Impacts of In-Cabin Exposure to Size-Fractionated Particulate Matters and Carbon Monoxide on Changes in Heart Rate Variability for Healthy Public Transit Commuters
Abstract
1. Introduction
2. Experiments
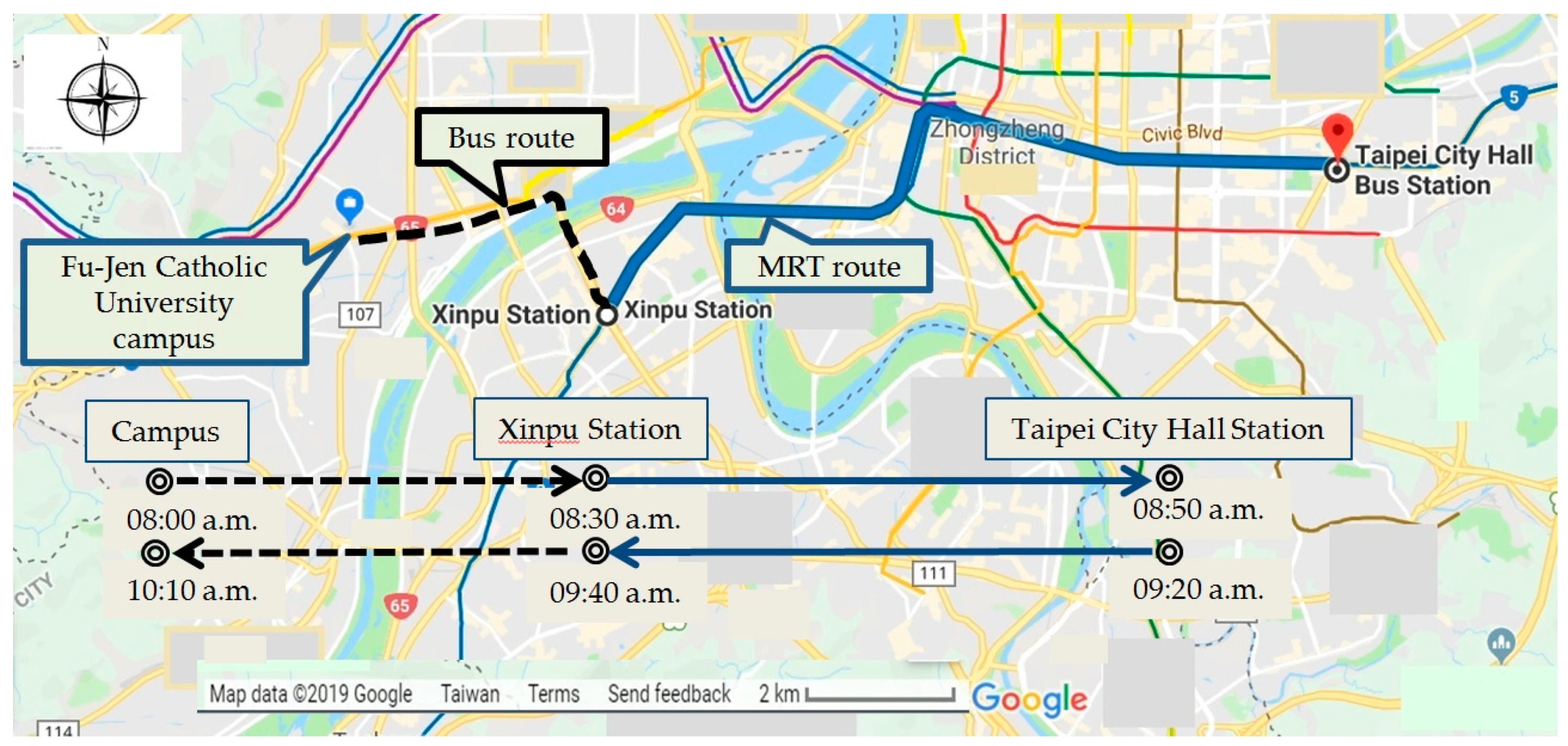
2.1. Study Design
2.2. HRV Indices
2.3. Air Pollution and Weather Conditions
2.4. Statistical Analysis
3. Results
3.1. Summary Statistics of the Participants
3.2. Impacts of PM and CO Exposures on HRV Indices
3.3. Short-Term Exposure Impacts on HRV Indices in Different Time Frames
3.4. Impact of Personal Exposures in Public Transportation Microenvironments on HRV Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | N | Mean ± SD | Minimum | Maximum |
|---|---|---|---|---|
| ln HR 1 (beats/min) | 5613 | 4.4 ± 0.2 | 3.6 | 5.1 |
| Time domain HRV 1 | ||||
| ln r-MSSD(ms) | 5609 | 3.4 ± 0.6 | 2.0 | 6.0 |
| ln SDNN(ms) | 5609 | 4.1 ± 0.5 | 2.0 | 6.0 |
| ln pNN50+1 (%) | 5609 | 1.8 ± 1.4 | −1.8 | 4.4 |
| Frequency domain HRV 1 | ||||
| ln LF (ms2) | 5599 | 6.5 ± 1.0 | 0.9 | 13.8 |
| ln HF (ms2) | 5599 | 5.4 ± 1.2 | 0.1 | 10.9 |
| ln TP(ms2) | 5599 | 7.8 ± 1.0 | 0.0 | 26.2 |
| ln LF/HF | 5599 | 1.1 ± 0.8 | −2.5 | 4.1 |
| Personal exposures 2 | ||||
| CO (ppm) | 7877 | 1.7 ± 2.8 | 0.0 | 22.8 |
| PM10 (μg/m3) | 7926 | 45.4 ± 50.6 | 2.4 | 1131.3 |
| PM2.5–10 (μg/m3) | 7926 | 16.3 ± 37.1 | 0.2 | 923.8 |
| PM2.5 (μg/m3) | 7926 | 29.0 ± 23.8 | 1.9 | 233.5 |
| PM1–2.5 (μg/m3) | 7926 | 4.9 ± 6.0 | 0.0 | 149.3 |
| PM 1 (μg/m3) | 7926 | 24.2 ± 19.7 | 1.1 | 206.2 |
| Temperature 2 (°C) | 7956 | 26.7 ± 3.7 | 12.8 | 34.9 |
| RH 2 (%) | 7955 | 59.6 ± 8.4 | 33.6 | 90.9 |
| Personal Exposures | Campus | MRT | Bus |
|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD + (Range) | Mean ± SD + (Range) | |
| CO (ppm) | 1.5 ± 2.6 | 2.4 ± 3.0 * | 7.0 ± 4.8 * |
| (0.0, 25.0) | (0.0, 14.0) | (0.0, 23.0) | |
| PM10 (μg/m3) | 43.1 ± 53.6 | 59.8 ± 40.2 * | 64.1 ± 50.9 * |
| (1.1, 1231.3) | (10.6, 363.6) | (11.0, 505.4) | |
| PM2.5–10 (μg/m3) | 15.2 ± 40.8 | 17.4 ± 27.1 * | 27.3 ± 44.3 * |
| (0.0, 1152.7) | (0.2, 305.4) | (0.6, 470.5) | |
| PM2.5 (μg/m3) | 27.9 ± 23.7 | 42.4 ± 23.2 * | 36.8 ± 22.8 * |
| (1.1, 382.9) | (4.4, 197.2) | (6.9, 189.4) | |
| PM1–2.5 (μg/m3) | 4.6 ± 6.2 | 9.2 ± 7.1 * | 5.6 ± 4.1 * |
| (0.0, 290.1) | (0.3, 41.8) | (0.2, 32.9) | |
| PM1 (μg/m3) | 23.3 ± 19.6 | 33.2 ± 18.6 * | 31.2 ± 20.2 * |
| (1.0, 211.4) | (4.1, 189.8) | (5.8, 183.9) | |
| Temperature (°C) | 26.8 ± 3.7 | 26.2 ± 2.6 * | 26.4 ± 4.4 |
| (13.2, 34.8) | (19.9, 33.6) | (13.1, 35.1) | |
| Relative humidity (%) | 59.6 ± 8.4 | 58.5 ± 6.0 * | 57.3 ± 13.4 * |
| (33.3, 95.4) | (36.2, 75.5) | (32.2, 90.8) |
References
- Travis, W.D.; Brambilla, E.; Muller-Hermelink, H.K.; Harris, C.C. (Eds.) World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart; IARC Press: Lyon, France, 2004. [Google Scholar]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Van Wijnen, J.H.; Verhoeff, A.P.; Jans, H.W.A.; van Bruggen, M. The exposure of cyclists, car drivers and pedestrians to traffic-related air pollutants. Int. Arch. Occup. Environ. Health 1995, 67, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, H.; Borgman, F.; Kamminga, J.; Hoek, G. Exposure to ultrafine and fine particles and noise during cycling and driving in 11 Dutch cities. Atmos. Environ. 2009, 43, 4234–4242. [Google Scholar] [CrossRef]
- Int Panis, L.; de Geus, B.; Vandenbulcke, G.; Willems, H.; Degraeuwe, B.; Bleux, N.; Mishra, V.; Thomas, I.; Meeusen, R. Exposure to particulate matter in traffic: A comparison of cyclists and car passengers. Atmos. Environ. 2010, 44, 2263–2270. [Google Scholar] [CrossRef]
- Karanasiou, A.; Viana, M.; Querol, X.; Moreno, T.; de Leeuw, F. Assessment of personal exposure to particulate air pollution during commuting in European cities—Recommendations and policy implications. Sci. Total Environ. 2014, 490, 785–797. [Google Scholar] [CrossRef]
- Nyhan, M.; McNabola, A.; Misstear, B. Comparison of particulate matter dose and acute heart rate variability response in cyclists, pedestrians, bus and train passengers. Sci. Total Environ. 2014, 468–469, 821–831. [Google Scholar] [CrossRef]
- Cepeda, M.; Schoufour, J.; Freak-Poli, R.; Koolhaas, C.M.; Dhana, K.; Bramer, W.M.; Franco, O.H. Levels of ambient air pollution according to mode of transport: A systematic review. Lancet Public Health 2017, 2, e23–e34. [Google Scholar] [CrossRef]
- Kaur, S.; Nieuwenhuijsen, M.J.; Colvile, R.N. Fine particulate matter and carbon monoxide exposure concentrations in urban street transport microenvironments. Atmos. Environ. 2007, 41, 4781–4810. [Google Scholar] [CrossRef]
- McNabola, A.; Broderick, B.M.; Gill, L.W. Relative exposure to fine particulate matter and VOCs between transport microenvironments in Dublin: Personal exposure and uptake. Atmos. Environ. 2008, 42, 6496–6512. [Google Scholar] [CrossRef]
- Thai, A.; McKendry, I.; Brauer, M. Particulate matter exposure along designated bicycle routes in Vancouver, British Columbia. Sci. Total Environ. 2008, 405, 26–35. [Google Scholar] [CrossRef]
- Hammond, D.; Jones, S.; Lalor, M. In-vehicle measurement of ultrafine particles on compressed natural gas, conventional diesel, and oxidation-catalyst diesel heavy-duty transit buses. Environ. Monit. Assess. 2007, 125, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Hansen, M.L.; Long, R.W.; Nielsen, K.R.; Eatough, N.L.; Wilson, W.E.; Eatough, D.J. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ. Health Perspect. 2004, 112, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rich, D.Q.; Schwartz, J.; Mittleman, M.A.; Link, M.; Luttmann-Gibson, H.; Catalano, P.J.; Speizer, F.E.; Dockery, D.W. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am. J. Epidemiol. 2005, 161, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.D.; Gold, D.R.; Coull, B.A.; Schwartz, J.; Stone, P.H.; Suh, H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology 2007, 18, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pieters, N.; Koppen, G.; Van Poppel, M.; De Prins, S.; Cox, B.; Dons, E.; Nelen, V.; Panis, L.I.; Plusquin, M.; Schoeters, G.; et al. Blood pressure and same-Day exposure to air pollution at school: Associations with nano-Sized to coarse PM in children. Environ. Health Perspect. 2015, 123, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Bashir, M.E.; Lee, D.G.; Li, M.; Bae, J.W.; Shon, H.S.; Cho, M.C.; Ryu, K.H. Trigger learning and ECG parameter customization for remote cardiac clinical care information system. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 561–571. [Google Scholar] [CrossRef]
- Lee, M.S.; Eum, K.D.; Rodrigues, E.G.; Magari, S.R.; Fang, S.C.; Modest, G.A.; Christiani, D.C. Effects of personal exposure to ambient fine particulate matter on acute change in nocturnal heart rate variability in subjects without overt heart disease. Am. J. Cardiol. 2016, 117, 151–156. [Google Scholar] [CrossRef]
- Ward, D.J.; Ayres, J.G. Particulate air pollution and panel studies in children: A systematic review. Occup. Environ. Med. 2004, 61, e13. [Google Scholar] [CrossRef]
- De Hartog, J.; Hoek, G.; Peters, A.; Timonen, K.; Ibald-Mulli, A.; Brunekreef, B.; Heinrich, J.; Tiittanen, P.; van Wijnen, J.H.; Kreyling, W.; et al. Effects of fine and ultrafine particles on cardiorespiratory symptoms in elderly subjects with coronary heart disease: The ULTRA study. Am. J. Epidemiol. 2003, 157, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, J.M.; Eisen, E.A.; Fang, S.C.; Schwartz, J.; Hauser, R.; Herrick, R.F.; Christiani, D.C. PM2.5 metal exposures and nocturnal heart rate variability: A panel study of boilermaker construction workers. Environ. Health 2008, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Ma, C.M.; Liu, I.J.; Han, B.C.; Chuang, H.C.; Chuang, K.J. Effects of commuting mode on air pollution exposure and cardiovascular health among young adults in Taipei, Taiwan. Int. J. Hyg. Environ. Health 2015, 218, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Bosner, M.S.; Kleiger, R.E.; Conger, B.M. Heart rate variability: A measure of cardiac autonomic tone. Am. Heart J. 1994, 127, 1376–1381. [Google Scholar] [CrossRef]
- Chang, L.T.; Tang, C.S.; Pan, Y.Z.; Chan, C.C. Association of heart rate variability of the elderly with personal exposure to PM1, PM1–2.5, and PM2.5–10. Bull. Environ. Contam. Toxicol. 2007, 79, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, K.; Svendsen, E.; Creason, J.; Herbst, M.; Scott, J.; Kupper, L.; Williams, R.; Neas, L.; Cascio, W.; Devlin, R.B.; et al. Coarse particulate matter (PM2.5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ. Health Perspect. 2007, 115, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.J.; Tsai, F.C.; Roger, L.; Woo, M.; Ostro, B.D. Coarse particles and heart rate variability among older adults with coronary artery disease in the Coachella Valley, California. Environ. Health Perspect. 2006, 114, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.J.; Chan, C.C.; Chen, N.T.; Su, T.C.; Lin, L.Y. Effects of particle size fractions on reducing heart rate variability in cardiac and hypertensive patients. Environ. Health Perspect. 2005, 113, 1693–1697. [Google Scholar] [CrossRef]
- De Paula Santos, U.; Braga, A.L.; Giorgi, D.M.; Pereira, L.A.; Grupi, C.J.; Lin, C.A.; Bussacos, M.A.; Zanetta, D.M.; do Nascimento Saldiva, P.H.; Filho, M.T. Effects of air pollution on blood pressure and heart rate variability: A panel study of vehicular traffic controllers in the city of São Paulo, Brazil. Eur. Heart J. 2005, 26, 193–200. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall: Boca Raton, FL, USA, 2006. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Hsu, K.H. Effects of Traffic-Related Pollution on the Cardiovascular Function of Healthy Young Adults in Taipei Urban Area. Master’s Thesis, Fu Jen Catholic University, New Taipei City, Taiwan, 2010. [Google Scholar]
- Wu, Y.H. Commuter Exposure to Submicrometer, Fine, Coarse Particles and Carbon Monoxide in Taipei City. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2005. [Google Scholar]
- Chen, N.T. Effect of Ambient Particulate Matter on Heart Rate Variability in Susceptible Population. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2003. [Google Scholar]
- Brunekreef, B.; Forsberg, B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 2005, 26, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Kuo, I.C.; Su, T.C.; Li, Y.R.; Lin, L.Y.; Chan, C.C.; Hsu, S.C. Effects of personal exposure to particulate matter and ozone on arterial stiffness and heart rate variability in healthy adults. Am. J. Epidemiol. 2010, 171, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Litonjua, A.; Suh, H.; Verrier, M.; Zanobetti, A.; Syring, M.; Nearing, B.; Verrier, R.; Stone, P.; MacCallum, G.; et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax 2005, 60, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Timonen, K.L.; Vanninen, E.; de Hartog, J.; Ibald-Mulli, A.; Brunekreef, B.; Gold, D.R.; Heinrich, J.; Hoek, G.; Lanki, T.; Peters, A.; et al. Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: The ULTRA study. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Riojas-Rodríguez, H.; Escamilla-Cejudo, J.A.; González-Hermosillo, J.A.; Téllez-Rojo, M.M.; Vallejo, M.; Santos-Burgoa, C.; Rojas-Bracho, L. Personal PM2.5 and CO exposure and heart rate variability in subjects with know ischemic heart rate disease in Mexico City. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tarkiainen, T.H.; Timonen, K.L.; Vanninen, E.J.; Alm, S.; Hartikainen, J.E.; Pekkanen, J. Effects of acute carbon monoxide exposure on heart rate variability in patients with coronary artery disease. Clin. Physiol. Funct. Imaging 2003, 23, 98–102. [Google Scholar] [CrossRef]
- Yasuma, F.; Hayano, J. Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm. Chest 2004, 125, 683–690. [Google Scholar] [CrossRef]
| Variable | Campus | Bus | MRT | Bus-Camp # | MRT-Camp # |
|---|---|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | |
| ln HR (beats/min) | 4.4 ± 0.2 | 4.5 ± 0.2 | 4.5 ± 0.1 | 3.1 ± 1.5 | 1.7 ± 1.5 |
| (3.6, 5.1) | (4.1, 5.0) | (4.2, 4.8) | (0.2, 5.6) | (−2.3, 3.6) | |
| ln r-MSSD (ms) | 3.5 ± 0.6 | 3.2 ± 0.7 | 3.2 ± 0.6 | −6.5 ± 15.2 | −7.2 ± 10.5 |
| (2.0, 6.0) | (2.0, 6.0) | (2.0, 5.0) | (−21.6, 31.3) | (−19.4, 20.0) | |
| ln SDNN (ms) | 4.1 ± 0.4 | 3.9 ± 0.5 | 3.9 ± 0.4 | −5.8 ± 6.9 | −5.5 ± 4.3 |
| (2.0, 6.0) | (3.0, 5.0) | (3.0, 5.0) | (−17.8, 11.1) | (−10.8, 3.3) | |
| ln pNN50 + 1 (%) | 1.9 ± 1.4 | 1.1 ± 1.5 | 1.1 ± 1.4 | −9.3 ± 152.5 | −42.6 ± 80.9 |
| (−1.8, 4.4) | (−1.8, 4.4) | (−1.8, 4.2) | (−139.6, 453.4) | (−150.4, 226.5) | |
| ln LF (ms2) | 6.6 ± 1.0 | 5.7 ± 1.2 | 6.2 ± 0.9 | −13.4 ± 7.1 | −6.8 ± 4.7 |
| (2.4, 13.8) | (0.9, 10.6) | (3.3, 9.7) | (−26.7, 7.1) | (−16.9, −0.8) | |
| ln HF (ms2) | 5.5 ± 1.2 | 4.5 ± 1.4 | 4.8 ± 1.1 | −17.3 ± 16.6 | −13.3 ± 10.9 |
| (0.1, 10.9) | (1.3, 10.1) | (1.7, 9.4) | (−34.7, 27.2) | (−30.8, 9.8) | |
| ln TP (ms2) | 7.8 ± 1.0 | 7.1 ± 1.1 | 7.3 ± 0.9 | −9.6 ± 6.5 | −6.7 ± 4.2 |
| (0.0, 26.2) | (2.6, 11.5) | (4.7, 10.4) | (−23.4, 6.2) | (−13.0, 2.0) | |
| ln LF/HF | 1.1 ± 0.8 | 1.2 ± 0.9 | 1.4 ± 0.8 | 22.1 ± 77.7 | 42.2 ± 68.9 |
| (−2.5, 4.1) | (−1.5, 3.2) | (−0.8, 3.2) | (−168.8, 217.1) | (−66.3, 257.5) |
| Variable | Multi-Pollutant Models * | |||
|---|---|---|---|---|
| PM2.5–10 | PM1–2.5 | PM1 | CO | |
| HR | 0.055† | −0.137† | 0.106† | −0.435† |
| (0.046, 0.064) | (−0.248, −0.026) | (0.063, 0.149) | (−0.670, −0.201) | |
| r–MSSD | 0.036 | −0.506 | 0.032 | −0.359 |
| (−0.018, 0.090) | (−1.012, 0.003) | (−0.132, 0.196) | (−1.515, 0.811) | |
| SDNN | 0.082† | −0.513† | 0.209† | 0.202 |
| (0.032, 0.131) | (−0.943, −0.081) | (0.081, 0.338) | (−0.783, 1.197) | |
| pNN50+1 | −0.047 | −0.262 | −0.128 | 0.294 |
| (−0.121, 0.026) | (−1.088, 0.571) | (−0.435, 0.181) | (−1.488, 2.108) | |
| LF | −0.086 | 0.664 | −0.060 | 1.576 |
| (−0.201, 0.029) | (−0.320, 1.656) | (−0.338, 0.218) | (−0.618, 3.819) | |
| HF | −0.180† | 0.415 | −0.034 | 1.473 |
| (−0.304, −0.056) | (−0.704, 1.546) | (−0.374, 0.307) | (−1.089, 4.102) | |
| TP | 0.034 | −0.074 | 0.189 | 1.119 |
| (−0.077, 0.145) | (−1.018, 0.880) | (−0.078, 0.456) | (−1.018, 3.301) | |
| LF/HF | 0.155† | 0.082 | 0.032 | 0.176 |
| (0.049, 0.261) | (−0.841, 1.014) | (−0.233, 0.298) | (−1.840, 2.234) | |
| Variable | Moving Average | Multi-Pollutant Models * | |||
|---|---|---|---|---|---|
| PM2.5–10 | PM1–2.5 | PM1 | CO | ||
| HR | 5 min | 0.055† | −0.137† | 0.106† | −0.435† |
| (0.046, 0.064) | (−0.248, −0.026) | (0.063, 0.149) | (−0.670, −0.201) | ||
| 30 min | 0.106† | −0.551† | 0.030 | −0.130 | |
| (0.072, 0.141) | (−0.822, −0.280) | (−0.043, 0.104) | (−0.655, 0.397) | ||
| 60 min | 0.169† | −0.994† | 0.125† | −0.222 | |
| (0.115, 0.222) | (−1.377, −0.608) | (0.045, 0.204) | (−0.993, 0.555) | ||
| r-MSSD | 5 min | 0.036 | −0.506 | 0.032 | −0.359 |
| (−0.018, 0.090) | (−1.012, 0.003) | (−0.132, 0.196) | (−1.515, 0.811) | ||
| 30 min | −0.084 | 0.454 | −0.047 | −0.348 | |
| (−0.208, 0.041) | (−0.483, 1.400) | (−0.261, 0.168) | (−2.090, 1.425) | ||
| 60 min | −0.155 | 1.038 | −0.032 | −2.548† | |
| (−0.312, 0.003) | (−0.120, 2.210) | (−0.274, 0.210) | (−4.676, −0.374) | ||
| SDNN | 5 min | 0.082† | −0.513† | 0.209† | 0.202 |
| (0.032, 0.131) | (−0.943, −0.081) | (0.081, 0.338) | (−0.783, 1.197) | ||
| 30 min | −0.083 | 0.480 | 0.102 | −0.745 | |
| (−0.179, 0.014) | (−0.240, 1.206) | (−0.059, 0.263) | (−2.062, 0.590) | ||
| 60 min | −0.194† | 1.139† | 0.104 | −1.844† | |
| (−0.313, −0.075) | (0.260, 2.026) | (−0.079, 0.286) | (−3.424, −0.238) | ||
| pNN50 + 1 | 5 min | −0.047 | −0.262 | −0.128 | 0.294 |
| (−0.121, 0.026) | (−1.088, 0.571) | (−0.435, 0.181) | (−1.488, 2.108) | ||
| 30 min | −0.210 | 0.757 | −0.027 | 1.621 | |
| (−0.449, 0.031) | (−1.100, 2.648) | (−0.499, 0.447) | (−1.944, 5.316) | ||
| 60 min | −0.472† | 2.702† | −0.352 | −2.521 | |
| (−0.817, −0.126) | (0.156, 5.314) | (−0.871, 0.168) | (−7.213, 2.409) | ||
| Variable | Moving Average | Multi-Pollutant Models * | |||
|---|---|---|---|---|---|
| PM2.5–10 | PM1–2.5 | PM1 | CO | ||
| LF | 5 min | −0.086 | 0.664 | −0.060 | 1.576 |
| (−0.201, 0.029) | (−0.320, 1.656) | (−0.338, 0.218) | (−0.618, 3.819) | ||
| 30 min | −0.459† | 2.779† | −0.147 | −1.849 | |
| (−0.666, −0.251) | (1.198, 4.385) | (−0.489, 0.196) | (−4.544, 0.932) | ||
| 60 min | −0.598† | 3.748† | −0.173 | −2.747 | |
| (−0.852, −0.343) | (1.834, 5.698) | (−0.559, 0.215) | (−5.914, 0.526) | ||
| HF | 5 min | −0.180† | 0.415 | −0.034 | 1.473 |
| (−0.304, −0.056) | (−0.704, 1.546) | (−0.374, 0.307) | (−1.089, 4.102) | ||
| 30 min | −0.625† | 3.539† | −0.315 | −0.161 | |
| (−0.880, −0.370) | (1.566, 5.549) | (−0.745, 0.117) | (−3.686, 3.494) | ||
| 60 min | −0.845† | 5.260† | −0.449 | −3.012 | |
| (−1.162, −0.527) | (2.827, 7.751) | (−0.936, 0.040) | (−7.181, 1.345) | ||
| TP | 5 min | 0.034 | −0.074 | 0.189 | 1.119 |
| (−0.077, 0.145) | (−1.018, 0.880) | (−0.078, 0.456) | (−1.018, 3.301) | ||
| 30 min | −0.319† | 1.890† | 0.056 | −1.568 | |
| (−0.516, −0.121) | (0.399, 3.403) | (−0.270, 0.382) | (−4.220, 1.157) | ||
| 60 min | −0.484† | 2.846† | 0.101 | −3.366† | |
| (−0.725, −0.242) | (1.042, 4.682) | (−0.268, 0.471) | (−6.480, −0.149) | ||
| LF/HF | 5 min | 0.155† | 0.082 | 0.032 | 0.176 |
| (0.049, 0.261) | (−0.841, 1.014) | (−0.233, 0.298) | (−1.840, 2.234) | ||
| 30 min | 0.235† | −0.730 | 0.208 | −2.235 | |
| (0.033, 0.439) | (−2.216, 0.779) | (−0.125, 0.543) | (−4.816, 0.415) | ||
| 60 min | 0.284† | −1.286 | 0.288 | −1.293 | |
| (0.034, 0.535) | (−3.066, 0.526) | (−0.091, 0.668) | (−4.395, 1.911) | ||
| Variable | Multi-Pollutant Models * | |||||||
|---|---|---|---|---|---|---|---|---|
| PM2.5–10 | PM1–2.5 | PM1 | CO | |||||
| Bus | MRT | Bus | MRT | Bus | MRT | Bus | MRT | |
| HR | 6.93 | 3.13 | 6.74 | 3.18 | 6.98 | 3.15 | 6.44 | 3.25 |
| r–MSSD | −19.94 | −22.26 | −20.49 | −22.03 | −19.95 | −22.21 | −20.34 | −21.69 |
| SDNN | −24.84 | −25.19 | −25.44 | −24.99 | −24.72 | −25.15 | −24.72 | −24.71 |
| pNN50+1 | −27.39 | −29.07 | −27.61 | −28.68 | −27.47 | −28.95 | −27.05 | −28.19 |
| LF | −58.60 | −46.90 | −57.85 | −46.46 | −58.58 | −46.82 | −56.94 | −45.84 |
| HF | −59.81 | −55.72 | −59.22 | −55.21 | −59.67 | −55.61 | −58.16 | −54.47 |
| TP | −53.37 | −47.84 | −53.48 | −47.42 | −53.22 | −47.77 | −52.29 | −46.81 |
| LF/HF | 4.80 | 18.82 | 4.72 | 19.24 | 4.67 | 18.90 | 4.82 | 19.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-S.; Wu, T.-Y.; Chuang, K.-J.; Chang, T.-Y.; Chuang, H.-C.; Lung, S.-C.C.; Chang, L.-T. Impacts of In-Cabin Exposure to Size-Fractionated Particulate Matters and Carbon Monoxide on Changes in Heart Rate Variability for Healthy Public Transit Commuters. Atmosphere 2019, 10, 409. https://doi.org/10.3390/atmos10070409
Tang C-S, Wu T-Y, Chuang K-J, Chang T-Y, Chuang H-C, Lung S-CC, Chang L-T. Impacts of In-Cabin Exposure to Size-Fractionated Particulate Matters and Carbon Monoxide on Changes in Heart Rate Variability for Healthy Public Transit Commuters. Atmosphere. 2019; 10(7):409. https://doi.org/10.3390/atmos10070409
Chicago/Turabian StyleTang, Chin-Sheng, Tzu-Yi Wu, Kai-Jen Chuang, Ta-Yuan Chang, Hsiao-Chi Chuang, Shih-Chun Candice Lung, and Li-Te Chang. 2019. "Impacts of In-Cabin Exposure to Size-Fractionated Particulate Matters and Carbon Monoxide on Changes in Heart Rate Variability for Healthy Public Transit Commuters" Atmosphere 10, no. 7: 409. https://doi.org/10.3390/atmos10070409
APA StyleTang, C.-S., Wu, T.-Y., Chuang, K.-J., Chang, T.-Y., Chuang, H.-C., Lung, S.-C. C., & Chang, L.-T. (2019). Impacts of In-Cabin Exposure to Size-Fractionated Particulate Matters and Carbon Monoxide on Changes in Heart Rate Variability for Healthy Public Transit Commuters. Atmosphere, 10(7), 409. https://doi.org/10.3390/atmos10070409









