Abstract
Palmes-type passive diffusion tubes (PDTs) are widely used to measure levels of nitrogen dioxide (NO2) in air quality studies. Molecules of NO2 diffuse down the concentration gradient established in the tube by their reactive conversion into nitrite (NO2−) with triethanolamine (TEA) absorbent at the inner end. The relatively low uptake rate for the tube geometry means that exposure-averaged NO2 concentration can be calculated from first principles using the diffusion coefficient, D, for NO2 in air. This review provides a critical assessment of the current understanding of sources and extent of potential bias in NO2 PDT measurements in each of the following methodological stages: preparation of the absorbent; quantification of the absorbed NO2−; deployment in the field; calculation of the exposure-average NO2 concentration from the absorbed NO2−; and assessment of PDT bias through comparison against a chemiluminescence NO2 analyser. The review has revealed strong evidence that PDT measurement of NO2 can be subject to bias from a number of sources. The most significant positive biases are ambient wind flow at the entrance of the tube potentially leading to bias of tens of percent, and within-tube chemical reaction between NO and O3 causing bias up to ~25% at urban background locations, but much less at roadside and rural locations. Sources of potentially significant negative bias are associated with deployment times of several weeks in warm and sunny conditions, and deployments in atmospheres with relative humidities <~75% which causes incomplete conversion of NO2 to NO2−. Evidence suggests that biases (positive or negative) can be introduced by individual laboratories in the PDT preparation and NO2− quantification steps. It is insufficiently acknowledged that the value of D is not accurately known—some controlled chamber experiments can be interpreted as indicating that the value of D currently used is too low, giving rise to a positive bias in PDT-derived NO2 concentration. More than one bias may be present in a given PDT deployment, and because the biases act independently the net effect on PDT NO2 determination is the linear sum of individual biases acting on that deployment. The effect of net bias can be reduced by application of a local “bias adjustment” factor derived from co-locations of PDTs with a chemiluminescence analyser. When this is carried out, the PDT is suitable as an indicative measure of NO2 for air quality assessments. However, it must be recognised that individual PDT deployments may be subject to unknown variation in the bias adjustment factor for that deployment.
1. Introduction
Nitrogen dioxide (NO2) is a major air pollutant [1,2] whose ambient concentrations in many urban areas continue to breach local statutory limits or the World Health Organization’s (WHO’s) air quality guidelines. For example, the European Environment Agency estimates that, in 2016, 8% of the EU-28 urban population lived in areas where NO2 concentrations exceeded the EU annual-mean limit value of 40 μg m−3, which is also the WHO’s air quality guideline [3]. NO2 is a particular issue in urban areas since a major source of NO2 is from vehicles, both as primary emissions from vehicle exhausts, but also from the fast reaction between ambient ozone (O3) and the nitric oxide (NO) also emitted by vehicle exhaust and all other combustion sources (e.g., domestic heating, power generation, industry, cooking). Rapid urbanisation worldwide means that concentrations of NO2 are high in many large cities, particularly in developing countries [4,5]. Within a city, the widespread sources and relatively short lifetime of NO2 also mean that its concentrations are often strongly spatially varied [6,7,8]. It is, therefore, a challenge to quantify spatial distributions of NO2 for air quality assessment and management or for health impact studies and similar.
In many places, national and local governments, researchers, environmental consultancies, third-sector organisations, etc., use the passive diffusion tube (PDT) (Figure 1) introduced by Palmes et al. [9] to measure levels of NO2. The PDT’s simplicity and lack of power and infrastructure requirements means large numbers can be deployed simultaneously and relatively cheaply.
Figure 1.
Schematic of the construction of a Palmes-type passive diffusion tube.
As with all passive samplers, the PDT operates on the principle of molecular diffusion of NO2 along a fixed-length path driven by the concentration gradient, from ambient concentration at the open end of the sampler to an assumed zero concentration at the chemical absorbent that is coated onto metal grids in the inner end of the tube [10]. Since, at most locations, compliance with an annual average objective for NO2 is more challenging than adherence to an hourly or daily objective, the inherent long averaging time of the passive diffusion tube methodology is not a disadvantage. Furthermore, evidence for the health risk associated with long-term average NO2 concentrations is strengthening [11].
The absorbent used to trap the ambient NO2 is triethanolamine (TEA, N(CH2CH2OH)3). The TEA is first dissolved in either water or acetone. Then either the grids are soaked in this solution prior to PDT assembly or a certain volume (typically 20 or 50 μL) of the solution is pipetted onto grids already placed inside a cap but before the tube is inserted. The TEA converts the NO2 molecules into nitrite (NO2−) ions. After exposure, the NO2− is extracted into a known volume of water and quantified either directly by ion chromatography or by a colorimetric procedure. The latter involves adding solutions of sulphanilamide (at acid pH) and N-1-naphthyl ethylene diamine dihydrochloride (NEDD) to form a purple-coloured azo dye whose absorbance intensity (measured at a wavelength of 540 nm) is proportional to the NO2− concentration in the extracted solution.
An advantage of the tube geometry of the PDT compared with other passive sampler designs (for example, badges) is that the large ratio of internal length L (typically 7.1 cm) to cross-sectional area A (typically 0.91 cm2), that forms the diffusion region, means that uptake rates should be minimally influenced by external factors that may perturb the assumption of diffusional mass transfer along the tube. Consequently, the exposure-averaged ambient concentration can be obtained directly from theoretical considerations without calibration, according to Equation (1):
where Q is the total amount of collected NO2−, t is the duration of the exposure and D is the diffusion coefficient for NO2 in air. A disadvantage of the high L/A ratio of the tube geometry is the comparatively low NO2 uptake rate. Hence PDTs for ambient measurement are typically exposed for one to four weeks at a time.
It is well accepted that passive samplers have potential for greater uncertainty than the chemiluminescence analyser reference method for NO2 determination: the EU Directive on air quality permits an overall measurement uncertainty of ±25% and ±15% for the two approaches, respectively [12]. Nevertheless, a number of studies over the years have suggested that the NO2 PDT methodology can be subject to intrinsic bias, rather than simply greater random uncertainties. The literature on the performance of PDTs for measurement of NO2 was reviewed in some detail over a decade ago by both EU [13] and UK [10,14] ad hoc expert groups. However, the principal aim of the former review was to evaluate the PDT method against the EU data uncertainty objective, and of the latter review to provide recommendations on PDT method harmonisation in the UK. In neither case was discussion or assessment of biases in the PDT method the focus. Further research has also been published since these expert group reports.
The purpose of this review, therefore, is to provide a state-of-the-art critical assessment of the current understanding of sources and extent of bias in PDT NO2 measurement.
Bias can potentially be introduced at many points in the PDT method, as summarised in Table 1 for each of the following methodological stages: (i) the preparation of the PDT, (ii) the post-exposure quantification of the absorbed NO2−, (iii) during the PDT exposure, (iv) in the calculation of the exposure-average NO2 concentration from the absorbed NO2−, and (v) in the assessment of PDT bias via comparison of the PDT-derived concentration of NO2 against a reference chemiluminescence analyser determination of NO2. The latter is relevant since assessments of PDT accuracy assume a co-located chemiluminescence analyser determination of NO2 represents the “true” NO2 concentration.

Table 1.
Potential factors influencing accuracy of quantification of ambient NO2 by passive diffusion tube (PDT).
This review analyses information only in respect of bias (inaccuracy) in the NO2 PDT methodology, not other aspects of uncertainty. The focus is on studies relating to the Palmes-type PDT, but relevant observations on bias derived from other designs of passive sampler are also reviewed. The review includes evaluation of findings from older studies in the light of subsequent research findings. The sources of potential bias are independent, so overall NO2 PDT accuracy is the net sum of all biases acting on a given PDT deployment. This makes it difficult to de-convolute the source and magnitude of individual biases.
The following section presents the findings of this review, subdivided into the five stages of the PDT methodology listed in Table 1 where bias may arise. This is followed by overall concluding remarks on the practical usage of PDTs. These sections are presented as “standalone” summaries without citation. The detailed review, discussion and citation of the literature evidence for the conclusions is presented as an Appendix A.
2. Conclusions from the Review of the Evidence
2.1. Bias in Preparation of the PDT
In principle, it should not matter how the TEA is transferred to the grids as long as sufficient TEA is permanently transferred for the TEA to be greatly in excess of the NO2 to be captured, which should be the case for all likely ambient PDT deployments.
A few studies have sought to evaluate whether factors such as choice of solvent for TEA (acetone or water), proportion of TEA in the solvent (10%, 20% or 50%) and method of application of the solution to the absorbent grids (dipping or pipetting) have significant influence on PDT accuracy. However, interpretation of influence of preparation is confounded by other sources of bias (for example, protection or not from wind, within-tube chemistry, ambient humidity, length of exposure) that influence the quantification of PDT accuracy assessed by comparison to reference analyser concentrations. PDT performance has also been shown to vary between laboratories using the same preparation methods.
Overall, this review has found no evidence to contradict the current UK recommendation that PDT preparation via dipping grids in 50% TEA in water or pipetting 50 μL of 20% TEA in water leads to the least bias.
Prepared PDTs that are suitably stored (cool and in the dark, e.g., a fridge), remain usable for several months at least.
2.2. Bias in Quantification of Absorbed Nitrite (NO2−)
There is a dearth of systematic investigation of potential bias arising from methods used to extract NO2− from the absorbent, and from the sulphanilamide and NEDD reagent ratios and concentrations used in its quantification. It must be assumed, however, that where a high standard of laboratory QC/QA procedures is maintained (including, for example, regular calibration of balances and pipettes and appropriate number and range of NO2− standards), and particularly where laboratories are subject to regular “round robins” and other external quality assurance procedures, the quantification of the trapped NO2− should not contribute a significant source of bias.
2.3. Bias through the Influence of Factors during PDT Exposure
2.3.1. Interference from Co-Pollutants
Although both peroxyacetyl nitrate (PAN) and nitrous acid (HONO) yield NO2− on reaction with TEA, this potential positive bias for NO2 PDTs is considered insignificant given the much lower abundances of these species relative to NO2, on average, in ambient air. The reaction between co-diffusing NO and O3 to produce additional NO2 along the diffusion path is, however, an important potential bias and is discussed separately in Section 2.3.5.
2.3.2. Variability in Ambient NO2 Concentrations
Fast fluctuations (of a few minutes) of high amplitude (for example, >200 μg m−3) in NO2 concentrations at the mouth of the tube can induce transient increases in PDT uptake compared with the standard equation derived from Fick’s fist law that assumes a steady-state concentration profile down the tube. However, model simulations show that the contribution of this variability to the mean NO2 concentration is negligible, less than a couple of percent even under unlikely unfavourable fluctuations.
2.3.3. Effect of Humidity on Stoichiometric Conversion of NO2 to NO2−
There is some evidence that for relative humidity (RH) less than ~75–80% the TEA is not fully hydrated, such that not all the NO2 reacting with TEA is converted to NO2−. Consequently, PDT concentrations calculated under the assumption of stoichiometric conversion of NO2 to NO2− will be biased low. For values of RH leading to full hydration of TEA, average NO2 concentrations calculated using the standard approach are correct (assuming no other biases present). An ambient RH of ~75–80% is quite high. Even for the moist climate of the UK, where average RH is around 80%, there are locations and/or periods during the year when RH during a PDT exposure is lower than 75% and, hence, potentially giving rise to negative bias. It is possible that in drier climates PDT negative bias from this cause may be substantial.
2.3.4. Effect of Ambient Wind Speed, Humidity and Temperature on Uptake Rate
Both chamber and field experiments provide some contradictory results on the significance of the effect of wind across the open end of the PDT (which leads to an effective shortening of the diffusion path length and a positive bias to the PDT measurement). However, it is clear from consideration of all the literature to date, and from scientific expectation, that positive bias from wind effects exists and can be very large, albeit that the extent of sensitivity of the bias to increasing wind speed is not clear. Under even moderate wind conditions, a number of chamber and field experiments suggest tens of percent positive bias. Close inspection of data across a number of chamber experiments suggests some consistency for an overestimation of the order of 20% compared with the theoretical uptake rate, even at the lowest wind speeds that will be routinely encountered in ambient deployments; however, as noted below, the observed bias could be caused by use of an incorrect value of NO2 diffusion coefficient, rather than to wind effects (or to both).
Results from chamber experiments also suggest that lower RHs reduce the NO2 uptake rate of PDTs, which is consistent with the argument that low RH reduces conversion of NO2 to NO2− to below 1:1 stoichiometry.
Of the three meteorological variables, evidence suggests smallest sensitivity of PDT uptake rate for temperature, of the order of a few percent per 10 °C. Temperature influences the rate of NO2 diffusion (but this is relatively small, see Section 2.4), the relative humidity for a given absolute humidity, and potentially also the physical phase of the TEA, although the latter is not believed to be important for ambient conditions. Because of the link between temperature and relative humidity, it is possible that effects attributed to temperature may be due to humidity.
It is difficult to pinpoint the individual effects of these factors on bias, because the bias between PDT and a reference analyser values may be the net effect of several factors acting together (e.g., wind, humidity, within-tube chemistry, and long-term degradation of absorbed NO2−). This is particularly the case for field evaluations where PDT exposures can vary between a few days to several weeks, and which are subject to varying environmental conditions during exposure that are usually not measured, or measured a long way from the PDT deployments. Chamber experiments have constant and known values of environmental variables and do not include ambient O3, so within-tube chemistry is not an issue for the chamber experiments.
An alternative explanation for chamber exposure data that suggest positive bias compared with the theoretical uptake rate of AD/L, even at low wind speeds, is that an inappropriate value for the diffusion coefficient of NO2 in air is being used for the theoretical uptake rate—one that is too low and, consequently, has the effect of giving rise to a positive bias in derived average NO2 concentration (see Section 2.4). This has not been discussed in the literature.
Considerable accumulated evidence indicates that positive bias from wind effects can be offset either by use of a coarse mesh across the tube and/or with the tubes placed within a shelter. Membranes across the mouth of the tube may overcompensate for wind-induced positive bias by providing resistance to free molecular diffusion and reducing uptake below its theoretical value derived via Fick’s first law.
2.3.5. Within-Tube Chemical Generation of Additional NO2
Model simulations of the diffusion and chemical reaction within a PDT clearly demonstrate potential for intrinsic positive bias from additional NO2 produced from the reaction between NO and O3 also diffusing within the tube. The bias arises because the timescale for this reaction (tens of seconds) is shorter than the average time of 2.8 min for diffusion down the tube, whereas the photolysis of NO2 back to NO and O3 has a timescale, even in daytime ambient air, of a few minutes (and is much longer inside non-UV transmitting tubes and does not happen at all at night). For locations where both NO and O3 are relatively high compared with NO2 (e.g., urban background), the simulations indicate the within-tube chemistry positive bias can average as high as ~25%. For roadside locations, where O3 concentrations may be low compared with NOx concentrations, and for rural locations where most NOx is already in the form of NO2, this bias may be only a few percent.
There is some experimental support of the chemical bias but interpretation is again confounded by the presence of other potential biases (wind and humidity effects, long-term absorbent degradation) that simultaneously impact on PDT performance.
2.3.6. Exposure-Duration “Loss” of Absorbed NO2−
Although evidence is sparse, it is consistent that there may be a small negative bias in PDT-derived NO2 concentrations associated with a slow degradation of the absorbed NO2−, of a few percent per week, particularly in sunnier, warmer conditions. This potential bias clearly becomes more relevant for longer PDT exposures, such as four or five weeks.
2.4. Bias in Calculation of Average Ambient NO2 from the Quantified NO2−: Uncertainty in the Value of the NO2 Diffusion Coefficient
The original Palmes value for the NO2 diffusion coefficient (sometimes with temperature correction) has been used in all subsequent PDT measurements, seemingly without further question. The value was derived from semi-empirical theoretical consideration of gas behaviour because it is very hard to measure experimentally. The one experimental value (from 1937) is a factor 0.89 of the Palmes value. Although semi-empirical methods for estimation of gas diffusion coefficients are well-established, a more recent calculated value is a factor 1.20 of the Palmes value.
The greater PDT uptake rates measured in some chamber experiments compared with uptake rates derived using the theoretical equation (AD/L) could be explained if D was greater than the standard Palmes value used. However, it is difficult to control for all variables that may influence uptake experimentally, even in a chamber study. If the true value of D was larger than the Palmes value currently used then NO2 concentrations currently calculated from PDT measurements are positively biased compared with the true NO2 concentrations, and vice versa.
There should be much greater acknowledgement that the value for D is not known with certainty, and particularly that it is not known to the precision implied by use of a value expressed to three significant figures. One evaluation suggests an uncertainty in D of ±35%. This does not mean random variability across individual PDT exposures in the range of several tens of percent because D has a single true value; instead it means that collectively all PDT-derived NO2 values may be a certain (currently unknown) percentage too high or a certain percentage too low. Any inaccuracy in D would proportionally apply universally to all NO2 passive sampler measurements. This particular potential source of PDT bias is not an issue for PDTs that are “bias adjusted” against a chemiluminescence analyser, since if this was the only source of PDT bias at all PDT exposure locations, including the co-location, then it would be accounted for through the bias adjustment factor.
2.5. Bias in Comparison of PDT NO2 with Chemiluminescence Analyser NO2
PDT bias is assessed by co-location with chemiluminescence analysers. The EC Air Quality Directive permits up to ±15% overall uncertainty in a chemiluminescence concentration. PDT values calculated using the value of D recommended in the UK (which assumes an average ambient temperature of 284 K) must be decreased by a factor 284/293 = 0.969 to compare against a chemiluminescence analyser that has been set up to report NO2 concentrations referenced to the EU reporting temperature of 293 K. Failure to make this adjustment means the PDT-derived value in the comparison has a positive bias of ~3%.
Chemiluminescence analysers using a heated molybdenum NOx-to-NO converter are subject to positive bias in NO2 measurement from HNO3, HONO and PAN also present in the air. The bias is much lower (maximum of a few %) for locations close to fresh emissions of NOx, such as roads, than for locations with more photochemically-aged air. For a location where this over-reporting of NO2 by a “thermal converter” chemiluminescence analyser exists, a co-located NO2 PDT would be deemed to have a negative bias. However, bias between chemiluminescence analyser and co-located PDT due to this issue would be offset if the other oxidised N-containing gases also gave rise to absorbed NO2− in the PDT, but this has not been sufficiently tested.
3. Overall Conclusions
The review has revealed strong evidence that measurement of NO2 by PDT can be subject to bias from a number of sources. The most significant positive biases in normal usage are ambient wind flow at the entrance of the tube potentially leading to bias of tens of percent, and within-tube chemical reaction between NO and O3 causing bias up to ~25% at urban background locations (but much less at roadside and rural locations). Sources of potentially significant negative bias are associated with deployments in atmospheres with relative humidities <~75% that cause incomplete conversion of NO2 to NO2−, and with long deployment times (i.e., several weeks) in warm and sunny conditions. There is also evidence to suggest that biases (positive or negative) can be introduced by individual laboratories in the PDT preparation and NO2− quantification steps.
More than one bias may be present in any given PDT deployment. The biases act independently so the net effect on PDT NO2 determination is the linear summation of individual biases acting in a particular deployment. For some PDT deployments, positive and negative biases may offset each other leading to smaller net bias.
The individual and net magnitude of bias that may impact NO2 determination in an individual PDT deployment cannot easily be predicted or quantified. In theory, laboratory-derived biases can be minimised by adherence to good QA/QC procedures and participation in inter-analyst comparisons. Positive bias from wind effects can be substantially reduced either by use of a coarse mesh across the tube and/or with the tubes placed within a shelter. Membranes across the mouth of the tube should not be used since these may overcompensate for wind-induced positive bias by providing resistance to free molecular diffusion. The positive bias from within-tube chemical reaction between NO and O3 can, in principle, be eliminated by use of tube material that fully transmits the UV wavelengths relevant to NO2 photolysis, but in practice this is hard to achieve and is likely incompatible with placing the tubes within a wind shelter.
There is also an unresolved question concerning the accuracy of the value of the diffusion coefficient for NO2 in air that is used to covert the mass of absorbed NO2− to average ambient NO2 concentration. Any inaccuracy in D would proportionally apply universally to all NO2 passive sampler measurements.
The effect of net bias can be reduced by application of a local “bias adjustment” factor derived from co-locations of PDTs with chemiluminescence analyser. When this is carried out, the PDT is suitable as an indicative measure of NO2 for air quality assessments. However, it must be recognised that individual PDT exposures may be subject to unknown variation in the true bias adjustment factor for that exposure.
Author Contributions
D.P.H.L. and B.B.M. led the bid that attracted funding for this work, with input from M.R.H. All authors conceived the design of this review. M.R.H. undertook the literature search and initial drafting of the review, with subsequent additional contributions and revisions from D.P.H.L. and B.B.M.
Funding
The Scottish Government providing financial support for this review through contract AQC/001/17.
Conflicts of Interest
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation or writing of the study.
Appendix A. Review and Discussion of the Evidence for Bias
Appendix A.1. Bias in PDT Preparation
Variables in the preparation of the PDT prior to exposure include the choice of solvent for TEA (acetone or water), proportion of TEA in the solvent (10%, 20% or 50%) and method of application of the solution to the grids (dipping or pipetting). Scientific expectation is that it should not matter how the TEA is transferred to the grids provided the amount of TEA applied is sufficient to avoid its saturation by NO2 during an exposure. This will be the case for all likely ambient PDT deployments. For example, preparation with 50 μL of 20% TEA solution delivers ~70 μmol TEA, which is in large excess to the ~0.1 μmol of NO2 that will be absorbed even for a four-week exposure with average ambient NO2 concentration of 100 μg m−3.
Studies examining experimental evidence for potential biases arising in PDT preparation have usually involved a spread of laboratories preparing and analysing the PDTs [15,16,17,18], and have concluded that PDT performance varied more with the laboratory than with any particular preparation variable. These observations were the driving factor in the establishment of the UK Working Group for harmonisation of NO2 PDT methods [14].
Almost no study has undertaken systematic investigation of PDT preparation with a single laboratory and analyst. From multiple preparation method comparisons, Kirby et al. [19] recommended pipetting 30–50 μL volumes of 10% or 20%, but not 50%, TEA in water; while Hamilton and Heal [20] likewise recommended against 50% TEA in water, but did support dipping as a preparation method, particularly 50% in acetone, in preference to pipetting.
Heal [21] reported a statistical evaluation of the effect of the absorbent grid preparation method using a dataset of 680 duplicated PDT exposures spanning 146 separate exposure periods, spread over five urban exposure locations in Edinburgh and a number of years (and a number of analysts). It was concluded that both PDT precision and accuracy (as quantified by maximum concentration across a set of co-located preparation methods) were both significantly better, on average, when the PDT grids were prepared by dipping the grid in TEA solution, and that neither solvent or percent of TEA used for the dipping solution were important. Where PDT preparation by pipetting TEA solution onto grids was used, better performance was obtained using 20% TEA in water.
Laxen et al. [22] undertook a similar “meta-analysis” of PDT preparation variables using data from 161 PDT/reference analyser co-location studies carried out by UK local authorities in the years 2003–2005. The dataset incorporated a range of preparation and analysis approaches spread across 21 laboratories. This evaluation similarly concluded that there was some evidence that dipping of grids in TEA solution provided better performance than pipetting of TEA solution onto grids, and that, for the former approach, there was a clear pattern that PDTs prepared with grids soaked in TEA solution for 10 min or more performed better than tubes with grids soaked in solution for less than 1 min. There was also a clear pattern that allowing the grids to dry before final tube assembly was associated with better performance. Laxen et al. [22] also concluded that tubes prepared using 20% TEA in water performed better than those prepared using 50% TEA in acetone, despite the evidence that preparation by dipping (which usually uses 50% TEA) yielded better performance than preparation by pipetting. However, the authors pointed out that by the nature of their “observational” study it was difficult to separate potential influence of different factors since there was incomplete data on all possible combinations of variables.
Tarvydaitė and Kazlauskienė [23] investigated use of three different absorbents, but in a custom-built passive sampler for NO2 with a geometry part-way between tube and badge, comprising a polypropylene tube 34 mm in length and 21 mm in inner diameter. Preparations of 10% TEA in water, 10% TEA in acetone and a solution of potassium iodide (KI) with sodium hydroxide (NaOH) (to enhance the capture of the acidic NO2) were deployed in Vilnius, Lithuania. The absorbent was held on a stainless steel grid for the first two solutions and a glass fibre filter for the third. The authors report that only the preparations with 10% TEA in water or acetone yielded uncertainty within the 25% requirement of the EU Directive. Their finding is consistent with current recommendations in the UK (see below).
Regardless of the nature of the study, as pointed out by Heal [21], interpretation of the influence of the preparation method is confounded by other sources of bias (for example, length of exposure, protection or not from wind, ambient humidity, within-tube chemistry, etc.) that may also affect extent of PDT agreement with a reference analyser.
For UK deployments, the Defra Working Group (WG) [14] recommended that the grids should be prepared either by dipping the grids in a solution of 50% TEA in acetone or by pipetting 50 µL of a solution of 20% TEA in water directly onto grids placed in the cap. No published evidence contradicts this recommendation.
Prepared PDT tubes suitably stored (cool and in the dark, e.g., a fridge) are reported to remain usable for several months [10].
Appendix A.2. Bias in Quantification of Absorbed Nitrite
No study has reported systematic investigation of the impact of the quantification of NO2− from the TEA absorbent arising from, for example, sulphanilamide and NEDD reagent concentrations and ratios. Furthermore, almost no publication provides any detail on the extraction and quantification steps, so it is also not possible to look for any observational associations between PDT performance and analytical methods.
The studies described above investigating evidence for impact of PDT preparation methods also noted that variation in NO2− quantification procedures may be one of the contributors for the observed inter-laboratory variation in PDT performance [15,16,17,18].
In principle, where high standards of analytical QC/QA are followed (including, for example, regular calibration of balances and pipettes and use of external reference standards), the extraction into solution of the absorbed NO2− and its quantification should not be a source of bias. To aid achievement of accurate NO2− determination, the UK NO2 PDT harmonisation group made detailed recommendations on the laboratory analytical steps (for both manual and automated analyses), based on their collective practical experience and results of small ad hoc in-house trials with varying analytical steps [14]. For example, extraction should be aided by use of a vortex mixer for at least 15 s or a vibrating tray for 10–30 min. The sulphanilamide and NEDD solutions should be prepared and stored separately. Once mixed, the colour reagents should be used the same day, not stored. The concentrations of the reagents should be such that, on mixing, the ratio of the two reagents in the extraction solution are 7 × 10−3 g of NEDD per 1 g of sulphanilamide with absolute reagent amounts per sample PDT of the order of 0.42 mg and 60 mg, respectively. The colour absorption should be measured within 2 h.
As part of a general evaluation of custom-built tube-like passive samplers for NO2, Bootdee et al. [24] evaluated extraction and analysis of NO2−. Their samplers comprised either a polyethylene tube (5.4 cm long and 1.4 cm i.d.) or polypropylene tubes (5.3 cm long, 1.3 cm i.d. or 7.7 cm long, 1.6 cm i.d.), with 50 μL of 20% TEA in water applied to either glass fibre or Whatman filters. The authors investigated extraction times and colour reagent development time in the range 5–25 min and reported optimum times of at least 15 min, yielding 94 ± 3 % recovery. Vardoulakis et al. [25] reported a 98% extraction efficiency of NO2− in their field study. These findings support current practices.
There is currently no published evidence to contradict the current UK recommendations [14] for NO2 PDT analysis.
Appendix A.3. Bias through the Influence of Factors during PDT Exposure
Appendix A.3.1. Interference from Co-Pollutants
Both peroxyacetyl nitrate (PAN) and nitrous acid (HONO) react with TEA to yield NO2−. The latter is reported to yield stoichiometric interference [26], but whilst PAN is reported to be stoichiometrically converted to NO2− by alkaline TEA [27], for operational PDTs the conversion was reported as <5% [28]. In practice, bias due to HONO and/or PAN is considered insignificant given the much lower abundances of these species relative to NO2, on average, in all but exceptional circumstances. The potential impact of HONO and PAN in the assessment of PDT bias via comparison against different types of chemiluminescence analyser is discussed in Appendix A.5.
The bias due to ambient NO and O3 molecules creating additional NO2 along the diffusion path within the PDT is discussed separately, in Appendix A.3.5.
Appendix A.3.2. Variability in Ambient NO2 Concentrations
Equation (1) that is used to calculate the exposure-average NO2 concentration is based on Fick’s first law of diffusion with an assumption of a time-independent linear concentration profile of NO2 along the tube. Bias in the derived NO2 occurs when this assumption breaks down. Plaisance [29] theoretically analysed the response of PDT uptake to fluctuating ambient concentrations relevant to likely ambient exposures using a mathematical methodology defined by Hearl and Manning [30] to resolve Fick's second law of diffusion. The errors of the PDT determination were computed numerically for different characteristics of the concentration variation encountered in outdoor environments, such as the peak duration and the ratio of the peak amplitude to the background concentration. Although fast fluctuations (of a few minutes) of high amplitude (e.g., >200 μg m−3) induce transient increases in PDT uptake compared with the standard equation, their contributions to the mean concentration estimated are negligible, less than a couple of percent even under unlikely unfavourable conditions [29].
Appendix A.3.3. Effect of Humidity on Stoichiometric Conversion of NO2 to NO2−
A fundamental assumption underpinning the PDT method is that molecules of NO2 are 100% converted to molecules of extractable NO2− at the absorbent. Palmes and Johnson [31] showed that a H2O:TEA molecular ratio of around 3.6, which is the amount absorbed by TEA in equilibrium with ambient air at 75% RH and 26 °C (18 g H2O m−3), provides 100% conversion of absorbed NO2 to NO2−.
The following stoichiometric reaction for the conversion is consistent with the observation that TEA needs to be hydrated [32].
2NO2 + N(CH2CH2OH)3 + 2OH− → 2NO2− + −O − +N(CH2CH2OH)3 + H2O
When the TEA is not sufficiently hydrated the reaction of NO2 with TEA yields N-nitroso-diethanolamine, (CH2CH2OH)2NNO, and no NO2− [33]. Cape [10] concluded (using other evidence also) that a minimum of around 3 g H2O m−3 (equivalent to an RH of 35% at 5 °C) is required for TEA to be effective and that this condition is normally readily met in the UK except in very cold, dry weather.
Poddubny and Yushketova [34] have explored, quantitatively, the expected stoichiometry of formation of NO2− from NO2 as a function of the water present. In their model there is 100% conversion of NO2 to NO2− with hydrated TEA (via R1), the fraction of hydrated TEA molecules depends on the humidity of the air at the time, and reaction of NO2 with non-hydrated TEA does not form NO2−. They also incorporated in their model the situation where all TEA is hydrated but there is insufficient TEA to react with all NO2, in which case the excess NO2 reacts with the excess water and conversion of NO2 to NO2− is 50% (R2).
2NO2 + H2O → 2H+ + NO2− + NO3−
However, in all likely ambient PDT deployments the amount of TEA will be in large excess of the NO2 captured.
Poddubny and Yushketova [34] cite Palmes and Johnson [31] and Kirby et al. [19] that the TEA in solution must be in protonated form (the proton deriving from dissociation of H2O), which is consistent with the need for OH− ions, which must also derive from H2O. So, the minimum is one molecule of H2O per conversion of NO2 to NO2−. Poddubny and Yushketova [34] also state that the ethanolic groups of the TEA also hydrate through hydrogen bond formation. Therefore, the number of water molecules to hydrate one TEA molecule may vary between one and four, or possibly more. It is assumed not relevant if TEA was initially applied in acetone, since this solvent readily evaporates and aqueous equilibrium of the TEA with ambient humidity is rapidly established.
Poddubny and Yushketova [34] denote the number of H2O molecules required for hydration of one TEA molecule as . Their calculation of the average concentration of NO2 during an exposure uses the standard Fick’s Law approach but with a stepwise summation over j time intervals (e.g., hourly intervals) with known values of RH and T, in each of which intervals there is explicit inclusion of the stoichiometry of conversion of NO2 to NO2−, represented by the term in Equation (A1), where represents the fraction RH in the interval and m is the mass of collected NO2−.
The value for the conversion coefficient for NO2 to NO2−, , depends as follows on whether , the number of H2O molecules present per TEA molecule in a time interval, is less than, equal to, or greater than , the number of H2O molecules required for full hydration of TEA.
Rearranging Raoult’s Law, assuming an ideal solution (and constant TEA amount),
gives
which is effectively an expression for as a function of RH.
The model was evaluated using 141 two-week measurements by PDTs prepared with 20% TEA in water spread across three years (2007–2009) and four sampling locations in the Middle Urals. Of these exposures, 106 were co-located with reference analysers and the authors fitted their model to the analyser data using different empirical values of . Their best fit was for = 3.9, close to the previously reported value of 3.6 [31], but their fit was very similar for any value in the range 3.6 to 4, so the authors suggested = 3.6 was appropriate.
The value of RH required to achieve is derived by substitution into Equation (A5).
Rearranging yields,
Therefore, conversion of NO2 to NO2− is less than unity when RH is <78%.
The requirement for ~78% RH is quite stringent. Poddubny and Yushketova [34] showed that for their own PDT measurements in the Urals (details above), during which there was substantial variation in RH, PDT NO2 concentrations calculated using the standard formula were significantly lower than the reference concentrations, but that there was no significant difference when the effect of RH per hourly time step was explicitly included. Even in a moist climate location such as the UK where average RH is around 80%, there are locations around the UK (e.g., east and south-east England) and/or periods during the year (e.g., early summer) when RH during a PDT exposure is lower than 75% and, hence, potentially causing negative bias.
Appendix A.3.4. Effect of Ambient Wind Speed, Humidity and Temperature on Uptake Rate
Of the three meteorological variables, wind speed, humidity and temperature, the first has received the most attention. Wind across the tube entrance causes bias by creating turbulent air movements within the upper part of the tube, which violates the assumption that mass transfer of NO2 is purely diffusive; that is, the diffusive path length becomes shorter in reality than the tube length L on which the theoretical uptake rate is based, leading to positive bias. The influence of humidity on the quantitative conversion of NO2 to NO2− in reaction with TEA has already been discussed. Temperature influences (i) the rate of NO2 diffusion (but this is relatively small, discussed in Appendix A.4), (ii) the RH for a given absolute humidity, and (iii) potentially also the phase (i.e., solid vs. liquid) of the TEA. The latter is not believed to be an issue under normal sampling conditions [10]. Because of the link between T and RH, it is possible that effects attributed to T may be due to concurrent change in RH.
Studies investigating the impact of these meteorological variables divide between those undertaken in chambers with controlled conditions and those in the field where all aspects of meteorology are varying. In both cases, multiple biases may be impacting simultaneously on PDT uptake rate. In addition, field studies are always limited by the absence of meteorological measurements immediately adjacent to each PDT entrance.
Chamber Studies
In a study by the UK National Physical Laboratory [35] seven designs of Palmes-type PDT (in replicate sets of six) were exposed for 28 days to different wind speeds in a controlled atmosphere test facility containing traceable concentrations of NO2, NO and water vapour. Temperature and RH were fixed at 20 °C and 80%, respectively. Of the seven types of PDT investigated, three were supplied by Gradko International (www.gradko.com) and four by ESG (now SOCOTEC, www.socotec.co.uk). One set from each manufacturer was exposed with an open end as normal; for the other sets either a mesh or membrane was put across the open end of the tube.
The absorbent grids in all samplers were prepared using 20% TEA in water, but it is not specified whether this was by a dipping or pipetting approach. The exposure chamber RH of 80% is sufficiently high that, according to the work of Poddubny and Yushketova [34], stoichiometric conversion of NO2 to NO2− is anticipated. Experiments were carried out for NO2 concentrations of 40, 60, 80 and 100 μg m−3 and at wind speeds in the range 0.5 to 2 m s−1 for the experiments with 40 μg m−3 NO2. The chamber also contained NO at the same mixing ratio as the NO2, but no O3. The absence of O3 means there is no within-tube chemical generation of additional NO2 from oxidation of NO.
No significant difference in NO2 uptake rate with wind speed was observed, even for the open tubes, which is in contrast to a number of earlier studies (both chamber and field, discussed below), although the non-turbulent nature of the wind flow in this study may have led to different outcomes compared with situations with variable and turbulent wind flow.
However, there were important differences in measured uptake rates between the tube designs and between measured and theoretically-calculated uptake rates. The measured uptake rate was calculated as the mean of the measurements across NO2 concentrations and wind speeds of that design. The theoretical uptake rate (AD/L) was calculated assuming the electrical resistance analogue that the total diffusion path resistance is the sum of diffusion path resistances in series (Equation (A8)).
For open tubes there is only the one main diffusion path length L to consider. For tubes fitted with meshes or membranes, two additional diffusion paths were also included: that associated with the thickness and pore area of the mesh itself, plus the small extra length by which the annular cap holding the mesh extended beyond the mesh. An uptake rate was not calculated for the tube with the polyethylene membrane since the total transmission area of the pores in the membrane was not known.
The theoretical uptake rate of 7.1 × 10−5 m3 h−1 presented by Martin et al. [35] for the open PDTs corresponds to an uptake rate of 1.18 cm3 min−1 or 0.0197 cm3 s−1. Since uptake rate corresponds to AD/L, applying L = 7.1 cm and A = 0.91 cm2 means these researchers were using a value for D of 0.154 cm2 s−1 for their chamber temperature of 20 °C, which is consistent with current recommendations [14].
The chamber data reported by Martin et al. [35] showed a measured uptake rate for the open Gradko and open ESG tubes that are 26% and 31% higher, respectively, than the theoretical uptake rate, even for the lowest wind speed of 0.5 m s−1 investigated (although, strictly speaking, in both cases the biases were not statistically significant because the confidence intervals for the measured and theoretical uptake rates overlap.) The higher uptake rate measured in practice means that application of the standard PDT equation (Equation (1)) leads to the equivalent positive bias on derived ambient NO2 concentration. The authors do not comment on this point.
The use of a very coarse mesh across the inlet (whose transmission area was 0.9 the tube cross-sectional area) still yielded a measured uptake rate substantially greater than the theoretical uptake rate (i.e., to an apparent positive bias in derived NO2 concentration compared with using the standard equation). However, where medium- or fine-weave polytetrafluoroethene (PTFE) or steel meshes were used, whose transmission areas were in the range 0.23–0.31 of the tube cross-sectional areas, the measured uptake rate was much closer to the theoretical uptake rate, which are themselves not much lower than the theoretical uptake rate for an open-ended tube. The use of the polyethylene membrane yielded a measured uptake rate lower than the normal open-ended tube theoretical uptake rate, as expected, since the membrane impedes the molecular diffusion.
Overall, the chamber data of Martin et al. [35] indicate that even at the lowest wind speed investigated (0.5 m s−1 (= 1.8 km h−1)) there is a positive bias in PDT measurement compared with that expected from the standard theoretical uptake rate, and that this can be substantially negated with meshes having a transmission area ratio of ~0.3 across the open end.
Plaisance [36] also used an exposure chamber to determine the effects of wind speed in the range 0 to 3.0 m s−1 on uptake by six diffusive samplers: a Palmes tube, a PASSAM tube (www.passam.ch), a badge with diffusion membrane, the EMD sampler (Ecole des Mines, Douai, France) and two radial diffusive samplers. Sensitivity to temperature and RH was not investigated. For all diffusive samplers tested, an increase in uptake rate was observed with increased air velocity usually following a logarithmic function, as shown in Figure A1, for three of the samplers tested. The two tube-type samplers were particularly affected by wind. According to Figure A1, the uptake rate for the PDT increases by a factor of two between the lowest wind speeds of ~0.1 m s−1 and wind speeds of 2 m s−1. Variation with wind speed for samplers equipped with a diffusion membrane was much lower (the latter data are not in Figure A1). This is consistent with Gerboles et al. [37] who demonstrated that fitting membranes to diffusion tubes removed their sensitivity to wind speed in both chamber and field experiments. Gerboles et al. [37] subsequently fitted an algorithm involving multiple environmental parameters to model the uptake rate of their membrane-closed Palmes tube, but this is not practical for normal PDT deployments which do not have the detailed measurements required.
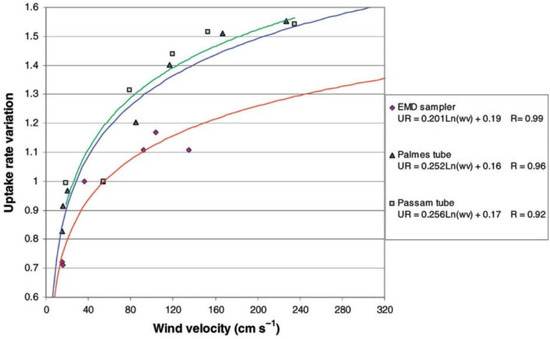
Figure A1.
Uptake rates as a function of wind velocity for three types of NO2 passive samplers in a controlled chamber at 20 °C, 40–50% relative humidity (RH) and 200 μg m−3 NO2 concentration. The uptake rates are expressed relative to that measured for a wind speed of 0.50 m s−1. Figure reproduced from Plaisance [36] with permission of Taylor and Francis. (EMD stands for Ecole des Mines.)
Although not discussed by Plaisance [36], Figure A1 shows a rapid increase in uptake rate for the Palmes PDT at the lowest wind speeds tested. The paper reports that the controlled chamber conditions gave an uptake rate for the standard Palmes PDT of 1.39 ± 0.07 cm3 min−1 (equivalent to 8.34 × 10−5 m3 h−1) for 24 h for conditions of 40–50% RH, 20°C, wind velocity of 0.50 m s−1 and NO2 concentration of 200 μg m−3. This is the uptake rate against which other uptake rates are normalised in the figure. Applying the tube dimensions of L = 7.116 cm and internal diameter = 1.091 cm (i.e., A = 0.9348 cm2) given in Plaisance [36], and a value of D = 0.154 cm2 s−1 appropriate for 293 K, yields a theoretical uptake rate (AD/L) of 1.21 cm3 min−1 (or 7.28 × 10−5 m3 h−1), which is only 0.873 of the measured uptake rate. This is approximately consistent with the Palmes PDT uptake rate shown for the lowest wind speeds in Figure A1. The greater-than-theoretical measured uptake rate at 0.5 m s−1 in this study means that application of the standard (theoretical) equation to the amount of captured nitrite would lead to a 15% positive bias in derived ambient NO2 concentration under the given conditions. As with the study by Martin et al. [35], Plaisance [36] does not discuss this significant discrepancy between measured and theoretical uptake rates. It can be speculated that either there is wind effect even at the wind speed of 0.50 m s−1, as is also inferred from the study of Martin et al. [35], and/or the value of diffusion coefficient being used is too small. The positive bias between measured and theoretical uptake rates for PDTs is 26% in Martin et al. [35] and 15% in Plaisance [36]. However, the former chamber experiments were conducted at 80% RH, but the latter only at 40–50% RH. The 80% RH is sufficiently high that, as per the work of Poddubny and Yushketova [34], stoichiometric conversion of NO2 to NO2− is anticipated. It is therefore possible that the lower positive bias in Plaisance [36] is the net outcome of positive bias from greater-than-theoretical uptake rate partially offset by negative bias from less-than-stoichiometric conversion of NO2 to NO2−.
In another chamber study, Sekine et al. [38] report on a sampler design comprising a 13 mm diameter Whatman no. 1 filter prepared by dipping into 10% v/v TEA in acetone, placed immediately behind a 13 mm diameter polyethylene membrane filter. Two kinds of polyethylene filter were used: one of thickness 0.75 mm and average pore size 54 μm, and one of thickness 1.0 mm and average pore size 43 μm. Although this sampler is not of tube design, the relevance to this review is that the authors include data on the effect of wind on uptake of their sampler and of a Palmes PDT. They report that their membranes gave constant mass transfer rates for wind speeds in the range 0.5–2 m s−1, and also reproduce data cited to a grouping of the same authors that the uptake rate of Palmes tubes increases from their lowest tested wind speed of 0.2 m s−1 to reach an over-read of around 50%, even at low wind speeds of <1.5 m s−1. However, these were measurements in a small chamber for less than 24 h. No other information or published sources of these data are available.
It is worth re-examining some of the earlier studies exposing PDTs in controlled environmental chambers in light of the interpretations of the more recent chamber studies discussed above. Plaisance et al. [39] exposed a total of 86 Palmes tubes to a gas stream with NO2 concentration of 200 ± 20 μg m−3 at various conditions: wind velocities ranging from 0.15–2.3 m s−1, temperatures from 2–40 °C and RHs from 20–85%. Tubes were prepared by pipetting on 30 μL of a 10% solution of TEA in water. The main observation was a strong increase of sampling rate with increasing wind velocity, as shown in Figure A2. Applying the stated PDT dimensions of L = 7.116 cm and internal diameter = 1.091 cm (i.e., A = 0.9348 cm2), and using a value of D = 0.154 cm2 s−1 relevant for 293 K, yields a theoretical uptake rate (AD/L) of 1.21 cm3 min−1 (or 72.8 cm3 h−1), marked as the horizontal dotted line on the figure. The measured uptake rate matches the theoretical uptake rate for the lowest wind speeds of 0.1–0.2 m s−1 tested. The wind effect causes a logarithmic-shaped increase in uptake rate, reaching a positive bias of nearly 50% for wind speed of 2 m s−1. The authors attributed this positive bias to the formation of eddies at the open end of the tube inducing a reduction in the effective length of diffusion.
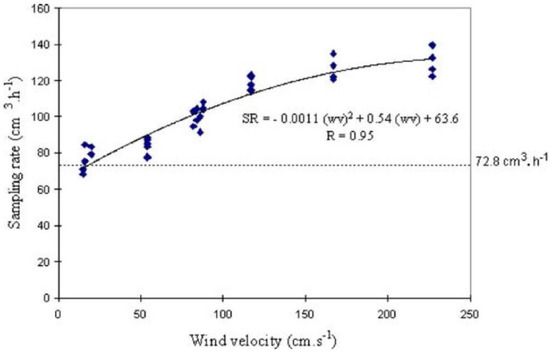
Figure A2.
Sampling rate (SR) as a function of wind velocity measured for Palmes PDTs exposed for 24 h in a chamber at 20 ± 1 °C, 50 ± 3% RH and 200 ± 20 μg m−3 NO2 concentration. Figure reproduced from Plaisance et al. [39] with permission of Elsevier.
In contrast to their results for wind speed, Plaisance et al. [39] concluded that temperature and RH had smaller influences on uptake rate, “exceeding 10% only under unusual conditions (T > 30 °C and RH > 80%)”. The authors plotted isocurves for sampling rate variation as a function of T and RH, as shown in Figure A3. The VSR (variability of sampling rate) variable in the figure is the ratio of the measured sampling rate at a given T and RH combination relative to the sampling rate at 20 °C and 50% RH, for a wind speed of 0.54 m s−1. (Note that according to Figure A2 this value of wind speed may itself be leading to a positive bias in uptake rate of ~10%.) The Plaisance et al. [39] results in Figure A3 show that PDT uptake rate decreases with decreasing RH, which is consistent with the work of Poddubny and Yushketova [34] that lower RHs give lower conversion of NO2 to NO2−. At a temperature of ~15 °C the uptake rate decreases by about 5%, between an RH of 80% and an RH of 20%. The decrease in uptake rate with decreasing RH is more marked at higher temperatures. Whilst these decreases in uptake rate with decreasing RH are lower than predicted by Poddubny and Yushketova [34], they are consistent in direction. Figure A3 also show that sampling rate increases by about 5% for each 10 °C increase in temperature.
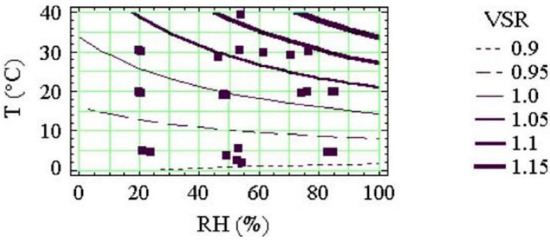
Figure A3.
Variability of sampling rate (VSR) as a function of temperature and relative humidity for Palmes PDTs exposed for 24 h in a chamber at a wind speed of 0.54 m s−1. VSR values are expressed relative to the sampling rate at T = 20 °C and RH = 50%. Figure reproduced from Plaisance et al. [39] with permission of Elsevier.
Buzica et al. [40] exposed standard Palmes PDTs, prepared with 40 μL of 10% v/v TEA in water, for two-week periods in a controlled laboratory chamber. Combinations of the following two levels per environmental condition were trialled: wind speed = 0.8 or 3.6 m s−1; T = 15.5 or 30.5 °C; RH = 29.9% or 72.5%; NO2 concentration = 21.1 or 70.2 ppb. The experimental design was not full factorial (16 different sets of conditions were used). The authors developed the following empirical expression for the measured variation of the uptake rate as a function of T, RH and wind speed (w).
U (ng ppb−1 min−1) = 7.40 × 10−4 + 2.72 × 10−5 T (°C) + 1.43 × 10−5 RH (%) + 5.81 × 10−4 w (m s−1)
Applying the Palmes PDT physical dimensions given in the paper (L = 10.94 mm, i.d. = 10.92 mm), and using a value for D of 0.154 cm2 s−1 (for T = 293 K) for comparison with the chamber studies of Plaisance et al. [39] and Plaisance [36], the theoretical PDT uptake rate (AD/L) = 0.02033 cm3 s−1 or 1.220 cm3 min−1. To convert this uptake into the units of ng ppb−1 min−1 used by Buzica et al. [40] in their empirical expression requires calculating the mass concentration of 1 ppb NO2 at the same temperature:
1 ppb NO2 = (10−9 × 1.01325 × 105)/(8.314 × 293) = 4.159 × 10−8 mol m−3 = 1.913 × 10−3 ng cm−3
∴ Theoretical Palmes PDT uptake rate, U = 1.220 cm3 min−1 × 1.913 × 10−3 ng cm−3 ppb−1 = 2.33 × 10−3 ng ppb−1 min−1
Under the diffusion-only conditions of no effect of wind speed (w = 0 m s−1), and T = 293 K and RH = 50%, the Buzica et al. [40] empirical expression gives an uptake rate of 2.00 × 10−3 ng ppb−1 min−1 which is about 14% lower than the theoretical calculated uptake rate of 2.33 × 10−3 ng ppb−1 min−1 under these conditions. For w = 0 m s−1, and for T = 284 K and RH = 78% which are more realistic T and RH conditions for the UK, the Buzica et al. [40] empirical expression gives an uptake rate of 2.15 × 10−3 ng ppb−1 min−1, which is only about 6% lower than the theoretical calculated uptake rate of 2.28 × 10−3 ng ppb−1 min−1 at this temperature.
However, the Buzica et al. [40] empirical expression incorporates strong sensitivity of uptake rate to changes in the three meteorological variables, particularly wind speed. Thus, for example, for T = 11 °C and RH = 78%, an increase in average wind speed from 0 to 3 m s−1 increases U from 2.15 × 10−3 (slightly below the theoretical uptake rate) to 3.90 × 10−3 ng ppb−1 min−1 (71% greater than the theoretical uptake rate), an 81% increase in uptake across this wind-speed range. Similarly, at an RH of 78% and wind speed of 1 m s−1, an increase in average T from 0 to 20 °C increases U from 2.44 × 10−3 to 2.98 × 10−3 ng ppb−1 min−1, a 22% increase in uptake across this temperature range, whilst at a T of 11°C and wind speed of 1 m s−1, an increase in RH from 45% to 95% increases U from 2.26 × 10−3 to 2.98 × 10−3 ng ppb−1 min−1, a 32% increase in uptake across this RH range. Overall, therefore, the Buzica et al. [40] empirical expression generally predicts a higher uptake value for anticipated average ambient meteorological conditions than the theoretical value even at relatively low wind speeds. Thus, if average meteorological conditions were T = 11°C, RH = 78%, w = 1 m s−1, the expression predicts U = 2.73 × 10−3 ng ppb−1 min−1, ~20% higher than the zero-wind theoretical value of 2.28 × 10−3 ng ppb−1 min−1.
The magnitudes of the Buzica et al. [40] empirically-derived changes in uptake rate with temperature, RH and wind speed are broadly consistent with those reported in the separate exposure chamber study of the same parameters by Plaisance et al. [39]. At T = 20°C and RH = 50%, Plaisance et al. [39] report an increase in uptake rate of 75% for an increase in wind speed from 0.2 to 2.2 m s−1. The Buzica et al. [40] expression predicts an increase in uptake rate of 55% for these conditions (from 2.11 × 10−3 to 3.28 × 10−3 ng ppb−1 min−1). For a change in temperature from 0 to 20 °C (at RH = 75%), Plaisance et al. [39] report an increase in uptake rate of 15% (compared with the 23% increase in uptake over this temperature range predicted by the Buzica et al. [40] expression), and for a change in RH from 45% to 95% (at T = 11 °C), Plaisance et al. [39] report an increase in uptake rate of 2% (compared with the 32% increase in uptake over this RH range predicted by the Buzica et al. [40] expression). Thus, the Plaisance et al. [39] work indicates a stronger effect of wind speed on sampling rate than the Buzica et al. [40] work, but a lower effect of temperature and much lower effect of RH on sampling rate. However, the increase of uptake with wind speed of both these earlier chamber studies is in contrast to the chamber study of Martin et al. [35], which observed no change in uptake with wind speed (although this does not mean that wind did not have any impact, only that there was invariance across the 0.5–2 m s−1 of wind speed that Martin et al. [35] investigated).
Overall, both Buzica et al. [40] and Plaisance et al. [39] show the effective uptake rate decreasing with decreasing RH, which is consistent with the work of Poddubny and Yushketova [34] that lower RHs give lower conversion of NO2 to NO2−; and both these earlier chamber studies imply that uptake rate, even at low wind speeds, is higher than theoretical calculations, of the order of ~20% or more (at high RHs). Retrospectively it can be seen that there is consistency between these older studies and the more recent chamber studies of Plaisance [36] and Martin et al. [35], and that all appear to show measured uptake rates of the order of 26–31% greater than theoretical uptake rate, even at low wind speed (even though the latter study did not also show an increase in uptake rate with increasing wind speed). One explanation for inconsistency between measured and theoretical uptake rates could be that an erroneous value of D is being used to calculate the latter (see Appendix A.4).
Gerboles et al. [41] carried out laboratory tests on different types of NO2 diffusive samplers in an exposure chamber under extreme conditions of controlling factors according to EN 13528-2, and showed that most of the NO2 samplers were affected by extreme exposure conditions. The majority of diffusive samplers overestimated the reference value when NO2 concentration, temperature, humidity and wind speed were set to their highest levels of, respectively, 80 μg m−3 NO2, 25 °C, 75% RH and 2.5 m s−1 wind speed. The reference value was underestimated by diffusive samplers when these parameters were set to their lowest levels (40 μg m−3 NO2, 5 °C, 30% RH and 1.0 m s−1 wind speed). The findings of this study are again consistent with generally strong positive bias due to wind speed and negative bias at low relative humidities. The influence of temperature cannot be isolated from the influences of the other variables from the data presented. In an earlier chamber study, Gerboles et al. [37] reported the effect of temperature on uptake was ±5% between 15 and 30 °C, which is consistent with the conclusion from the other chamber studies described above that the influence of temperature is relatively small.
De Santis et al. [42] reported some limited experiments with a lab chamber to test effects of wind on both standard Palmes PDTs, and ones with a stainless-steel mesh across the open end. They report that the protective mesh substantially attenuated the effect of wind turbulence in their test range of 2–4 m s−1: at maximum wind speed the NO2 overestimation was 48 ± 3% and 7 ± 0.8% for the tubes without and with the stainless-steel mesh, respectively. However, no data to support these statements are presented and many experimental details are lacking, including the nature of the mesh used and, importantly, the humidity and temperature of the air in their chamber experiments.
Field Studies
The different PDT designs used in the controlled chamber study of Martin et al. [35], described in detail above, were also exposed for between six and eight four-week exposures at a central London location (which is not identified but is presumed to be Marylebone Road). There is very little discussion of the field comparison data in the paper, and the discussion focuses on correlation coefficients and PDT precision, both of which are generally better for the PDT designs with meshes. It is not possible to get a full view of the extent of the agreement between PDT measurements and reference analyser concentrations. The gradients forced through the origin are in the range 0.90 to 0.98 across the PDT designs, but the measured NO2 concentrations are high (all > 60 μg m−3), which may lead to a distortion of the view of absolute PDT agreements. In general, however, the field data suggest the PDTs yield slightly lower NO2 concentrations than the analyser, in contrast to their chamber tests that suggest a general positive bias in PDT performance. There is no comment in the paper on this discrepancy between chamber and field outcomes. It can be speculated that perhaps this is associated with long-term degradation of trapped NO2− in field conditions (see Appendix A.3.6).
Masey et al. [43] assessed the precision and accuracy of NO2 concentrations measured with standard Palmes PDTs (as well as other variants of passive sampler) over 32 separate two-day, three-day and seven-day exposure periods at an urban background site in Glasgow. PDTs were prepared by dipping grids in 50% TEA in acetone, and analysis followed the Defra WG [14] procedures. The authors noted that uptake rates for PDT measurements linearly increased with wind speed. The positive bias ranged from around zero for exposure-mean wind speeds <~2 m s−1 to about 100% for exposure-mean wind speeds of ~8 m s−1. The wind data were for Glasgow airport several km away from the PDT exposures in the centre of Glasgow. The average positive bias compared with the theoretical uptake rate for a PDT in the Masey et al. [43] study was ~25% across the 32 exposures of exposure-mean wind speeds ranging from ~1 m s−1 to ~8 m s−1. The magnitude of this mean bias is similar to that reported by Martin et al. [35] from their chamber experiments, but Martin et al. [35] did not observe variation in their bias across their tested wind speed range of 0.5–2 m s−1, whilst Masey et al. [43] report substantial variation with wind speed.
Sánchez Jiménez et al. [44] deployed NO2 PDTs adjacent to chemiluminescence analysers for twelve onw-week exposures at kerbside, urban centre and urban background locations in Glasgow as part of a study to evaluate performance of NOx and NO2 PDTs. The NO2 PDTs were prepared (by dipping in 50% v/v TEA in acetone solution) and analysed according to the UK recommendations [14]. For the PDT exposures at the urban centre location, where hourly mean concentrations of O3 were also available, it was possible to model the anticipated positive bias in PDT NO2 concentration due to within-tube reaction between NO and O3 also present in the ambient air during an individual exposure, as per Heal and Cape [45] and Heal et al. [46].
There was significant positive bias between PDT and analyser NO2 at the kerbside and urban centre locations, but duplicate PDT precision was good and correlation between PDT and analyser across the 12 exposures at all three sites extremely high (R ranging from 0.87 to 0.96), indicating that bias was systematic. Mean positive bias at the urban background site was a further ~50% greater than the mean bias of 28% simulated for within-tube NO + O3 reaction. The authors attributed this to additional wind-induced positive bias at this site, supported by the observation that the co-located NOx PDT tubes (which will not be subject to within-tube chemical bias because the reaction converts between NO and NO2) also had a positive bias of around 50%. Allowing for the possibility also of an exposure-duration-dependent negative bias over one-week of ~8%, based on earlier work [47], the authors concluded that wind bias at this site could have been as high as 55–60%. They noted that these particular tubes were deployed in an exposed location, mounted at 3 m height without shelter on an air intake duct in the middle of a relatively open square, in one of the UK’s windiest urban areas.
Although simulation of chemical overestimation bias was not possible at the kerbside location, based on unpublished simulations at other urban locations, Sánchez Jiménez et al. [44] estimated that chemical overestimation at this high NOx (thus low O3) site would likely be comparatively small, ~5–10%. The excess PDT NO2 positive bias of ~45% at this site the authors again attributed to wind effects, noting that the kerbside location of these samplers was subject to considerable air turbulence from the high density of bus and other traffic continually passing within a metre or two of the PDTs.
Sánchez Jiménez et al. [44] further estimated that the chemical overestimation for their urban background site would likely be approximately the same magnitude as the potential exposure-dependent negative bias. The generally comparable PDT and analyser NO2 values at this location therefore indicated a lack of any significant wind-induced bias at this site, consistent with the location of the PDTs in a sheltered second-floor window recess not subject to wind or to air turbulence from passing traffic.
Vardoulakis et al. [25] evaluated the performance of co-located NO2, NOx and O3 passive diffusion tubes relative to their respective continuous analyser measurements in just over one year of monthly exposures at one urban background and two roadside sites in Birmingham, UK. All PDT measurements were in triplicate. Wind speed and direction, ambient temperature and relative humidity were also available, but for another location. PDTs were prepared via pipetting 30 μL of 50% TEA in water. Tubes were exposed for either four weeks or occasionally five weeks, and no protective shelter was used. To convert NO2− to ambient NO2 concentration, the authors used an uptake rate (AD/L) of 74.2 × 10−6 m3 h−1, which is equivalent to 1.24 cm3 min−1. Taking the authors’ stated tube dimensions of L = 71 mm and internal diameter 11.0 mm, this uptake rate implies the authors were using a value for D of 0.154 cm2 s−1, which is about 2% greater than the value recommended by the Defra WG [14] for UK PDT data reporting to EU standard conditions and, hence, their reported PDT values will be 2% greater than they ought to be. These authors reported no significant difference between NO2 PDT and chemiluminescence analyser, with PDTs reading lower on an annual-average basis by 3.2–6.4% across the three sites (plus a further 2% underestimated because of the authors’ use of too high a value for D). A very slightly increasing NO2 bias with increasing exposure-average O3 concentration was attributed to within-tube NO + O3 reaction. The authors reported no significant trend of NO2 bias with wind speed (which ranged from 2.7–4.7 m s−1), temperature (which ranged from 4–20 °C) or RH (which ranged from 65–90%), although they acknowledge that meteorological measurements were made some distance from the sampling sites, as also in the Masey et al. [43] study, which could mean that wind speed in particular was different at the PDT locations.
As part of a laboratory and field validation of a combined NO2–SO2 Radiello radial-type diffusive sampler, Swaans et al. [48] also exposed Gradko Palmes-type combined NO2–SO2 PDTs adjacent to reference analysers for three two-week exposures in Ghent and Borgerhout, Belgium. Two sets of triplicates were deployed, with one set sent to each of Gradko and VITO to analyse. PDTs were prepared with 50% TEA in water and contained a filter across the open end to prevent ingress of particles. The Gradko samplers significantly under-read the analyser, with ratio of sampler:analyser ranging from 0.6 to 1 for individual exposures and averaging around 0.8 overall. The authors do not discuss any reason for the negative bias but simply noted that agreement is to within the 30% criteria for accuracy for passive samplers. (N.B. the EU data quality objective for accuracy for NO2 is actually 25%.) It is not possible to draw any conclusions from these data as the number of comparisons is very limited and the PDTs included a filter across the open end (that will likely impact on uptake rate), which is not the case for standard NO2 PDTs.
Ozden and Dogeroglu [49] describe a field evaluation in Eskisehir, Turkey, of a tailor-made glass passive sampler for NO2. This is not a Palmes-type PDT but is similar in concept, and the authors also deployed standard Gradko PDTs. The authors’ sampler was 3.98 cm in length and 1.2 cm in diameter, and contained a fibre filter paper impregnated using 20% v/v TEA in water, which is one of the preparation methods recommended by the Defra WG [14]. They trialled both transparent and dark-coloured glass. Exposures were generally for one-week duration, although the exposures including Gradko PDTs were for two weeks. It is important to note that the authors’ own tubes were exposed with a metal mesh across the open end of the tube and within a shallow rain protection shelter which would also have afforded some protection from the wind. Another important note is that the authors’ reference NO2 data was not from a chemiluminescence analyser but from an actively-sampled Griess-Saltzman ASTM D 1607 standard test method. The authors state the theoretical uptake rate (AD/L) for their sampler is 2.63 cm3 min−1. Taking their stated tube dimensions of L = 3.98 cm and diameter 1.2 cm this uptake rate implies the authors were using a value for D of 0.154 cm2 s−1, which is the value of D appropriate to a temperature of 293 K and may be appropriate for this study’s climate. Their measured uptake rate determined from comparison against their standard method was 2.49 cm3 min−1, a difference of ~6%, which the authors report as good agreement with the theoretical rate. Their data show about 10% greater NO2 from the co-located Gradko PDTs compared with their own shorter tubes. They do not discuss this but it can be speculated that it may be a consequence of the Gradko tubes not having the mesh across their open end and consequently being subject to a wind-induced positive bias. Although not having the mesh, the Gradko tubes were exposed underneath their shallow rain-protection shelter used for their own tubes.
Two further field studies involving custom-built tube-like passive samplers for NO2 provide some information relevant to meteorology-associated biases in field deployments. A study by Bootdee et al. [24] exposed three geometries of tube (a polyethylene tube 5.4 cm long, 1.4 cm i.d.; and two sizes of polypropylene tube, 5.3 cm long, 1.3 cm i.d. and 7.7 cm long, 1.6 cm i.d.) in protective shelters in Chiang Mai, Thailand. The authors report that concentrations of NO2 measured over short-duration exposures (≤7 days) were not significantly different between the tube lengths, which suggests that the use of a protective shelter mitigated any potential bias from wind speed. Tarvydaitė and Kazlauskienė [23] exposed their passive sampler comprising a polypropylene tube 34 mm in length and 21 mm in inner diameter, also in protective shelters, for two-week periods in Vilnius, Lithuania. The authors report that NO2 concentrations were within the permitted EU uncertainty of ±25% and that there was no significant correlations between accuracy and temperature, RH or wind speed. However, their dataset is very small.
Appendix A.3.5. Within-Tube Chemical Generation of Additional NO2
In ambient air, NO and O3 react to produce NO2 which, in daylight hours, photolyses back to NO and to O3, the latter formed by the very fast reaction between the oxygen atom O from the photolysis and O2. The reaction that produces NO2 generally occurs on faster timescales (tens of seconds) than the photolysis reaction (a few minutes) that removes this NO2. In addition, the material from which the PDT is constructed (acrylic or polyethylene) is relatively opaque to the wavelengths of UV light needed for the NO2 photolysis, and during night-time there is no photolysis at all. The consequence of these factors is that additional NO2 can be generated within the PDT from the reaction between NO and O3 compared with the NO2 in the ambient air at the mouth of the tube. There is time for this to be a significant source of additional NO2, because the average time for a molecule to diffuse the length of PDT is around 2.8 min [45] and the chemical loss of the NO and O3 creates a concentration gradient that drives diffusion into the tube for these two species also. Heal and Cape [45] developed a numerical model of the coupled diffusion and chemistry processes within a PDT and showed that the model predicted positive bias of 28% using example hourly values of NO2, NO and O3 from an urban background site in summer in Edinburgh. The model simulations revealed that, as expected, the magnitude of positive bias due to within-tube chemistry varied with the average relative abundances of NO2, NO and O3 at a given location, being greatest when NO and O3 abundances exceeded that of NO2. Thus, estimated positive bias was a lot lower (a few percent) using data from a rural background site where the majority of NOx was already in the form of NO2.
The model overestimations were subsequently compared against real PDT exposures at urban background sites in Edinburgh [46] and Cambridge [47], with average positive biases due to within-tube chemistry across all exposures at each location of 22% and 31%, respectively. The former study also showed that positive bias was lower for PDTs constructed of quartz glass, which transmit wavelengths for NO2 photolysis, compared with standard acrylic PDTs. In a similar experiment using quartz tubes, Kirby et al. [50] concluded there was evidence for presence of within-tube chemical reaction bias. The magnitude of bias increased as the exposure average NO:NO2 ratio increased to ~1 and the exposure average O3:NO2 ratio increased to ~2.
In a study primarily to investigate potential influence of preparation method on PDT performance, Hamilton and Heal [20] reported that for 14 one-week PDT exposures at an urban background location in Edinburgh the mean model-simulated chemical bias was +26%.
The study of Sánchez Jiménez et al. [44] that deployed PDTs at three sites in Glasgow (described in detail above in relation to wind effects) included model-simulated estimates of bias due to chemical overestimation for exposures at the urban centre location. The mean modelled chemical bias was +28% across 12 one-week exposures. Sánchez Jiménez et al. [44] also referred to unpublished model simulations of chemical overestimation bias using hourly measured NO2, NO and O3 data from other locations at which all three species were measured. These model simulations covered a range of relative and absolute NO2, NO and O3 concentrations. The results indicated that positive bias from within-tube chemistry is smaller at roadside (high NOx) sites than at urban background sites because the O3 to NO ratio is lower closer to roadside.
The study by Vardoulakis et al. [25] for a year of monthly deployments in Birmingham (also described in detail above) did not explicitly simulate chemical overestimation; however, the authors speculated that the slightly smaller underestimation they observed at their roadside sites compared with at their urban background site could be due to additional NO + O3 reaction, which they supported by referring to the very slightly increasing NO2 bias with increasing exposure-average O3 concentration.
An analysis by Laxen and Marner [51] of bias in PDTs with distance from road utilising UK local authority PDT/reference analyser co-location data drew the same conclusion. They examined the results of 252 long-term co-location studies at roadside and background sites across the UK between 2000 and 2005. Results were compared with a model of in-tube chemistry developed by Bush et al. [52] and showed broad agreement, with the model showing higher bias at intermediate concentrations of NOx and lower relative bias at higher and lower concentrations (near source and away from source, respectively).
Appendix A.3.6. Exposure-Duration “Loss” of Trapped NO2−
Evidence for an exposure-duration-associated negative bias in PDT determination of NO2 is derived from co-located comparisons of concentrations derived from a single long-duration exposure (e.g., of four weeks, versus the average of consecutive co-located one-week or two-week exposures [46,47]) and from differences in these comparisons between summer and winter seasons. These studies very approximately estimate a “loss” rate for absorbed NO2− of a few percent (e.g., 5–8%) per week, speculatively ascribed to (photo)chemical reaction of the NO2−.
The study by Vardoulakis et al. [25] for a year of monthly deployments in Birmingham (described in detail above) concluded from a comparison of four- and five-week exposures that photolysis, or other exposure-duration-dependent losses, at the absorbent was not a factor. However, the authors had only few data to make this evaluation of exposure duration and were only comparing between exposures differing in duration by a small proportion.
The study of Ozden and Dogeroglu [49] using custom built samplers (also described in more detail above) trialled dark-coloured glass samplers alongside their transparent glass samplers. The dark-coloured glass samplers gave about 25% higher reading than their transparent samplers for exposures from spring to mid-summer. The difference was only 6% for samplers exposed during winter. The authors attributed this to possible photodegradation of NO2−–TEA complex during the summer season. However, it is not clear from the data presented whether it is the transparent or dark coloured tubes that yield the NO2 concentrations that are consistent with their standard method for NO2 (i.e., whether dark tubes had net positive bias, or transparent tubes had a net negative bias). The authors do not pick up on this point.
De Santis et al. [42] investigated the stability of the NO2−–TEA adduct as determined by the proportion of nitrite extracted after increasing lengths of storage time, including at room temperature and in a fridge, and with or without shielding with aluminium foil. They reported decreases in extractable NO2− that ranged from almost no decrease up to ~20% after a month or two of storage, with storage in ambient T and light conditions yielding greater loss. The authors attributed this decrease to photodegradation of the NO2−–TEA adduct.
Appendix A.4. Bias in Calculation of Average NO2 from the Quantified NO2−: Uncertainty in the Value of the NO2 Diffusion Coefficient
The diffusion coefficient is the only variable in Equation (1) with potential to contribute significant bias to the exposure-average NO2 concentration calculated from the mass of absorbed NO2−. The value of D originally recommended by Palmes et al. [9] was 0.154 cm2 s−1, for a temperature of 293 K. The diffusion coefficient for NO2 is very difficult to measure directly because of its dimerization to N2O4 at concentrations at which experiments need to be conducted. (The dimerization is not important at the very low NO2 abundances in ambient air.) Palmes et al. [9] derived their value for D using a semi-empirical gas-theory expression incorporating molecular masses, molecular cross-sections and strength of molecular interactions. The Palmes et al. value of D has been used in all subsequent PDT measurements (albeit sometimes with a temperature correction—see below), seemingly without further question.
In a review of molecular diffusivities, Massman [53] refers only to measurements made by Chambers and Sherwood [54] for N2O4 in N2 (quoting an experimental value for NO2 of 0.121 cm2 s−1 at 273 K), and to measurements by Sviridenko et al. [55]. Massman [53] used these data and further gas-kinetic theoretical considerations to derive his recommendation of D = 0.1361 cm2 s−1 for NO2 in air at 273 K, with a T-dependence factor of 1.81 (for temperatures in kelvin), which yields a value for D at 293 K of 0.154 cm2 s−1, the same as the Palmes et al. [9] value. Massman [53] provided a nominal estimation of uncertainty of ±10% in his recommended value, but the text also states that the “results suggest that the diffusivity of NO2 is relatively uncertain and that experiments should be repeated.”
The UK NO2 PDT working group [14] used the temperature-dependence for D reported by Massman [53] to recommend that from 2009 the value for UK PDT measurements be changed to D = 0.146 cm2 s−1, which assumes the average ambient temperature during sampling is 284 K, more realistic for the UK. The Defra WG also noted that, since the EU reporting temperature for mass concentration values is 293 K, when PDT data are to be compared with air quality objectives or reference chemiluminescence analyser data, the PDT values need to be decreased by a factor 284/293 = 0.969.
In a more recent evaluation of diffusivities of trace gases, Tang et al. [56] refer to the Chambers and Sherwood [54] measurements as the only experimental data for NO2, also quoting, as does Massman [53], an experimental value of 0.121 cm2 s−1 at 273 K. They also quote an experimental value from Chambers and Sherwood [54] of 0.129 cm2 s−1 at 283 K. Although the relative magnitudes of these two experimental values are consistent with a T-dependence factor of 1.75 quoted by Tang et al. [56] for the “Fuller” semi-empirical estimation method for gas-phase diffusion coefficients [57,58], the absolute values are substantially smaller than the value of D = 0.163 cm2 s−1 at 273 K that these authors derive from the Fuller estimation method. The recommendation of Tang et al. [56] is to use the experimental value, with a ± uncertainty of 35%. These authors appear not to be aware of the earlier review by Massman [53].
No other independent value for D for NO2 has been uncovered in the literature. The following summarises all available values for D at 284 K, the temperature used as the average for the UK.
| Experimental value from Chambers and Sherwood: | 0.129 cm2 s−1; |
| Palmes et al. (using Massman T dependence): | 0.146 cm2 s−1; |
| Massman recommendation (using Massman T dependence): | 0.148 ± 0.015 cm2 s−1; |
| Tang et al. recommendation (using “Fuller” T dependence): | 0.130 ± 0.045 cm2 s−1; |
| Tang et al. “Fuller” estimate (using “Fuller” T dependence): | 0.175 cm2 s−1. |
These reported values and uncertainties span a substantial range. The one experimental value (from 1937) is a factor 0.89 of the Palmes value at 284 K; and, whilst semi-empirical methods for estimation of gas diffusion coefficients are well-established, the more recent calculated value from Tang et al. [56] is a factor 1.20 of the Palmes value (although these authors actually recommend using the value that is 0.89 the Palmes value).
In principle, the value of D can be back-calculated from measurements of the amount of NO2− captured in PDT exposures in controlled chamber experiments, assuming that the conditions for Fick’s law of diffusion hold perfectly. As discussed in earlier sections of this review, some chamber studies have yielded PDT uptake rates (= AD/L) that are higher than the theoretical uptake rate derived using the Palmes value of D. The interpretation of higher-than-theoretical uptake rates would disappear if D was actually greater than the standard Palmes value used. However, it is difficult even in chamber experiments to exclude, or quantitatively correct for, factors that break the assumption of Fick’s law (e.g., non-diffusive air movements) or that affect NO2− capture and quantification (e.g., humidity levels, uncertainties in laboratory analyses).
If the true value of D was larger than the Palmes value currently used then NO2 concentrations currently calculated from PDT measurements are positively biased with respect to the true NO2 concentrations, and vice versa.
The above review indicates that there should be much greater acknowledgement that the value for D is not known with certainty, and particularly that its value is not known to the precision implied by use of a value for D expressed to three significant figures. The Tang et al. [56] evaluation suggests an uncertainty in D spanning several tens of percent. This does not mean random variability across individual PDT exposures in the range of several tens of percent because D must have a single true value (at a given T); instead it means that collectively all PDT-derived NO2 values may be a certain (currently unknown) percentage too high or a certain percentage too low. It is important to note, however, that this particular potential source of PDT bias is not an issue for PDTs that are “bias adjusted” against a chemiluminescence analyser, since if this was the only source of PDT bias at all PDT exposure locations, including the co-location, then it would be accounted for through the bias adjustment factor.
Appendix A.5. Bias in Comparison of PDT NO2 with Chemiluminescence Analyser NO2
Bias in PDT measurement is assessed by its comparison against a reference chemiluminescence analyser determination of NO2. The EC Air Quality Directive permits up ±15% overall uncertainty in a chemiluminescence concentration [12].
When comparing PDT and analyser NO2 concentrations expressed gravimetrically (e.g., as μg m−3 units), rather than as volumetric mixing ratios (e.g., ppb), it is important to ensure that both concentrations are being expressed relative to the same values of pressure and temperature. For PDT values calculated using the Defra WG [14] recommended value for D, which assumes an average ambient temperature of 284 K, the PDT values then need to be decreased by a factor of 284/293 = 0.969 to compare against a chemiluminescence analyser that has been set up to report NO2 concentrations referenced to the EU reporting temperature of 293 K. Failure to make this adjustment means the PDT-derived value in the comparison is too high, although only by ~3%.
The chemiluminescence analyser does not determine NO2 directly, but as the difference between successive measurements of NO and NOx, where, for the latter determination, the NO2 is first quantitatively converted to NO using either a heated molybdenum oxide catalyst or UV photolysis. The chemiluminescence analysers are potentially subject to their own biases in NO2 determination: (i) negative bias from incomplete conversion of NO2 to NO; and (ii) positive bias from NO produced in the converter from other N-containing oxidised species in the air such as HNO3, HONO and PAN, collectively referred to as NOz. The photolytic converter is far more specific in its conversion of only NO2 to NO. However, the vast majority of analysers in the UK utilise the thermal converter.
Gerboles et al. [59] applied the methods of the Guide to the Expression of Uncertainty in Measurement [60] to assess the ability of the chemiluminescence method to measure ambient NO2, with an accuracy within 15%, as stipulated in the EU data quality objective [12]. They reported that the contribution of accuracy of calibration standard, linearity, converter efficiency and drift of the analyser between calibration checks to the overall uncertainty is less important than the contribution of interference, mainly humidity and PAN in rural areas. They assessed that the data quality objective is not met at the annual limit value of 40 μg m−3 if NO is greater than 100 μg m−3, but could be met by correcting the measurements for the bias due to interference.
Steinbacher et al. [61] compared long-term co-located NO2 measurements using analysers with thermal and photolytic converters at two rural sites in Switzerland. On a monthly basis only 70% to 83% of the measured NO2 was due to genuine NO2 at their non-elevated site (and less for a higher elevations site), with greatest discrepancy in spring/summer, consistent with photochemical aging of the rural air mass creating significant relative amounts of NOz species such as HNO3 and PAN.
Xu et al. [62] similarly compared instruments with the two types of converter at four differently polluted sites in China. (The measurement period at each site was only a few weeks and not contemporaneous). The thermal converter worked well at the urban site, which was greatly affected by fresh emissions, but, on average, overestimated NO2 by 30% to 50% at the two suburban sites and by more than 130% at the mountain-top site during afternoon hours, with a much larger positive bias seen during the highest ozone events. The degree of overestimation depended on both air-parcel age and the composition of the NOz species. Additionally, in East Asia, Jung et al. [63] reported that a thermal-converter instrument overestimated NO2 levels, determined by a photolytic-converter instrument, by 20.4 ± 14.7% for a monitoring period of several months at a suburban site in Daejeon, Korea, downwind of the Asian continental outflow.
In the study of Leston and Ollison [64], comparison was made between a thermal-converter instrument and a direct NO2-reading photometer at a “near-road” site (15 m from an interstate carriageway edge) in Hartford, USA. On average, hourly averages from the former instrument were 10% higher than for the latter. The comparison was over winter (late November to mid-March).
In summary, in some types of air mass there is considerable potential for other oxidised N-containing species (NOz) to cause thermal-converter chemiluminescence analysers to over-report NO2, and hence for co-located NO2 PDTs to appear negatively biased. However, if the PDT also quantifies some or all NOz species as absorbed nitrite (see Appendix A.3) then this would offset this bias between PDT and chemiluminescence analyser, but this has not been adequately tested.
References
- WHO. Air Quality Guidelines. Global Update 2005. Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide; World Health Organisation Regional Office for Europe: Copenhagen, Denmark, 2006; ISBN 92-890-2192-6. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/78638/E90038.pdf (accessed on 5 June 2019).
- WHO. Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project: Technical Report; World Health Organisation: Copenhagen, Denmark, 2013; Available online: http://www.euro.who.int/ __data/assets/pdf_file/0004/193108/REVIHAAP-Final-technical-report-final-version.pdf (accessed on 5 June 2019).
- EEA. Air Quality in Europe—2018 Report; EEA Report No 12/2018; European Environment Agency: København, Denmark, 2018; ISSN 1977-8449. Available online: https://www.eea.europa.eu//publications/air-quality-in-europe-2018 (accessed on 5 June 2019).
- Baldasano, J.M.; Valera, E.; Jimenez, P. Air quality data from large cities. Sci. Total Environ. 2003, 307, 141–165. [Google Scholar] [CrossRef]
- Larkin, A.; Geddes, J.A.; Martin, R.V.; Xiao, Q.; Liu, Y.; Marshall, J.D.; Brauer, M.; Hystad, P. Global land use regression model for nitrogen dioxide air pollution. Environ. Sci. Technol. 2017, 51, 6957–6964. [Google Scholar] [CrossRef] [PubMed]
- Cyrys, J.; Eeftens, M.; Heinrich, J.; Ampe, C.; Armengaud, A.; Beelen, R.; Bellander, T.; Beregszaszi, T.; Birk, M.; Cesaroni, G.; et al. Variation of NO2 and NOx concentrations between and within 36 European study areas: Results from the ESCAPE study. Atmos. Environ. 2012, 62, 374–390. [Google Scholar] [CrossRef]
- Lin, C.; Feng, X.; Heal, M.R. Temporal persistence of intra-urban spatial contrasts in ambient NO2, O3 and Ox in Edinburgh, UK. Atmos. Pollut. Res. 2016, 7, 734–741. [Google Scholar] [CrossRef]
- Weissert, L.F.; Salmond, J.A.; Miskell, G.; Alavi-Shoshtari, M.; Williams, D.E. Development of a microscale land use regression model for predicting NO2 concentrations at a heavy trafficked suburban area in Auckland, NZ. Sci. Total Environ. 2018, 619–620, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Palmes, E.D.; Gunnison, A.F.; DiMattio, J.; Tomczyk, C. Personal sampler for nitrogen dioxide. Am. Ind. Hyg. Assoc. J. 1976, 37, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Cape, J.N. The use of passive diffusion tubes for measuring concentrations of nitrogen dioxide in air. Crit. Rev. Anal. Chem. 2009, 39, 289–310. [Google Scholar] [CrossRef]
- COMEAP. Associations of Long-Term Average Nitrogen Dioxide with Mortality; PHE Report No. 2018238; UK Department of Health Committee on the Medical Effects of Air Pollutants: London, UK, 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/734799/COMEAP_NO2_Report.pdf (accessed on 5 June 2019).
- EC Directive. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; European Parliament, Council of the European Union: Bruxelles, Belgium, 2008; Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32008L0050:EN:NOT (accessed on 5 June 2019).
- Hafkenscheid, T.; Fromage-Mariette, A.; Goelen, E.; Hangartner, M.; Pfeffer, U.; Plaisance, H.; De Santis, F.; Saunders, K.; Swaans, W.; Tang, Y.S.; et al. Review of the Application of Diffusive Samplers in the European Union for the Monitoring of Nitrogen Dioxide in Ambient Air; JRC Scientific and Technical Report EUR23793; Office for Official Publications of the European Communities: Luxembourg, 2010; ISBN 978-92-79-12052-7. [Google Scholar]
- Defra Working Group (WG). Diffusion Tubes for Ambient NO2 Monitoring: Practical Guidance for Laboratories and Users. A Report by the Defra Working Group on Harmonisation of Diffusion Tube Methods; Report No. AEAT/ENV/R/2504; AEA Energy & Environment: Didcot, UK, 2008; Available online: www.airquality.co.uk/ archive/reports/cat05/0802141004_NO2_WG_PracticalGuidance_Issue1a.pdf (accessed on 5 June 2019).
- AEAT, Summary Results from the UK NO2 Network Field Intercomparison Exercise 1999; AEA Technology Environment: Culham, UK, 2000. Available online: https://uk-air.defra.gov.uk/assets/documents/reports/empire/no2rep2/no2comp.htm (accessed on 25 June 2019).
- Loader, A.; Mooney, D.; Bush, T. UK Nitrogen Dioxide Network 2000; AEAT/ENV/R/0669; AEA Technology Environment: Culham, UK, 2001. Available online: https://uk-air.defra.gov.uk/assets/documents/reports/empire/no2_report_2000.pdf (accessed on 25 June 2019).
- Loader, A. Investigation of the Effects of Preparation Technique on Performance of Nitrogen Dioxide Diffusion Tubes; Report No. AEAT/ENV/R/0563; AEA Technology: Harwell, UK, 2001. [Google Scholar]
- Laxen, D.H.P.; Wilson, P. Compilation of diffusion tube collocation studies carried out by local authorities. In UK Air Quality Archive; Air Quality Consultants: Bristol, UK, 2002; Available online: http://www.airquality.co.uk/archive/reports/cat06/NO2DiffusionTubePerformance(Final).pdf (accessed on 5 June 2019).
- Kirby, C.; Fox, M.; Waterhouse, J. Reliability of nitrogen dioxide passive diffusion tubes for ambient measurement: In situ properties of the triethanolamine absorbent. J. Environ. Monit. 2000, 2, 307–312. [Google Scholar] [CrossRef]
- Hamilton, R.P.; Heal, M.R. Evaluation of method of preparation of passive diffusion tubes for measurement of ambient nitrogen dioxide. J. Environ. Monit. 2004, 6, 12–17. [Google Scholar] [CrossRef][Green Version]
- Heal, M.R. The effect of absorbent grid preparation method on precision and accuracy of ambient nitrogen dioxide measurement using Palmes passive diffusion tubes. J. Environ. Monit. 2008, 10, 1363–1369. [Google Scholar] [CrossRef]
- Laxen, D.H.P.; Marner, B.; Donovan, S. Analysis of Factors Influencing Diffusion Tube Performance. A Report Prepared for Defra and the Devolved Administrations by Air Quality Consultants Ltd.; Report No. 504/2/F1; AEA Energy & Environment: Defra, UK, 2008. Available online: http://www.airquality.co.uk /archive/reports/cat05/0807101007_Factors_Influencing_Diff_Tube_Performance.pdf (accessed on 25 June 2019).
- Tarvydaite, J.; Kazlauskiene, A. Research of effectiveness of diffusive samplers determining nitrogen dioxide in air. In Proceedings of the ICEE International Conference on Environmental Engineering, Vilnius, Lithuania, 22–23 May 2014. [Google Scholar]
- Bootdee, S.; Chalemrom, P.; Chantara, S. Validation and field application of tailor-made nitrogen dioxide passive samplers. Int. J. Environ. Sci. Technol. 2012, 9, 515–526. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Lumbreras, J.; Solazzo, E. Comparative evaluation of nitrogen oxides and ozone passive diffusion tubes for exposure studies. Atmos. Environ. 2009, 43, 2509–2517. [Google Scholar] [CrossRef]
- Sickles, J.E.; Grohse, P.M.; Hodson, L.L.; Salmons, C.A.; Cox, K.W.; Turner, A.R.; Estes, E.D. Development of a method for the sampling and analysis of sulfur dioxide and nitrogen dioxide from ambient air. Anal. Chem. 1990, 62, 338–346. [Google Scholar] [CrossRef]
- Hisham, M.W.M.; Grosjean, D. Sampling of atmospheric nitrogen dioxide using triethanolamine: Interference from peroxyacetyl nitrate. Atmos. Environ. Part. A Gen. Top. 1990, 24, 2523–2525. [Google Scholar] [CrossRef]
- Gair, A.J.; Penkett, S.A.; Oyola, P. Development of a simple passive technique for the determination of nitrogen dioxide in remote continental locations. Atmos. Environ. 1991, 25, 1927–1939. [Google Scholar] [CrossRef]
- Plaisance, H. Response of a Palmes tube at various fluctuations of concentration in ambient air. Atmos. Environ. 2004, 38, 6115–6120. [Google Scholar] [CrossRef]
- Hearl, F.J.; Manning, M.P. Transient response of diffusion dosimeters. Am. Ind. Hyg. Assoc. J. 1980, 41, 778–783. [Google Scholar] [CrossRef]
- Palmes, E.D.; Johnson, E.R. Explanation of pressure effects on a nitrogen dioxide (NO2) sampler. Am. Ind. Hyg. Assoc. J. 1987, 48, 73–76. [Google Scholar] [CrossRef]
- Glasius, M.; Carlsen, M.F.; Hansen, T.S.; Lohse, C. Measurements of nitrogen dioxide on Funen using diffusion tubes. Atmos. Environ. 1999, 33, 1177–1185. [Google Scholar] [CrossRef]
- Aoyama, T.; Yashiro, T. Analytical study of low-concentration gases: IV. Investigation of the reaction by trapping nitrogen dioxide in air using the triethanolamine method. J. Chromatogr. A 1983, 265, 69–78. [Google Scholar] [CrossRef]
- Poddubny, V.A.; Yushketova, N.A. A physicochemical model of sorption processes in NO2 passive sampling with air humidity effects. Environ. Monit. Assess. 2013, 185, 3819–3829. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.A.; Helmore, J.J.; White, S.; Barker Snook, I.L.; Parish, A.; Gates, L.S. Measurement of nitrogen dioxide diffusive sampling rates for Palmes diffusion tubes using a controlled atmosphere test facility (CATFAC). Atmos. Environ. 2014, 94, 529–537. [Google Scholar] [CrossRef]
- Plaisance, H. The effect of the wind velocity on the uptake rates of various diffusive samplers. Int. J. Environ. Anal. Chem. 2011, 91, 1341–1352. [Google Scholar] [CrossRef]
- Gerboles, M.; Buzica, D.; Amantini, L. Modification of the Palmes diffusion tube and semi-empirical modelling of the uptake rate for monitoring nitrogen dioxide. Atmos. Environ. 2005, 39, 2579–2592. [Google Scholar] [CrossRef]
- Sekine, Y.; Watts, S.F.; Rendell, A.; Butsugan, M. Development of highly sensitive passive sampler for nitrogen dioxide using porous polyethylene membrane filter as turbulence limiting diffuser. Atmos. Environ. 2008, 42, 4079–4088. [Google Scholar] [CrossRef]
- Plaisance, H.; Plechocki-Minguy, A.; Garcia-Fouque, S.; Galloo, J.C. Influence of meteorological factors on the NO2 measurements by passive diffusion tube. Atmos. Environ. 2004, 38, 573–580. [Google Scholar] [CrossRef]
- Buzica, D.; Gerboles, M.; Amantini, L.; Ballesta, P.P.; de Saeger, E. Modelling of the uptake rate of the nitrogen dioxide Palmes diffusive sampler based on the effect of environmental factors. J. Environ. Monit. 2005, 7, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Gerboles, M.; Buzica, D.; Amantini, L.; Lagler, F. Laboratory and field comparison of measurements obtained using the available diffusive samplers for ozone and nitrogen dioxide in ambient air. J. Environ. Monit. 2006, 8, 112–119. [Google Scholar] [CrossRef] [PubMed]
- De Santis, F.; Fino, A.; Tiwari, S.; Vazzana, C.; Allegrini, I. Advances in Air Pollution Series; Wit Press: Southampton, UK, 2000; ISBN 1-85312-822-8. [Google Scholar]
- Masey, N.; Gillespie, J.; Heal, M.R.; Hamilton, S.; Beverland, I.J. Influence of wind-speed on short-duration NO2 measurements using Palmes and Ogawa passive diffusion samplers. Atmos. Environ. 2017, 160, 70–76. [Google Scholar] [CrossRef]
- Sánchez Jiménez, A.; Heal, M.R.; Beverland, I.J. Intercomparison study of NOx passive diffusion tubes with chemiluminescence analysers and evaluation of bias factors. Atmos. Environ. 2011, 45, 3062–3068. [Google Scholar]
- Heal, M.R.; Cape, J.N. A numerical evaluation of chemical interferences in the measurement of ambient nitrogen dioxide by passive diffusion samplers. Atmos. Environ. 1997, 31, 1911–1923. [Google Scholar] [CrossRef]
- Heal, M.R.; O’Donoghue, M.A.; Cape, J.N. Overestimation of urban nitrogen dioxide by passive diffusion tubes: A comparative exposure and model study. Atmos. Environ. 1999, 33, 513–524. [Google Scholar] [CrossRef]
- Heal, M.R.; Kirby, C.; Cape, J.N. Systematic biases in measurement of urban nitrogen dioxide using passive diffusion samplers. Environ. Monit. Assess. 2000, 62, 39–54. [Google Scholar] [CrossRef]
- Swaans, W.; Goelen, E.; De Fre, R.; Damen, E.; Van Avermaet, P.; Roekens, E.; Keppens, V. Laboratory and field validation of a combined NO2-SO2 Radiello passive sampler. J. Environ. Monit. 2007, 9, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Ozden, O.; Dogeroglu, T. Field evaluation of a tailor-made new passive sampler for the determination of NO2 levels in ambient air. Environ. Monit. Assess. 2008, 142, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.; Fox, M.; Waterhouse, J.; Drye, T. Influence of environmental parameters on the accuracy of nitrogen dioxide passive diffusion tubes for ambient measurement. J. Environ. Monit. 2001, 3, 150–158. [Google Scholar] [CrossRef]
- Laxen, D.H.P.; Marner, B. The Relationship Between Diffusion Tube Bias and Distance from the Road. A Report Prepared for Defra and the Devolved Administrations by Air Quality Consultants Ltd; Air Quality Consultants Ltd: Bristol, UK, 2006; Available online: http://www.aqconsultants.co.uk/AQC/media/Reports/Bias-Adj-Dist-Road-Rept-Final.pdf (accessed on 5 June 2019).
- Bush, T.; Jenkin, M.E.; Banham, J.; Loader, A. Investigation of NO2 Diffusion Tube Over-Read Resulting from Reduced Photolysis by UV Light; AEA Technology Report AEAT/ENV/R/0562; AEA Technology: Harwell, UK, 2001. [Google Scholar]
- Massman, W.J. A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO and NO2 in air, O2 and N2 near STP. Atmos. Environ. 1998, 32, 1111–1127. [Google Scholar] [CrossRef]
- Chambers, F.S.; Sherwood, T.K. Absorption of nitrogen dioxide by aqueous solutions. Ind. Eng. Chem. 1937, 29, 1415–1422. [Google Scholar] [CrossRef]
- Sviridenko, Y.; Makhin, V.A.; Shandorov, G.S. Determining the diffusivity of nitrogen tetroxide. J. Eng. Phys. 1973, 24, 351–353. [Google Scholar] [CrossRef]
- Tang, M.J.; Cox, R.A.; Kalberer, M. Compilation and evaluation of gas phase diffusion coefficients of reactive trace gases in the atmosphere: Volume 1. Inorganic compounds. Atmos. Chem. Phys. 2014, 14, 9233–9247. [Google Scholar] [CrossRef]
- Fuller, E.N.; Schettler, P.D.; Giddings, J.C. New method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. 1966, 58, 18–27. [Google Scholar] [CrossRef]
- Fuller, E.N.; Ensley, K.; Giddings, J.C. Diffusion of halogenated hydrocarbons in helium. The effect of structure on collision cross sections. J. Phys. Chem. 1969, 73, 3679–3685. [Google Scholar] [CrossRef]
- Gerboles, M.; Lagler, F.; Rembges, D.; Brun, C. Assessment of uncertainty of NO2 measurements by the chemiluminescence method and discussion of the quality objective of the NO2 European Directive. J. Environ. Monit. 2003, 5, 529–540. [Google Scholar] [CrossRef] [PubMed]
- ISO. Guide to the Expression of Uncertainty in Measurement. International Standards Organisation; ISO: Geneva, Switzerland, 1995; ISBN 92-67-10188-9. [Google Scholar]
- Steinbacher, M.; Zellweger, C.; Schwarzenbach, B.; Bugmann, S.; Buchmann, B.; Ordonez, C.; Prevot, A.S.H.; Hueglin, C. Nitrogen oxide measurements at rural sites in Switzerland: Bias of conventional measurement techniques. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Xue, L.K.; Louie, P.K.K.; Luk, C.W.Y.; Gao, J.; Wang, S.L.; Chai, F.H.; Wang, W.X. Evaluating the uncertainties of thermal catalytic conversion in measuring atmospheric nitrogen dioxide at four differently polluted sites in China. Atmos. Environ. 2013, 76, 221–226. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.; Kim, B.; Oh, S. Seasonal variations in the NO2 artifact from chemiluminescence measurements with a molybdenum converter at a suburban site in Korea (downwind of the Asian continental outflow) during 2015–2016. Atmos. Environ. 2017, 165, 290–300. [Google Scholar] [CrossRef]
- Leston, A.R.; Ollison, W.M. Field evaluations of newly available “interference-free” monitors for nitrogen dioxide and ozone at near-road and conventional National Ambient Air Quality Standards compliance sites. J. Air Waste Manag. Assoc. 2017, 67, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).