Air/Surface Exchange of Gaseous Elemental Mercury at Different Landscapes in Mississippi, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Descriptions
2.1.1. In Situ Measurements
Wetland
Pond and Mowed Field
Forest and Residential Lawn
2.1.2. Mesocosm Measurements
2.2. Gaseous Elemental Mercury (GEM) Flux Measurements
2.3. Meteorological and Solar Radiation Measurements
2.4. Total-Hg and Organic Matter Content Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Total-Hg Concentrations in the Soils
3.2. Total GEM Concentrations in the Ambient Air
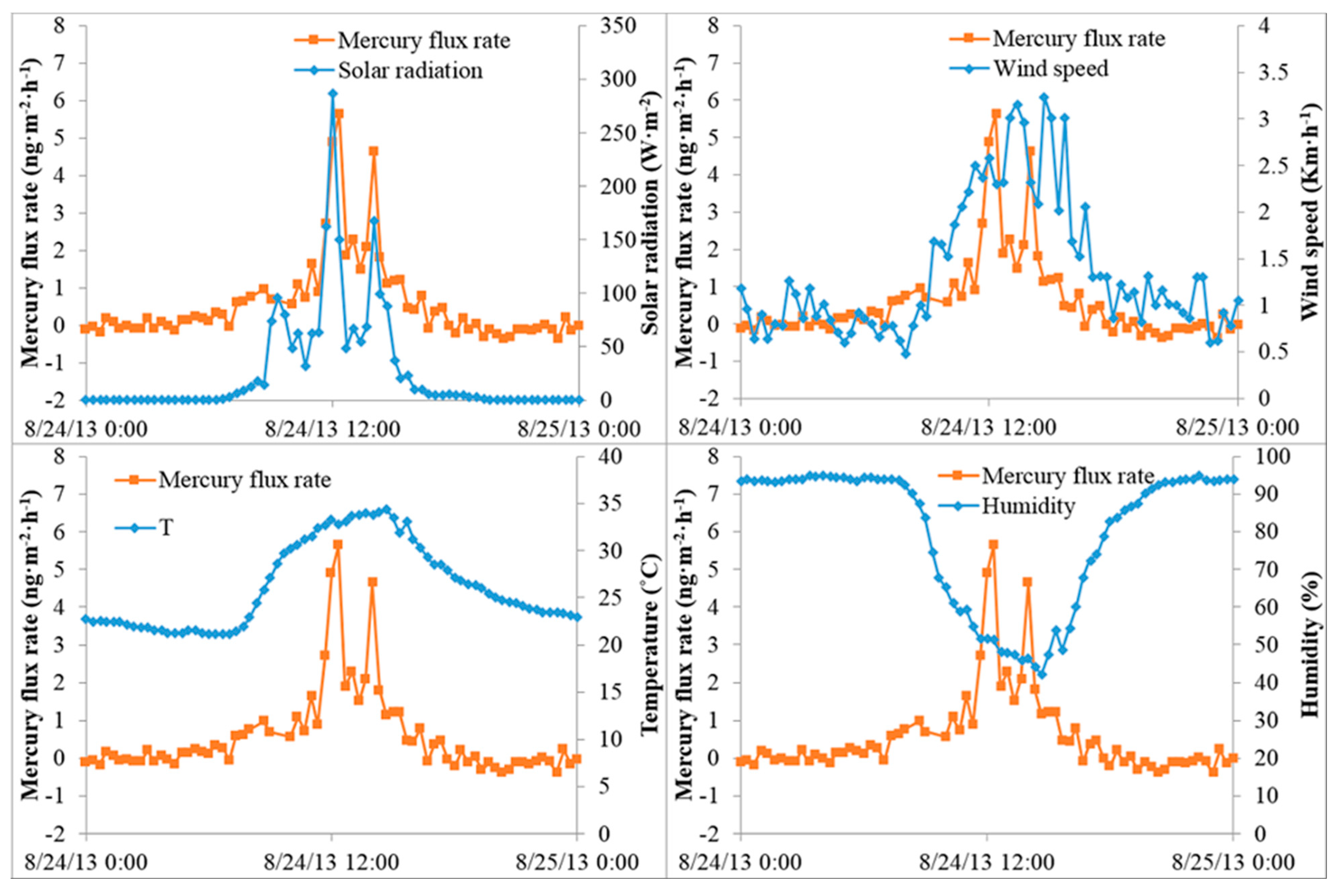
3.3. Hg Fluxes over a Loblolly Pine Forest Floor
3.4. GEM Fluxes over a Residential Grass Lawn
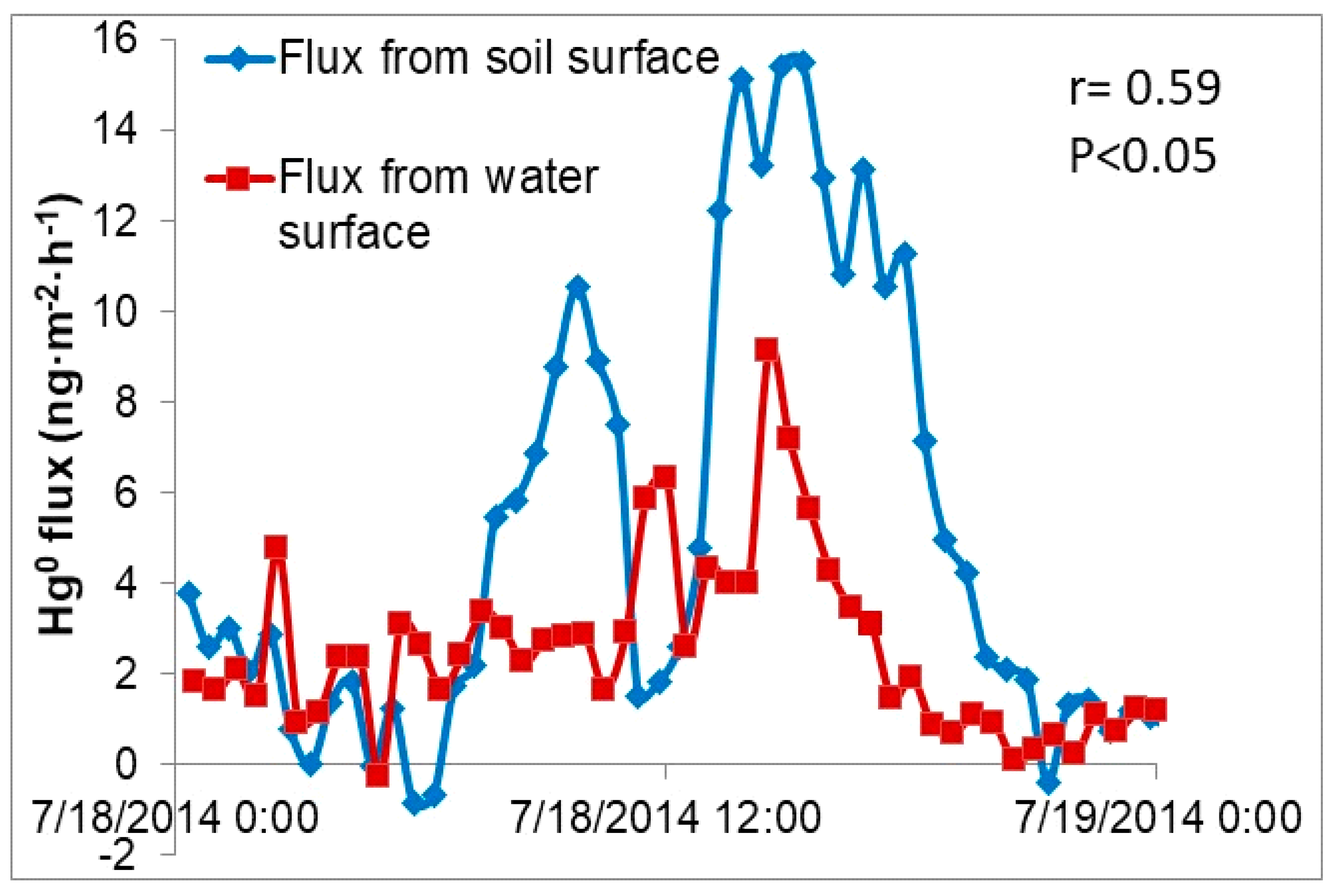
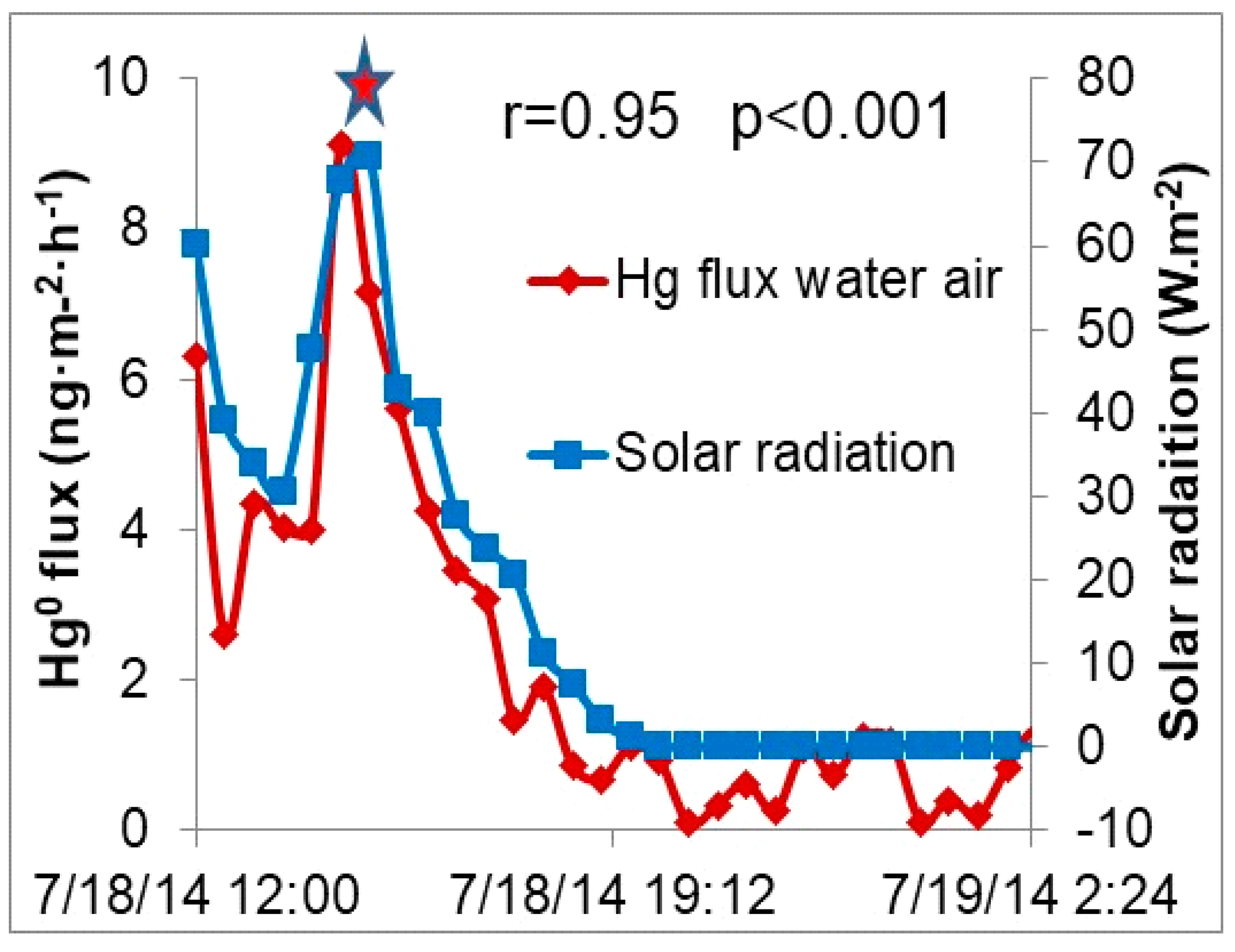
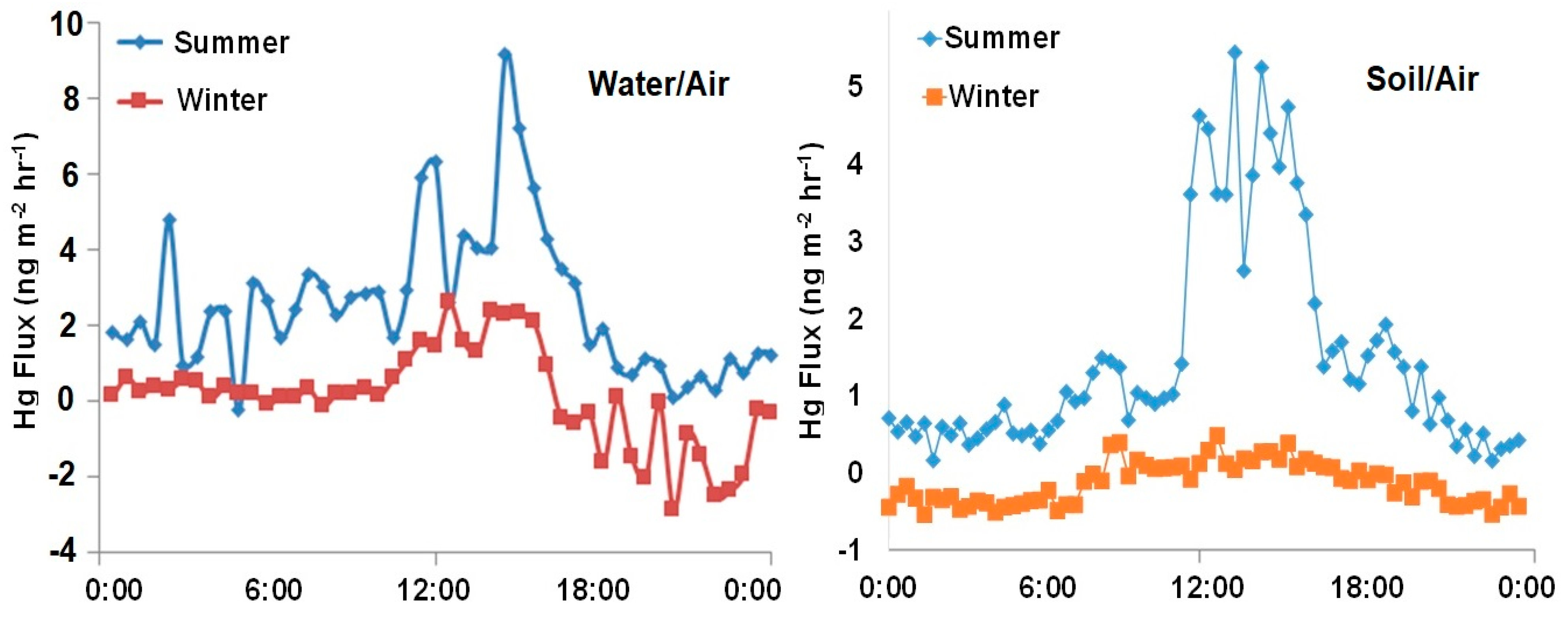
3.5. GEM Fluxes over Wetland Water, Wetland Soil, a Pond and a Mowed Field
3.6. GEM Fluxes over Agriculture Soils
3.7. Summary of Seasonal Differences for GEM Fluxes
3.8. Comparison of GEM Fluxes between Landscapes and with Other Studies Worldwide
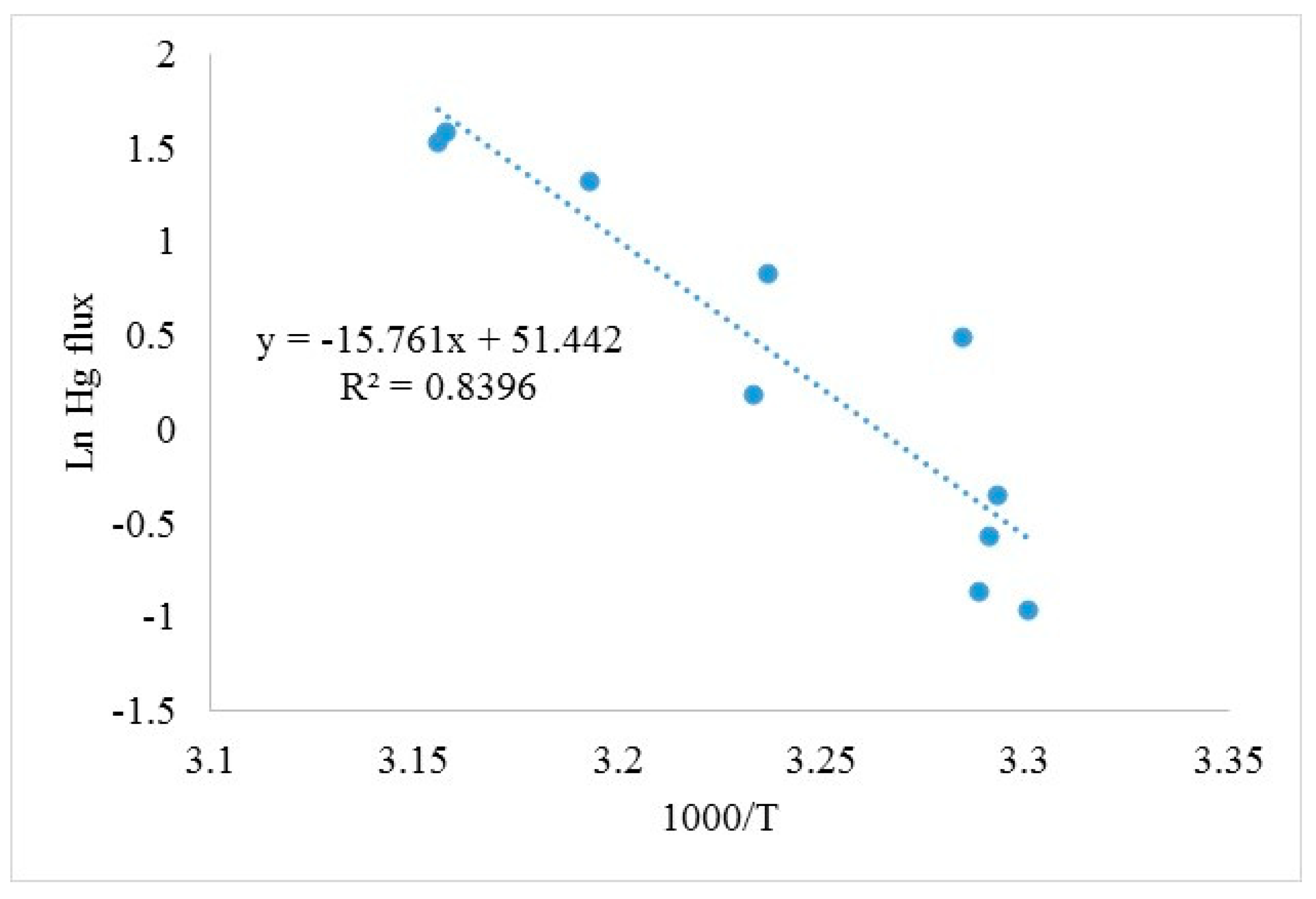
3.9. Impact of Soil Temperature on Hg Air–Surface Exchange
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Gustin, S.M.; Coolbaugh, M.; Engle, M.; Fitzgerald, B.; Keislar, R.; Lindberg, S.; Nacht, D.; Quashnick, J.; Rytuba, J.; Sladek, C.; et al. Atmospheric mercury emissions from mine wastes and surrounding geologically enriched terrains. Environ. Geol. 2003, 43, 339–351. [Google Scholar] [CrossRef]
- Engle, M.A.; Gustin, M.S.; Goff, F.; Counce, D.A.; Janik, C.J.; Bergfeld, D.; Rytuba, J.J. Atmospheric mercury emissions from substrates and fumaroles associated with three hydrothermal systems in the western United States. J. Geophys. Res. 2006, 111, D17304. [Google Scholar] [CrossRef]
- Sommar, J.; Zhu, W.; Lin, C.-J.; Feng, X. Field approaches to measure Hg exchange between natural surfaces and the atmosphere—A Review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1657–1739. [Google Scholar] [CrossRef]
- Gustin, M.S.; Kolker, A.; Gårdfeldt, K. Transport and fate of mercury in the environment. Appl. Geochem. 2008, 23, 343–344. [Google Scholar] [CrossRef]
- Fitzgerald, W.F.; Engstrom, D.R.; Mason, R.P.; Nater, E.A. The case for atmospheric mercury contamination in remote areas. Environ. Sci. Technol. 1998, 32, 1–7. [Google Scholar] [CrossRef]
- Davidson, G.R.; Carnley, M.; Lange, T.; Galicki, S.J.; Douglas, A. Changes in sediment accumulation rate in an Oxbow Lake following late 19th century clearing of land for agricultural use: A 210Pb, 137Cs, and 14C study in Mississippi, USA. Radiocarbon 2004, 46, 755–764. [Google Scholar] [CrossRef]
- Davidson, G.R.; Laine, B.C.; Galicki, S.J.; Threlkeld, S.T. Root-zone hydrology: Why bald cypress in flooded wetlands grow more when it rains. Tree-Ring Res. 2006, 62, 3–13. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Major Land Resource Regions Custom Report. In USDA Agricultural Handbook; USDA: Washington, DC, USA, 2006; p. 296. [Google Scholar]
- Carpi, A.; Lindberg, S.E. Sunlight-mediated emission of elemental mercury from soil amended with municipal sewage sludge. Environ. Sci. Technol. 1997, 31, 2085–2091. [Google Scholar] [CrossRef]
- Carpi, A.; Lindberg, S.E. Application of a TeflonTM dynamic flux chamber for quantifying soil mercury flux: Tests and results over background soil. Atmos. Environ. 1998, 32, 873–882. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Price, J.L. Airborne emissions of mercury from municipal landfill operations: A short-term measurement study in Florida. J. Air Waste Manag. Assoc. 1999, 49, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Eckley, C.S.; Gustin, M.; Lin, C.J.; Li, X.; Miller, M.B. The influence of dynamic chamber design and operating parameters on calculated surface-to-air mercury fluxes. Atmos. Environ. 2010, 44, 194–203. [Google Scholar] [CrossRef]
- Cizdziel, J.V.; Hinners, T.A.; Heithmar, E.M. Determination of total Hg in fish tissues using combustion atomic absorption spectrometry with gold amalgamation. Water Air Soil Pollut. 2002, 135, 357–372. [Google Scholar] [CrossRef]
- Chen, J.; Chakravarty, P.; Davidson, G.R.; Wren, D.G.; Locke, M.A.; Zhou, Y.; Brown, G., Jr.; Cizdziel, J.V. Simultaneous Determination of Mercury and Organic Carbon using a Direct Mercury Analyzer based on Thermal Decomposition—Atomic Absorption Spectrophotometry. Anal. Chim. Acta 2015, 871, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; Zhang, H.; Gustin, M.; Lindberg, S. Mercury emission from terrestrial background surfaces in the eastern USA. II: Air/surfaces exchange of mercury within forests from South Carolina to New England. Appl. Geochem. 2008, 23, 356–368. [Google Scholar] [CrossRef]
- Nacht, D.M.; Gustin, M.S. Mercury emissions from background and altered geologic units throughout Nevada. Water Air Soil Pollut. 2004, 151, 179–193. [Google Scholar] [CrossRef]
- Poissant, L.; Pilote, M.; Constant, P.; Beauvais, C.; Zhang, H.H.; Xu, X. Mercury gas exchanges over selected bare soil and flooded sites in the bay St. Francois wetlands (Quebec, Canada). Atmos. Environ. 2004, 38, 4205–4214. [Google Scholar] [CrossRef]
- Zhang, H.; Lindberg, S.E.; Marsik, F.J.; Keeler, G.J. Mercury air/surface exchange kinetics of background soils of the Tahquamenon river watershed in the Michigan upper peninsula. Water Air Soil Pollut. 2001, 126, 151–169. [Google Scholar] [CrossRef]
- Edwards, G.C.; Howard, D.A. Air-surface exchange measurements of gaseous elemental mercury over naturally enriched and background terrestrial landscapes in Australia. Atmos. Chem. Phys. 2013, 13, 5325–5336. [Google Scholar] [CrossRef]
- Fu, X.; Feng, X.; Wang, S. Exchange fluxes of Hg between surfaces and atmosphere in the eastern flank of Mount Gongga, Sichuan province, southwestern China. J. Geophys. Res. 2008, 113, D20306. [Google Scholar] [CrossRef]
- Fu, X.; Feng, X.; Zhang, H.; Yu, B.; Chen, L. Mercury emissions from natural surfaces highly impacted by human activities in Guangzhou province, South China. Atmos. Environ. 2012, 54, 185–193. [Google Scholar] [CrossRef]
- Ericksen, J.A.; Gustin, M.S.; Xin, M.; Weisberg, P.J.; Fernandez, G.C.J. Air/soil exchange of mercury from background soils in the United States. Sci. Total Environ. 2006, 366, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Holsen, T.M. Gaseous mercury fluxes from the forest floor of the Adirondacks. Environ. Pollut. 2009, 157, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Amyot, M.; Mierle, G.; Lean, D. Sunlight-induced formation of dissolved gaseous Hg in lake waters. Environ. Sci. Technol. 1994, 28, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, D.Y.; Ming, M.A. Mercury release flux and its influencing factors at the air-water interface in paddy field in Chongqing, China. Chin. Sci. Bull. 2013, 58, 266–274. [Google Scholar] [CrossRef]
- Nriagu, J.O. Mechanistic steps in the photoreduction of mercury in natural waters. Sci. Total Environ. 1994, 149, 167–181. [Google Scholar] [CrossRef]
- Cost, M.; Liss, P. Photoreduction of mercury in seawater and its possible implications for Hg air-sea fluxes. Mar. Chem. 1999, 68, 87–95. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Beauchamp, S.; Edwards, G.; Poissant, L.; Rasmussen, P.; Tordon, R.; Dias, G.; Kemp, J.; Van Heyst, B.; Banic, C.M. Gaseous mercury emissions from natural sources in Canadian landscapes. J. Geophys. Res. 2005, 110, D18302. [Google Scholar] [CrossRef]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef]
- Poissant, L.; Casimir, A. Water–air and soil–air exchange rate of total gaseous mercury measured at background sites. Atmos. Environ. 1998, 32, 883–893. [Google Scholar] [CrossRef]
- Kyllönen, K.; Hakola, H.; Hellén, H.; Korhonen, M.; Verta, M. Atmospheric Mercury Fluxes in a Southern Boreal Forest and Wetland. Water Air Soil Poll. 2012, 223, 1171–1182. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Qiu, G.; Hou, Y.; Tang, S. Total gaseous mercury emissions from soil in Guiyang, Guizhou, China. J. Geophys. Res. 2005, 110, D14306. [Google Scholar] [CrossRef]
- Moore, C.; Carpi, A. Mechanisms of the emission of mercury from soil: Role of UV radiation. J. Geophys. Res. 2005, 110, D24302. [Google Scholar] [CrossRef]
- Gustin, M.S.; Ericksen, J.A.; Schorran, D.E.; Johnson, D.W.; Lindberg, S.E.; Coleman, J.S. Application of controlled mesocosms for understanding mercury air/soil-plant exchange. Environ. Sci. Technol. 2004, 38, 6044–6050. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, K. Review: Evaporation of mercury from soils. An integration and synthesis of current knowledge. Environ. Geol. 2000, 39, 249–271. [Google Scholar] [CrossRef]
- Kocman, D.; Horvat, M. A laboratory based experimental study of mercury emission from contaminated soils in the River Idrijca catchment. Atmos. Chem. Phys. 2010, 10, 1417–1426. [Google Scholar] [CrossRef]
- Bahlmann, E.; Ebinghaus, R.; Ruck, W. Development and application of a laboratory flux measurement system (LFMS) for the investigation of the kinetics of mercury emissions from soils. J. Environ. Manag. 2006, 81, 114–125. [Google Scholar] [CrossRef]
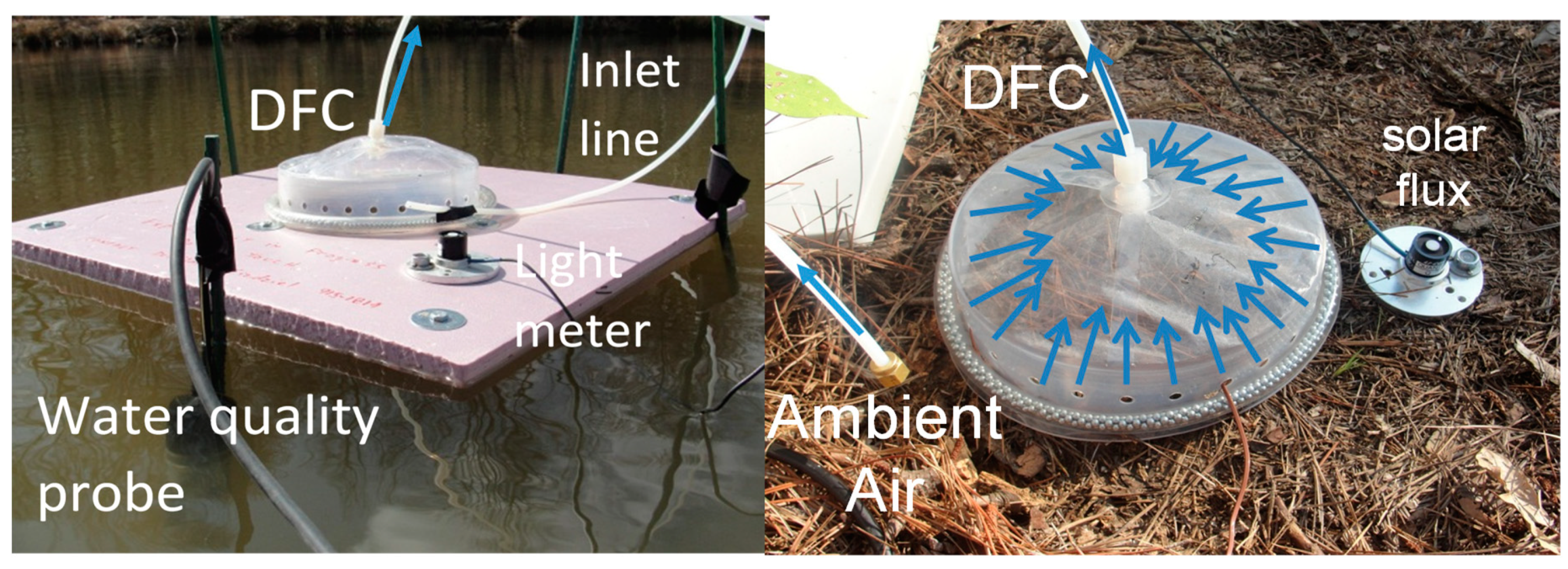
| Surface | Description | Location | Latitude | Longitude |
|---|---|---|---|---|
| Grass a | Residential lawn | Lafayette County, MS | 34.379189 | −89.601822 |
| Agricultural Soil b | Cotton field | 34.272125 | −89.522294 | |
| Pond Water a | UMFS c | 34.432188 | −89.391557 | |
| Mowed Field a | UMFS | 34.432190 | −89.391559 | |
| Wetland Soil b | UM Campus Trail | 34.351175 | −89.555886 | |
| Forest a | Loblolly Pine | 34.379406 | −89.601697 | |
| Agricultural Soil b | Dundee Series | Mississippi Delta | 33.395056 | −90.682278 |
| Agriculture Soil b | Dowling Series | 33.389972 | −90.682028 | |
| Wetland Soil a | Sky Lake | 33.228820 | −90.487100 | |
| Wetland Water a | Sky Lake | 33.228822 | −90.487102 |
| Season | Site | Temp. | Pressure | Humidity | Wind | Solar Rad. | GEM |
|---|---|---|---|---|---|---|---|
| Summer | Forest | 0.66 | −0.15 | −0.69 | 0.53 | 0.90 | 0.61 |
| Grass | 0.25 | 0.08 | −0.34 | 0.07 | 0.76 | 0.18 | |
| Wetland soil | 0.58 | 0.52 | NA (not available) | 0.05 | 0.36 | NA | |
| Wetland water | 0.31 | 0.22 | NA | 0.07 | 0.08 | NA | |
| Pond | 0.12 | 0.38 | NA | 0.34 | 0.95 | NA | |
| Mowed field | 0.67 | 0.91 | NA | 0.13 | 0.81 | NA | |
| Agriculture | 0.77 | 0.32 | −0.80 | 0.67 | 0.90 | 0.63 | |
| Delta (Dundee) | 0.77 | −0.06 | −0.71 | 0.28 | 0.77 | 0.29 | |
| Delta (Dowling) | 0.81 | −0.08 | −0.80 | 0.62 | 0.92 | 0.62 | |
| Winter | Forest | −0.09 | 0.08 | −0.06 | 0.05 | NA * | −0.47 |
| Grass | 0.58 | 0.26 | −0.26 | 0.30 | 0.40 | −0.64 | |
| Pond | 0.04 | −0.80 | NA | 0.10 | 0.38 | NA | |
| Agriculture | 0.80 | −0.35 | −0.80 | 0.47 | 0.75 | 0.69 | |
| Delta (Dundee) | −0.15 | 0.30 | −0.36 | −0.11 | 0.36 | −0.56 | |
| Delta (Dowling) | 0.52 | −0.07 | −0.55 | 0.05 | 0.53 | 0.43 |
| Site | Hg Flux (ng m−2 h−1) | n Emission | ||
|---|---|---|---|---|
| Range | Median | Mean ± SD * | (Deposition) | |
| Summer | ||||
| Forest | (−0.93) to 6.71 | 0.04 | 0.50 ± 1.20 | 73 (66) |
| Grass (lawn) | 1.34 to 11.41 | 4.18 | 4.38 ± 1.71 | 125 (0) |
| Wetland soil | 1.74 to 13.1 | 4.10 | 4.92 ± 2.8 | 54 (0) |
| Wetland water | (−0.87) to 11.8 | 0.26 | 0.25 ± 0.49 | 37 (16) |
| Field Station Pond | (−0.25) to 9.1 | 1.82 | 2.3 ± 1.9 | 54 (1) |
| Mowed field | (−0.84) to 15.5 | 2.20 | 4.52 ± 4.8 | 50 (5) |
| Agriculture | 0.08 to 5.46 | 1.10 | 1.58 ± 1.37 | 157 (0) |
| Delta (Dundee) | (−0.58) to 2.47 | 0.18 | 0.32 ± 0.57 | 54 (31) |
| Delta (Dowling) | (−0.52) to 4.87 | −0.07 | 0.55 ± 1.48 | 33 (36) |
| Winter | ||||
| Forest | (−0.50) to 0.12 | −0.17 | −0.19 ± 0.14 | 6 (69) |
| Grass | (−0.94) to 0.22 | −0.32 | −0.32 ± 0.25 | 9 (64) |
| Field Station Pond | (−2.87) to 2.58 | 0.12 | 0.12 ± 0.71 | 125 (73) |
| Agriculture | (−0.52) to 0.50 | −0.09 | −0.11 ± 0.26 | 27 (50) |
| Delta (Dundee) | (−0.43) to 0.30 | −0.03 | −0.03 ± 0.14 | 33 (45) |
| Delta (Dowling) | (−0.64) to 0.24 | −0.2 | −0.20 ± 0.23 | 17 (54) |
| Site | Hg Flux (ng m−2 h−1) | Solar Rad. (W m−2) | Air Temp. (°C) | Relative Humidity (%) | Wind Speed (km∙h−1) |
|---|---|---|---|---|---|
| Summer | |||||
| Forest | 0.50 ± 1.20 | 25 ± 52 | 26.4 ± 4.2 | 79 ± 18 | 1.54 ± 0.79 |
| Grass (lawn) | 4.38 ± 1.71 | 33 ± 70 | 23.8 ± 4.8 | 78 ± 18 | 1.82 ± 0.97 |
| Wetland soil | 4.92 ± 2.80 | 1.5 ± 2.7 | 23.2 ± 3.5 | 87 ± 12 | 0.63 ± 0.21 |
| Wetland water | 0.25 ± 0.49 | 1.5 ± 2.7 | 23.2 ± 3.5 | 87 ± 12 | 0.63 ± 0.21 |
| Field Station Pond | 2.3 ± 1.9 | 38 ± 71 | 19.1 ± 0.7 | 92 ± 14 | 2.11 ± 1.54 |
| UMFS Mowed field | 4.52 ± 4.8 | 38 ± 71 | 19.1 ± 0.7 | 92 ± 14 | 2.11 ± 1.54 |
| Agriculture | 1.58 ± 1.37 | 84 ± 134 | 24.9 ± 5.7 | 70 ± 22 | 1.90 ± 0.89 |
| Delta (Dundee) | 0.32 ± 0.57 | 59 ± 88 | 24.5 ± 3.1 | 77 ± 12 | 2.72 ± 1.20 |
| Delta (Dowling) | 0.55 ± 1.48 | 53 ± 100 | 24.5 ± 4.6 | 77 ± 19 | 2.16 ± 1.14 |
| Winter | |||||
| Forest | −0.19 ± 0.14 | NA * | 10.4 ± 2.1 | 63 ± 7 | 1.84 ± 0.50 |
| Grass (lawn) | −0.32 ± 0.25 | 66 ± 124 | 6.8 ± 1.6 | 74 ± 24 | 2.02 ± 0.84 |
| Pond | 0.12 ± 0.71 | 46.7 ± 85.6 | 7.1 ± 6.9 | 75 ± 22 | 1.31 ± 0.79 |
| Agriculture | −0.11 ± 0.26 | 36 ± 45 | 5.7 ± 7.1 | 71 ± 23 | 1.40 ± 0.91 |
| Delta (Dundee) | −0.03 ± 0.14 | 45 ± 77 | 3.3 ± 1.8 | 77 ± 5 | 1.63 ± 0.47 |
| Delta (Dowling) | −0.20 ± 0.23 | 35 ± 50 | 3.4 ± 4.1 | 70 ± 24 | 1.92 ± 0.51 |
| Mississippi Soils | LS Mean Day | Significance | LS Mean Night | Significance |
|---|---|---|---|---|
| Grass | 2.04 | A | 1.86 | A |
| Wetland (Lafeyette) a | 1.60 | B | 1.30 | B |
| Agriculture a | 1.41 | C | 1.12 | C |
| Delta (Dundee) a | 1.31 | D | 0.99 | D |
| Delta (Dowling) a | 1.36 | C, D | 0.75 | E |
| Forest | 1.21 | D | 0.82 | E |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cizdziel, J.V.; Jiang, Y.; Nallamothu, D.; Brewer, J.S.; Gao, Z. Air/Surface Exchange of Gaseous Elemental Mercury at Different Landscapes in Mississippi, USA. Atmosphere 2019, 10, 538. https://doi.org/10.3390/atmos10090538
Cizdziel JV, Jiang Y, Nallamothu D, Brewer JS, Gao Z. Air/Surface Exchange of Gaseous Elemental Mercury at Different Landscapes in Mississippi, USA. Atmosphere. 2019; 10(9):538. https://doi.org/10.3390/atmos10090538
Chicago/Turabian StyleCizdziel, James V., Yi Jiang, Divya Nallamothu, J. Stephen Brewer, and Zhiqiang Gao. 2019. "Air/Surface Exchange of Gaseous Elemental Mercury at Different Landscapes in Mississippi, USA" Atmosphere 10, no. 9: 538. https://doi.org/10.3390/atmos10090538
APA StyleCizdziel, J. V., Jiang, Y., Nallamothu, D., Brewer, J. S., & Gao, Z. (2019). Air/Surface Exchange of Gaseous Elemental Mercury at Different Landscapes in Mississippi, USA. Atmosphere, 10(9), 538. https://doi.org/10.3390/atmos10090538





