1. Introduction
People recognise that indoor air quality may be more important than outdoor air quality because they spend over 70% [
1] of their time indoors, either at home or in work environments.
Despite this, the majority of the data on breathable particle material is based on the values obtained in the exterior, meaning that they may not really be good indicators of the real exposure of inhabitants to contaminants. Numerous outdoor air quality studies exist [
2,
3,
4,
5,
6], however, such studies of indoor atmospheric particle levels are scarce. This is especially the case in the area of the Mediterranean coast.
In Spain, studies are carried out in indoor working environments because it is stipulated by law, but very few exist for homes, indoor leisure facilities or public buildings. When considering the different sectors of the population, it is necessary to point out that those most affected by the levels of atmospheric contamination are children (3–12 years in the studied primary schools), the elderly and people with respiration or cardiovascular illnesses [
7,
8]. Children present certain characteristics that make them more susceptible to atmospheric contamination than adults [
8]. On the one hand, their respiration frequency is greater [
8,
9], on the other, the respiratory system of children under five is not fully developed, and their immunological system has not matured fully [
10,
11]. Despite this evidence, the majority of the work is done with the general population (where all age groups are considered), and studies of the infant population are scarce. For this reason, the study of air quality in primary schools was considered to be of interest, given that the children spend more time in school than any other indoor environment outside of the home.
Among the factors that determine the damage done to health by particle contaminants, particle size stands out. The size conditions the grade of penetration in the lungs, with particles less than 10 μm being considered as dangerous. In concrete terms according to Parker [
12], around 50% of the particle load introduced on the respiratory system, made up of sizes ranging from 0.01 μm to 0.1 μm, penetrate the lung cavity and are deposited permanently. Studies carried out by Pope [
13] show that there is a significant increase in daily mortality rates when the concentration of fine particles is increased.
In epidemiological studies, positive correlations between elevated levels of inhaled airborne particulate matter (PM) especially those with aerodynamic diameters less than 2.5 μm (PM
2.5), and acute adverse health effects have been established [
14,
15].
Apart from the size, the morphology of the particles also greatly conditions the capacity of the particles in the respiratory system.
The scanning electron microscope (SEM) was used as a complementary technique to complete the analysis of the indoor samples obtained from the schools. Different authors have used this technique in studies to characterise particles to establish their morphologies and origins [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. The morphology is addressed to detect possible sources of emission of airborne nanoparticles, fine particle matter from coal combustion, spherical particles as tracers of atmospheric ceramic industry or other polluting industries. Several studies had been published on SEM morphology of PM
2.5 particles in school classrooms or in school playgrounds [
28,
29]. However, this is the first publication that establishes a catalogue that brings together the morphologic characteristics of the main types of particles found among the indoor airborne fine particles captured in the primary schools in a strategic area in the framework of the European Union (EU) pollution control.
The gravimetric and chemical study of particles allows us to determine the concentration levels and their composition, however, when it comes to discerning their origin, it is necessary to identify the different types of compounds that make up the sample [
2,
28]. The techniques of X-ray diffraction and fluorescence allow the identification of the different mineral compounds, vitreous phases and combustion compounds, etc. present in the samples of particulate matter.
The primary advantage of the SEM technique is that apart from being applicable to a very small quantity of a sample, it allows for the individual analysis of particles, one by one, whether they are isolated or if they form groups, thus, if morphological differences exist they may be classified, and later their origin can also be deduced [
3,
22,
26].
Another key aspect of SEM is that it stands out as the best technique when it comes to carrying out morphological studies of particles. Soot and carbonaceous particles are the compounds that are of greatest importance in studies on the effects of particulate contaminants on health [
21]. These particles produced by combustion while still generally having a homogeneous composition with mainly carbon content can present morphologies that are very varied in function with their origin (type of combustion, combustibles, temperature, etc.). The SEM is, therefore, the ideal technique for their study. For example, the soot particles with dendritic structures are the ones that present a greater tendency to interact with the nasal system, the thorax and the bronchioles of humans [
17,
23]. Furthermore, the scanning electron microscopy allows for the identification of individual particle sizes and consequently establishes ranks that frame said particles.
Spain is one of the main ceramics producers of Europe and, in spite of the world economic crisis, the exporting market and the production of advanced ceramics are strengthening many Spanish ceramic areas again. In this sense, the efforts should now be directed to the consecution of new ceramic formulations that optimise the technological properties of the products and contribute to significant changes in the fabrication technology. Some advances should be directed to improve the drying process, to reduce the firing temperature, etc. and even to reduce contamination effects of ceramic production, if possible [
30]. The schools studied are located in a coastal area of the Mediterranean (province of Castellon, Spain) that possesses an important industrial nucleus dedicated to the treatment of mineral materials. The study area is a strategic zone in the framework of EU pollution control. Around 80% of the European Union ceramic tile and ceramic frit manufacturers are concentrated in two areas, forming the so-called ceramic clusters—in Modena (Italy) and in Castellón (Spain). Based on the studies in the literature [
30] and on the data shown on the Internet by the regional authorities derived from the air quality networks in these areas, it can be deduced that metals and PM are the two parameters of most concern regarding EU legal requirements [
31].
The main highlights of this paper are: a) to establish a catalogue that brings together the morphologic characteristics of the main types of particles found among the indoor airborne fine particles (less than 2.5 μm) captured in the primary schools in a strategic area in the framework of the EU pollution control (ceramic cluster of Castellón area) and b) to assess the influence of outside emission sources, on the particles found inside of schools with natural ventilation.
2. Material and Methods
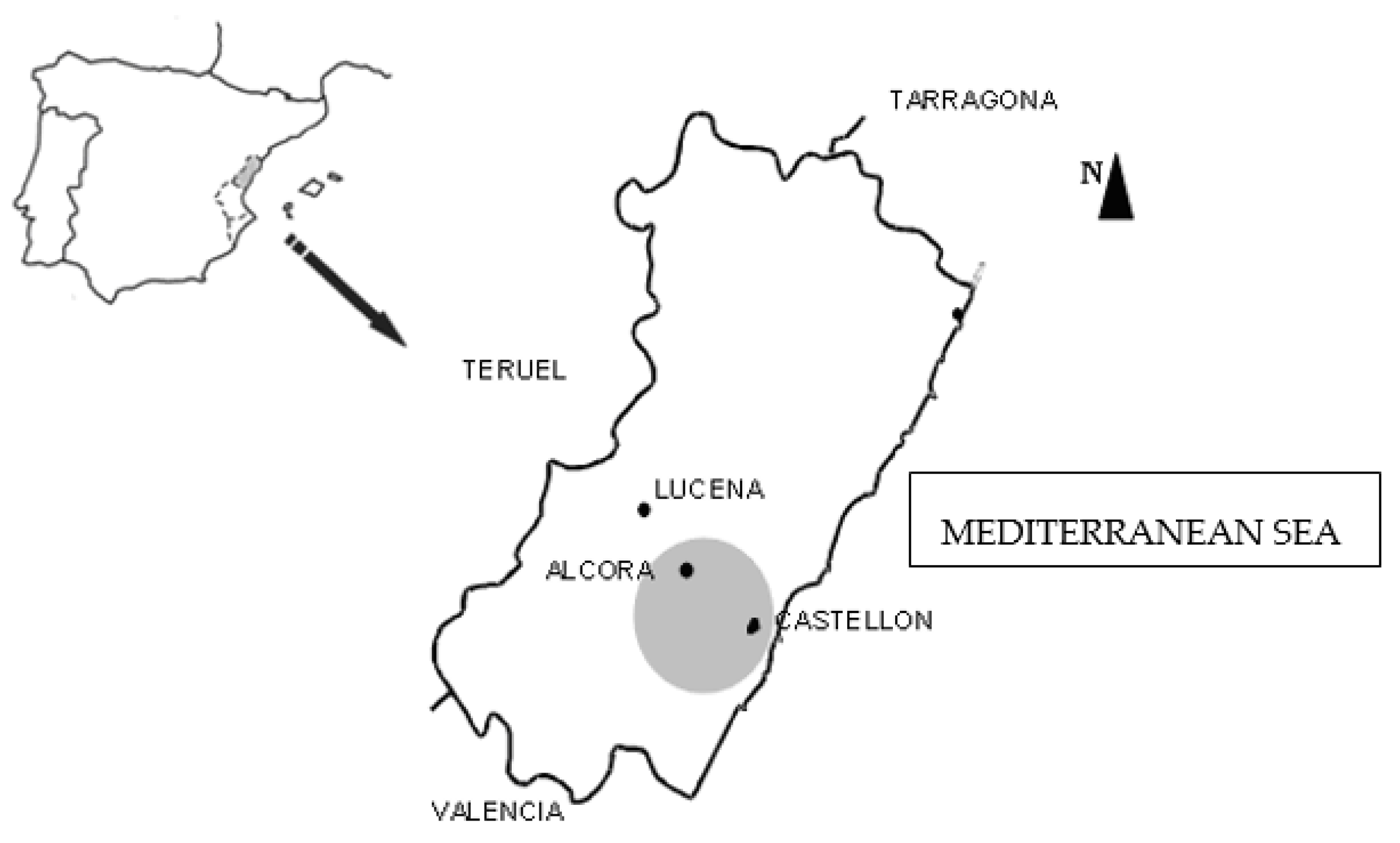
2.1. Site Description
This study concentrates on a strip of land in the province of Castellón (Spain) that ranges from its capital (Castellón), located on the
Plana de Castellón where there are no natural barriers, passing through the locality of Alcora situated on the slope of a mountain, and ending in the village of Lucena located in the mountainous interior (
Figure 1). These localities (Castellón, Alcora, and Lucena) represent three different environments: urban, industrial and rural, respectively.
In the city of Castellón, three schools were selected: S1, S2 and S3. As a fundamental characteristic, all are located near road networks with a high density of traffic. The three locations selected are situated in different zones of the city, so as to analyse whether, the orientation with regards to the regimes of air currents, and the distinct urban morphologies influence the concentration levels of indoor particles.
The three schools in the locality of Alcora share as a principal feature, their greater proximity to an important industrial belt. As in the case of the urban schools, three primary schools were chosen: S4, S5 and S6, in different points with differing orientations and surroundings, to analyse the influence of these characteristics in the concentration levels.
In the municipality of Lucena, a village with a low density of traffic and a low concentration of industry, the school selected was S7.
Table 1 summarises the main characteristics of the seven schools studied. In all schools, manual ventilation of classrooms was performed.
2.2. Sample Collection
Samples were taken for a duration of 5 to 8 h depending on the school hours to obtain a monthly sample for each of the schools.
The indoor airborne samples were collected using RespiCon TM. This collector is a multi-stage, virtual impactor that traps airborne particles on three individual collection filters. At a flow rate of 3.11 L/min., the first stage separates out and collects the particles that are smaller than 2.5 μm. The second stage collects particles between 2.5 and 10 μm, while the third stage collects the remaining particles.
Quartz fibre and PTFE (Polytetrafluoroethylene) filters (37-mm diameter) were used to collect indoor particles. PTFE filters were only used on the first and second stage of RespiconTM and then examined using scanning electron microscopy (SEM) to determine physical particle size distribution.
2.3. SEM Analysis
Approximately 1 cm2 of the PTFE filter was cut off and mounted onto an aluminium SEM stub using a conducting carbon tab and then coated with at least 30 nm of Au/Pd to ensure sufficient electrical conductivity, to suppress the massive background for carbon and oxygen and to get a higher quality secondary electron image.
Suspended particles were analysed by the SEM technique, using a scanning electron microscope type LEO model 440. In addition to this, OXFORD EDX micro-analysis equipment was incorporated into the microscope to allow us to study these particles in detail. The geometric shapes of these particles showed a possible anthropogenic source. The size and morphology of aerosol particles were studied by secondary electrons which allow (improving images) improved imaging. Micrographs were taken at magnifications of ×7500. All measurements were carried out at an accelerating voltage of 20.00 kV and with a beam current of 10 nA. The possibility to analyse the data available for every single particle is an important outcome of these techniques that could also be used to identify possible sources emission or specific particle characterisation by mean of clustering algorithms. The data are elaborated to obtain the number concentration of the particle as a function of the particles mean diameters. The first step in Scanning Electron Microscopy-Energy Dispersive Spectroscopy (SEM/EDS) utomated particle analysis is the acquisition of an image with sufficient contrast between the background and the particles that an image analysis algorithm is capable of differentiating between them. The analysis system saves the location of each particle and two dimensional size and shape parameters for every particle are determined. Typical parameters include maximum, minimum and average diameters, perimeter and aspect ratio. Once the particles in the field of view are recognised, the automation system of the microscope acquires a chemical analysis of each particle in the form of an EDS spectrum. A peak in the EDS spectrum indicates the presence of the corresponding element in the particle and the particle can be classified based on its composition. Finally, the results are tabulated, giving a complete picture of the particle types, their size and their shapes. And this tabulation is entirely customisable since all of the data (size, shape and chemistry) is stored for each individual particle.
Due to the fact that the analysis was carried out over PTFE filters, the peaks corresponding to C and F appear in all of the EDX microanalyses.
3. Results and Discussion
In general, particulate matter present in indoor air has some differences with respect to outdoor environments. The emissions of kitchens, heating systems, tobacco, air fresheners and cleaning products produce contaminants, among them particles. However, the emissions produced outdoors (traffic, construction and industry) penetrate the interiors of buildings, and these make the primary contribution to indoor air quality [
20,
27]. This effect is even greater in buildings, as is the case with the classrooms of the schools where there are no important indoor emission sources; furthermore, many activities take place in the playgrounds adjacent to the classrooms.
The particle matter identified by SEM in the airborne fine particle samples (<2.5 μm) in this study are grouped into two main groups: mineral phases and particles produced by combustion processes. In addition, spheres emitted by processes at high temperatures were found in lower proportion.
The majority of the mineral particles were found in the schools situated in the industrial zone (S4, S5, and S6), a result that was associated with the proximity of these stations to an industrial nucleus for the treatment of mineral materials. Despite this the neoformation compounds (cubes of halite and gypsum) that were to be found in the stations situated in the industrial and rural settings. The schools situated in urban areas (S1, S2 and S3) presented greater concentrations of particles resulting from combustion due to these sampling points greater proximity to roads with a high density of traffic. The particles from processes at high temperatures, found in less concentration than the mineral or combustion ones, were identified in all of the schools studied.
3.1. Mineral Particles
This type of particle is as common in natural emissions as it is in industrial processes. Their sizes varied between 0.5 and 2.5 μm. The mineral phases found in the particle samples from the interior of the schools were classified in function with their morphologies into the following groups:
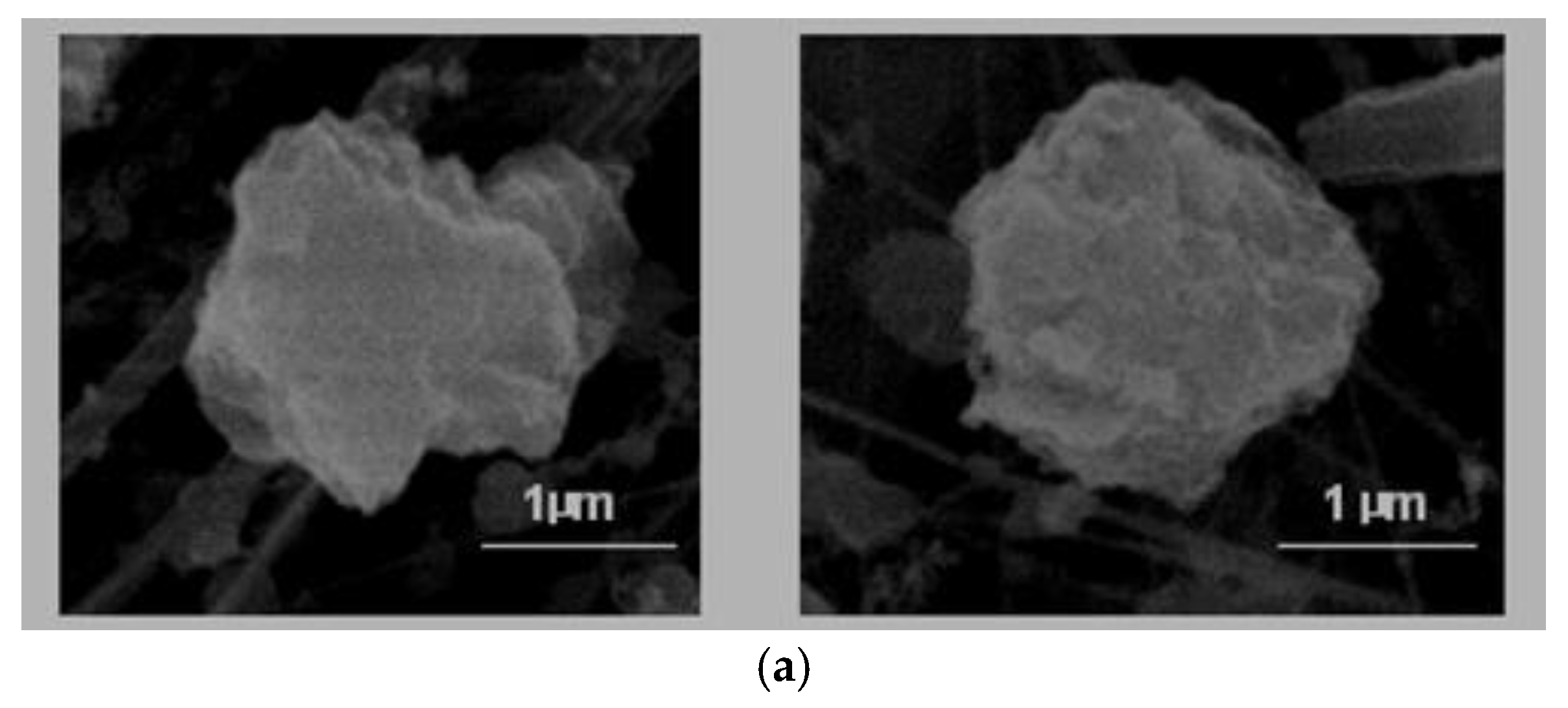
A. Isometric alotriomorphs or subidiomorphs. These are particles that do not present predominant growth in any direction and whose faces, either do not present a crystalline outer appearance, such as non-defined ridges (alotriomorphous,
Figure 2a) or show some sign of crystallisation on its exterior (subidiomorphous,
Figure 2b)
The alotriomophous particles are generally natural carbonates or silicates because they do not present defined forms. Instead, they have rounded borders that clearly indicated signs of erosion.
On the other hand, the origin of the subidiomorphous particles, even their composition is based in carbonates or silicates, was associated with mechanical fracturing processes that are employed in the ceramics industry (milling, crushing, etc.), given that they show clear cuts and defined edges.
B. Acicular Morphology (
Figure 3). They are crystals of a very elongated habit. In other studies, carried out in the zone, particles were found to be of an acicular habit, presenting compositions that follow two patrons. Some of them had sulphur, calcium and potassium while others contained sulphur, calcium and sodium [
2].
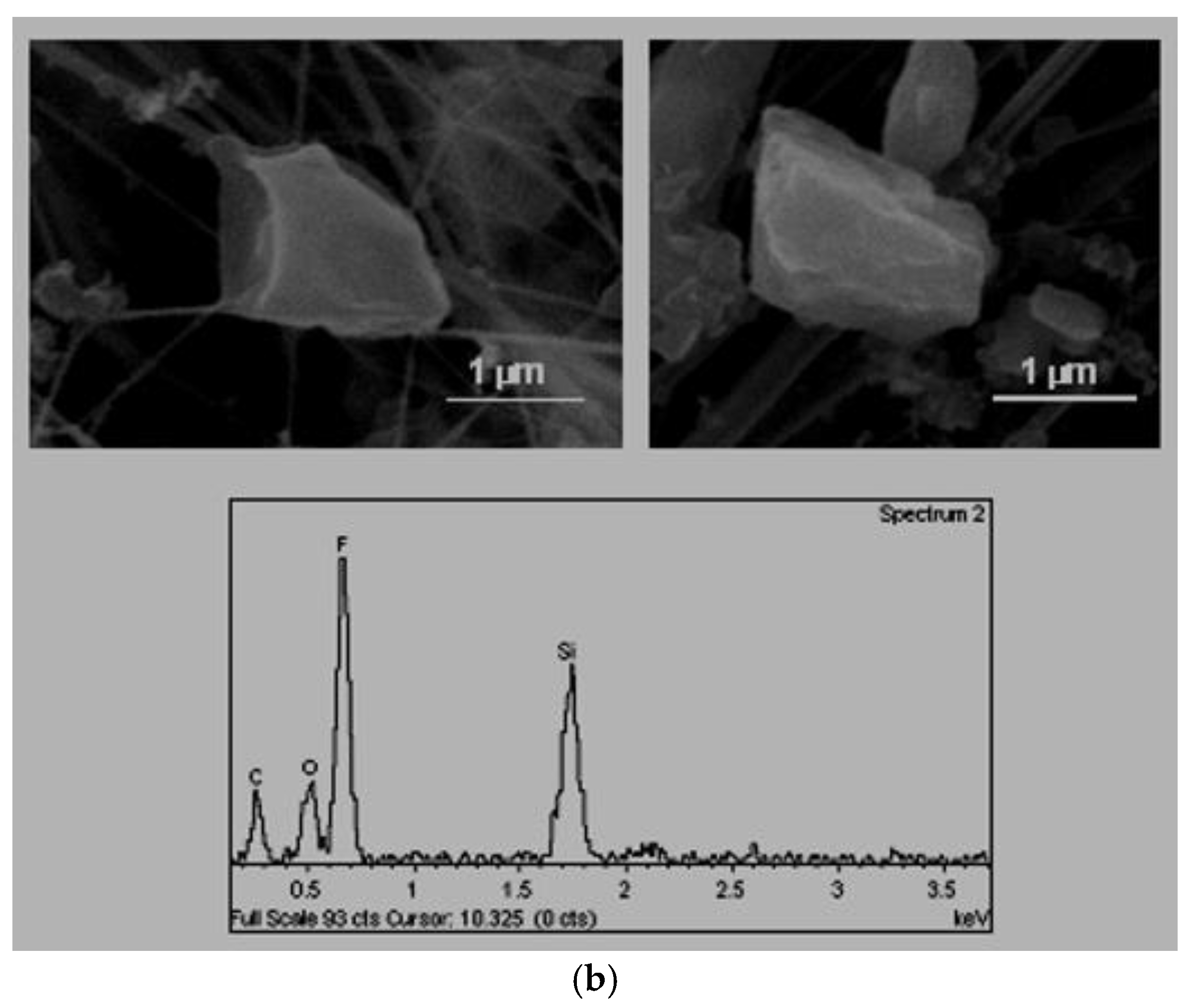
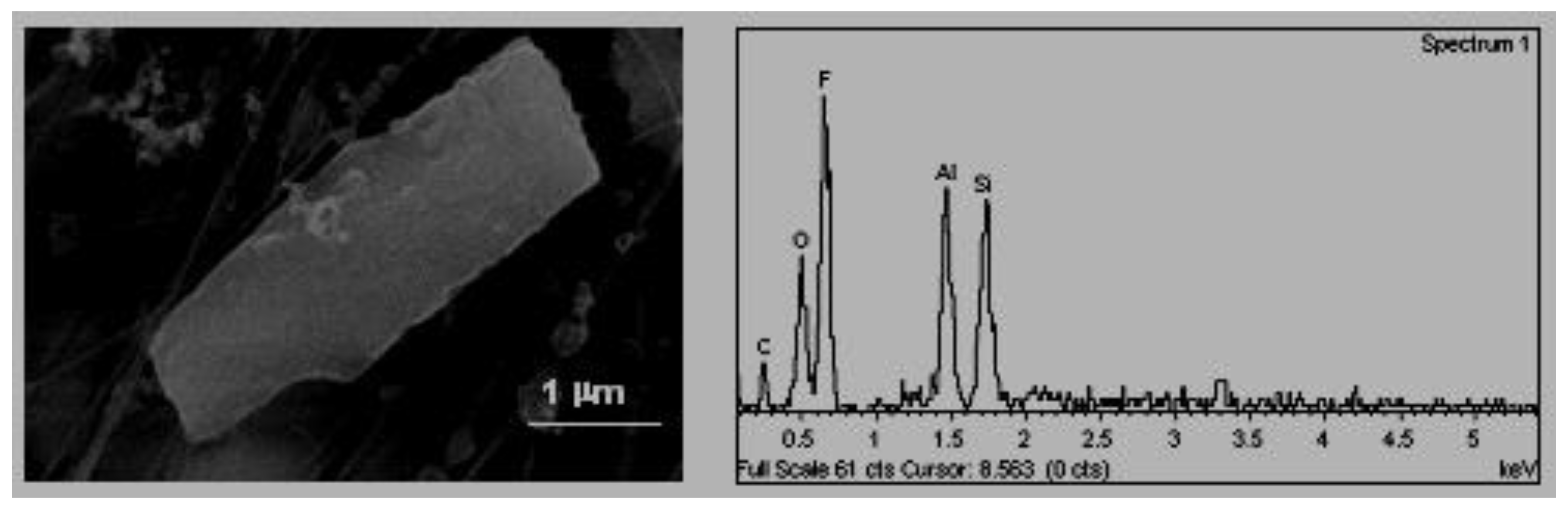
C. Tabular habit (
Figure 4). These types of particles were also identified by Boix [
2] and their composition, in their majority aluminium–silicates, was associated with emissions from natural or industrial clays treatments. In some cases, they were found as agglomerates, in these cases the sulphates and the chlorates were present as bonding materials.
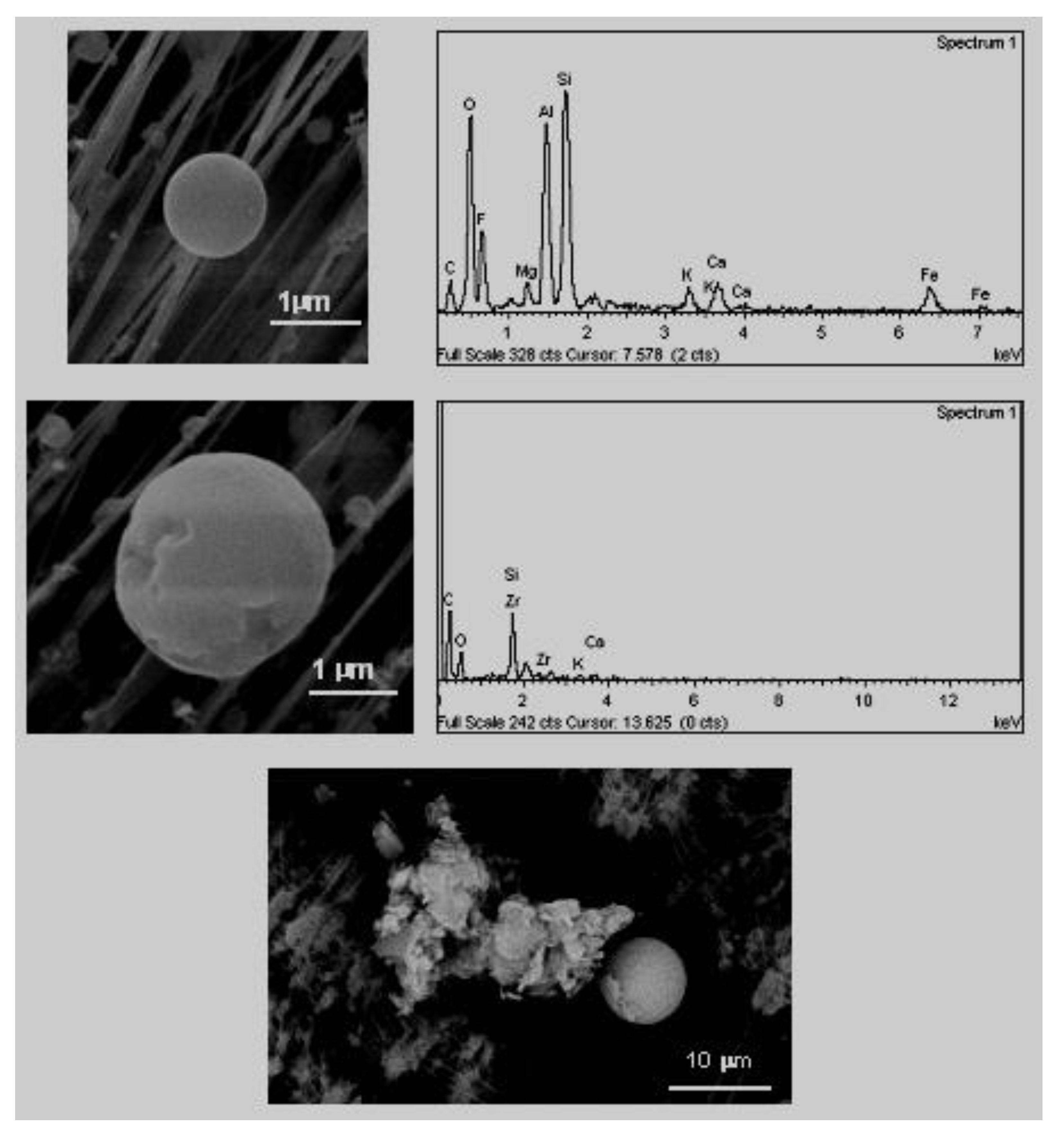
D. Pure crystalline forms (unaltered). These particles, in the majority of cases, crystallise in the atmosphere after the emission, and, therefore, they are denominated neoformation particles. For this reason, they do not show signs of erosion and their symmetrical elements can be clearly observed. In the samples of this study, it was possible to identify crystals of halite and gypsum.
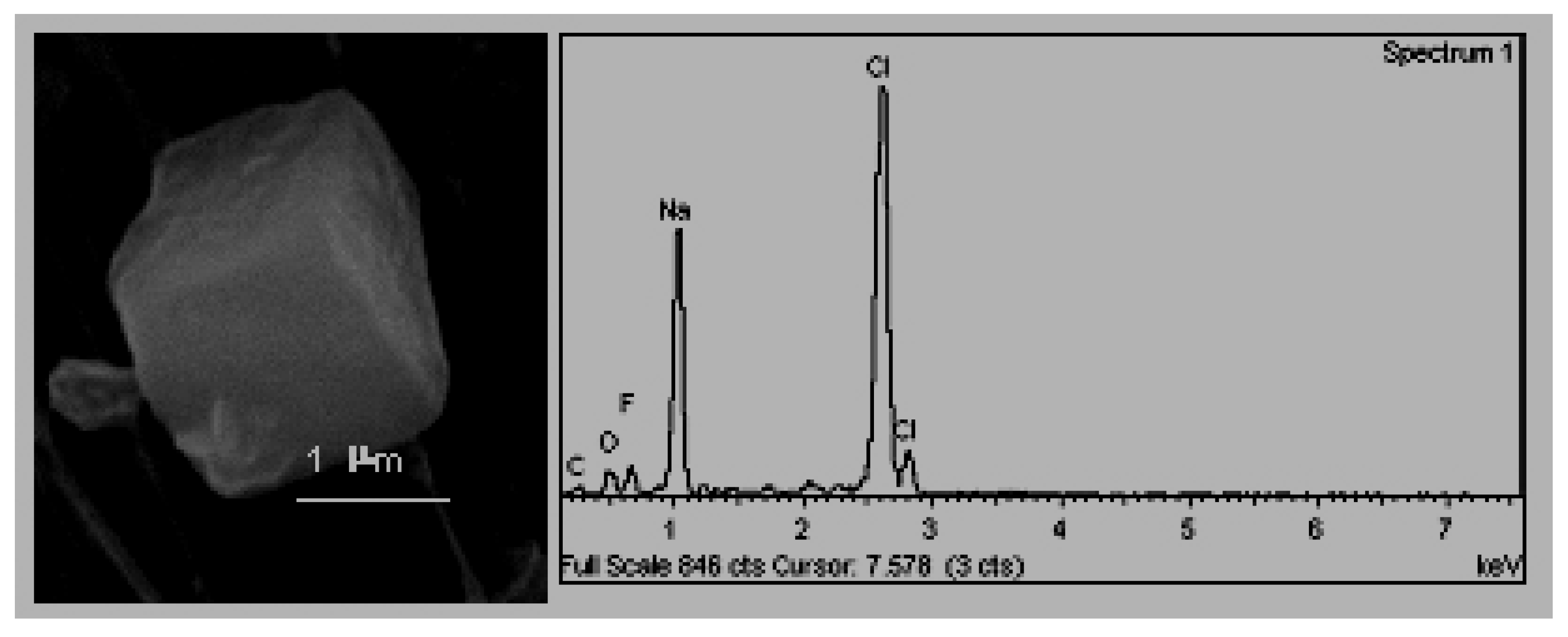
The cubic crystals (
Figure 5), associated with halite crystals (NaCl) that crystallise in the cubic system and whose exterior appearance is of a cube with rounded vertices [
32]. The presence of halite in atmospheric samples has been traditionally attributed to being of a natural origin, as a component of marine aerosols [
33].
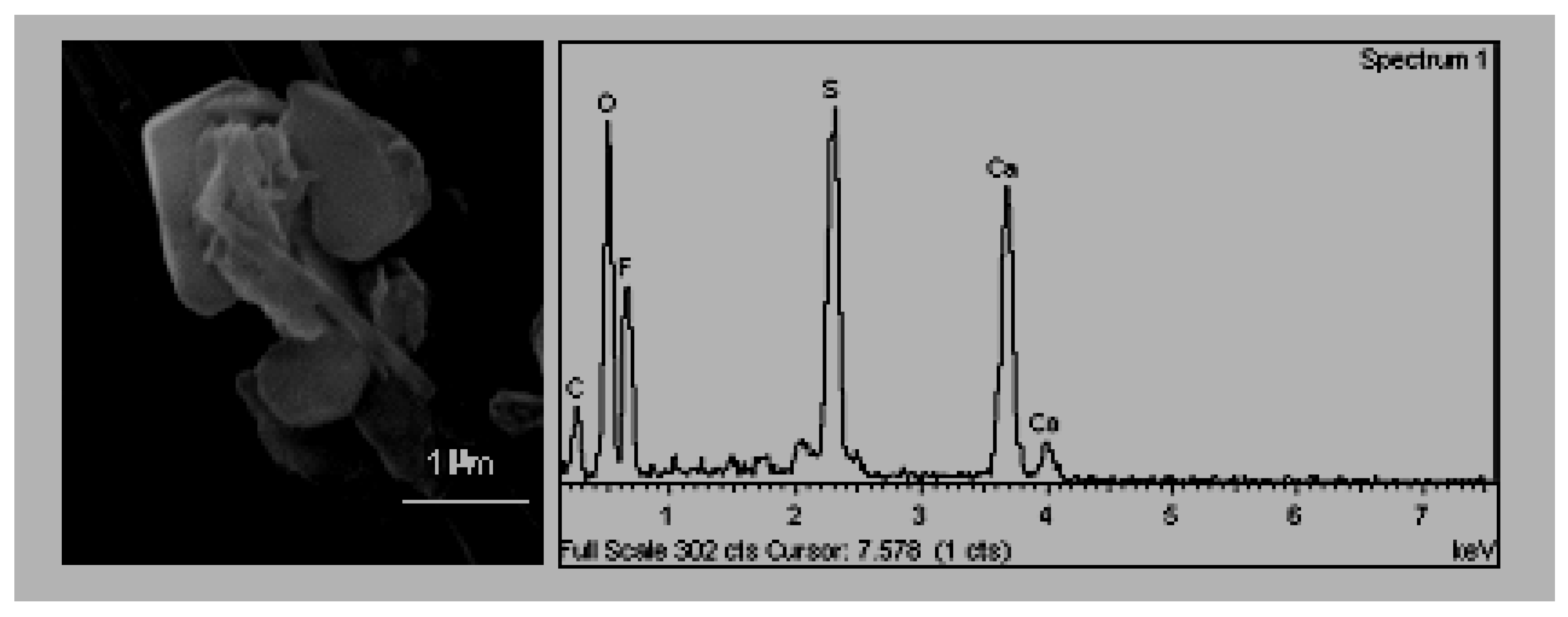
Gypsum crystal with rosette shape (
Figure 6). The neoformation of secondary gypsum does not occur directly, rather they are produced during the transporting of contaminants from the oxidation of sulphate compounds along with their subsequent precipitation or conversion with calcite [
34,
35,
36]. The primary sources of the SOx in the area are the petrochemical complex [
2] and traffic, given that the combustion of natural gas in the ceramics industry is not characterised by high emissions of sulphur compounds [
37].
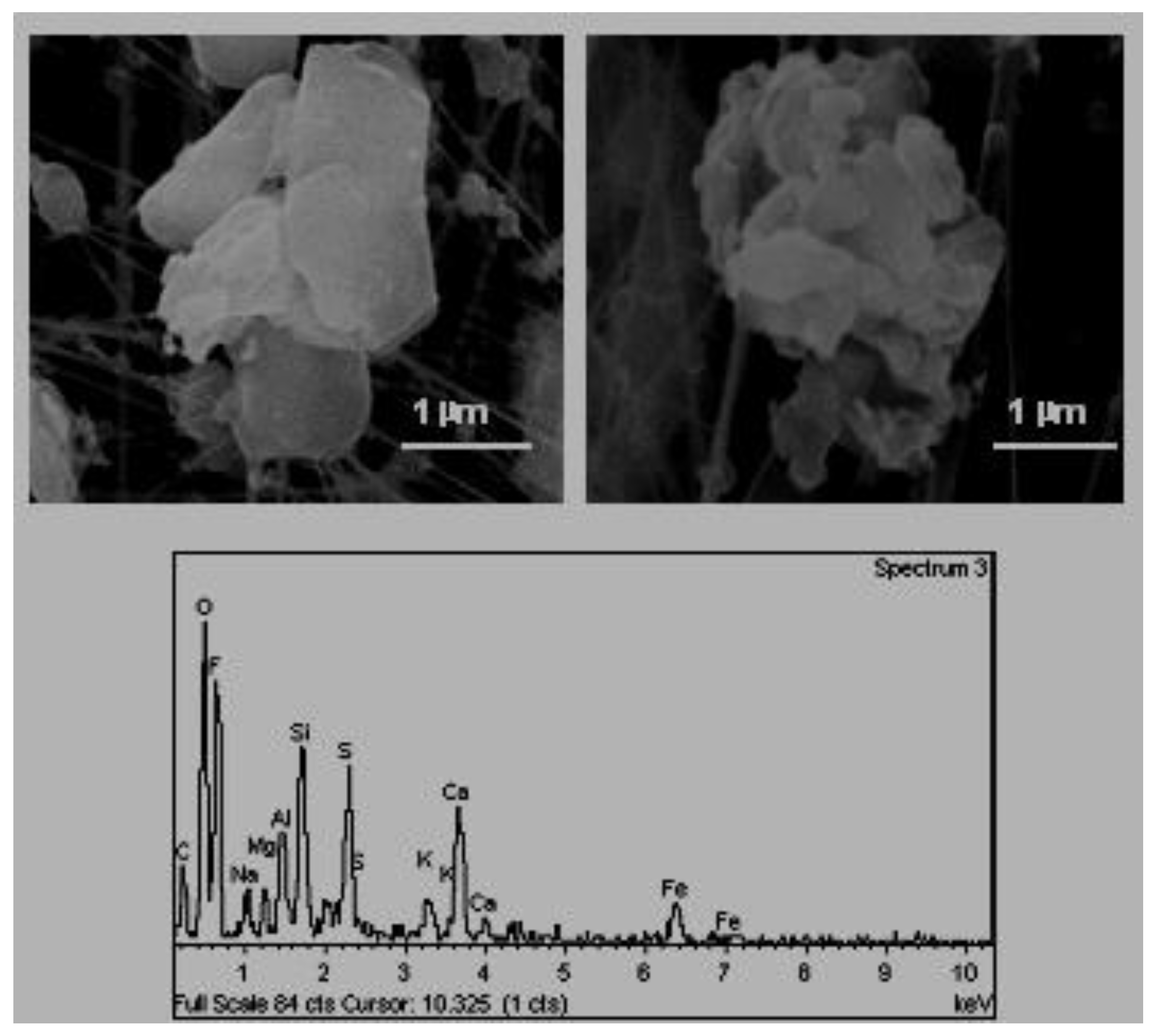
E. Mineral aggregates of varied composition (
Figure 7). These aggregates originate primarily in the sector of industrial ceramics where they use mixtures of different primary mineral materials.
The locations situated in the industrial environment present the highest concentrations of mineral particles due to the fact that the industry located there is based on the treatment of primary minerals. Previous studies carried out in this town determined that the enrichment of these particles ranges between 2 and 4 μm [
3]. Moreover, the size of the mineral particles found in schools situated in the industrial environment is greater than those identified in urban locations and rural areas. This fact is associated with more proximity of the first to the industrial sites.
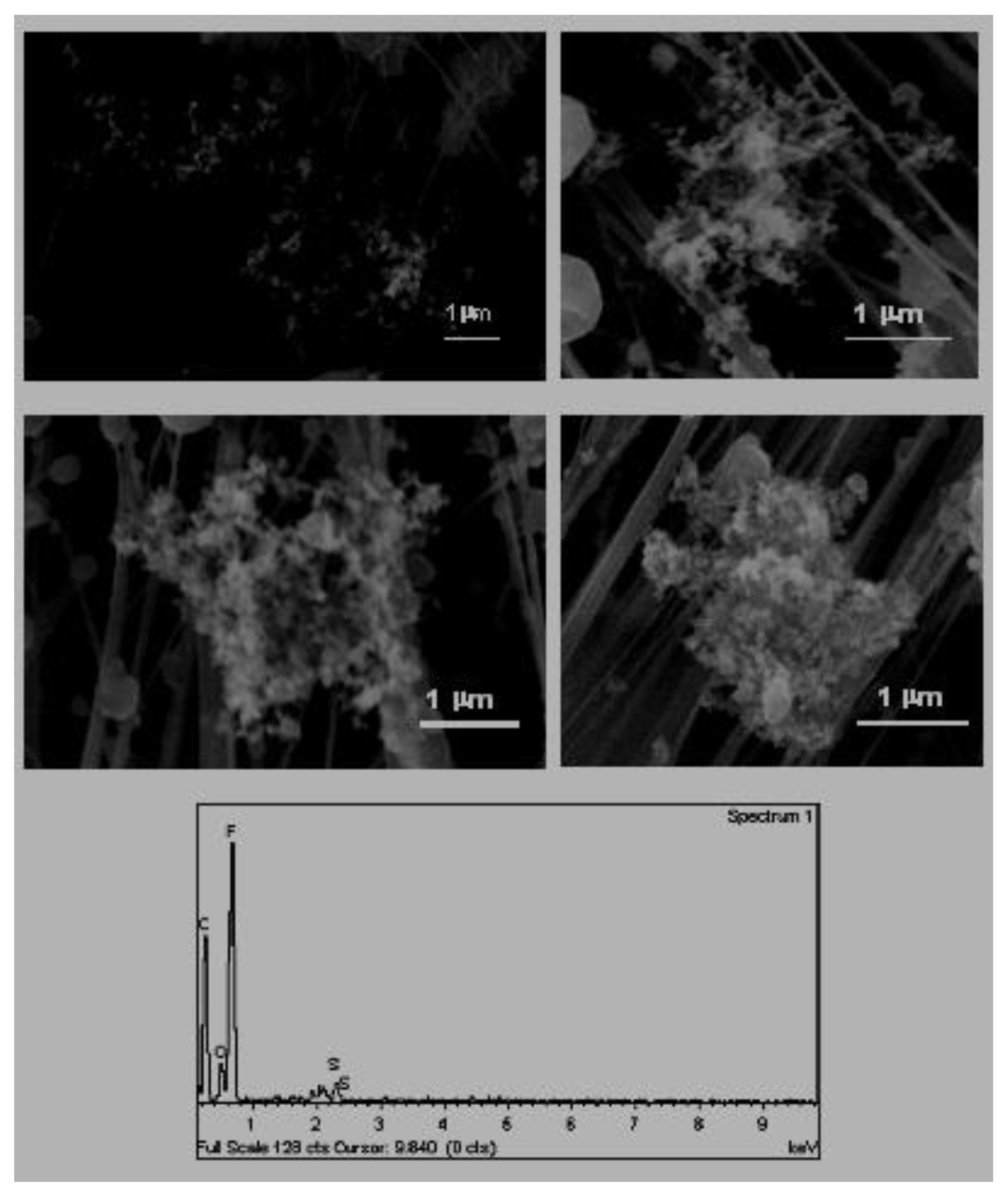
3.2. Particles Originating from Combustion Processes
This group is constituted by particles with high carbon content and exhibit a great variety of morphologies. The main ones identified in this work were sphere-like particles and dendritic soot aggregates. The origin of these particles and groupings is the same; they are associated primarily with the partial combustion of fossil fuels [
21,
23]. Normally, these two morphologies are found together [
27], the study of particles of combustion originating from the refining of nickel, both types of particles were identified, with soot representing approximately 50% of the total particles.
A. Sphere-like particles or rounded forms (
Figure 8). These include rounded particles consisting of a carbon matrix with trace elements (S, Na, K, Fe, etc.) absorbed or included in phases with greater carbon crystallinity, such as graphite [
38]. The remains of carbon with a compact morphology and the spheroids are believed to originate from carbonised particles (de-volatilisation, fluidification and posterior solidification) derived from processes of pyrolysis and incomplete oxidation from different combustible sources (carbon, gasoline, etc.) [
38]. Their sizes varied between 100 and 800 nm. These small sizes did not allow individual composition analyses to be carried out by EDX.
The formation of these types of particles is primarily associated with the incomplete combustion of fuels formed by long-chain hydrocarbons, by, for example, diesel motors or aeroplane turbines. The incomplete combustion of these fuels gives rise to, among other things, particle dehydrogenation [
23].
B. Dendritic soot aggregates (
Figure 9). The mechanisms of vaporisation and condensation during the processes of combustion (diesel and gasoline in vehicles, carbon, residual petrol, biomass, etc.) are responsible for the formation of these dendritic soot aggregates [
38]. After combustion, organic compounds condense in the air to form spheres of a very reduced size (less than 100 nm) that subsequently bond to give rise to dendritic chains of soot that can contain hundreds or thousands of these primary nano-components [
16,
21] In the samples obtained, these groupings reached 2.5 μm in size.
In previous studies in the zone [
3] when these groupings were analysed compositions were obtained with high contents of C and lesser proportions of Si, S, K, and Fe.
The difference in content and distribution between compact masses of carbon, sphere-like particles, and dendritic soot aggregates, is due to, among other factors, the proximity to traffic routes (the number of particles diminishes by 50% within a distance of 100 m from the route of circulation [
36], the velocity of the vehicles on these nearby roads (the greater velocity, the greater the possibility to produce nucleation phenomenons and, thereby, increasing the possibility of giving rise to the formation of soot) and the transport capacity of the different types of morphologies. The soot aggregates due to their small size and low density can travel great distances in the air [
21].
Working on the basis of these observations, it may be deduced that the reason for finding a greater number of particles originating from combustion processes of the sphere-like type in the urban stations was because there was more traffic and, furthermore, they were produced at slower velocities (traffic jams, traffic lights, etc.).
On the other hand, the size of the dendritic soot aggregates increased as we distance ourselves from the sources of traffic, this is due to the formation mechanism of these types of aggregates. After the combustion organic compounds condense in the air to form spheres of a very small size (less than 100 nm) that later bond in the air giving rise to dendritic chains of soot [
21]. Thus, by increasing the transport time will increase the size of these aggregates, hence, they in the rural location, greater chains were found than in the industrial one, just as these present a greater size than those found in the samples taken from the urban setting.
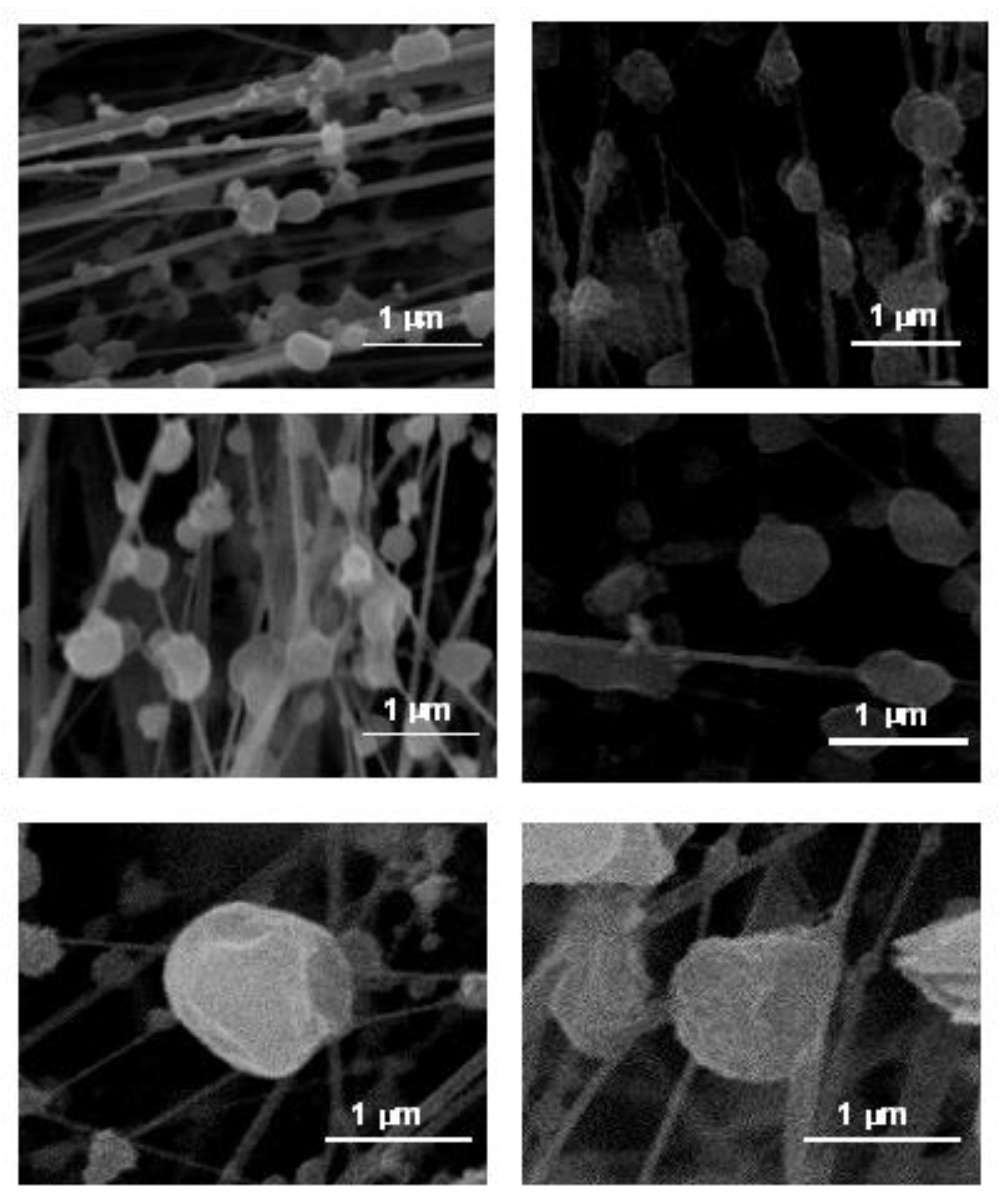
3.3. Compounds Originating from High-Temperature Industrial Processes
These particles have perfect spherical morphology (
Figure 10), geometrical shape which excludes any possible explanation based on a crystalline genesis process. Their sizes varied between 0.5 and 2.5 μm. The origin of these particles was associated with emissions of ceramic tile firing and frit melting processes. Due to the strong transfer of gases and the high temperatures reached in the interior of the frits fusion or ceramic tile cooking kiln, a small proportion of the fused material can be dragged in the form of fine aerosol from the interior to the atmosphere. The cooling that takes place when leaving the chimney occurs more rapidly than the formation in crystalline phases, thereby, being able to re-base the temperature at which fusion takes place (the temperature that overcomes the crystallisation of the material) without producing any crystals. Consequentially, a sub frozen liquid of greater or lesser viscosity is obtained, that in the centre will adopt a spherical morphology, rigid, lacking an ordered grouping of its constituent elements [
3].
Most of these particles had a seemingly plain texture on the surface, lined with a thin mesh of small cracks [
39]. A possible justification for the existence of these networks can be determined by the cooling rate inside the transformation interval. If the cooling is rapid (the gas evacuation stream temperature is much lower than the melting temperature), the added constituents will be limited in their mobility by the sharp increase in viscosity. The consequential glass will reach its rigidity with a more open structure, and, therefore, with a low contraction level (low cracking probability). However, if the cooling is slower (if the gas evacuation stream temperature is higher than the melting temperature) the increase in viscosity will be gradual and the reticulated units will have more time to gather into a closer and compact form that will lead to a higher degree of concentration (high cracking probability) [
5,
18,
38,
39,
40,
41]. Some of these spherical nodules were part of the particle agglomerates.
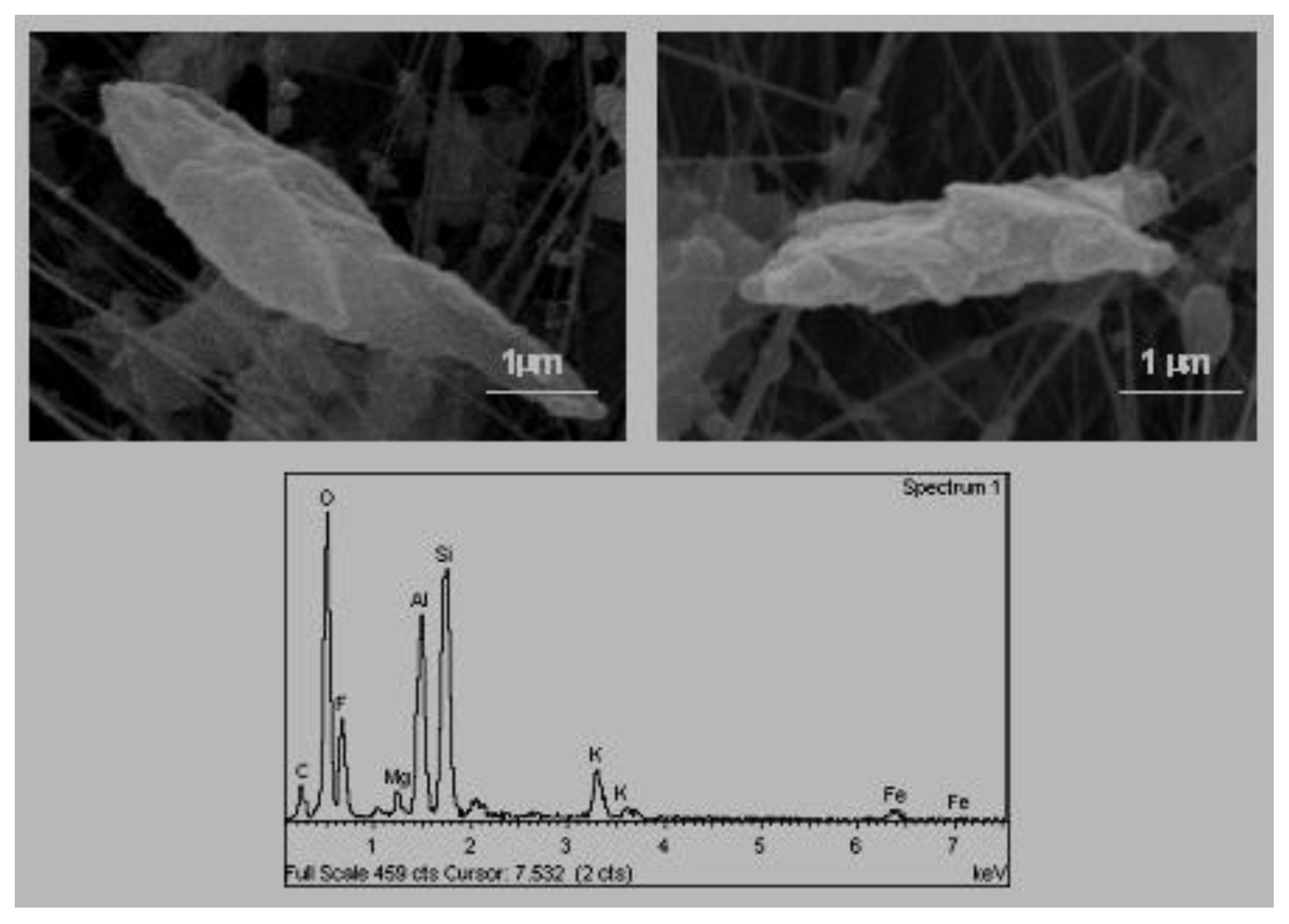
3.4. Fibres
Small quantities of fibres were identified punctually in one of the schools. The term fibre is applied to those particles that have an elongated form, with significantly greater dimensions than the other two. Because of this elongation, the fibres can have aerodynamics and other properties that are notably different from the other more compact particles [
38]. The fibres are normally classified according to their organic or inorganic composition. The size of these fibres was approximately 0.5 μm wide and from 2.5 μm to 6 μm in length.
The analysis of one of these fibres in isolation showed that they were composed primarily of carbon. Their composition, together with their morphology, permitted to relate these fibres with compounds of an organic nature.
The analysis of the fine airborne particle samples (less than 2.5 μm) obtained in the interior of the schools in the area of the study proved the existence of two main groups of particles: minerals and those originating from combustion processes. In addition, particles emitted by high -temperature processes were found in less concentration. In conclusion, a summary table was created (
Table 2) that gathers the characteristics of the principle particles identified in the fine airborne particles captured indoor of the seven primary schools located in three different environments (urban, industrial and rural) in a coastal area of the Mediterranean.
The types of particles and the differences of these types of classified particles (
Table 2) between schools clearly showed that the external emission sources, mainly industry and traffic, have a great influence on particulate levels recorded inside the schools with natural ventilation.
4. Conclusions
The paper aimed to provide a detailed study of fine particles found indoors which is hoped to serve as a basic reference when it comes to carrying out indoor air quality studies in areas that have the same contamination problem (overlapping of natural and anthropogenic emission sources).
Mineral particles were more abundant in locations near the industrial area. In addition, the size of mineral particles identified indoor at industrial locations was greater than those corresponding to the urban and rural environment due to the proximity to emission focus. The composition and morphology of these particles were associated the with raw material used in ceramic industries sector. So, dust emissions (classified inside diffuse emissions) influenced both the number and size of mineral particles found inside schools. The particles from combustion processes were found at the most amount in the urban locations. In these locations sphere-like particles were in greater proportion in schools while more soot dendritic aggregates were identified located in the industrial and rural environment. In addition, the size of dendritic soot aggregates increases as we distance ourselves from the sources which is due to the formation mechanism of these types of aggregates. The differences in quantity, morphology and size of the particles from combustion processes, found inside schools, confirmed that traffic (external source) is the main emission source of these particles. Furthermore, the differences in carbonaceous particles also indicated that particles emitted in gaseous discharges of combustion processes had influence mainly in the morphology of particles identified inside schools.
Spherical particles coming from high-temperature industrial processes were identified at urban and industrial buildings in the same proportion. However, the size of these particles was greater at industrial areas due to the proximity to formation and emission focus. So, particles emitted in gaseous discharges of industrial processes at high temperature had influence principally in the size of particles found inside schools.
This paper has established a catalogue that brings the morphologic characteristics of the main types of particles found among the indoor airborne fine particles (less than 2.5 μm) captured in the primary schools in a strategic area in the framework of the EU pollution control. However, a quantitative study carried out in this strategic studied area is necessary and urgent.