 95, 18489–18500. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, B.; Kang, H.; Gao, J.; Wang, Y.; Jiang, Q. Analysis of the impact of two typical air pollution events on the air quality of Nanjing. Environ. Sci. 2012, 33, 3647–3655. (In Chinese) [Google Scholar]
- Makkonen, U.; Virkkula, A.; Mäntykenttä, J.; Hakola, H.; Keronen, P.; Vakkari, V.; Aalto, P.P. Semi-continuous gas and inorganic aerosol measurements at a Finnish urban site: Comparisons with filters, nitrogen in aerosol and gas phases, and aerosol acidity. Atmos. Chem. Phys. 2012, 12, 5617–5631. [Google Scholar] [CrossRef]
- Wang, H.; An, J.; Shen, L.; Zhu, B.; Pan, C.; Liu, Z.; Liu, X.; Duan, Q.; Liu, X.; Wang, Y. Mechanism for the formation and microphysical characteristics of submicron aerosol during heavy haze pollution episode in the Yangtze River Delta, China. Sci. Total Environ. 2014, 490, 501–508. [Google Scholar] [CrossRef]
- Zou, J.; An, J.; Wang, H.; Shao, P.; Duan, Q.; Xue, G.; Pang, B. Distribution Characteristics of Pollution Gases and Water Soluble Ion in Aerosol During the Asian Youth Games of Nanjing. Environ. Sci. 2014, 35, 4044–4051. (In Chinese) [Google Scholar]
- Liao, B.; Wu, D.; Chang, Y.; Lin, Y.; Wang, S.; Li, F. Characteristics of particulate SO42−, NO3−, NH4+ and related gaseous pollutants in Guangzhou. Acta Sci. Cir. 2014, 34, 1551–1559. (In Chinese) [Google Scholar]
- Liu, A.; Wang, H.; Chen, K.; Lu, W.; Shi, S.; Liu, Z. Distribution characteristics of water-soluble ions during a haze pollution process in Nanjing. China Environ. Sci. 2019, 39, 1793–1803. (In Chinese) [Google Scholar]
- Huang, J.; Chen, Z.; Mo, Z.; Li, H.; Liu, H.; Li, H.; Liang, G.; Yang, J.; Zhang, D.; Li, Y. Analysis of characteristics of water-soluble ions in PM2.5 in Guilin based on the MARGA. China. Environ. Sci. 2019, 39, 1390–1404. (In Chinese) [Google Scholar]
- Li, Y.; Zhou, H.; Zhang, Z.; Wang, Q.; Luo, L. Pollution characteristics of secondary water-soluble inorganic ions of PM2.5 in urban Chengdu. Environ. Sci. 2014, 35, 4439–4445. (In Chinese) [Google Scholar]
- Liu, Y.; Yan, C.; Zheng, M. Source apportionment of black carbon during winter in Beijing. Sci. Total Environ. 2018, 618, 531–541. [Google Scholar] [CrossRef]
- Jing, A.; Zhu, B.; Wang, H.; Yu, X.; An, J.; Kang, H. Source apportionment of black carbon in different seasons in the northern suburb of Nanjing, China. Atmos. Environ. 2019, 201, 190–200. [Google Scholar] [CrossRef]
- Sisler, J.F.; Malm, W.C. Interpretation of trends of PM2.5 and reconstructed visibility from the IMPROVE network. J. Air Waste Manage. Assoc. 2000, 50, 775–789. [Google Scholar] [CrossRef]
- Malm, W.C.; Day, D.E. Estimates of aerosol species scattering characteristics as a function of relative humidity. Atmos. Environ. 2001, 35, 2845–2860. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Z.; Zhu, B.; Shen, Y.; Wang, H.; Shi, S.; Sha, D. Comparison of parameterizations for the atmospheric extinction coefficient in Lin’an, China. Sci. Total Environ. 2018, 621, 507–515. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Y.; Li, Y.; Zhu, B.; Yu, X. Analysis of extinction properties as a function of relative humidity using a κ-EC-Mie model in Nanjing. Atmos. Chem. Phys. 2017, 17, 4147–4157. [Google Scholar] [CrossRef]
- McCartney, E.J.; Pan, N.X. Optics of the Atmosphere: Scattering by Molecules and Particle; Science Press: Beijing, China, 1988; pp. 1–413. (In Chinese) [Google Scholar]
- Schichtel, B.A.; Husar, R.B.; Falke, S.R.; Wilson, W.E. Haze trends over the United States, 1980–1995. Atmos. Environ. 2001, 35, 5205–5210. [Google Scholar] [CrossRef]
- Lillis, D.; Cruz, C.N.; Collett, J., Jr.; Richards, L.W.; Pandis, S.N. Production and removal of aerosol in a polluted fog layer: Model evaluation and fog effect on PM. Atmos. Environ. 1999, 33, 4797–4816. [Google Scholar]
- Zhang, L.; Sun, J.; Shen, X.; Zhang, Y.; Che, H.; Ma, Q.; Zhang, Y.; Ogren, J.A. Observations of relative humidity effects on aerosol light scattering in the Yangtze River Delta of China. Atmos. Chem. Phys. 2015, 15, 8439–8454. [Google Scholar] [CrossRef]
- Kulmala, M.; Laaksonen, A.; Charlson, R.J.; Korhonen, P. Clouds without supersaturation. Nature 1997, 388, 336–337. [Google Scholar] [CrossRef]
- Yin, Y.; Dong, Y.; Wei, Y.; Wang, T.; Li, J.; Yang, W.; Fan, S. The analysis of chemistry composition of fine mode particles in Nanjing. Tran. Atmos. Sci. 2009, 32, 723–733. (In Chinese) [Google Scholar]
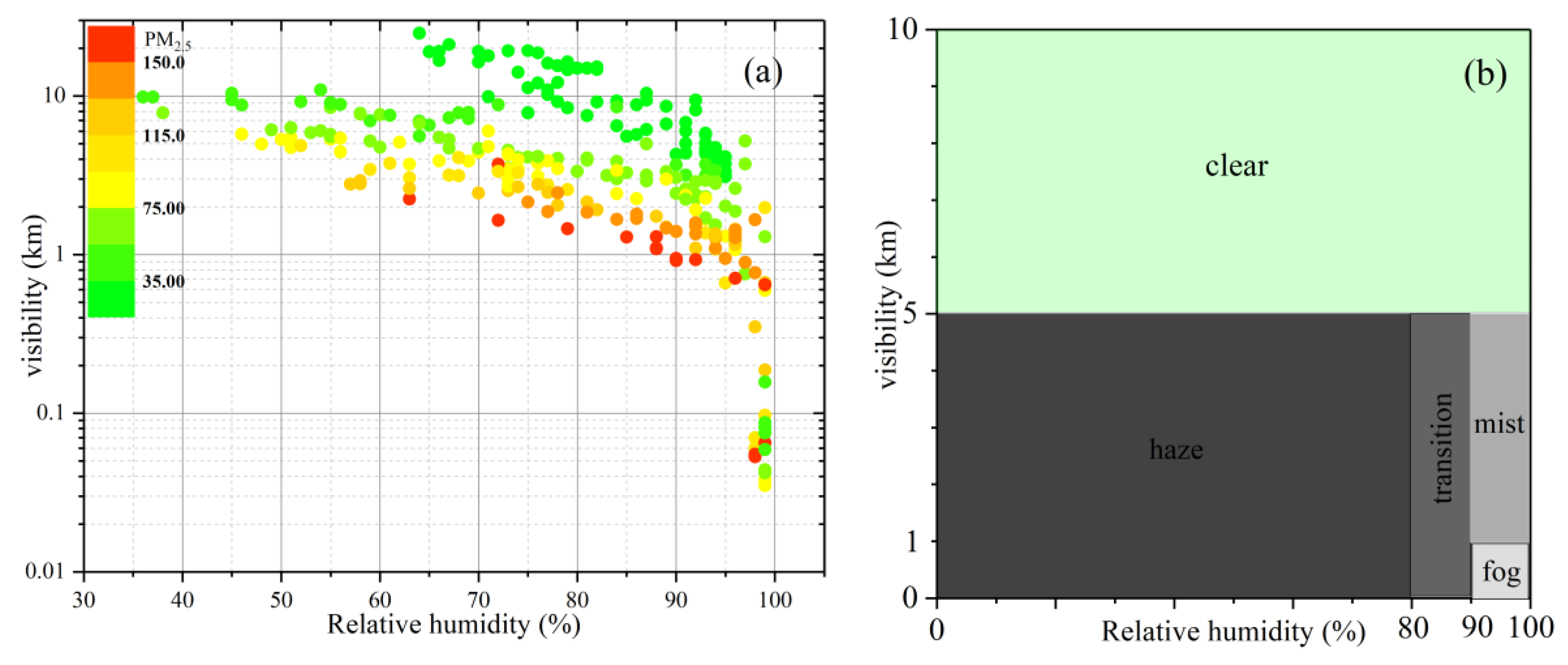
| Type | Visibility (km) | Relative Humidity (%) | PM2.5 (μg/m3) | Frequency (%) | Hours (h) |
|---|---|---|---|---|---|
| Clear | >5 | — | 42.47 | 31.76 | 81 |
| Mist | 1–5 | >90 | 81.51 | 21.96 | 56 |
| Haze | <5 | <80 | 101.11 | 22.75 | 58 |
| Mix | <5 | 80–90 | 110.88 | 12.94 | 33 |
| Fog | <1 | >90 | 107.21 | 10.59 | 27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Wang, H.; Cui, Y.; Shen, L.; Yin, Y.; Wu, Z.; Guo, S.; Shi, S.; Chen, K.; Zhu, B.; et al. Characteristics of Aerosol during a Severe Haze-Fog Episode in the Yangtze River Delta: Particle Size Distribution, Chemical Composition, and Optical Properties. Atmosphere 2020, 11, 56. https://doi.org/10.3390/atmos11010056
Liu A, Wang H, Cui Y, Shen L, Yin Y, Wu Z, Guo S, Shi S, Chen K, Zhu B, et al. Characteristics of Aerosol during a Severe Haze-Fog Episode in the Yangtze River Delta: Particle Size Distribution, Chemical Composition, and Optical Properties. Atmosphere. 2020; 11(1):56. https://doi.org/10.3390/atmos11010056
Chicago/Turabian StyleLiu, Ankang, Honglei Wang, Yi Cui, Lijuan Shen, Yan Yin, Zhijun Wu, Song Guo, Shuangshuang Shi, Kui Chen, Bin Zhu, and et al. 2020. "Characteristics of Aerosol during a Severe Haze-Fog Episode in the Yangtze River Delta: Particle Size Distribution, Chemical Composition, and Optical Properties" Atmosphere 11, no. 1: 56. https://doi.org/10.3390/atmos11010056
APA StyleLiu, A., Wang, H., Cui, Y., Shen, L., Yin, Y., Wu, Z., Guo, S., Shi, S., Chen, K., Zhu, B., Wang, J., & Kong, X. (2020). Characteristics of Aerosol during a Severe Haze-Fog Episode in the Yangtze River Delta: Particle Size Distribution, Chemical Composition, and Optical Properties. Atmosphere, 11(1), 56. https://doi.org/10.3390/atmos11010056







