Is Contact Nucleation Caused by Pressure Perturbation?
Abstract
:1. Introduction
2. Proposed Mechanisms for Contact Nucleation
2.1. Local Cooling
2.2. Etching
2.3. Ice Embryo Formed Before Contact
2.4. Transient Energy Zone During Wetting
2.5. Line Tension
2.6. Pressure Perturbation
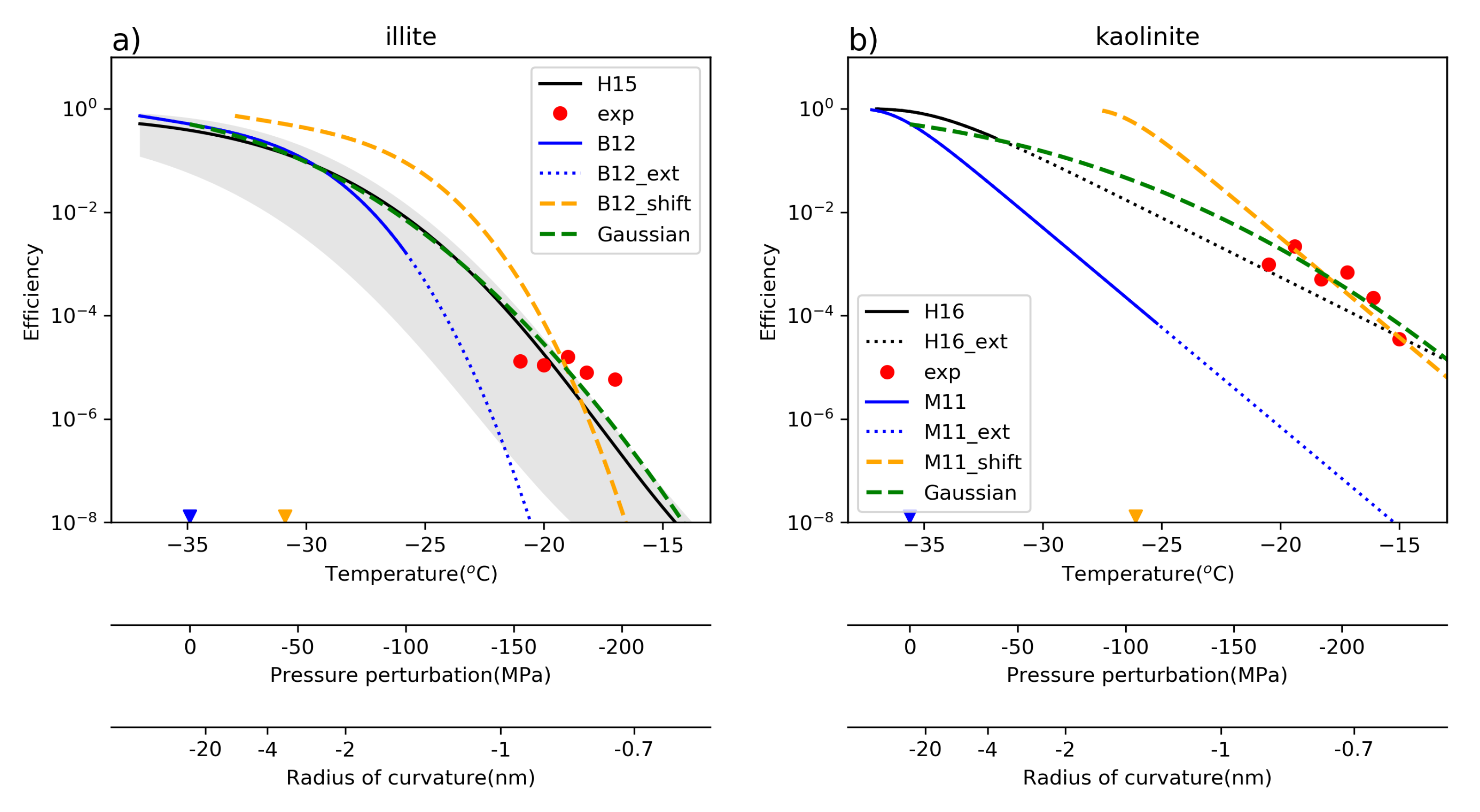
3. Estimation of the Pressure Perturbation Responsible for Contact Nucleation
3.1. Approach
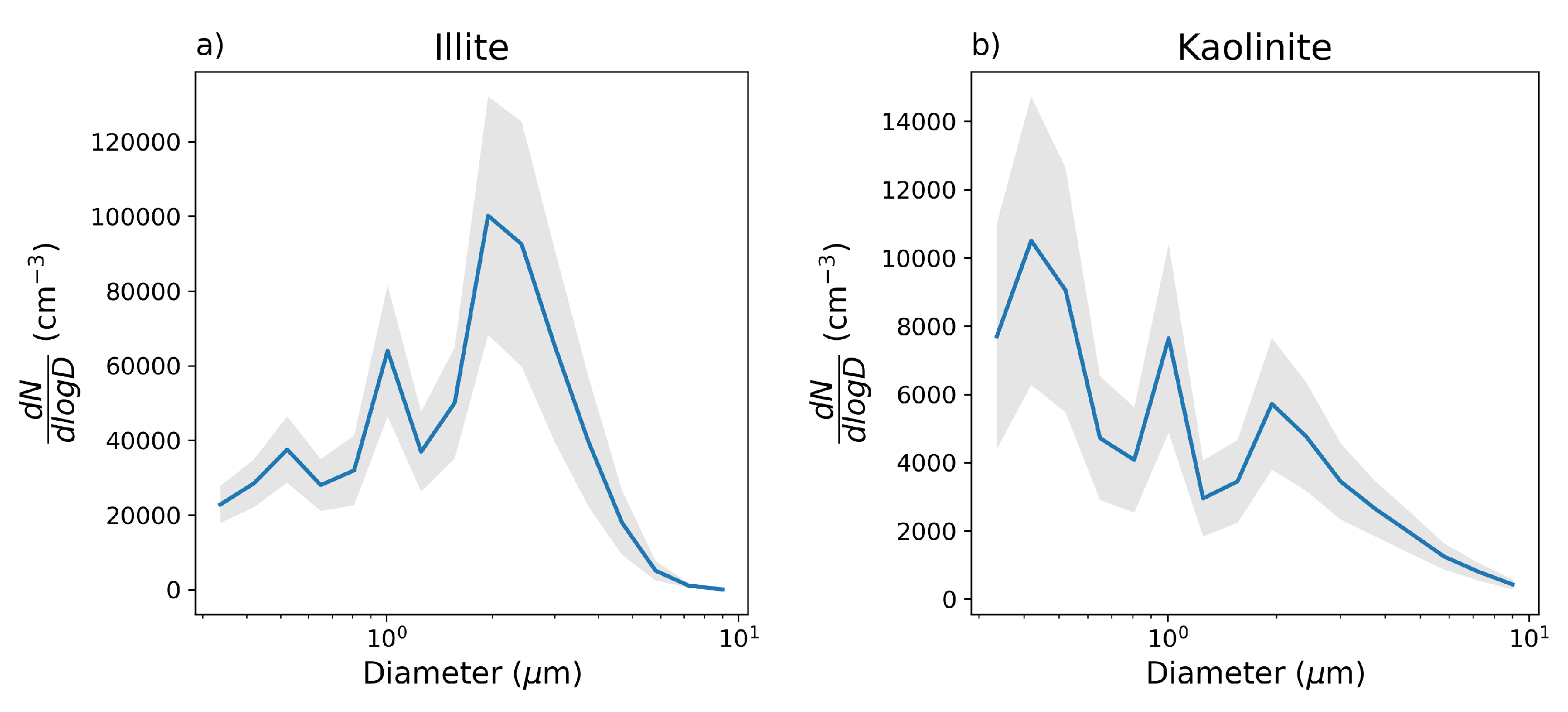
3.2. Data
3.3. Results and Discussion
4. Conclusions and Suggestions
- It is important to measure the corresponding immersion nucleation temperature for each contact nucleation event. This will provide the actual immersion freezing temperature for each contact nucleation event, based on direct measurements rather than indirect estimations using the parameterization equations. It can be accomplished by melting ice after contact freezing and then slightly decreasing temperature until immersion nucleation occurs. A superhydrophobic substrate might be used to avoid substrate freezing in cold stage experiments.
- It will be useful to perform contact nucleation experiments for a broader range of temperatures, such that the contact nucleation efficiency varies from to . This will extend our understanding of the behavior of contact nucleation and also benefit parameterizations of contact nucleation in cloud-resolving models.
- It will be a major breakthrough if pressure perturbation can be precisely measured or controlled. Experiments such as that in Bird et al. [64], in which the neck region arising from the coalescence of two droplets was well controlled and quantified, might be useful for investigating the effect of negative Laplace pressures on ice nucleation.
- Numerical simulation might be another useful tool with which to investigate the pressure perturbation hypothesis. If indeed pressure perturbation is a valid mechanism for inducing freezing, then it may be possible to have freezing just through droplet-droplet collisions or droplet breakup. A multi-phase fluid model has been applied to simulate the development of pressure perturbation in the neck region for two collided droplets [65]. The next step is to find a way to realistically simulate the collision process, instead of setting up the neck region initially as in Bartlett et al. [65].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kikuchi, K.; Kameda, T.; Higuchi, K.; Yamashita, A.; Working Group Members for New Classification of Snow Crystals. A global classification of snow crystals, ice crystals, and solid precipitation based on observations from middle latitudes to polar regions. Atmos. Res. 2013, 132, 460–472. [Google Scholar] [CrossRef]
- Braham, R.R., Jr. Meteorological bases for precipitation development. Bull. Am. Meteorol. Soc. 1968, 49, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Lubin, D.; Vogelmann, A.M. The influence of mixed-phase clouds on surface shortwave irradiance during the Arctic spring. J. Geophys. Res. Atmos. 2011, 116. [Google Scholar] [CrossRef] [Green Version]
- Macke, A. Scattering of light by polyhedral ice crystals. Appl. Opt. 1993, 32, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, W.; Illingworth, A. Charge transfer accompanying individual collisions between ice particles and its role in thunderstorm electrification. Q. J. R. Meteorol. Soc. 1980, 106, 841–854. [Google Scholar] [CrossRef]
- Lamb, D.; Verlinde, J. Physics and Chemistry of Clouds; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Cantrell, W.; Heymsfield, A. Production of ice in tropospheric clouds: A review. Bull. Am. Meteorol. Soc. 2005, 86, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Vali, G.; DeMott, P.; Möhler, O.; Whale, T. A proposal for ice nucleation terminology. Atmos. Chem. Phys. 2015, 15, 10263–10270. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, N. Active sites and ice crystal nucleation. J. Atmos. Sci. 1969, 26, 1266–1271. [Google Scholar] [CrossRef]
- Kiselev, A.; Bachmann, F.; Pedevilla, P.; Cox, S.J.; Michaelides, A.; Gerthsen, D.; Leisner, T. Active sites in heterogeneous ice nucleation—The example of K-rich feldspars. Science 2017, 355, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Holden, M.A.; Whale, T.F.; Tarn, M.D.; O’Sullivan, D.; Walshaw, R.D.; Murray, B.J.; Meldrum, F.C.; Christenson, H.K. High-speed imaging of ice nucleation in water proves the existence of active sites. Sci. Adv. 2019, 5, eaav4316. [Google Scholar] [CrossRef] [Green Version]
- Rau, W. Unterkühlbarkeit des Wassers und atmosphärische Eisbildung. Wetter Klima 1949, 2, 81–92. [Google Scholar]
- Gokhale, N.R.; Goold, J. Droplet freezing by surface nucleation. J. Appl. Meteorol. 1968, 7, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Gokhale, N.R.; Lewinter, O. Microcinematographic studies of contact nucleation. J. Appl. Meteorol. 1971, 10, 469–473. [Google Scholar] [CrossRef]
- Ladino Moreno, L.A.; Stetzer, O.; Lohmann, U. Contact freezing: A review of experimental studies. Atmos. Chem. Phys. 2013, 13, 9745–9769. [Google Scholar] [CrossRef] [Green Version]
- Niehaus, J.; Cantrell, W. Contact freezing of water by salts. J. Phys. Chem. Lett. 2015, 6, 3490–3495. [Google Scholar] [CrossRef]
- Yang, F.; Shaw, R.A.; Gurganus, C.W.; Chong, S.K.; Yap, Y.K. Ice nucleation at the contact line triggered by transient electrowetting fields. Appl. Phys. Lett. 2015, 107, 264101. [Google Scholar] [CrossRef]
- Yang, F.; Cruikshank, O.; He, W.; Kostinski, A.; Shaw, R.A. Nonthermal ice nucleation observed at distorted contact lines of supercooled water drops. Phys. Rev. E 2018, 97, 023103. [Google Scholar] [CrossRef] [Green Version]
- Knollenberg, R.G. Urea as an ice nucleant for supercooled clouds. J. Atmos. Sci. 1966, 23, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Knollenberg, R.G. A laboratory study of the local cooling resulting from the dissolution of soluble ice nuclei having endothermic heats of solution. J. Atmos. Sci. 1969, 26, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Knollenberg, R.G. The local cooling ice nucleation model. J. Atmos. Sci. 1969, 26, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, N. On contact nucleation. J. Atmos. Sci. 1970, 27, 1098–1099. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.A.; Durant, A.J.; Mi, Y. Heterogeneous surface crystallization observed in undercooled water. J. Phys. Chem. B 2005, 109, 9865–9868. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.A. A possible mechanism for contact nucleation. J. Atmos. Sci. 1974, 31, 1832–1837. [Google Scholar] [CrossRef] [Green Version]
- Page, A.J.; Sear, R.P. Heterogeneous nucleation in and out of pores. Phys. Rev. Lett. 2006, 97, 065701. [Google Scholar] [CrossRef] [Green Version]
- David, R.O.; Marcolli, C.; Fahrni, J.; Qiu, Y.; Sirkin, Y.A.P.; Molinero, V.; Mahrt, F.; Brühwiler, D.; Lohmann, U.; Kanji, Z.A. Pore condensation and freezing is responsible for ice formation below water saturation for porous particles. Proc. Natl. Acad. Sci. USA 2019, 116, 8184–8189. [Google Scholar] [CrossRef] [Green Version]
- Fukuta, N. Comments on “A possible mechanism for contact nucleation”. J. Atmos. Sci. 1975, 32, 2371–2373. [Google Scholar] [CrossRef] [Green Version]
- Fukuta, N. A study of the mechanism of contact ice nucleation. J. Atmos. Sci. 1975, 32, 1597–1603. [Google Scholar] [CrossRef] [Green Version]
- Djikaev, Y.; Ruckenstein, E. Thermodynamics of heterogeneous crystal nucleation in contact and immersion modes. J. Phys. Chem. A 2008, 112, 11677–11687. [Google Scholar] [CrossRef] [Green Version]
- Djikaev, Y.; Tabazadeh, A.; Hamill, P.; Reiss, H. Thermodynamic conditions for the surface-stimulated crystallization of atmospheric droplets. J. Phys. Chem. A 2002, 106, 10247–10253. [Google Scholar] [CrossRef]
- Sear, R.P. Nucleation at contact lines where fluid–fluid interfaces meet solid surfaces. J. Phys. Condens Matter 2007, 19, 466106. [Google Scholar] [CrossRef]
- Gurganus, C. Investigating the Role of the Contact Line in Heterogeneous Nucleation with High Speed Imaging. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2014. [Google Scholar]
- Gurganus, C.; Kostinski, A.B.; Shaw, R.A. Fast imaging of freezing drops: No preference for nucleation at the contact line. J. Phys. Chem. Lett. 2011, 2, 1449–1454. [Google Scholar] [CrossRef]
- Gurganus, C.; Kostinski, A.B.; Shaw, R.A. High-speed imaging of freezing drops: Still no preference for the contact line. J. Phys. Chem. C 2013, 117, 6195–6200. [Google Scholar] [CrossRef]
- Gurganus, C.; Charnawskas, J.; Kostinski, A.; Shaw, R. Nucleation at the contact line observed on nanotextured surfaces. Phys. Rev. Lett. 2014, 113, 235701. [Google Scholar] [CrossRef] [Green Version]
- Winkler, P.; McGraw, R.; Bauer, P.; Rentenberger, C.; Wagner, P. Direct determination of three-phase contact line properties on nearly molecular scale. Sci. Rep. 2016, 6, 26111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, R.P. The effect of an external electric field on the supercooling of water drops. J. Geophys. Res. 1963, 68, 4463–4474. [Google Scholar] [CrossRef]
- Hobbs, P. The aggregation of ice particles in clouds and fogs at low temperatures. J. Atmos. Sci. 1965, 22, 296–300. [Google Scholar] [CrossRef]
- Alkezweeny, A. Freezing of supercooled water droplets due to collision. J. Appl. Meteorol. 1969, 8, 994–995. [Google Scholar] [CrossRef] [Green Version]
- Czys, R.R. Ice initiation by collision-freezing in warm-based cumuli. J. Appl. Meteorol. 1989, 28, 1098–1104. [Google Scholar] [CrossRef]
- Durant, A.J.; Shaw, R.A. Evaporation freezing by contact nucleation inside-out. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Donadio, D.; Galli, G. Ice nucleation at the nanoscale probes no man’s land of water. Nat. Commun. 2013, 4, 1887. [Google Scholar] [CrossRef]
- Marcolli, C. Ice nucleation triggered by negative pressure. Sci. Rep. 2017, 7, 16634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickling, R. Nucleation of freezing by cavity collapse and its relation to cavitation damage. Nature 1965, 206, 915–917. [Google Scholar] [CrossRef]
- Hunt, J.; Jackson, K. Nucleation of the solid phase by cavitation in an undercooled liquid which expands on freezing. Nature 1966, 211, 1080–1081. [Google Scholar] [CrossRef]
- Hunt, J.; Jackson, K. Nucleation of solid in an undercooled liquid by cavitation. J. Appl. Phys. 1966, 37, 254–257. [Google Scholar] [CrossRef]
- Li, B.; Sun, D.W. Novel methods for rapid freezing and thawing of foods—A review. J. Food Eng. 2002, 54, 175–182. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. The principles of ultrasound and its application in freezing related processes of food materials: A review. Ultrason. Sonochem. 2015, 27, 576–585. [Google Scholar] [CrossRef]
- Schremb, M.; Roisman, I.V.; Tropea, C. Transient effects in ice nucleation of a water drop impacting onto a cold substrate. Phys. Rev. E 2017, 95, 022805. [Google Scholar] [CrossRef]
- Pallares, G.; Azouzi, M.E.M.; González, M.A.; Aragones, J.L.; Abascal, J.L.; Valeriani, C.; Caupin, F. Anomalies in bulk supercooled water at negative pressure. Proc. Natl. Acad. Sci. USA 2014, 111, 7936–7941. [Google Scholar] [CrossRef] [Green Version]
- Barrow, M.S.; Williams, P.R.; Chan, H.H.; Dore, J.C.; Bellissent-Funel, M.C. Studies of cavitation and ice nucleation in ‘doubly-metastable’ water: Time-lapse photography and neutron diffraction. Phys. Chem. Chem. Phys. 2012, 14, 13255–13261. [Google Scholar] [CrossRef]
- Kostinski, A.; Cantrell, W. Entropic aspects of supercooled droplet freezing. J. Atmos. Sci. 2008, 65, 2961–2971. [Google Scholar] [CrossRef]
- Niehaus, J.; Becker, J.G.; Kostinski, A.; Cantrell, W. Laboratory measurements of contact freezing by dust and bacteria at temperatures of mixed-phase clouds. J. Atmos. Sci. 2014, 71, 3659–3667. [Google Scholar] [CrossRef]
- Murray, B.; O’sullivan, D.; Atkinson, J.; Webb, M. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedermeier, D.; Shaw, R.; Hartmann, S.; Wex, H.; Clauss, T.; Voigtländer, J.; Stratmann, F. Heterogeneous ice nucleation: Exploring the transition from stochastic to singular freezing behavior. Atmos. Chem. Phys. 2011, 11, 8767–8775. [Google Scholar] [CrossRef]
- Ervens, B.; Feingold, G. Sensitivities of immersion freezing: Reconciling classical nucleation theory and deterministic expressions. Geophys. Res. Lett. 2013, 40, 3320–3324. [Google Scholar] [CrossRef]
- Murray, B.J.; Broadley, S.; Wilson, T.; Atkinson, J.; Wills, R. Heterogeneous freezing of water droplets containing kaolinite particles. Atmos. Chem. Phys. 2011, 11, 4191–4207. [Google Scholar] [CrossRef] [Green Version]
- Broadley, S.; Murray, B.; Herbert, R.; Atkinson, J.; Dobbie, S.; Malkin, T.; Condliffe, E.; Neve, L. Immersion mode heterogeneous ice nucleation by an illite rich powder representative of atmospheric mineral dust. Atmos. Chem. Phys. 2012, 12, 287–307. [Google Scholar] [CrossRef] [Green Version]
- DeMott, P.J.; Möhler, O.; Cziczo, D.J.; Hiranuma, N.; Petters, M.D.; Petters, S.S.; Belosi, F.; Bingemer, H.G.; Brooks, S.D.; Budke, C.; et al. The Fifth International Workshop on Ice Nucleation phase 2 (FIN-02): laboratory intercomparison of ice nucleation measurements. Atmos. Meas. Tech. 2018, 11, 6231–6257. [Google Scholar] [CrossRef] [Green Version]
- Hiranuma, N.; Augustin-Bauditz, S.; Bingemer, H.; Budke, C.; Curtius, J.; Danielczok, A.; Diehl, K.; Dreischmeier, K.; Ebert, M.; Frank, F.; et al. A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: A comparison of 17 ice nucleation measurement techniques. Atmos. Chem. Phys. 2015, 15, 2489–2518. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Wex, H.; Clauss, T.; Augustin-Bauditz, S.; Niedermeier, D.; Rösch, M.; Stratmann, F. Immersion freezing of kaolinite: Scaling with particle surface area. J. Atmos. Sci. 2016, 73, 263–278. [Google Scholar] [CrossRef]
- Niehaus, J.; Bunker, K.W.; China, S.; Kostinski, A.; Mazzoleni, C.; Cantrell, W. A technique to measure ice nuclei in the contact mode. J. Atmos. Ocean. Technol. 2014, 31, 913–922. [Google Scholar] [CrossRef]
- Borzsák, I.; Cummings, P.T. Electrofreezing of water in molecular dynamics simulation accelerated by oscillatory shear. Phys. Rev. E 1997, 56, R6279. [Google Scholar] [CrossRef]
- Bird, J.C.; Ristenpart, W.D.; Belmonte, A.; Stone, H.A. Critical angle for electrically driven coalescence of two conical droplets. Phys. Rev. Lett. 2009, 103, 164502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, C.T.; Généro, G.A.; Bird, J.C. Coalescence and break-up of nearly inviscid conical droplets. J. Fluid Mech. 2015, 763, 369–385. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Cantrell, W.H.; Kostinski, A.B.; Shaw, R.A.; Vogelmann, A.M. Is Contact Nucleation Caused by Pressure Perturbation? Atmosphere 2020, 11, 1. https://doi.org/10.3390/atmos11010001
Yang F, Cantrell WH, Kostinski AB, Shaw RA, Vogelmann AM. Is Contact Nucleation Caused by Pressure Perturbation? Atmosphere. 2020; 11(1):1. https://doi.org/10.3390/atmos11010001
Chicago/Turabian StyleYang, Fan, Will H. Cantrell, Alexander B. Kostinski, Raymond A. Shaw, and Andrew M. Vogelmann. 2020. "Is Contact Nucleation Caused by Pressure Perturbation?" Atmosphere 11, no. 1: 1. https://doi.org/10.3390/atmos11010001




