Differences in Model Performance and Source Sensitivities for Sulfate Aerosol Resulting from Updates of the Aqueous- and Gas-Phase Oxidation Pathways for a Winter Pollution Episode in Tokyo, Japan
Abstract
1. Introduction
2. Modeling Design
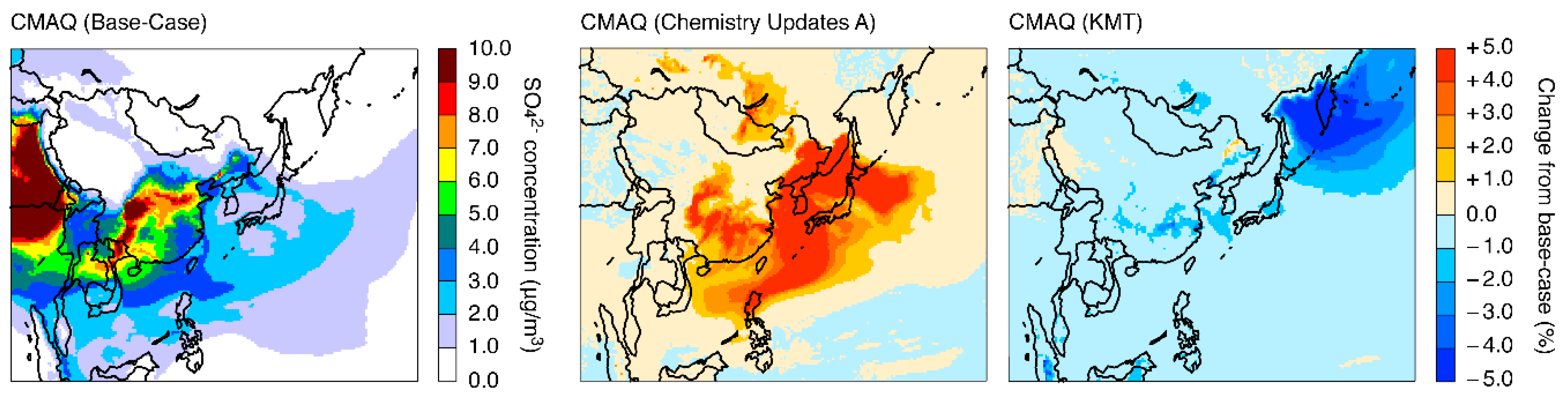
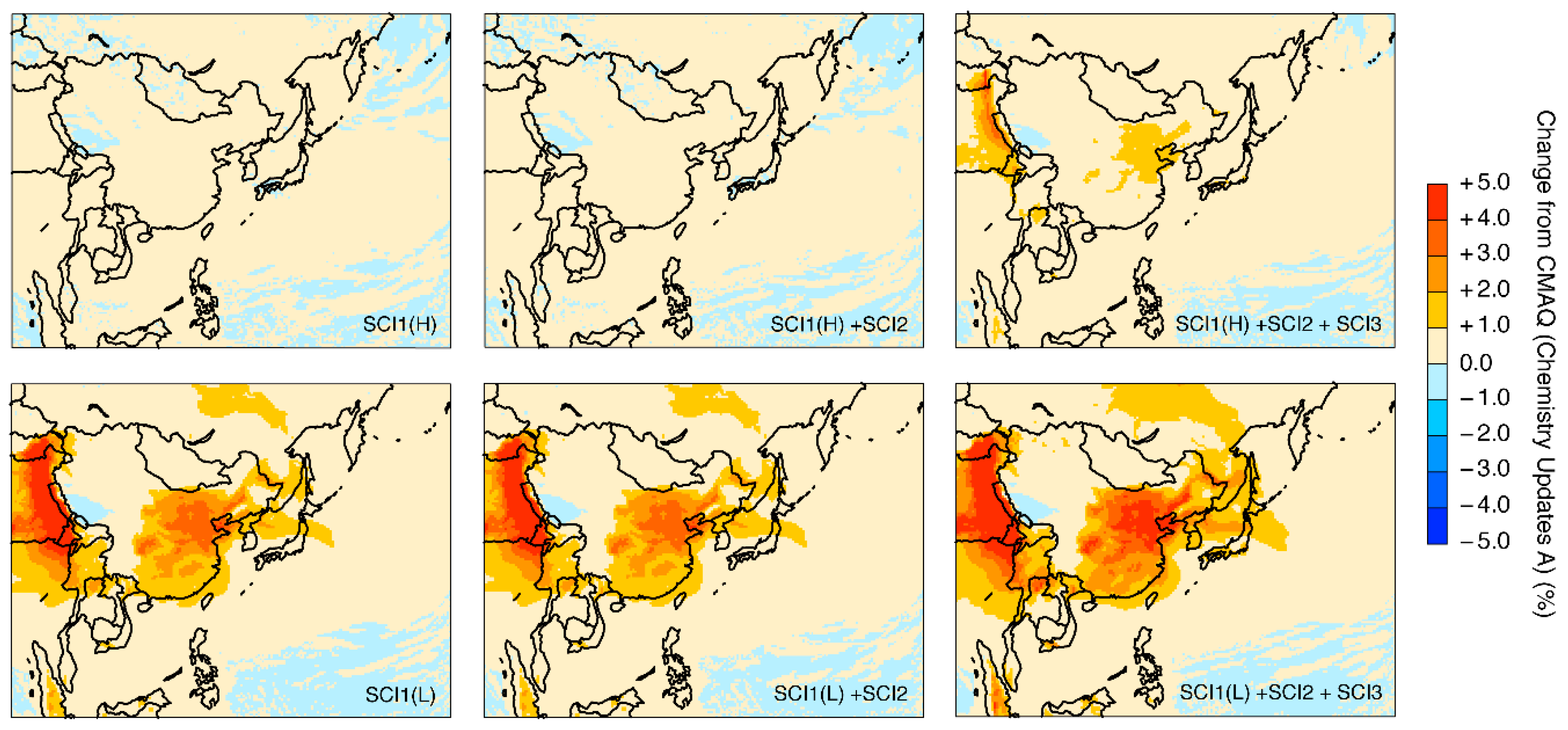
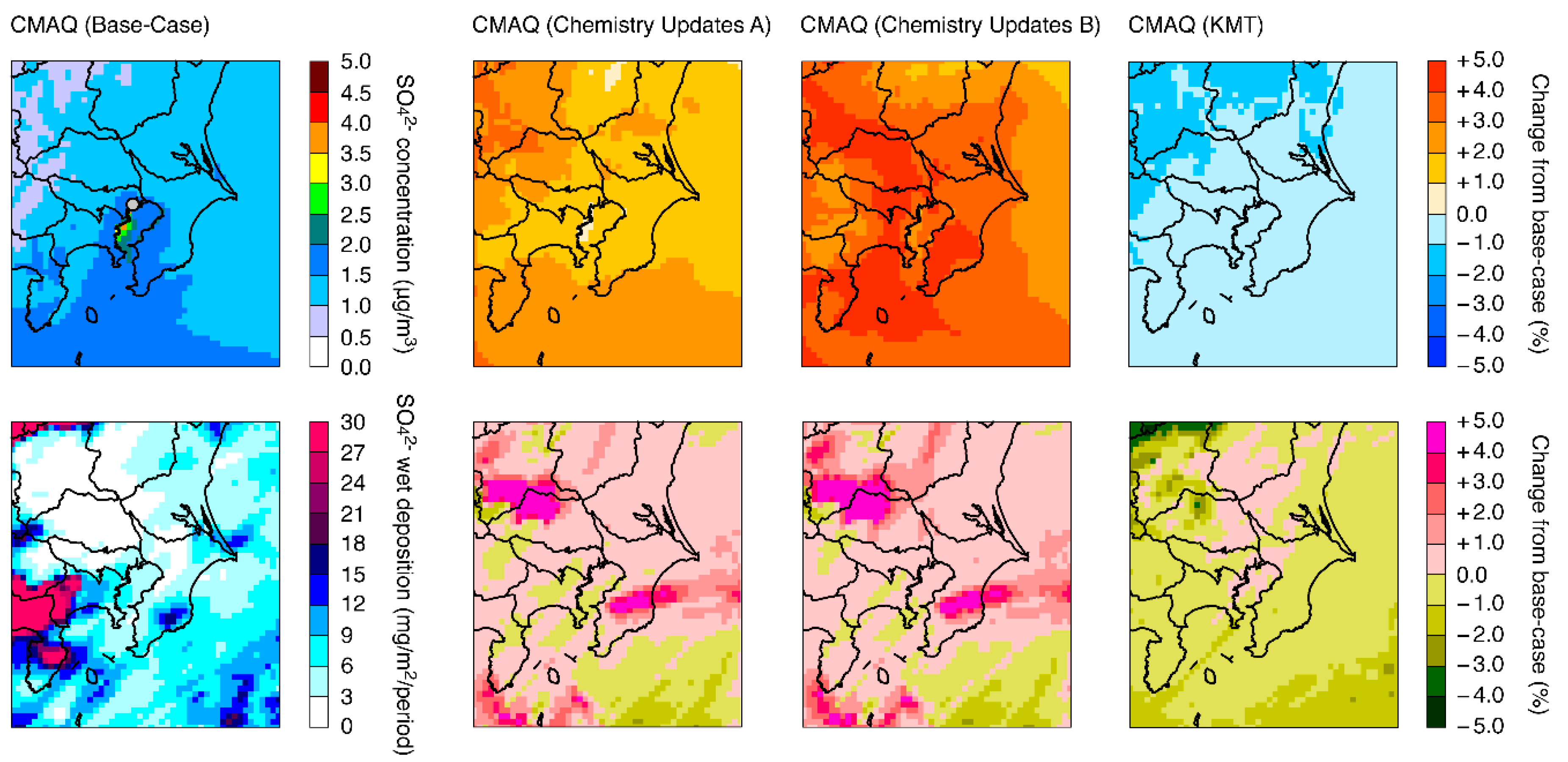
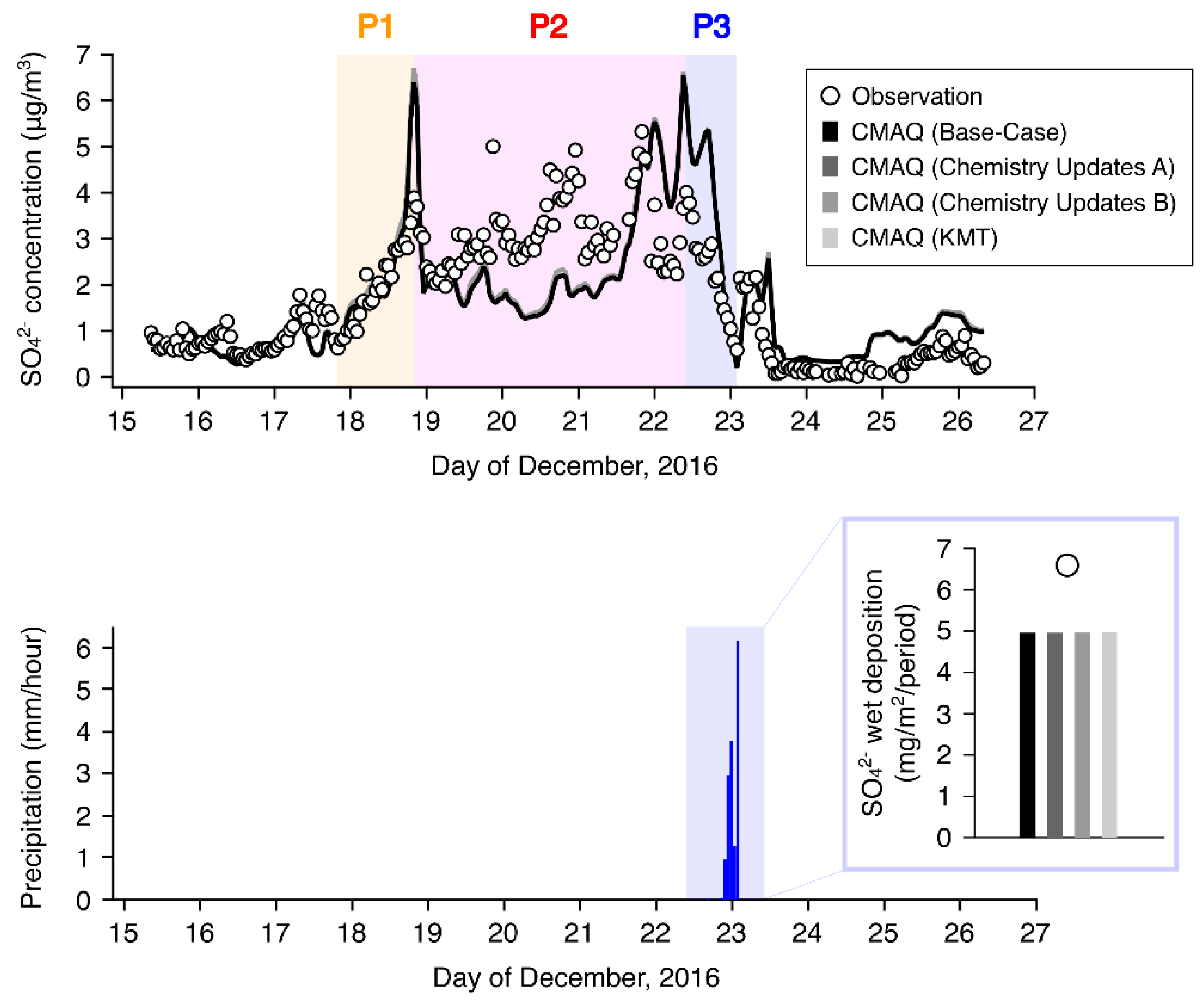
3. Results and Discussion
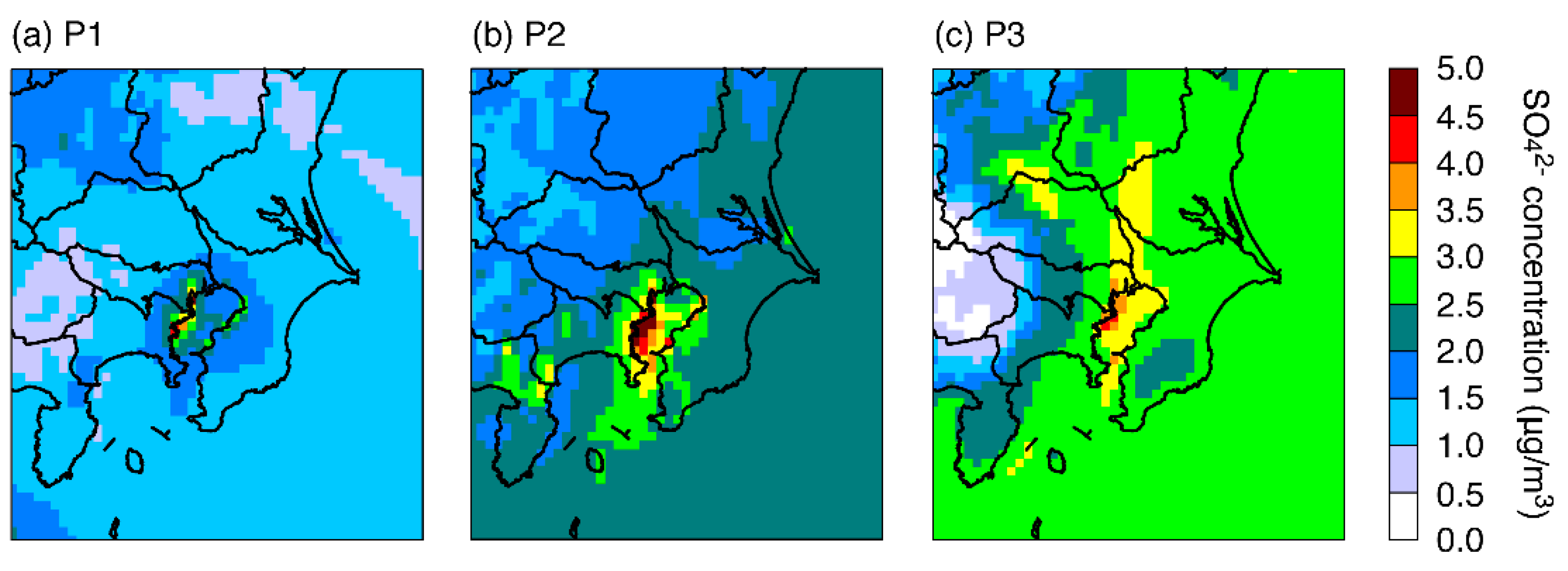
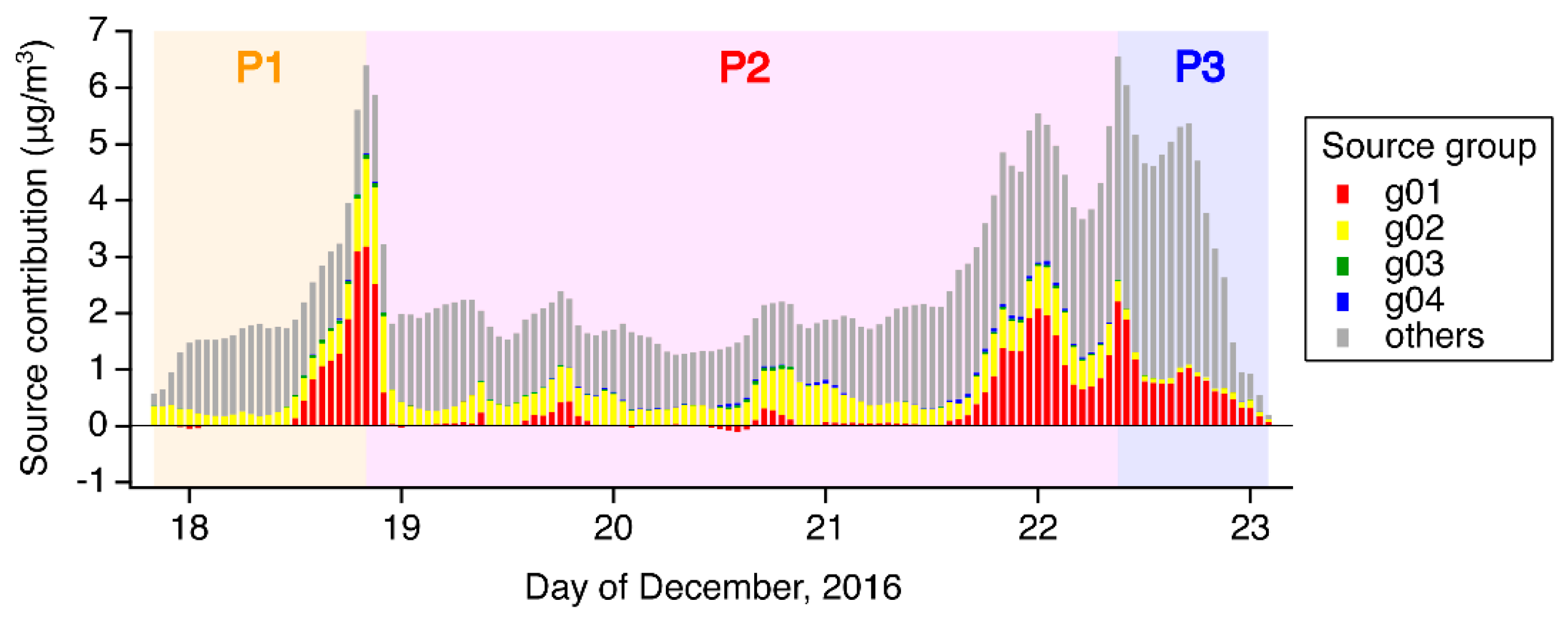
3.1. Model Performance
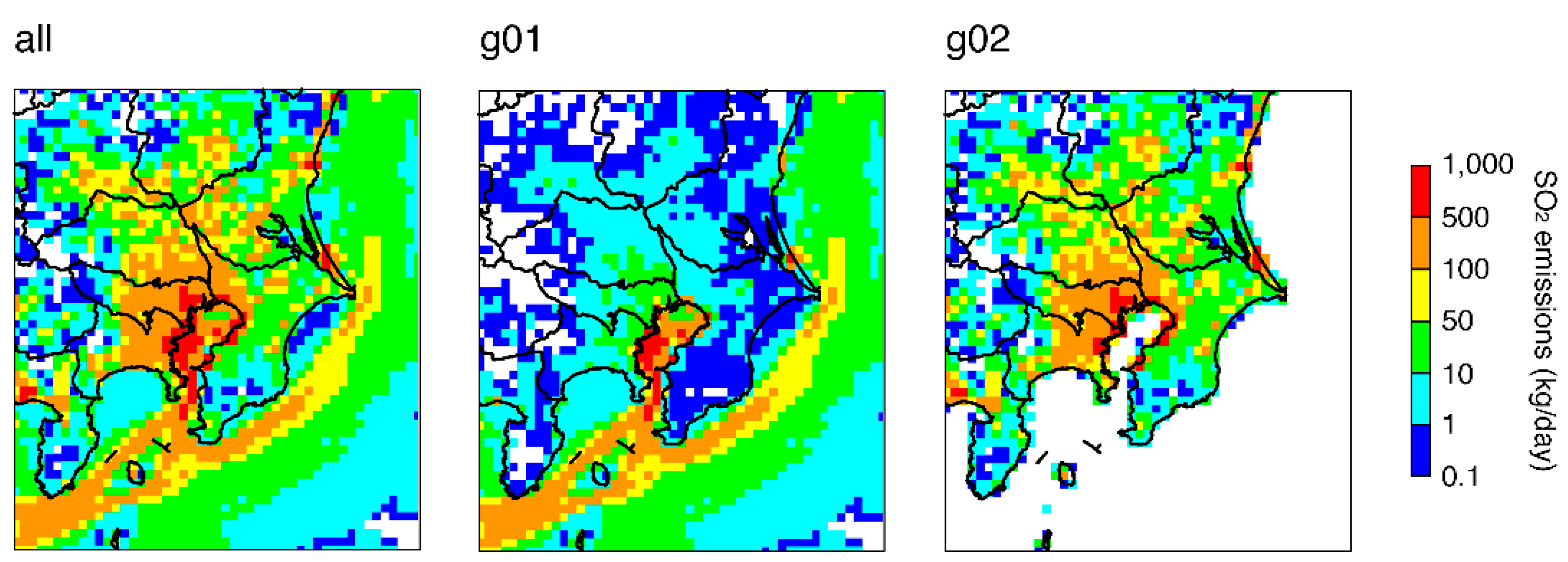
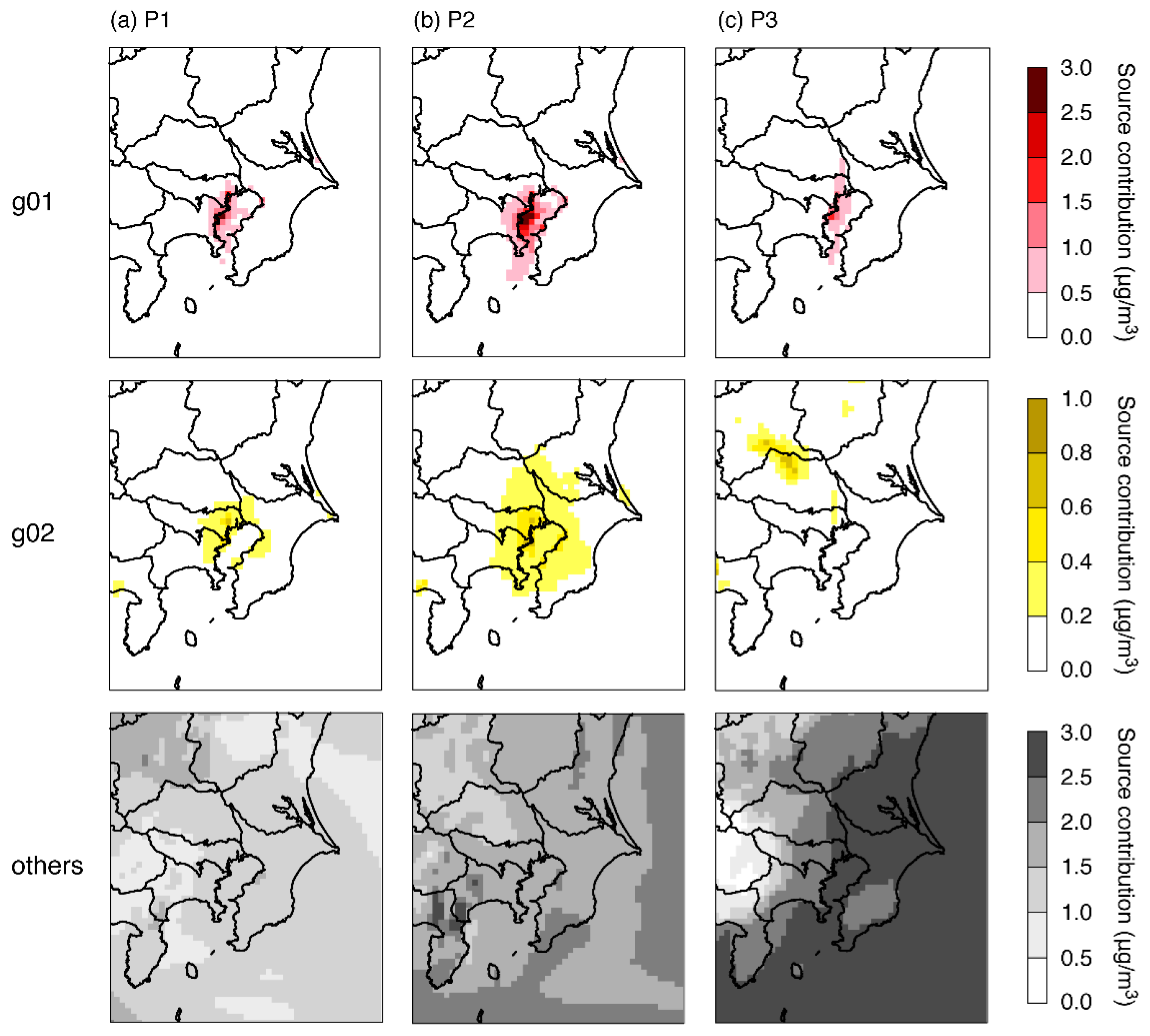
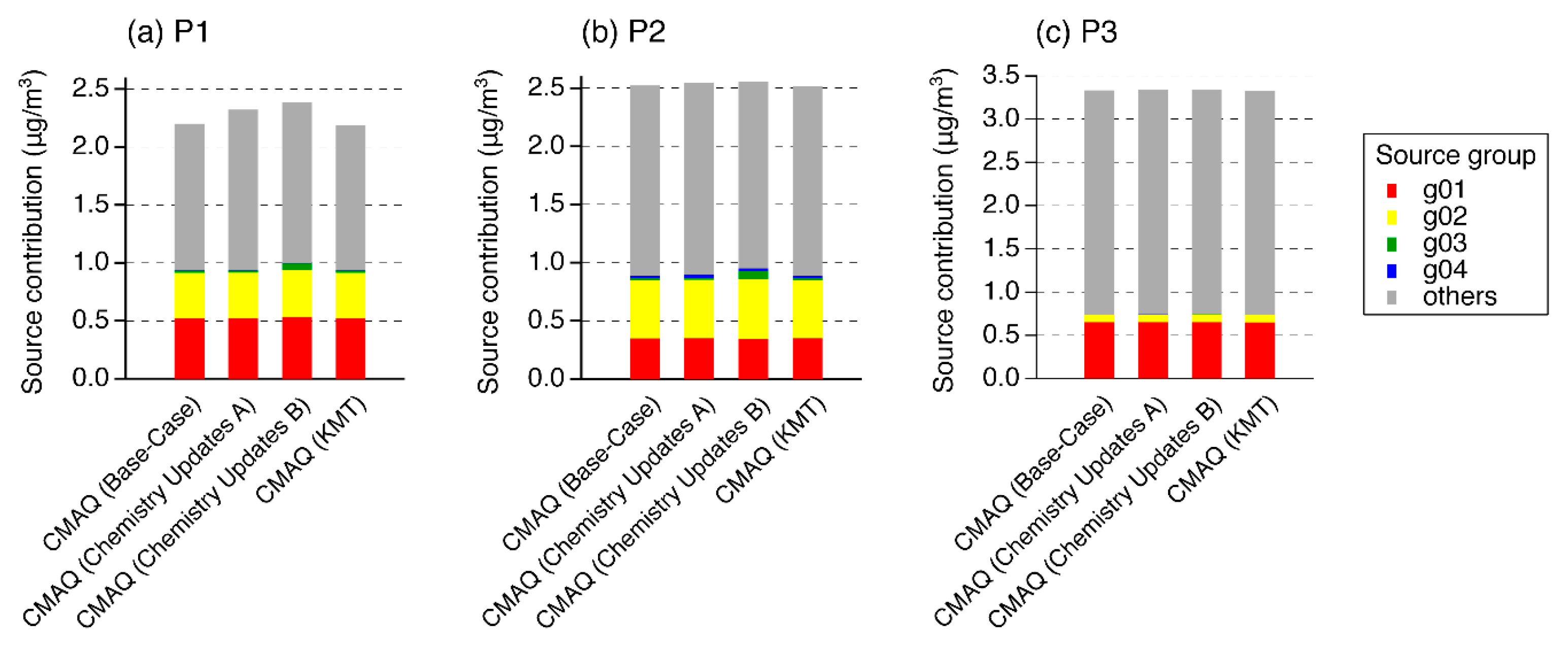
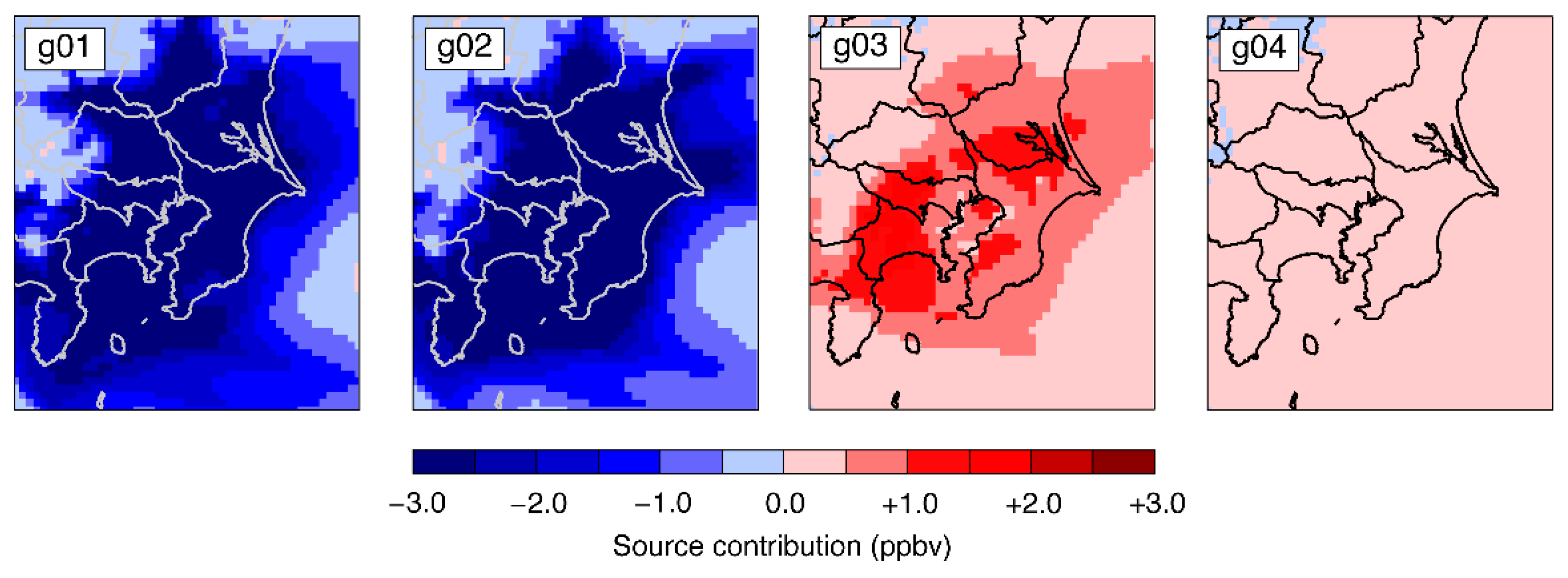
3.2. Model Sensitivities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chatani, S.; Yamaji, K.; Sakurai, T.; Itahashi, S.; Shimadera, H.; Kitayama, K.; Hayami, H. Overview of model inter-comparison in Japan’s study for reference air quality modeling (J-STREAM). Atmosphere 2018, 9, 19. [Google Scholar] [CrossRef]
- Chatani, S.; Okumura, M.; Shimadera, H.; Yamaji, K.; Kitayama, K.; Matsunaga, S. Effects of a detailed vegetation database on simulated meteorological fields, biogenic VOC emissions, and ambient pollutant concentrations over Japan. Atmosphere 2018, 9, 179. [Google Scholar] [CrossRef]
- Itahashi, S.; Yamaji, K.; Chatani, S.; Hayami, H. Refinement of modeled aqueous-phase sulfate production via the Fe- and Mn-catalyzed oxidation pathway. Atmosphere 2018, 9, 132. [Google Scholar] [CrossRef]
- Itahashi, S.; Yamaji, K.; Chatani, S.; Hisatsune, K.; Saito, S.; Hayami, H. Model performance differences in sulfate aerosol in winter over Japan based on regional chemical transport models of CMAQ and CAMx. Atmosphere 2018, 9, 488. [Google Scholar] [CrossRef]
- Kitayama, K.; Morino, Y.; Yamaji, K.; Chatani, S. Uncertainties in O3 concentrations simulated by CMAQ over Japan using four chemical mechanisms. Atmos. Environ. 2019, 198, 448–462. [Google Scholar] [CrossRef]
- Fu, X.; Wang, S.; Zhao, B.; Xing, J.; Cheng, Z.; Liu, H.; Hao, J. Emission inventory of primary pollutants and chemical speciation in 2010 for the Yangtze River Delta region, China. Atmos. Environ. 2013, 70, 39–50. [Google Scholar] [CrossRef]
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. 2018, 18, 14095–14111. [Google Scholar] [CrossRef]
- Skamarock, W.C.; Klemp, J.B.; Dudhia, J.; Gill, D.O.; Barker, D.M.; Duda, M.G.; Huang, X.Y.; Wang, W.; Power, J.G. A Description of the Advanced Research WRF Version 3; NCAR/TN-475+STR; National Center for Atmospheric Research: Boulder, CO, USA, 2008. [Google Scholar]
- National Centers for Environmental Prediction/National Weather Service/NOAA/U.S. Department of Commerce. NCEP GDAS/FNL 0.25 Degree Global Tropospheric Analyses and Forecast Grids, Research Data Archive at the National Center for Atmospheric Research, Computational and Information Systems Laboratory, Boulder, Colo. 2015. Available online: https://rda.ucar.edu/datasets/ds083.3/ (accessed on 7 May 2019).
- Group for High Resolution Sea Surface Temperature (GHRSST). Available online: https://www.ghrsst.org (accessed on 7 July 2019).
- Iacono, M.J.; Delamere, J.S.; Mlawer, E.J.; Shephard, M.W.; Clough, S.A.; Collins, W.D. Radiative forcing by long-lived greenhouse gases: Calculations with the AER radiative transfer models. J. Geophys. Res. 2008, 113, D13103. [Google Scholar] [CrossRef]
- Morrison, H.; Thompson, G.; Tatarskii, V. Impacts of cloud microphysics on the development of trailing stratiform precipitation in a simulated squall line: Comparison of one- and two-moment schemes. Mon. Weather Rev. 2009, 137, 991–1007. [Google Scholar] [CrossRef]
- Grell, G.A.; Devenyi, D. A generalized approach to parameterizing convection combining ensemble and data assimilation techniques. Geophys. Res. Lett. 2002, 29. [Google Scholar] [CrossRef]
- US EPA Office of Research and Development. Community Multiscale Air Quality (CMAQ) Model Version 5.2; US EPA Office of Research and Development: Washington, DC, USA, 2017. [CrossRef]
- Carter, W.P.L. Development of the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5336–5345. [Google Scholar] [CrossRef]
- CMAQ v5.0 Sulfur Chemistry. Available online: https://www.airqualitymodeling.org/index.php/CMAQv5.0_Sulfur_Chemistry (accessed on 10 May 2019).
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics—From Air Pollution to Climate Change, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Akimoto, H. Atmospheric Reaction Chemistry; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hatakeyama, S.; Akimoto, H. Reactions of Criegee intermediates in the gas phase. Res. Chem. Intermed. 1994, 20, 503–524. [Google Scholar] [CrossRef]
- Welz, O.; Savee, J.D.; Osborn, D.L.; Vasu, S.S.; Percival, C.J.; Shallcross, D.E.; Taatjes, C.A. Direct kinetic measurements of Criegee Intermediate (CH2OO) formed by reaction of CH2I with O2. Science 2012, 335, 204–207. [Google Scholar] [CrossRef]
- Stone, D.; Blitz, M.; Daubney, L.; Howes, N.U.M.; Seakins, P. Kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO as a function of pressure. Phys. Chem. Chem. Phys. 2014, 16, 1139. [Google Scholar] [CrossRef]
- Tadayon, S.V.; Foreman, E.S.; Murray, C. Kinetics of the reactions between the Criegee intermediate CH2OO and alcohols. J. Phys. Chem. A 2018, 122, 258–268. [Google Scholar] [CrossRef]
- Taatjes, C.A.; Welz, O.; Eskola, A.J.; Savee, J.D.; Scheer, A.M.; Shallcross, D.E.; Rotavera, B.; Lee, E.P.F.; Dyke, J.M.; Mok, D.K.W.; et al. Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. Science 2013, 340, 177–180. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chao, W.; Lin, J.M. Kinetics of a Criegee intermediate that would survice high humidity and may oxidize atmospheric SO2. Proc. Natl. Acad. Sci. USA 2015, 112, 10857–10862. [Google Scholar] [CrossRef]
- Sarwar, G.; Fahey, K.; Kwok, R.; Gilliam, R.C.; Roselle, S.J.; Mathur, R.; Xue, J.; Yu, J.; Carter, W.P.L. Potential impacts of two SO2 oxidation pathways on regional sulfate concentrations: aqueous-phase oxidation by NO2 and gas-phase oxidation by Stabilized Criegee Intermediates. Atmos. Environ. 2013, 68, 186–197. [Google Scholar] [CrossRef]
- Li, J.; Ying, Q.; Yi, B.; Yang, P. Role of stabilized Criegee Intermediates in the formation of atmospheric sulfate in eastern United States. Atmos. Environ. 2013, 79, 442–447. [Google Scholar] [CrossRef]
- CMAQ v5.1 Aqueous Chemistry. Available online: https://www.airqualitymodeling.org/index.php/CMAQv5.0_Sulfur_Chemistry (accessed on 28 June 2018).
- Fahey, K.M.; Carlton, A.G.; Pye, H.O.T.; Baek, J.; Hutzell, W.T.; Stanier, C.O.; Baker, K.R.; Appel, K.W.; Jaoui, M.; Offenberg, J.H. A framework for expanding aqueous chemistry in the Community Multiscale Air Quality (CMAQ) model version 5.1. Geosci. Model Dev. 2017, 10, 1587–1605. [Google Scholar] [CrossRef]
- Damian, V.; Sandu, A.; Damian, M.; Potra, F.; Carmichael, G.R. The kinetic preprocessor KPP—A software environment for solving chemical kinetics. Comput. Chem. Eng. 2002, 26, 1567–1579. [Google Scholar] [CrossRef]
- Itahashi, S.; Uno, I.; Kim, S.-T. Source contributions of sulfate aerosol over East Asia estimated by CMAQ-DDM. Environ. Sci. Technol. 2012, 46, 6733–6741. [Google Scholar] [CrossRef]
- Itahashi, S.; Hayami, H.; Yumimoto, K.; Uno, I. Chinese province-scale source apportionments for sulfate aerosol in 2005 evaluated by the tagged tracer method. Environ. Pollut. 2017, 220, 1366–1375. [Google Scholar] [CrossRef]
- Itahashi, S.; Hatakeyama, S.; Shimada, K.; Tatsuta, S.; Taniguchi, Y.; Chan, C.K.; Kim, Y.-P.; Lin, N.-H.; Takami, A. Model estimation of sulfate aerosol source collected at Cape Hedo during an intensive campaign in October-November, 2015. Aerosol Air Qual. Res. 2017, 17, 3079–3090. [Google Scholar] [CrossRef]
- Itahashi, S. Toward synchronous evaluation of source apportionments for atmospheric concentration and deposition of sulfate aerosol over East Asia. J. Geophys. Res. Atmos. 2018, 123, 2927–2953. [Google Scholar] [CrossRef]
- Itahashi, S.; Hatakeyama, S.; Shimada, K.; Takami, A. Sources of high sulfate aerosol concentration observed at Cape Hedo in spring 2012. Aerosol Air Qual. Res. 2019, 19, 587–600. [Google Scholar] [CrossRef]
- Itahashi, S.; Uno, I.; Osada, K.; Kamiguchi, Y.; Yamamoto, S.; Tamura, K.; Wang, Z.; Kurosaki, Y.; Kanaya, Y. Nitrate transboundary heavy pollution over East Asia in winter. Atmos. Chem. Phys. 2017, 17, 3823–3843. [Google Scholar] [CrossRef]
- EANET. Technical Manual for Wet Deposition Monitoring in East Asia. Available online: http://www.eanet.asia/product/manual/techwet.pdf (accessed on 3 September 2018).
- Emery, C.; Liu, Z.; Russell, A.G.; Odman, M.T.; Yarwood, G.; Kumar, N. Recommendations on statistics and benchmarks to assess photochemical model performance. J. Air Waste Manag. Assoc. 2017, 67, 582–598. [Google Scholar] [CrossRef]
- Boylan, J.W.; Russell, A.G. PM and light extinction model performance metrics, goals, and criteria for three-dimensional air quality models. Atmos. Environ. 2006, 40, 4946–4959. [Google Scholar] [CrossRef]
- Fiore, A.M.; Dentener, F.J.; Wild, O.; Cuvelier, C.; Schultz, M.G.; Hess, P.; Textor, C.; Schulz, M.; Doherty, R.M.; Horowitz, L.W.; et al. Multimodel estimates of intercontinental source-receptor relationships for ozone pollution. J. Geophys. Res. 2009, 114, D04301. [Google Scholar] [CrossRef]
- Itahashi, S.; Uno, I.; Kim, S.-T. Seasonal source contributions of tropospheric ozone over East Asia based on CMAQ-HDDM. Atmos. Environ. 2013, 70, 204–217. [Google Scholar] [CrossRef]
| Reaction | Rate Constant | Reference |
|---|---|---|
| O3 + ETHE → … + 0.370 × SCI1 | [15] | |
| O3 + PRPE → … + 0.185 × SCI1 + 0.075 × SCI2 | [15] | |
| O3 + BD13 → … + 0.185 × SCI1 | [15] | |
| O3 + OLE1 → … + 0.185 × SCI1 + 0.159 × SCI3 | [15] | |
| O3 + OLE2 → … + 0.024 × SCI1 + 0.065 × SCI2 + 0.235 × SCI3 | [15] | |
| O3 + ISOP → … + 0.204 × SCI1 | [15] | |
| O3 + IPRD → … + 0.100 × SCI1 + 0.372 × SCI3 | [15] | |
| O3 + TERP → … + 0.172 × SCI1 + 0.068 × SCI3 | [15] | |
| O3 + SESQ → … + 0.172 × SCI1 + 0.058 × SCI3 | [15] | |
| SCI1 + SO2 → HCHO + SULF | 3.9 × 10−11 | [20] |
| SCI1 + NO2 → HCHO + NO3 | 1.5 × 10−12 | [21] |
| SCI1 + NO → HCHO + NO2 | 2.0 × 10−13 | [21] |
| SCI1 + H2O → | 2.4 × 10−15 9.0 × 10−17 | [20] [21] |
| SCI1 + MEOH → | 1.4 × 10−13 | [22] |
| SCI1 + ETOH → | 2.3 × 10−13 | [22] |
| SCI1 + ALK4 → | 1.9 × 10−13 | [22] |
| SCI2 + SO2 → CCHO + SULF | 4.55 × 10−11 | [23] |
| SCI2 + H2O → | 7.0 × 10−14 | [23] |
| SCI3 + SO2 → RCHO + SULF | 1.3 × 10−10 | [24] |
| SCI3 + H2O → | 1.5 × 10−16 | [24] |
| Name | Description |
|---|---|
| Chemistry Updates A | Fe and Mn solubilities are increased and the rate constant expression for the Fe- and Mn-catalyzed oxidation by O2 includes a pH dependency. Addition of an NO2 aqueous-phase reaction (a total of six aqueous-phase reactions were treated). |
| Chemistry Updates B | Same as sensitivity Simulation A, but with addition of gas-phase oxidation pathways related to SCI (see Table 1). |
| Kinetic Mass Transfer (KMT) | Selection of the AQCHEM-KMT option |
| Base-Case | Chemistry Updates A | Chemistry Updates B | KMT | |
|---|---|---|---|---|
| N | 247 | |||
| Mean (observation) [μg/m3] | 1.70 | |||
| Mean (model) [μg/m3] | 1.68 | 1.70 | 1.74 | 1.66 |
| R | 0.68 * (p < 0.001) | 0.68 * (p < 0.001) | 0.69 * (p < 0.001) | 0.68 * (p < 0.001) |
| NMB [%] | −1.4 ** | 0.0 ** | +2.6 ** | −2.1 ** |
| NME [%] | 45.0 * | 45.1 * | 44.1 * | 44.9 * |
| MFB [%] | +10.7 ** | +12.0 ** | +14.4 ** | +9.8 ** |
| MFE [%] | 52.1 * | 52.1 * | 51.4 * | 51.9 * |
| % within a factor of 2 | 69.6 | 69.2 | 70.5 | 70.4 |
| % within a factor of 3 | 87.9 | 87.9 | 87.9 | 88.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itahashi, S.; Yamaji, K.; Chatani, S.; Hayami, H. Differences in Model Performance and Source Sensitivities for Sulfate Aerosol Resulting from Updates of the Aqueous- and Gas-Phase Oxidation Pathways for a Winter Pollution Episode in Tokyo, Japan. Atmosphere 2019, 10, 544. https://doi.org/10.3390/atmos10090544
Itahashi S, Yamaji K, Chatani S, Hayami H. Differences in Model Performance and Source Sensitivities for Sulfate Aerosol Resulting from Updates of the Aqueous- and Gas-Phase Oxidation Pathways for a Winter Pollution Episode in Tokyo, Japan. Atmosphere. 2019; 10(9):544. https://doi.org/10.3390/atmos10090544
Chicago/Turabian StyleItahashi, Syuichi, Kazuyo Yamaji, Satoru Chatani, and Hiroshi Hayami. 2019. "Differences in Model Performance and Source Sensitivities for Sulfate Aerosol Resulting from Updates of the Aqueous- and Gas-Phase Oxidation Pathways for a Winter Pollution Episode in Tokyo, Japan" Atmosphere 10, no. 9: 544. https://doi.org/10.3390/atmos10090544
APA StyleItahashi, S., Yamaji, K., Chatani, S., & Hayami, H. (2019). Differences in Model Performance and Source Sensitivities for Sulfate Aerosol Resulting from Updates of the Aqueous- and Gas-Phase Oxidation Pathways for a Winter Pollution Episode in Tokyo, Japan. Atmosphere, 10(9), 544. https://doi.org/10.3390/atmos10090544





