Stable Isotopes in Greenhouse Gases from Soil: A Review of Theory and Application
Abstract
1. Introduction
2. Theory of Stable Isotope Techniques
2.1. Stable Isotopic Compositions
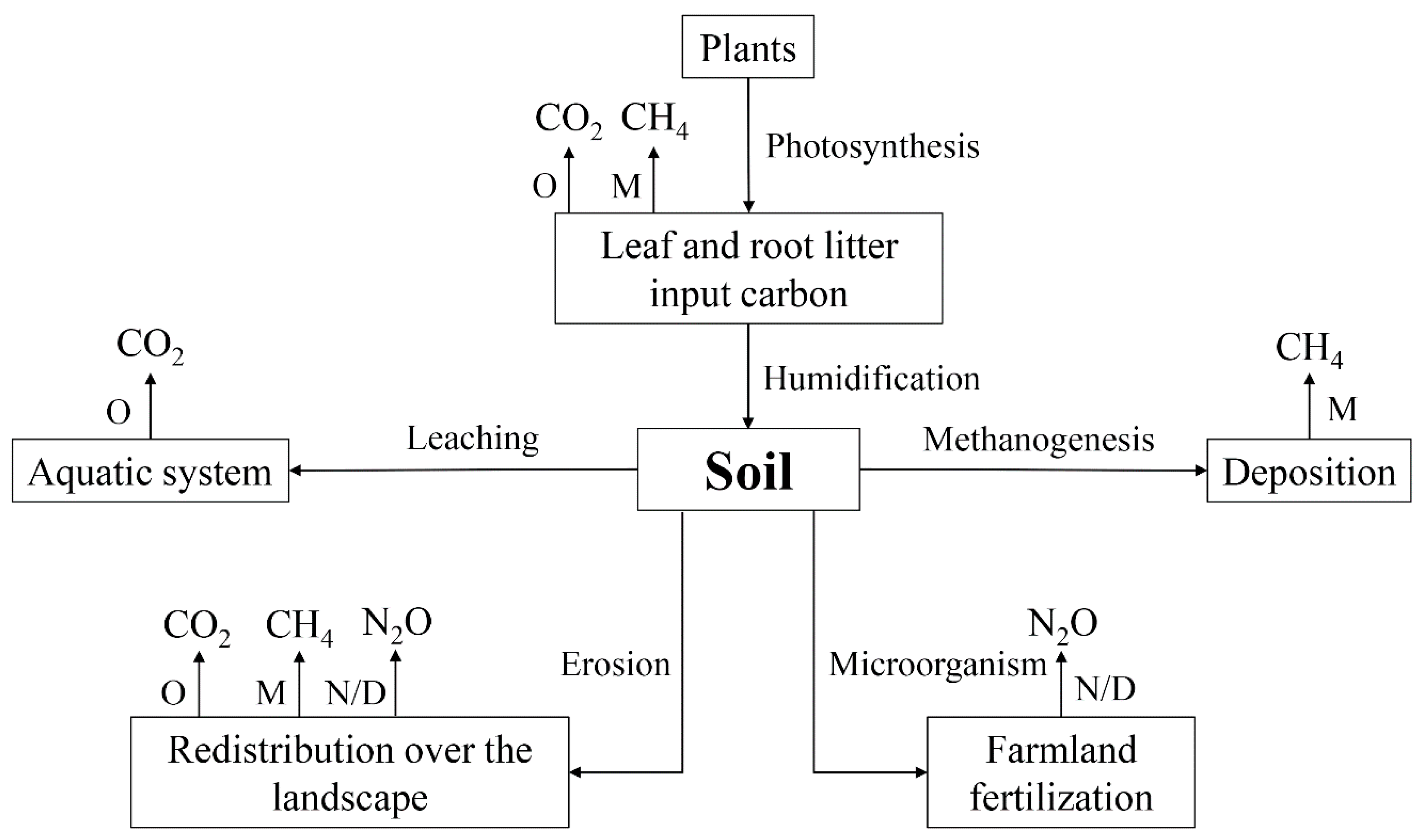
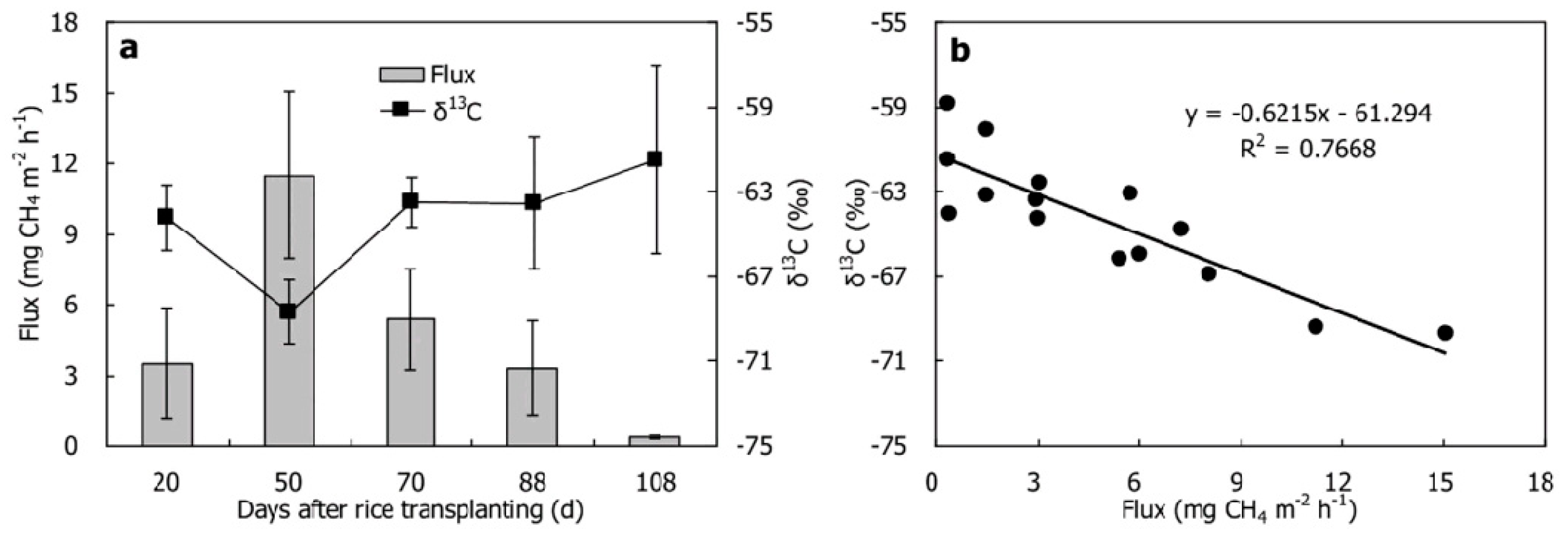
2.2. Soil CO2, CH4 and N2O Stable Isotope Signatures
2.3. Environmental Factors Affect the Isotope Signal of Soil Greenhouse Gases
2.3.1. Temperature and Precipitation
2.3.2. Soil Physical Factors
2.3.3. Soil Microbial Process
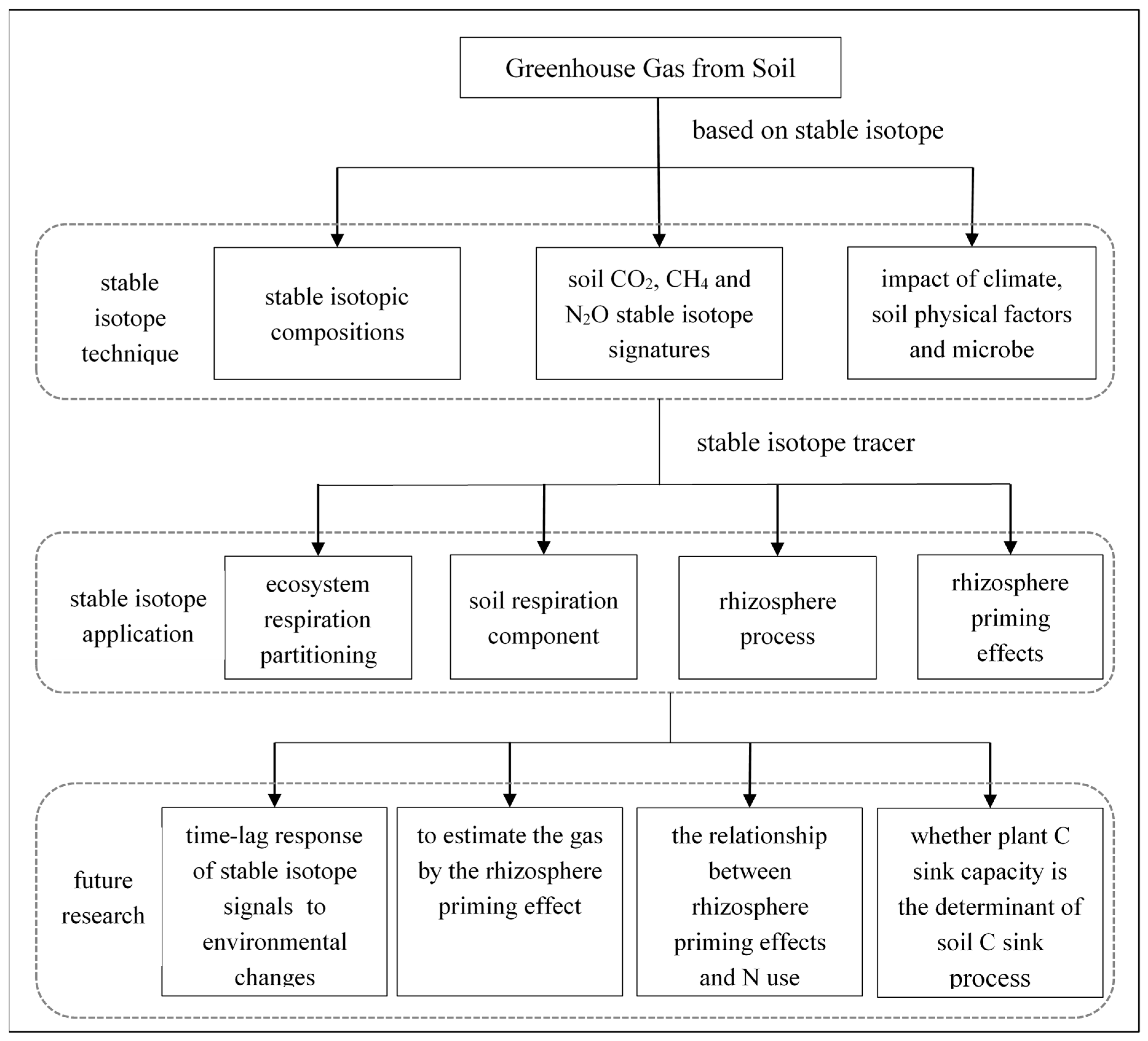
3. Stable Isotope Application
3.1. Stable Isotope Tracer
3.2. Application of Stable Isotopes in a Soil System
4. Future Research for Soil Greenhouse Gas Emissions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crutzen, P.J.; Heidt, L.E.; Krasnec, J.P.; Pollock, W.H.; Seiler, W. Biomass burning as a source of atmospheric gases CO, H2, N2O, NO, CH3Cl and COS. Nature 1979, 282, 253. [Google Scholar] [CrossRef]
- Gu, L.; Chen, J.; Xu, C.; Kim, J.; Chen, H.; Xia, J.; Zhang, L. The contribution of internal climate variability to climate change impacts on droughts. Sci Total Environ. 2019, 684, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Metz, B.; Davidson, O.; Bosch, P.; Dave, R.; Meyer, L. Climate change 2007: Mitigation of climate change. In Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lashof, D.A.; Ahuja, D.R. Relative contributions of greenhouse gas emissions to global warming. Nature 1990, 344, 529–531. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.; Ye, Y. Coastal vegetation invasion increases greenhouse gas emission from wetland soils but also increases soil carbon accumulation. Sci. Total Environ. 2015, 526, 19–28. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Alexander, L.V.; Allen, S.K.; Bindoff, N.L.; Bréon, F.-M.; Church, J.A.; Cubasch, U.; Emori, S. Technical summary. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 33–115. [Google Scholar]
- Anderegg, W.R.; Prall, J.W.; Harold, J.; Schneider, S.H. Expert credibility in climate change. Proc. Natl. Acad. Sci. USA 2010, 107, 12107–12109. [Google Scholar] [CrossRef] [PubMed]
- Venturi, S.; Tassi, F.; Magi, F.; Cabassi, J.; Ricci, A.; Capecchiacci, F.; Caponi, C.; Nisi, B.; Vaselli, O. Carbon isotopic signature of interstitial soil gases reveals the potential role of ecosystems in mitigating geogenic greenhouse gas emissions: Case studies from hydrothermal systems in Italy. Sci. Total Environ. 2019, 655, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Mueller, T.; Tate, K.; Ross, D.; Magid, J.; Nielsen, N. Soil surface CO2 flux as an index of soil respiration in situ: A comparison of two chamber methods. Soil. Biol. Biochem. 1996, 28, 1297–1306. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chem. Erde Geochem. 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H. Response of soil respiration to a severe drought in Chinese Eucalyptus plantations. J. For. Res. 2017, 28, 841–847. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Cui, Y.Q.; Jianying, M.A.; Sun, W. Application of stable isotope techniques to the study of soil salinization. J. Arid Land 2011, 3, 285–291. [Google Scholar] [CrossRef]
- Tiunov, A. Stable isotopes of carbon and nitrogen in soil ecological studies. Biol. Bull. 2007, 34, 395–407. [Google Scholar] [CrossRef]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Boutton, T.W.; Archer, S.R.; Midwood, A.J.; Zitzer, S.F.; Bol, R. δ13C values of soil organic carbon and their use in documenting vegetation change in a subtropical savanna ecosystem. Geoderma 1998, 82, 5–41. [Google Scholar] [CrossRef]
- Busari, M.; Salako, F.; Tuniz, C. Stable isotope technique in the evaluation of tillage and fertilizer effects on soil carbon and nitrogen sequestration and water use efficiency. Eur. J. Agron. 2016, 73, 98–106. [Google Scholar] [CrossRef]
- Snider, D.M.; Venkiteswaran, J.J.; Schiff, S.L.; Spoelstra, J. A new mechanistic model of δ18O-N2O formation by denitrification. Geochim. Cosmochim. Acta 2013, 112, 102–115. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef]
- Koba, K.; Osaka, K.; Tobari, Y.; Toyoda, S.; Ohte, N.; Katsuyama, M.; Suzuki, N.; Itoh, M.; Yamagishi, H.; Kawasaki, M. Biogeochemistry of nitrous oxide in groundwater in a forested ecosystem elucidated by nitrous oxide isotopomer measurements. Geochim. Cosmochim. Acta 2009, 73, 3115–3133. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Vogel, J.C. Fractionation of the carbon isotopes during photosynthesis. In Fractionation of the Carbon Isotopes During Photosynthesis; Springer: New York, NY, USA, 1980; pp. 5–29. [Google Scholar]
- O’Leary, M.H. Carbon isotopes in photosynthesis. Bioscience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Bernoux, M.; Cerri, C.C.; Neill, C.; de Moraes, J.F. The use of stable carbon isotopes for estimating soil organic matter turnover rates. Geoderma 1998, 82, 43–58. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil. Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, W.; Luo, J.; Donnison, A. Changes in soil organic carbon dynamics in an Eastern Chinese coastal wetland following invasion by a C4 plant Spartina alterniflora. Soil. Biol. Biochem. 2010, 42, 1712–1720. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.; Van Beusichem, M.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil. Boil. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Shi, W.-Y.; Tateno, R.; Zhang, J.-G.; Wang, Y.-L.; Yamanaka, N.; Du, S. Response of soil respiration to precipitation during the dry season in two typical forest stands in the forest–grassland transition zone of the Loess Plateau. Agric. For. Meteorol. 2011, 151, 854–863. [Google Scholar] [CrossRef]
- Ramachandran Nair, P.K.; Mohan Kumar, B.; Nair, V.D. Agroforestry as a strategy for carbon sequestration. J. Plant Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
- de Stefano, A.; Jacobson, M.G. Soil carbon sequestration in agroforestry systems: A meta-analysis. Agrofor. Syst. 2018, 92, 285–299. [Google Scholar] [CrossRef]
- Burras, C.L.; Kimble, J.M.; Lal, R.; Mausbach, M.J.; Uehara, G.; Cheng, H.; Kissel, D.E.; Luxmoore, R.J.; Rice, C.W.; Wilding, L.P. Carbon sequestration: Position of the Soil Science Society of America. Carbon 2001, 25, 10. [Google Scholar]
- Amundson, R.; Stern, L.; Baisden, T.; Wang, Y. The isotopic composition of soil and soil-respired CO2. Geoderma 1998, 82, 83–114. [Google Scholar] [CrossRef]
- Balesdent, J.; Mariotti, A.; Guillet, B. Natural 13C abundance as a tracer for studies of soil organic matter dynamics. Soil Biol. Biochem. 1987, 19, 25–30. [Google Scholar] [CrossRef]
- Drewer, J.; Dufossé, K.; Skiba, U.M.; Gabrielle, B. Changes in isotopic signatures of soil carbon and CO2 respiration immediately and one year after Miscanthus removal. Gcb Bioenergy 2016, 8, 59–65. [Google Scholar] [CrossRef]
- Pendall, E.; King, J.Y. Soil organic matter dynamics in grassland soils under elevated CO2: Insights from long-term incubations and stable isotopes. Soil Biol. Biochem. 2007, 39, 2628–2639. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Fan, X.; Ma, J.; Xu, H. Carbon isotope fractionation reveals distinct process of CH4 emission from different compartments of paddy ecosystem. Sci. Rep. 2016, 6, 27065. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 465–570. [Google Scholar]
- Lelieveld, J.; Crutzen, P.J.; Dentener, F.J. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B 1998, 50, 128–150. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Fan, X.; Liu, G.; Ma, J.; Xu, H. Effect of rice straw application on stable carbon isotopes, methanogenic pathway, and fraction of CH4 oxidized in a continuously flooded rice field in winter season. Soil Biol. Biochem. 2015, 84, 75–82. [Google Scholar] [CrossRef]
- Ogle, S.M.; Breidt, F.J.; Paustian, K. Agricultural management impacts on soil organic carbon storage under moist and dry climatic conditions of temperate and tropical regions. Biogeochemistry 2005, 72, 87–121. [Google Scholar] [CrossRef]
- Bilek, R.S.; Tyler, S.C.; Sass, R.L.; Fisher, F.M. Differences in CH4 oxidation and pathways of production between rice cultivars deduced from measurements of CH4 flux and δ13C of CH4 and CO2. Glob. Biogeochem. Cycles 1999, 13, 1029–1044. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Krüger, M.; Eller, G.; Conrad, R.; Frenzel, P. Seasonal variation in pathways of CH4 production and in CH4 oxidation in rice fields determined by stable carbon isotopes and specific inhibitors. Glob. Chang. Biol. 2002, 8, 265–280. [Google Scholar] [CrossRef]
- Uzaki, M.; Mizutani, H.; Wada, E. Carbon isotope composition of CH4 from rice paddies in Japan. Biogeochemistry 1991, 13, 159–175. [Google Scholar] [CrossRef]
- Tyler, S.C.; Brailsford, G.W.; Yagi, K.; Minami, K.; Cicerone, R.J. Seasonal variations in methane flux andδl3CH4 values for rice paddies in Japan and their implications. Glob. Biogeochem. Cycles 1994, 8, 1–12. [Google Scholar] [CrossRef]
- Bergamaschi, P. Seasonal variations of stable hydrogen and carbon isotope ratios in methane from a Chinese rice paddy. J. Geophys. Res. Atmos. 1997, 102, 25383–25393. [Google Scholar] [CrossRef]
- Chanton, J.; Whiting, G.; Blair, N.; Lindau, C.; Bollich, P. Methane emission from rice: Stable isotopes, diurnal variations, and CO2 exchange. Glob. Biogeochem. Cycles 1997, 11, 15–27. [Google Scholar] [CrossRef]
- Tyler, S.; Bilek, R.; Sass, R.; Fisher, F. Methane oxidation and pathways of production in a Texas paddy field deduced from measurements of flux, δl3C, and δD of CH4. Glob. Biogeochem. Cycles 1997, 11, 323–348. [Google Scholar] [CrossRef]
- Marik, T.; Fischer, H.; Conen, F.; Smith, K. Seasonal variations in stable carbon and hydrogen isotope ratios in methane from rice fields. Glob. Biogeochem. Cycles 2002, 16, 41-1–41-11. [Google Scholar] [CrossRef]
- Krüger, M.; Frenzel, P. Effects of N-fertilisation on CH4 oxidation and production, and consequences for CH4 emissions from microcosms and rice fields. Glob. Chang. Biol. 2003, 9, 773–784. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M. Effect of potassium phosphate fertilization on production and emission of methane and its 13C-stable isotope composition in rice microcosms. Soil Biol. Biochem. 2005, 37, 2099–2108. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, Y.; Ma, J.; Xu, H.; Cai, Z.; Yagi, K. Intermittent irrigation changes production, oxidation, and emission of CH4 in paddy fields determined with stable carbon isotope technique. Soil Biol. Biochem. 2012, 52, 108–116. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, Y.; Liu, G.; Ma, J.; Xu, H. Carbon isotope fractionation during CH4 transport in paddy fields. Sci. China Earth Sci. 2014, 57, 1664–1670. [Google Scholar] [CrossRef]
- Ravishankara, A.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Xu, C.; Li, Q.; Lin, W. Isotope signatures of N2O emitted from vegetable soil: Ammonia oxidation drives N2O production in NH4+-fertilized soil of North China. Sci. Rep. 2016, 6, 29257. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Sawamoto, T.; Akiyama, H.; Sudo, S.; Cheng, W.; Yagi, K. Continuous, automated nitrous oxide measurements from paddy soils converted to upland crops. Soil Sci. Soc. Am. J. 2005, 69, 1977–1986. [Google Scholar] [CrossRef]
- Bateman, E.; Baggs, E. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Lewicka-Szczebak, D.; Well, R.; Köster, J.R.; Fuß, R.; Senbayram, M.; Dittert, K.; Flessa, H. Experimental determinations of isotopic fractionation factors associated with N2O production and reduction during denitrification in soils. Geochim. Cosmochim. Acta 2014, 134, 55–73. [Google Scholar] [CrossRef]
- Firestone, M.; Davidson, E. Microbial basis of NO and N2O production and consumption. In Exchange of Trace Gases between Ecosystems and the Atmosphere: Report of the Dahlem Workshop; Andreae, M.O., Schimel, D.S., Eds.; Wiley: New York, NY, USA, 1989; pp. 7–21. [Google Scholar]
- Toyoda, S.; Yoshida, N.; Koba, K. Isotopocule analysis of biologically produced nitrous oxide in various environments. Mass Spectr. Rev. 2017, 36, 135–160. [Google Scholar] [CrossRef]
- Schmidt, H.L.; Werner, R.A.; Yoshida, N.; Well, R. Is the isotopic composition of nitrous oxide an indicator for its origin from nitrification or denitrification? A theoretical approach from referred data and microbiological and enzyme kinetic aspects. Rapid Commun. Mass Spectrom. 2004, 18, 2036–2040. [Google Scholar]
- Smemo, K.A.; Ostrom, N.E.; Opdyke, M.R.; Ostrom, P.H.; Bohm, S.; Robertson, G.P. Improving process-based estimates of N2O emissions from soil using temporally extensive chamber techniques and stable isotopes. Nutr. Cycl. Agroecosyst. 2011, 91, 145–154. [Google Scholar] [CrossRef]
- Toyoda, S.; Mutobe, H.; Yamagishi, H.; Yoshida, N.; Tanji, Y. Fractionation of N2O isotopomers during production by denitrifier. Soil Biol. Biochem. 2005, 37, 1535–1545. [Google Scholar] [CrossRef]
- Sutka, R.L.; Ostrom, N.; Ostrom, P.; Breznak, J.; Gandhi, H.; Pitt, A.; Li, F. Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. Appl. Environ. Microb. 2006, 72, 638–644. [Google Scholar] [CrossRef]
- Pérez, T.; Garcia-Montiel, D.; Trumbore, S.; Tyler, S.; de Camargo, P.; Moreira, M.; Piccolo, M.; Cerri, C. Nitrous oxide nitrification and denitrification 15N enrichment factors from Amazon forest soils. Ecol. Appl. 2006, 16, 2153–2167. [Google Scholar] [CrossRef]
- Barton, L.; McLay, C.; Schipper, L.; Smith, C. Annual denitrification rates in agricultural and forest soils: A review. Soil Res. 1999, 37, 1073–1094. [Google Scholar] [CrossRef]
- Snider, D.M.; Schiff, S.L.; Spoelstra, J. 15N/14N and 18O/16O stable isotope ratios of nitrous oxide produced during denitrification in temperate forest soils. Geochim. Cosmochim. Acta 2009, 73, 877–888. [Google Scholar] [CrossRef]
- Peri, P.L.; Ladd, B.; Pepper, D.A.; Bonser, S.P.; Laffan, S.W.; Amelung, W. Carbon (δ13C) and nitrogen (δ15N) stable isotope composition in plant and soil in S outhern P atagonia’s native forests. Glob. Chang. Biol. 2012, 18, 311–321. [Google Scholar] [CrossRef]
- Lee, X.; Feng, Z.; Guo, L.; Wang, L.; Jin, L.; Huang, Y.; Chopping, M.; Huang, D.; Jiang, W.; Jiang, Q. Carbon isotope of bulk organic matter: A proxy for precipitation in the arid and semiarid central East Asia. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Lei, Y.; Yang, F.; Zhang, D.; Zhang, K.; Zhang, Q.; Cheng, X. Agricultural land use change impacts soil CO2 emission and its 13C-isotopic signature in central China. Soil Tillage Res. 2018, 177, 105–112. [Google Scholar] [CrossRef]
- Vitória, A.P.; Ávila-Lovera, E.; de Oliveira Vieira, T.; do Couto-Santos, A.P.L.; Pereira, T.J.; Funch, L.S.; Freitas, L.; de Miranda, L.D.A.P.; Rodrigues, P.J.P.; Rezende, C.E. Isotopic composition of leaf carbon (δ13C) and nitrogen (δ15N) of deciduous and evergreen understorey trees in two tropical Brazilian Atlantic forests. J. Trop. Ecol. 2018, 34, 145–156. [Google Scholar] [CrossRef]
- Brüggemann, N.; Gessler, A.; Kayler, Z.; Keel, S.; Badeck, F.; Barthel, M.; Boeckx, P.; Buchmann, N.; Brugnoli, E.; Esperschütz, J. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: A review. Biogeosciences 2011, 8, 3457–3489. [Google Scholar] [CrossRef]
- Dijkstra, P.; Ishizu, A.; Doucett, R.; Hart, S.C.; Schwartz, E.; Menyailo, O.V.; Hungate, B.A. 13C and 15N natural abundance of the soil microbial biomass. Soil Biol. Biochem. 2006, 38, 3257–3266. [Google Scholar] [CrossRef]
- Wynn, J.G.; Bird, M.I. Environmental controls on the stable carbon isotopic composition of soil organic carbon: Implications for modelling the distribution of C3 and C4 plants, Australia. Tellus B Chem. Phys. Meteorol. 2008, 60, 604–621. [Google Scholar] [CrossRef]
- Koch, P.L.; Fox, L.R. Browsing impacts on the stable isotope composition of chaparral plants. Ecosphere 2017, 8, e01686. [Google Scholar] [CrossRef]
- Radajewski, S.; Ineson, P.; Parekh, N.; Murrell, J. Stable-isotope probing as a tool in microbial ecology. Nature 2000, 403, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, M.; Firestone, M. Seasonal dynamics of microbial community composition and function in oak canopy and open grassland soils. Microb. Ecol. 2006, 52, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Hornibrook, E.R.; Longstaffe, F.J.; Fyfe, W.S. Spatial distribution of microbial methane production pathways in temperate zone wetland soils: Stable carbon and hydrogen isotope evidence. Geochim. Cosmochim. Acta 1997, 61, 745–753. [Google Scholar] [CrossRef]
- Morse, J.L.; Bernhardt, E.S. Using 15N tracers to estimate N2O and N2 emissions from nitrification and denitrification in coastal plain wetlands under contrasting land-uses. Soil Biol. Biochem. 2013, 57, 635–643. [Google Scholar] [CrossRef]
- Bowling, D.R.; Pataki, D.E.; Randerson, J.T. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 2008, 178, 24–40. [Google Scholar] [CrossRef]
- Conrad, R. Quantification of methanogenic pathways using stable carbon isotopic signatures: A review and a proposal. Org. Geochem. 2005, 36, 739–752. [Google Scholar] [CrossRef]
- Andersson, R.A.; Meyers, P.; Hornibrook, E.; Kuhry, P.; Mörth, C.M. Elemental and isotopic carbon and nitrogen records of organic matter accumulation in a Holocene permafrost peat sequence in the East European Russian Arctic. J. Quat. Sci. 2012, 27, 545–552. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, T.; Wu, Q.; Peng, X.; Zhang, P.; Yang, Y.; Hou, Y.; Zhang, X.; Cheng, G. Dissolved organic carbon, CO2, and CH4 concentrations and their stable isotope ratios in thermokarst lakes on the Qinghai-Tibetan Plateau. J. Limnol. 2016, 75, 313–319. [Google Scholar] [CrossRef]
- Chen, C.; Wei, J.; Wen, X.; Sun, X.; Guo, Q. Photosynthetic Carbon Isotope Discrimination and Effects on Daytime NEE Partitioning in a Subtropical Mixed Conifer Plantation. Agric. For. Meteorol. 2019, 272, 143–155. [Google Scholar] [CrossRef]
- Cisneros-Dozal, L.; Trumbore, S.E.; Hanson, P. Partitioning sources of soil-respired CO2 and their seasonal variation using a unique radiocarbon tracer. Glob. Chang. Biol. 2006, 12, 194–204. [Google Scholar] [CrossRef]
- Ogée, J.; Peylin, P.; Cuntz, M.; Bariac, T.; Brunet, Y.; Berbigier, P.; Richard, P.; Ciais, P. Partitioning net ecosystem carbon exchange into net assimilation and respiration with canopy-scale isotopic measurements: An error propagation analysis with 13CO2 and CO18O data. Glob. Biogeochem. Cycles. 2003, 17. [Google Scholar] [CrossRef]
- Badeck, F.W.; Tcherkez, G.; Nogues, S.; Piel, C.; Ghashghaie, J. Post-photosynthetic fractionation of stable carbon isotopes between plant organs—A widespread phenomenon. Rapid Commun. Mass Spectrom. 2005, 19, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Bowling, D.R.; Tans, P.P.; Monson, R.K. Partitioning net ecosystem carbon exchange with isotopic fluxes of CO2. Glob. Chang. Biol. 2001, 7, 127–145. [Google Scholar] [CrossRef]
- Fassbinder, J.J.; Griffis, T.J.; Baker, J.M. Evaluation of carbon isotope flux partitioning theory under simplified and controlled environmental conditions. Agric. For. Meteorol. 2012, 153, 154–164. [Google Scholar] [CrossRef]
- Baldocchi, D.; Bowling, D. Modelling the discrimination of 13CO2 above and within a temperate broad-leaved forest canopy on hourly to seasonal time scales. Plant Cell Environ. 2003, 26, 231–244. [Google Scholar] [CrossRef]
- Griffis, T.J. Tracing the flow of carbon dioxide and water vapor between the biosphere and atmosphere: A review of optical isotope techniques and their application. Agric. For. Meteorol. 2013, 174, 85–109. [Google Scholar] [CrossRef]
- Baggs, E. Partitioning the components of soil respiration: A research challenge. Plant Soil 2006, 284, 1–5. [Google Scholar] [CrossRef]
- Conrad, R. Methanogenic microbial communities associated with aquatic plants. In Plant Surface Microbiology; Springer: New York, NY, USA, 2008; pp. 35–50. [Google Scholar]
- Shrestha, M.; Abraham, W.R.; Shrestha, P.M.; Noll, M.; Conrad, R. Activity and composition of methanotrophic bacterial communities in planted rice soil studied by flux measurements, analyses of pmoA gene and stable isotope probing of phospholipid fatty acids. Environ. Microb. 2008, 10, 400–412. [Google Scholar] [CrossRef]
- Lu, Y.; Murase, J.; Watanabe, A.; Sugimoto, A.; Kimura, M. Linking microbial community dynamics to rhizosphere carbon flow in a wetland rice soil. FEMS Microb. Ecol. 2004, 48, 179–186. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Li, X.; Liu, C.; Lin, W.; Zheng, W.; Chen, Y.; Yang, Y. Nitrogen addition accelerates the nitrogen cycle in a young subtropical Cunninghamia lanceolata (Lamb.) plantation. Ann. For. Sci. 2019, 76, 31. [Google Scholar] [CrossRef]
- Vallano, D.M.; Sparks, J.P. Foliar δ15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 2013, 172, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Parton, W.J.; Gonzalez-Meler, M.A.; Phillips, R.; Asao, S.; McNickle, G.G.; Brzostek, E.; Jastrow, J.D. Synthesis and modeling perspectives of rhizosphere priming. New Phytol. 2014, 201, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Subke, J.-A.; Hahn, V.; Battipaglia, G.; Linder, S.; Buchmann, N.; Cotrufo, M.F. Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 2004, 139, 551–559. [Google Scholar] [CrossRef]
- Murphy, C.J.; Baggs, E.M.; Morley, N.; Wall, D.P.; Paterson, E. Rhizosphere priming can promote mobilisation of N-rich compounds from soil organic matter. Soil Biol. Biochem. 2015, 81, 236–243. [Google Scholar] [CrossRef]
- Pausch, J.; Loeppmann, S.; Kühnel, A.; Forbush, K.; Kuzyakov, Y.; Cheng, W. Rhizosphere priming of barley with and without root hairs. Soil Biol. Biochem. 2016, 100, 74–82. [Google Scholar] [CrossRef]
- Prévost-Bouré, N.C.; Soudani, K.; Damesin, C.; Berveiller, D.; Lata, J.-C.; Dufrêne, E. Increase in aboveground fresh litter quantity over-stimulates soil respiration in a temperate deciduous forest. Appl. Soil Ecol. 2010, 46, 26–34. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Tang, C. Wheat and white lupin differ in rhizosphere priming of soil organic carbon under elevated CO2. Plant Soil 2017, 421, 43–55. [Google Scholar] [CrossRef]
- Lloyd, D.A.; Ritz, K.; Paterson, E.; Kirk, G.J. Effects of soil type and composition of rhizodeposits on rhizosphere priming phenomena. Soil Biol. Biochem. 2016, 103, 512–521. [Google Scholar] [CrossRef]
- Thurgood, A.; Singh, B.; Jones, E.; Barbour, M.M. Temperature sensitivity of soil and root respiration in contrasting soils. Plant Soil 2014, 382, 253–267. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, X.; Kuzyakov, Y. Mechanisms of rhizosphere priming effects and their ecological significance. J. Plant Ecol. 2014, 38, 62–75. [Google Scholar]
- Lin, W.; Fang, F.; Zhang, W.; Ding, J.; Li, Y.; Xu, C.; Li, Q. A review on development of stable isotope technique in the studies of N2O formation mechanism. J. Appl. Ecol. 2017, 28, 2344–2352. [Google Scholar]
- Yin, L.; Dijkstra, F.; Wang, P.; Zhu, B.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. New Phytol. 2018, 218, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Ciotoli, G.; Etiope, G.; Marra, F.; Florindo, F.; Giraudi, C.; Ruggiero, L. Tiber delta CO2-CH4 degassing: A possible hybrid, tectonically active sediment-hosted geothermal system near rome. J. Geophys. Res. Soil Eerth 2016, 121, 48–69. [Google Scholar] [CrossRef]
| Location | Seasonal Coverage | δ13CH4 (‰) | Reference |
|---|---|---|---|
| Japan | throughout the season | −68 to −48 | Uzaki et al., 1991 [45] |
| Japan | throughout the season | −72 to −56 | Tyler et al., 1994 [46] |
| China | throughout the season | −71 to −52 | Bergamaschi, 1997 [47] |
| throughout the season | −71 to −58 | ||
| America | throughout the season | −66 to −61 | Chanton et al., 1997 [48] |
| America | throughout the season | −58 to −53 | Tyler et al., 1997 [49] |
| America | throughout the season | −63 to −46 | Bilek et al., 1999 [42] |
| throughout the season | −62 to −48 | ||
| Italy | throughout the season | −67 to −47 | Marik et al., 2002 [50] |
| throughout the season | −65 to −53 | ||
| Italy | throughout the season | −73 to −58 | Krüger and Frenzel, 2003 [51] |
| Germany | throughout the season | −68 to −61 | Conrad and Klose, 2005 [52] |
| China | throughout the season | −71 to −47 | Zhang et al., 2012 [53] |
| China | throughout the season | −61 to −59 | Zhang et al., 2014 [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.-c.; Di, D.-r.; Ma, M.-g.; Shi, W.-y. Stable Isotopes in Greenhouse Gases from Soil: A Review of Theory and Application. Atmosphere 2019, 10, 377. https://doi.org/10.3390/atmos10070377
Zhu X-c, Di D-r, Ma M-g, Shi W-y. Stable Isotopes in Greenhouse Gases from Soil: A Review of Theory and Application. Atmosphere. 2019; 10(7):377. https://doi.org/10.3390/atmos10070377
Chicago/Turabian StyleZhu, Xiao-cong, Dong-rui Di, Ming-guo Ma, and Wei-yu Shi. 2019. "Stable Isotopes in Greenhouse Gases from Soil: A Review of Theory and Application" Atmosphere 10, no. 7: 377. https://doi.org/10.3390/atmos10070377
APA StyleZhu, X.-c., Di, D.-r., Ma, M.-g., & Shi, W.-y. (2019). Stable Isotopes in Greenhouse Gases from Soil: A Review of Theory and Application. Atmosphere, 10(7), 377. https://doi.org/10.3390/atmos10070377





