A Test Stand Study on the Volatile Emissions of a Passenger Car Brake Assembly
Abstract
:1. Introduction
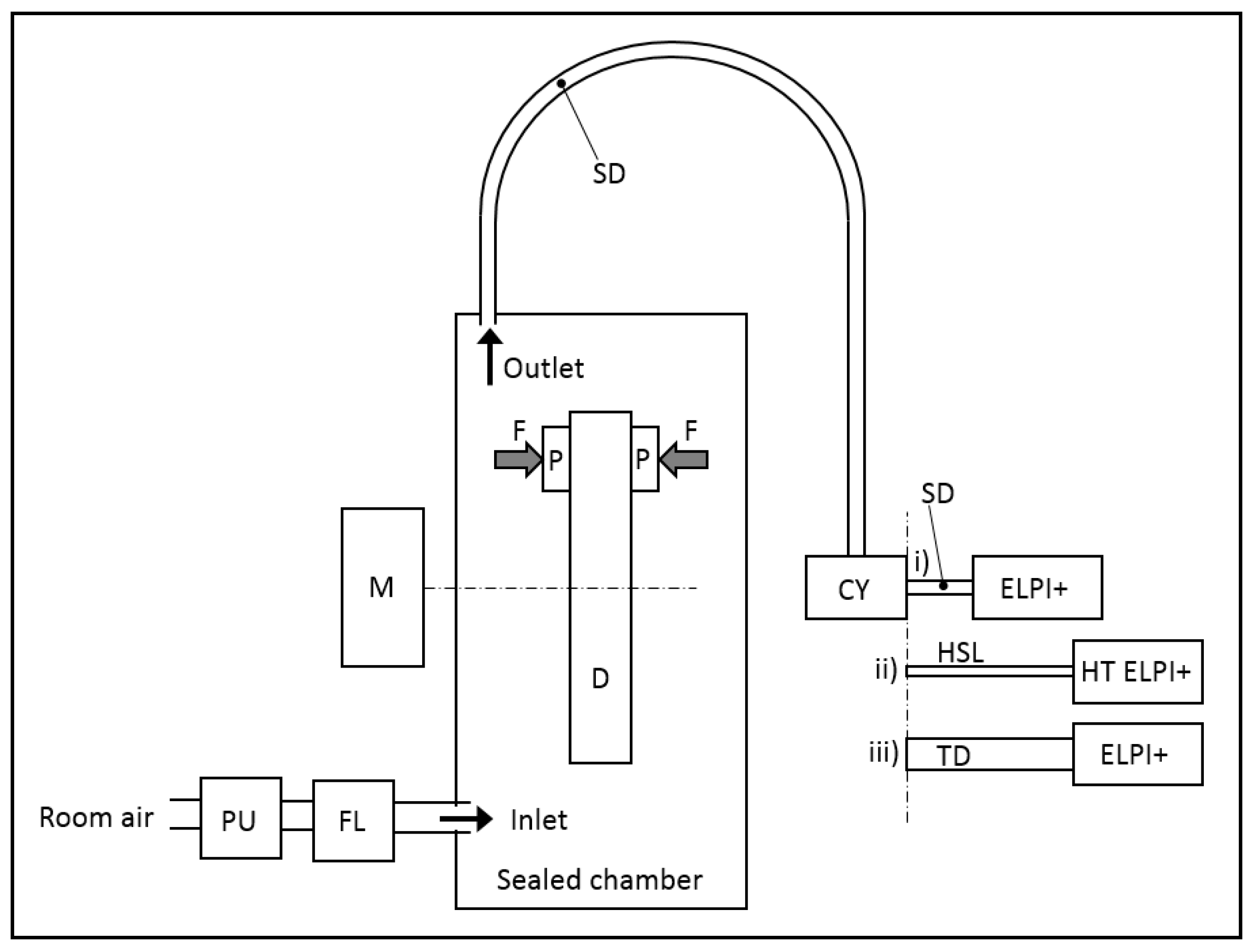
2. Materials and Methods
3. Sampling Efficiency in the Thermodenuder and in the Heated Sampling Line (HSL)
3.1. Aerosol Losses for the Thermodenuder
3.2. Aerosol Losses for the Heated Sampling Line
3.2.1. Thermophoretic Losses for the HSL
3.2.2. Sedimentation Losses for the HSL
3.2.3. Diffusive Losses for the HSL
4. Emission Factors
5. Results
6. Discussion
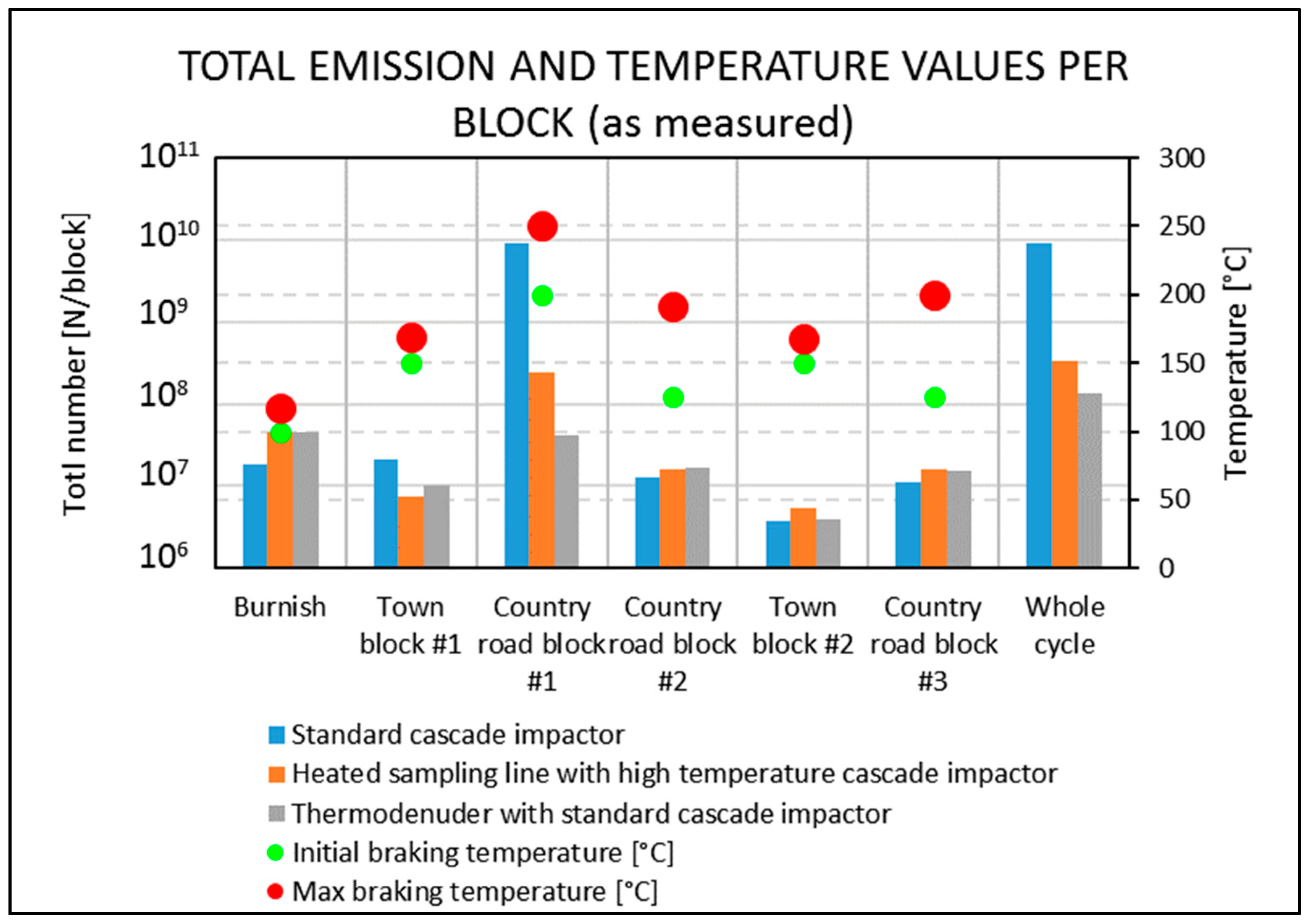
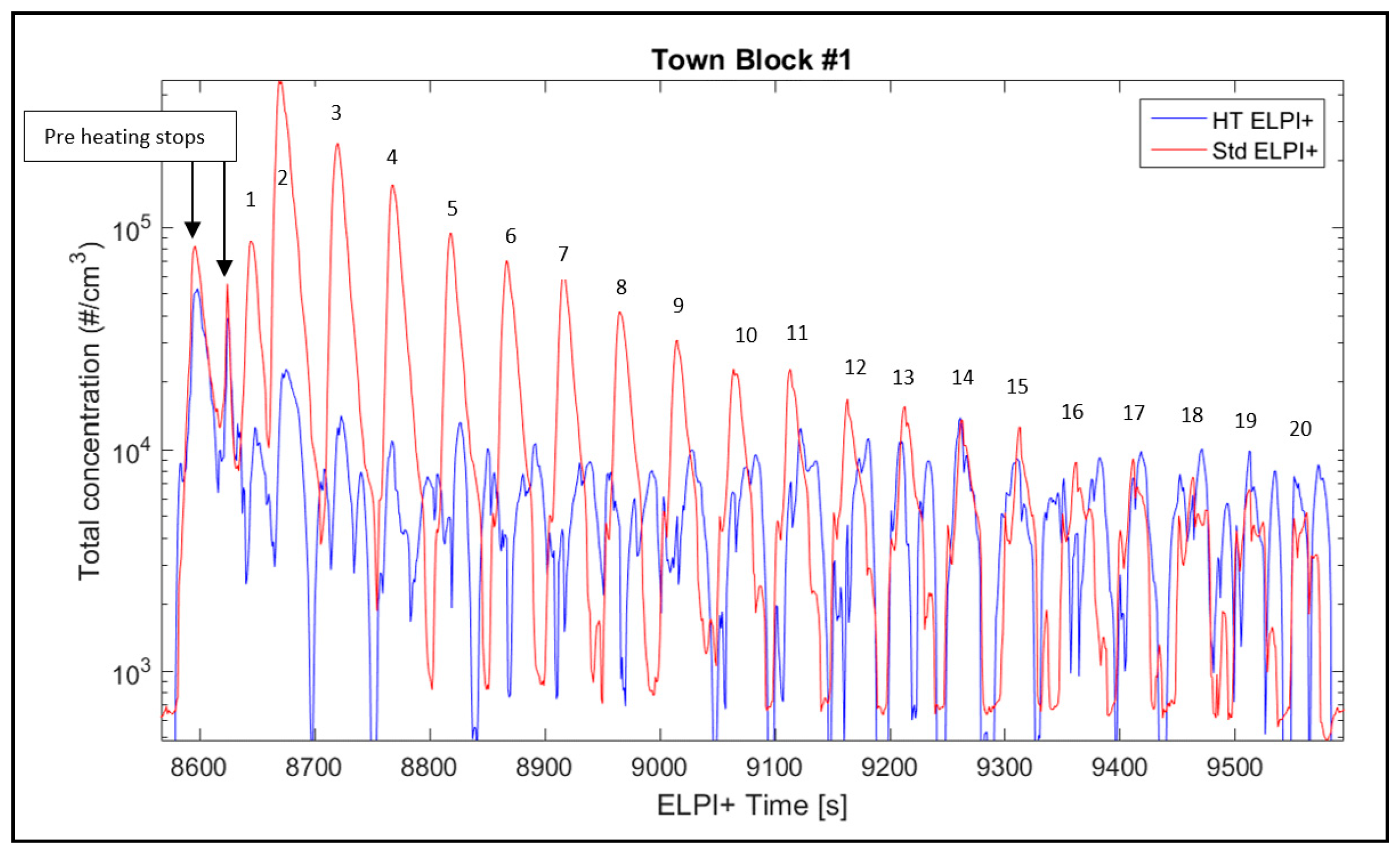
- When comparing the two tests executed at the same time in this work using the standard cascade impactor (std. ELPI+) and the one operating at high temperature (HT ELPI+), a 28-fold higher EFPN can be detected for the standard cascade impactor, as reported in Table 4. This difference is due mainly to the count of particles smaller than 200 nm in the case of std. ELPI+, as can be seen in Figure 2. This means that 200 °C heating of the incoming air sample results in a decrease in particle count below 200 nm because organics and other easily volatilized compounds are lost. For example, an initial decrease in particle number may result from a loss of moisture in the chamber with the clean air blowing in, as also reported in [23] for natural aerosols. On the other hand, there may be a competitive phenomenon for which some larger particles can release smaller coagulated particles on their surface and so increase the total number of particles. Plachà et al. [19] reported that more than 150 different organic compounds were found in an unused milled brake pad, whereas only 49 confirmed compounds were found in actual-wear debris. This means that several organic compounds are released during the friction process, and as a result we can expect significant amounts of volatile organic compounds (VOCs) and semi-volatile organic compounds (SVOCs) to be released into the atmosphere as real VOCs or be absorbed in airborne particulate matter during braking. Note that in the heated sampling line (HT ELPI+) experiment, mass should be conserved because no device was used to absorb the volatilized fraction. For this reason, the volatile fraction should remain in the air flow but with different sizes; this could not be seen in Figure 2, where it seemed that a net loss of particles smaller than 200 nm occurred. The reason for this discrepancy could be that the particles were disaggregated in particles smaller than 6 nm (the lowest instrument detection limit), or heating changed some electrostatic, density, or shape properties of the particle as measured from the instrument. Further research is necessary to better address this. From Figure 4 it is possible to observe that in the first 13 stops out the 20 that make up Town block #1, the presence of volatile emissions that decrease stop-by-stop until becoming negligible in the last seven stops is clear. This means that volatile emissions are a transient phenomenon once disc braking parameters are kept constant. From Figure 5 it becomes clear that total particle emissions depend on experimental conditions such as the starting braking velocity, temperature, and the existence of some run-in effects as also described in [42]. In fact, considering the emission ratio we can observe that:
- (i)
- In the Burnish block, where initial stopping velocity and temperature are 50 km/h and 100 °C, respectively, the emission ratio is 0.5. This means that the HT ELPI+ measures more particles than the standard cascade impactor, and this can be ascribed to some poorly understood artefacts. Regarding the braking conditions, we can surely conclude that there are no volatiles.
- (ii)
- Increasing only the initial stopping temperature to 150 °C in Town block #1 leads to a ratio equal to 3.5. In this case, the standard ELPI+ measured 3.5 times more particles compared to the HT ELPI+, as is also qualitatively visible stop-by-stop from Figure 4.
- (iii)
- Increasing both the braking starting velocity and temperature to 80 km/h and 200 °C, respectively, as in Country road block #1, results in clearly visible volatile emissions, and the HT ELPI+ measures almost 50 times less particles than the standard one.
- (iv)
- Passing to Country road block #2, and so increasing the braking starting velocity to 125 km/h while at the same time decreasing the disc stopping starting temperature to 100 °C, does not trigger pronounced release of volatiles, and the ratio of emissions measured by the two cascade impactors equals 1: it is possible to believe that previous blocks have provoked the running in of the braking system.
- (v)
- The repetition of Town block #1, and namely carrying out Town block #2, showed that braking conditions in “soft” blocks did not cause release of volatile emissions.
- (vi)
- Repeating the conditions of Country road block #2 with the last Country road block #3 confirms again that the system is already run-in because no volatile emissions are observed.
- When comparing the two tests that focused on heat conditioning of the sampled aerosol (one with the heated sampling line used in connection with the HT ELPI+ and one with the thermodenuder and the standard cascade impactor), a 2.5-fold higher EFPN was calculated for the HT ELPI+ setup, as reported in Table 4. This was due mainly to the number of particles smaller than around 20 nm, as shown in Figure 2. This indicates that the thermodenuder operating at 300 °C is more effective in removing the smallest particle fraction. This hypothesis is also supported by the fact that this experiment provided the particles with the biggest geometric mean diameter (see Table 4). One possible explanation for this phenomenon is that the compounds that volatilize at 300 °C are prevented from re-entering the aerosol stream thanks to the active charcoal that absorbs them when using the thermodenuder. If, however, VOCs are still in the aerosol stream after the aerosol has heated, as is the case in the heated sampling line, they can act as nucleation sites, or they can coagulate to form larger particles that can be detected in the cascade impactor of ELPI+. This is confirmed by the fact that the configuration with the heated sampling line is the one showing the smallest particle geometric mean diameter, as reported in Table 4, even though the related EFPN is much lower than the ones measured using the standard cascade impactor.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, J.; Olofsson, U. A field study of airborne particle emissions from automotive disc brakes. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2014, 229, 747–757. [Google Scholar] [CrossRef]
- Abbasi, S.; Jansson, A.; Olander, L.; Olofsson, U.; Sellgren, U. A pin-on-disc study of the rate of airborne wear particle emissions from railway braking materials. Wear 2012, 284–285, 18–29. [Google Scholar] [CrossRef]
- Alemani, M.; Oleksii, N.; Ibrahim, M.; Olofsson, U. A study on emission of airborne wear particles from car brake friction pairs. Sae Int. J. Mater. Manuf. 2015, 9, 147–157. [Google Scholar] [CrossRef]
- Nosko, O.; Vanhanen, J.; Olofsson, U. Emission of 1.3–10 nm airborne particles from brake materials. Aerosol Sci. Technol. 2016, 51, 91–96. [Google Scholar] [CrossRef]
- Namgung, H.G.; Kim, J.B.; Woo, S.H.; Park, S.; Kim, M.; Kim, M.S.; Bae, G.N.; Park, D.; Kwon, S.B. Generation of nanoparticles from friction between railway brake disks and pads. Environ. Sci. Technol. 2016, 50, 3453–3461. [Google Scholar] [CrossRef]
- Nosko, O.; Alemani, M.; Olofsson, U. Temperature effect on emission of airborne wear particles from car brakes. In Proceedings of the Europe’s Braking Conference and Exhibition, Dresden, Germany, 4–6 May 2015; FISITA: London, UK, 2015. paper EB2015-TEF-014. [Google Scholar]
- Alemani, M.; Gialanella, S.; Straffelini, G.; Ciudin, R.; Olofsson, U.; Perricone, G.; Metinoz, I. Dry sliding of a low steel friction material against cast iron at different loads: Characterization of the friction layer and wear debris. Wear 2017, 376–377, 1450–1459. [Google Scholar] [CrossRef]
- Alemani, M.; Wahlström, J.; Olofsson, U. On the influence of car brake system parameters on particulate matter emissions. Wear 2018, 396–397, 67–74. [Google Scholar] [CrossRef]
- Wahlström, J.; Olander, L.; Olofsson, U. A pin-on-disc study focusing on how different load levels affect the concentration and size distribution of airborne wear particles from the disc brake materials. Tribol. Lett. 2012, 46, 195–204. [Google Scholar] [CrossRef]
- Mathissen, M.; Grochowicz, J.; Schmidt, C.; Vogt, R.; Zum Hagen, F.H.F.; Grabiec, T.; Steven, H.; Grigoratos, T. A novel real-world braking cycle for studying brake wear particle emissions. Wear 2018, 414, 219–226. [Google Scholar] [CrossRef]
- Garg, B.; Cadle, S.; Mulawa, P.; Groblicki, P.; Laroo, C.; Parr, G. Brake wear particulate matter emissions. Environ. Sci. Technol. 2000, 34, 4463–4469. [Google Scholar] [CrossRef]
- Kukutschová, J.; Moravec, P.; Tomášek, V.; Matějka, V.; Smolík, J.; Schwarz, J.; Seidlerová, J.; Šafářová, K.; Filip, P. On airborne nano/micro-sized wear particles released from low-metallic automotive brakes. Environ. Pollut. 2011, 159, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Nosko, O.; Olofsson, U. Effective density of airborne wear particles from car brake materials. J. Aerosol Sci. 2017, 107, 94–106. [Google Scholar] [CrossRef]
- Nosko, O.; Borrajo-Pelaez, R.; Hedström, P.; Olofsson, U. Porosity and shape of airborne wear microparticles generated by sliding contact between a low-metallic friction material and a cast iron. J. Aerosol Sci. 2017, 111, 130–140. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Chirico, R.; DeCarlo, P.F.; Clairotte, M.; Adam, T.; Martini, G.; Heringa, M.F.; Richter, R.; Prevot, A.S.H.; Baltensperger, U.; et al. Evaluation of the particle measurement programme (PMP) protocol to remove the vehicles’ exhaust aerosol volatile phase. Sci. Total Environ. 2010, 408, 5106–5116. [Google Scholar] [CrossRef]
- Khalek, I.A.; Bougher, T. Development of a solid exhaust particle number measurement system using a catalytic stripper technology. SAE Int. J. Eng. 2011, 4, 639–649. [Google Scholar] [CrossRef]
- Plachá, D.; Peikertova, P.; Kukutschova, J.; Lee, P.W.; Čabanová, K.; Karas, J.; Kuchařová, J.; Filip, P. Identification of organic compounds released from low-metallic automotive model brake pad and its non-airborne wear particles. Sae Int. J. Mater. Manuf. 2015, 9, 123–132. [Google Scholar] [CrossRef]
- Plachá, D.; Vaculík, M.; Mikeska, M.; Dutko, O.; Peikertová, P.; Kukutschová, J.; Kutláková, K.M.; Růžičková, J.; Tomášek, V.; Filip, P. Release of volatile organic compounds by oxidative wear of automotive friction materials. Wear 2017, 376–377, 705–716. [Google Scholar]
- Gramstat, S.; Cserhati, A.; Schroeder, M.; Lugovyy, D. Brake Particle Emission Measurements - Testing Method and Results. SAE Int. J. Eng. 2017, 10, 1841–1846. [Google Scholar] [CrossRef]
- Amato, F.; Alastuey, A.; Karanasiou, A.; Lucarelli, F.; Nava, S.; Calzolai, G.; Severi, M.; Becagli, S.; Gianelle, V.; Colombi, C.; et al. AIRUSE-LIFE+: A harmonized PM speciation and source apportionment in five southern European cities. Atmos. Chem. Phys. 2016, 16, 3289–3309. [Google Scholar] [CrossRef]
- Husar, R.B.; Shu, W.R. Thermal analyses of the LA smog aerosol. J. Appl. Meteorol. 1975, 14, 1558–1565. [Google Scholar] [CrossRef]
- Pueschel, R.F.; Bodhaine, B.A.; Mendonca, B.G. The proportion of volatile aerosol on the island of Hawaii. J. Appl. Meteorol. 1972, 12, 308–315. [Google Scholar] [CrossRef]
- European Commission. Directive 2008/692/EC of the European Parliament and of the Council of 18 July 2008 on Type-Approval of Motor Vehicles with Respect to Emissions from Light Passenger and Commercial Vehicles (Euro 5 and Euro 6) and on Access to Vehicle Repair and Maintenance Information. Off. J. Eur. Union 2008, L199, 1–135. Available online: http://eur-lex.europa.eu/search.html?qid=1490711490766&whOJ=NO_OJ%3D199,YEAR_OJ%3D2008&type=advanced&lang=en&SUBDOM_INIT=ALL_ALL&DB_COLL_OJ=oj-l (accessed on 17 May 2018).
- Giechaskiel, B.; Mamakos, A.; Andersson, J.; Dilara, P.; Martini, G.; Schindler, W.; Bergmann, A. Measurement of automotive nonvolatile particle number emissions within the European legislative framework: A review. Aerosol Sci. Technol. 2012, 46, 719–749. [Google Scholar] [CrossRef]
- Mikkanen, M.P.; Ntziachristos, L.; Keskinen, J. Conditioning the automotive exhaust sample with a thermodenuder. In Proceedings of the European Aerosol Conference, Leipzig, Germany, 3–7 September 2001; Elesevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Burtscher, H.; Baltensperger, U.; Bukowiecki, N.; Cohn, P.; Hüglin, C.; Mohr, M.; Matter, U.; Nyeki, S.; Schmatloch, V.; Streit, N.; et al. Separation of volatile and non-volatile aerosol fractions by thermodesorption: Instrumental development and applications. J. Aerosol Sci. 2001, 32, 427–442. [Google Scholar] [CrossRef]
- Wehner, B.; Philippin, S.; Wiedensohler, A. Design and calibration of a thermodenuder with an improved heating unit to measure the size-dependent volatile fraction of aerosol particles. J. Aerosol Sci. 2002, 33, 1087–1093. [Google Scholar] [CrossRef]
- Park, D.; Kim, S.; Choi, N.K.; Hwang, J. Development and performance test of a thermo-denuder for separation of volatile matter from submicron aerosol particles. J. Aerosol Sci. 2008, 39, 1099–1108. [Google Scholar] [CrossRef]
- Alemani, M.; Perricone, G.; Olofsson, U.; Söderberg, A.; Wahlström, J.; Ciotti, A. A proposed dyno bench test cycle to study particle. In Proceedings of the Eurobrake 2014 conference, Lille, France, 13–15 May 2014; FISITA: London, UK, 2014. Paper EB2014-SE-001. [Google Scholar]
- Sanders, P.G.; Xu, N.; Dalka, T.M.; Maricq, M.M. Airborne brake wear debris: Size distributions, composition, and a comparison of dynamometer and vehicle tests. Environ. Sci. Technol. 2003, 37, 4060–4069. [Google Scholar] [CrossRef]
- Cox, R.L. Engineered Tribological Composites, 1st ed.; SAE International: Warrendale, PA, USA, 2011; Chapter 6. [Google Scholar]
- Perricone, G.; Alemani, M.; Metinöz, I.; Matĕjka, V.; Wahlström, J.; Olofsson, U. Towards the ranking of airborne particle emissions from car brakes—A system approach. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2016, 231, 781–797. [Google Scholar] [CrossRef]
- Perricone, G.; Wahlström, J.; Olofsson, U. Towards a test stand for standardized measurements of the brake emissions. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2015, 230, 1521–1528. [Google Scholar] [CrossRef]
- Järvinen, A.; Aitomaa, M.; Rostedt, A.; Keskinen, J.; Yli-Ojanperä, J. Calibration of the new electrical low pressure impactor (ELPI+). J. Aerosol Sci. 2014, 69, 150–159. [Google Scholar] [CrossRef]
- Kannosto, J. Dekati® High Temperature ELPI+TM. Presentation for the Royal Technical University of Stockholm, Sweden. 2015. Available online: http://scielutions.com/data/documents/4-HT-ELPI+-JOK-05112015.pdf (accessed on 21 July 2018).
- Brockmann, J.E. Aerosol transport in sampling lines and inlets. In Aerosol Measurement; Kulkarni, P., Baron, P.A., Willeke, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 88–98. [Google Scholar]
- Hinds, W.C. Aerosol Technology, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999; Chapters 3, 7, 8. [Google Scholar]
- Dekati. Dekati® Thermodenuder User Manual. 2015. Available online: http://www.dekati.com/products/Aerosol%20Sample%20Conditioning/Dekati%C2%AE%20Thermodenuder (accessed on 12 August 2017).
- Lin, J.S.; Tsai, C.J. Thermophoretic deposition efficiency in a cylindrical tube taking into account developing flow at the entrance region. J. Aerosol Sci. 2003, 34, 569–583. [Google Scholar] [CrossRef]
- Ramachandran, G.; Cooper, D.W. Size distribution data analysis and presentation. In Aerosol Measurement; Kulkarni, P., Baron, P.A., Willeke, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 480–485. [Google Scholar]
- Matějka, V.; Metinöz, I.; Wahlström, J.; Alemani, M.; Perricone, G. On the running-in of brake pads and discs for dyno bench tests. Tribol. Int. 2017, 115, 424–431. [Google Scholar] [CrossRef]
- Perricone, G.; Alemani, M.; Wahlström, J.; Olofsson, U. A proposed driving cycle for brake emissions investigation for test stand. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2019. [Google Scholar] [CrossRef]


| Sample | Mg | Si | Al | S | Ca | Fe | Cu | Zn | Cr | Zr | Sn | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brake Pads | 11.1 | 6.3 | 9.8 | 5.6 | 5.2 | 7.6 | 5.8 | 13.4 | 3.5 | 0.1 | 9.3 | 22.3 |
| Sample | C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|
| Disk 1 | 3.40 | 1.70 | 0.57 | 0.03 | 0.26 | 93.6 |
| Number | Section | Initial Speed (km/h) | Final Speed (km/h) | Initial Rotor Brake Temperature (°C) | Braking Deceleration (units of g) | Number of Stops in SAE Test Cycle NSAE | Number of Stops in Present Test Npresent |
|---|---|---|---|---|---|---|---|
| Cleaning for 5′ | |||||||
| 1 | Burnish | 50 | 4 | 100 | 0.25 | 100 | 100 |
| Cleaning for 5′ | |||||||
| 2 | Town block #1 | 50 | 4 | 150 | 0.25 | 200 | 20 |
| Cleaning for 5′ | |||||||
| 4 | Country road block #1 | 80 | 4 | 200 | 0.35 | 200 | 20 |
| Cleaning for 5′ | |||||||
| 5 | Country road block #2 | 100 | 4 | 125 | 0.4 | 200 | 20 |
| Cleaning for 5′ | |||||||
| 7 | Town block #2 | 50 | 4 | 150 | 0.25 | 200 | 20 |
| Cleaning for 5′ | |||||||
| 8 | County road block #3 | 100 | 4 | 125 | 0.4 | 200 | 20 |
| Standard Cascade Impactor | Cascade Impactor Operating at High Temperature with Heated Sampling Line | Standard Cascade Impactor with Thermodenuder | |
|---|---|---|---|
| EFPN (N × 109 per stop per brake) as from Equation (8) | 90.7 | 3.27 | 1.3 |
| EFPN [N × 107 per stopper brake] for particle count ≥ 200 nm | 7.59 | 5.2 | 4.8 |
| Geometric mean diameter [nm] as from Equation (10) | 13.49 | 12.28 | 16.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perricone, G.; Matĕjka, V.; Alemani, M.; Wahlström, J.; Olofsson, U. A Test Stand Study on the Volatile Emissions of a Passenger Car Brake Assembly. Atmosphere 2019, 10, 263. https://doi.org/10.3390/atmos10050263
Perricone G, Matĕjka V, Alemani M, Wahlström J, Olofsson U. A Test Stand Study on the Volatile Emissions of a Passenger Car Brake Assembly. Atmosphere. 2019; 10(5):263. https://doi.org/10.3390/atmos10050263
Chicago/Turabian StylePerricone, Guido, Vlastimil Matĕjka, Mattia Alemani, Jens Wahlström, and Ulf Olofsson. 2019. "A Test Stand Study on the Volatile Emissions of a Passenger Car Brake Assembly" Atmosphere 10, no. 5: 263. https://doi.org/10.3390/atmos10050263
APA StylePerricone, G., Matĕjka, V., Alemani, M., Wahlström, J., & Olofsson, U. (2019). A Test Stand Study on the Volatile Emissions of a Passenger Car Brake Assembly. Atmosphere, 10(5), 263. https://doi.org/10.3390/atmos10050263







