Recent Advances in Quantifying Wet Scavenging Efficiency of Black Carbon Aerosol
Abstract
:1. Introduction
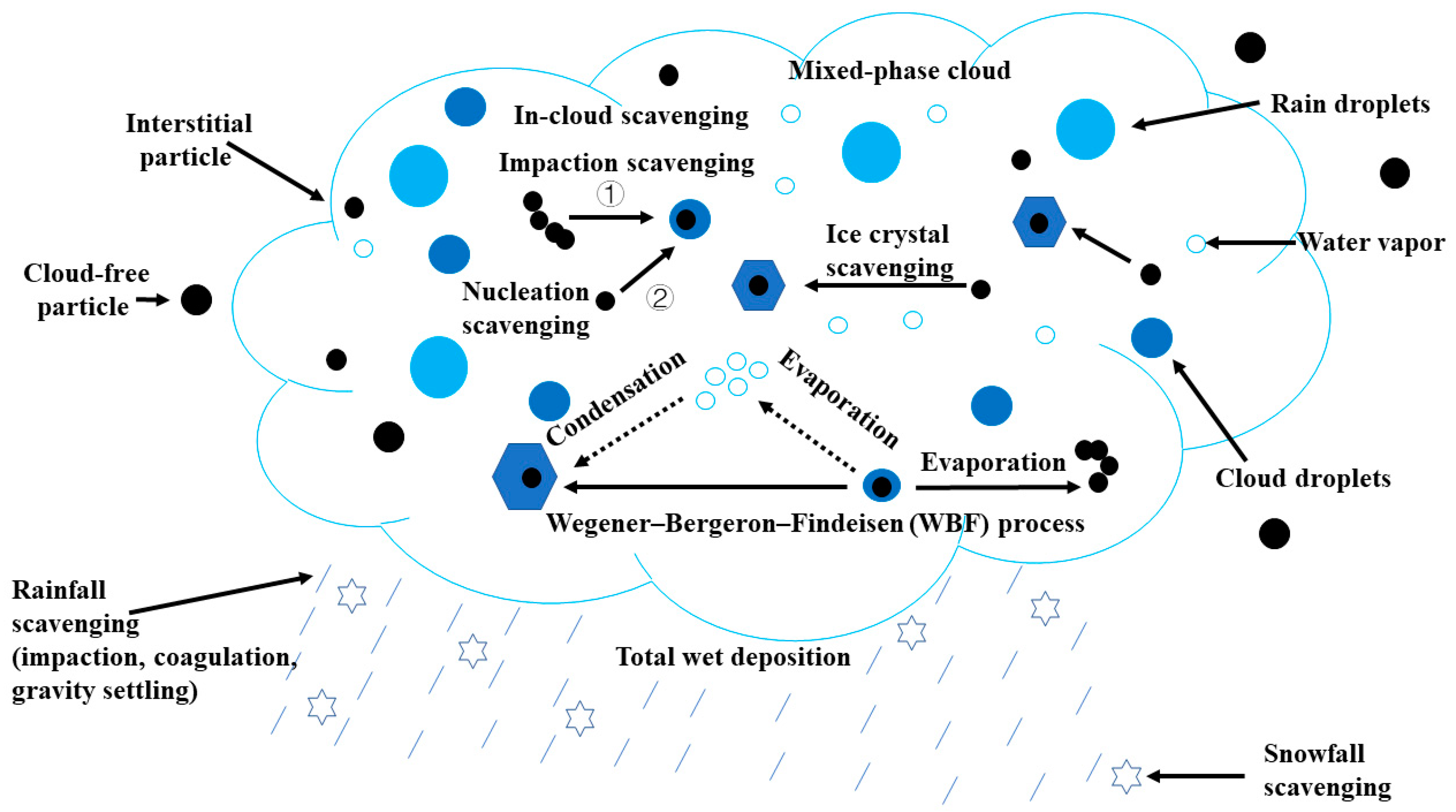
2. Methods to Investigate Wet Scavenging of BC
2.1. In-Cloud Scavenging of BC
2.2. Below-Cloud and Total Wet Scavenging of BC
3. Current State of Knowledge of Wet Scavenging of BC
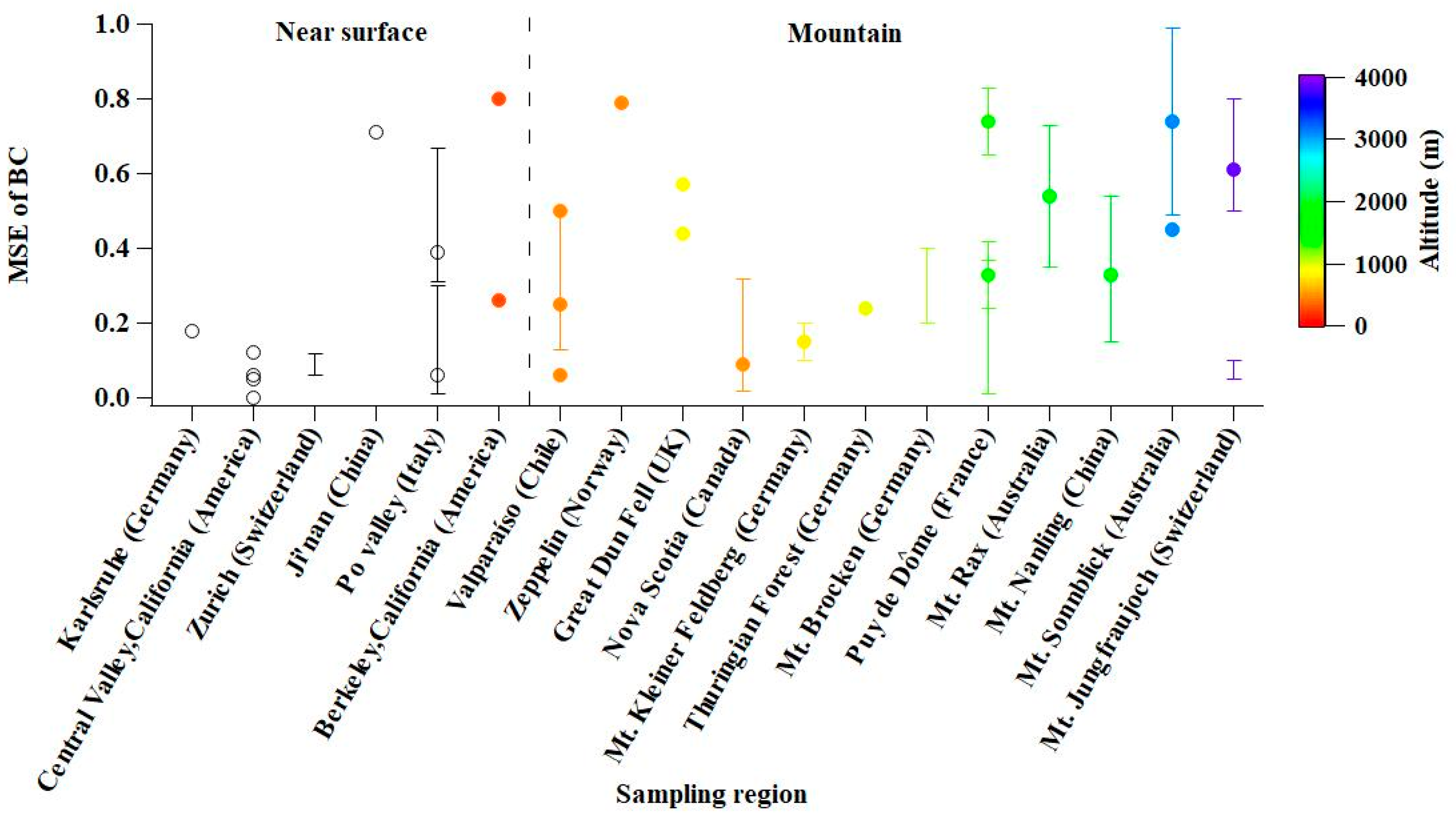
3.1. In-Cloud Mass Scavenging Efficiency of BC
3.2. Factors that Influence the In-Cloud SE of BC
3.3. Total Wet Scavenging of BC by Precipitation
3.3.1. SE of Total BC Wet Scavenging
3.3.2. Scavenging of BC by In-Cloud or Below-Cloud Processes
3.4. Modeling Results on the Wet Scavenging of BC
4. Research Needs
4.1. More Field Measurements on the Direct Links between SEs of BC and the Physical and Chemical Properties of BC-Containing Particles
4.2. High Time-Resolved Investigations of the NSE of BC-Containing Particles
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Liousse, C.; Penner, J.E.; Chuang, C.; Walton, J.J.; Eddleman, H.; Cachier, H. A global three-dimensional model study of carbonaceous aerosols. J. Geophys. Res. Atmos. 1996, 101, 19411–19432. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). The Physical Science Basis. In Contribution of Working Group I to Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Koch, D.; Del Genio, A.D. Black carbon semi-direct effects on cloud cover: Review and synthesis. Atmos. Chem. Phys. 2010, 10, 7685–7696. [Google Scholar] [CrossRef]
- Conant, W.C.; Nenes, A.; Seinfeld, J.H. Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect—1. Extended Kohler theory. J. Geophys. Res. Atmos. 2002, 107, AAC 23-1–AAC 23-9. [Google Scholar] [CrossRef]
- Nenes, A.; Conant, W.C.; Seinfeld, J.H. Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect—2. Cloud microphysics. J. Geophys. Res. Atmos. 2002, 107, AAC 24-1–AAC 24-11. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Effects of externally-through-internally-mixed soot inclusions within clouds and precipitation on global climate. J. Phys. Chem. A 2006, 110, 6860–6873. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhang, G.; Peng, L.; Bi, X.; Wang, X.; Brechtel, F.J.; Li, M.; Chen, D.; Peng, P.; Sheng, G.; et al. In situ chemical composition measurement of individual cloud residue particles at a mountain site, southern China. Atmos. Chem. Phys. 2017, 17, 8473–8488. [Google Scholar] [CrossRef]
- Bi, X.; Lin, Q.; Peng, L.; Zhang, G.; Wang, X.; Brechtel, F.J.; Chen, D.; Li, M.; Peng, P.; Sheng, G.; et al. In situ detection of the chemistry of individual fog droplet residues in the Pearl River Delta region, China. J. Geophys. Res. Atmos. 2016, 121, 9105–9116. [Google Scholar] [CrossRef]
- Levin, E.J.T.; McMeeking, G.R.; DeMott, P.J.; McCluskey, C.S.; Carrico, C.M.; Nakao, S.; Jayarathne, T.; Stone, E.A.; Stockwell, C.E.; Yokelson, R.J.; et al. Ice-nucleating particle emissions from biomass combustion and the potential importance of soot aerosol. J. Geophys. Res. Atmos. 2016, 121, 5888–5903. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Xu, L.; Yuan, Q.; Huang, D.; Chen, J.; Shi, Z.; Sun, Y.; Fu, P.; Wang, Z.; et al. Cloud scavenging of anthropogenic refractory particles at a mountain site in North China. Atmos. Chem. Phys. 2018, 18, 14681–14693. [Google Scholar] [CrossRef]
- Vergara-Temprado, J.; Holden, M.A.; Orton, T.R.; O’Sullivan, D.; Umo, N.S.; Browse, J.; Reddington, C.; Baeza-Romero, M.T.; Jones, M.J.; Lea-Langton, A.; et al. Is Black Carbon an Unimportant Ice-Nucleating Particle in Mixed-Phase Clouds? J. Geophys. Res. Atmos. 2018, 123, 4273–4283. [Google Scholar] [CrossRef]
- Lohmann, U. A glaciation indirect aerosol effect caused by soot aerosols. Geophys. Res. Lett. 2002, 29, 11. [Google Scholar] [CrossRef]
- Wang, C. A modeling study on the climate impacts of black carbon aerosols. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Poschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. Ed. Engl. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- Highwood, E.J.; Kinnersley, R.P. When smoke gets in our eyes: The multiple impacts of atmospheric black carbon on climate, air quality and health. Environ. Int. 2006, 32, 560–566. [Google Scholar] [CrossRef]
- Luben, T.J.; Nichols, J.L.; Dutton, S.J.; Kirrane, E.; Owens, E.O.; Datko-Williams, L.; Madden, M.; Sacks, J.D. A systematic review of cardiovascular emergency department visits, hospital admissions and mortality associated with ambient black carbon. Environ. Int. 2017, 107, 154–162. [Google Scholar] [CrossRef]
- Geng, F.; Hua, J.; Mu, Z.; Peng, L.; Xu, X.; Chen, R.; Kan, H. Differentiating the associations of black carbon and fine particle with daily mortality in a Chinese city. Environ. Res. 2013, 120, 27–32. [Google Scholar] [CrossRef]
- Nichols, J.L.; Owens, E.O.; Dutton, S.J.; Luben, T.J. Systematic review of the effects of black carbon on cardiovascular disease among individuals with pre-existing disease. Int. J. Public Health 2013, 58, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Chen, J. Formation, features and controlling strategies of severe haze-fog pollutions in China. Sci. Total Environ. 2017, 578, 121–138. [Google Scholar] [CrossRef]
- Cooke, W.F.; Liousse, C.; Cachier, H.; Feichter, J. Construction of a 1° × 1° fossil fuel emission data set for carbonaceous aerosol and implementation and radiative impact in the ECHAM4 model. J. Geophys. Res. Atmos. 1999, 104, 22137–22162. [Google Scholar] [CrossRef]
- Bond, T.C.; Streets, D.G.; Yarber, K.F.; Nelson, S.M.; Woo, J.H.; Klimont, Z. A technology-based global inventory of black and organic carbon emissions from combustion. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd ed.; Wiley and Sons: Hoboken, NJ, USA, 2006; pp. 60–61. ISBN 978-0-471-72018-8. [Google Scholar]
- Jurado, E.; Dachs, J.; Duarte, C.M.; Simo, R. Atmospheric deposition of organic and black carbon to the global oceans. Atmos. Environ. 2008, 42, 7931–7939. [Google Scholar] [CrossRef]
- Bourgeois, Q.; Bey, I. Pollution transport efficiency toward the Arctic: Sensitivity to aerosol scavenging and source regions. J. Geophys. Res. Atmos. 2011, 116. [Google Scholar] [CrossRef]
- Ding, M.; Tian, B.; Zhang, T.; Tang, J.; Peng, H.; Bian, L.; Sun, W. Shipborne observations of atmospheric black carbon aerosol from Shanghai to the Arctic Ocean during the 7th Chinese Arctic Research Expedition. Atmos. Res. 2018, 210, 34–40. [Google Scholar] [CrossRef]
- Tedesco, M.; Doherty, S.; Fettweis, X.; Alexander, P.; Jeyaratnam, J.; Stroeve, J. The darkening of the Greenland ice sheet: Trends, drivers, and projections (1981–2100). Cryosphere 2016, 10, 477–496. [Google Scholar] [CrossRef]
- Qi, L.; Li, Q.; Li, Y.; He, C. Factors controlling black carbon distribution in the Arctic. Atmos. Chem. Phys. 2017, 17, 1037–1059. [Google Scholar] [CrossRef]
- Ikeda, K.; Tanimoto, H.; Sugita, T.; Akiyoshi, H.; Kanaya, Y.; Zhu, C.; Taketani, F. Tagged tracer simulations of black carbon in the Arctic: Transport, source contributions, and budget. Atmos. Chem. Phys. 2017, 17, 10515–10533. [Google Scholar] [CrossRef]
- Miyakawa, T.; Oshima, N.; Taketani, F.; Komazaki, Y.; Yoshino, A.; Takami, A.; Kondo, Y.; Kanaya, Y. Alteration of the size distributions and mixing states of black carbon through transport in the boundary layer in east Asia. Atmos. Chem. Phys. 2017, 17, 5851–5864. [Google Scholar] [CrossRef]
- Liou, K.N.; Takano, Y.; He, C.; Yang, P.; Leung, L.R.; Gu, Y.; Lee, W.L. Stochastic parameterization for light absorption by internally mixed BC/dust in snow grains for application to climate models. J. Geophys. Res. Atmos. 2014, 119, 7616–7632. [Google Scholar] [CrossRef]
- He, C.; Liou, K.N.; Takano, Y.; Yang, P.; Qi, L.; Chen, F. Impact of Grain Shape and Multiple Black Carbon Internal Mixing on Snow Albedo: Parameterization and Radiative Effect Analysis. J. Geophys. Res. Atmos. 2018, 123, 1253–1268. [Google Scholar] [CrossRef]
- Furutani, H.; Dall’osto, M.; Roberts, G.C.; Prather, K.A. Assessment of the relative importance of atmospheric aging on CCN activity derived from field observations. Atmos. Environ. 2008, 42, 3130–3142. [Google Scholar] [CrossRef]
- Baumgardner, D.; Subramanian, R.; Twohy, C.; Stith, J.; Kok, G. Scavenging of black carbon by ice crystals over the northern Pacific. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Cozic, J.; Verheggen, B.; Mertes, S.; Connolly, P.; Bower, K.; Petzold, A.; Baltensperger, U.; Weingartner, E. Scavenging of black carbon in mixed phase clouds at the high alpine site Jungfraujoch. Atmos. Chem. Phys. 2007, 7, 1797–1807. [Google Scholar] [CrossRef]
- Herckes, P.; Valsaraj, K.T.; Collett, J.L., Jr. A review of observations of organic matter in fogs and clouds: Origin, processing and fate. Atmos. Res. 2013, 132, 434–449. [Google Scholar] [CrossRef]
- Sellegri, K.; Laj, P.; Dupuy, R.; Legrand, M.; Preunkert, S.; Putaud, J.P. Size-dependent scavenging efficiencies of multicomponent atmospheric aerosols in clouds. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Heintzenberg, J.; Cereceda-Balic, F.; Vidal, V.; Leck, C. Scavenging of black carbon in Chilean coastal fogs. Sci. Total Environ. 2016, 541, 341–347. [Google Scholar] [CrossRef]
- Schroder, J.C.; Hanna, S.J.; Modini, R.L.; Corrigan, A.L.; Kreidenwies, S.M.; Macdonald, A.M.; Noone, K.J.; Russell, L.M.; Leaitch, W.R.; Bertram, A.K. Size-resolved observations of refractory black carbon particles in cloud droplets at a marine boundary layer site. Atmos. Chem. Phys. 2015, 15, 1367–1383. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, Q.; Peng, L.; Bi, X.; Chen, D.; Li, M.; Li, L.; Brechtel, F.J.; Chen, J.; Yan, W.; et al. The single-particle mixing state and cloud scavenging of black carbon: A case study at a high-altitude mountain site in southern China. Atmos. Chem. Phys. 2017, 17, 14975–14985. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Zheng, J.; Khalizov, A.; Wang, X.; Wang, Z.; Zhou, Y. Size-resolved measurements of mixing state and cloud-nucleating ability of aerosols in Nanjing, China. J. Geophys. Res. Atmos. 2017, 122, 9430–9450. [Google Scholar] [CrossRef]
- Liu, D.; Allan, J.; Whitehead, J.; Young, D.; Flynn, M.; Coe, H.; McFiggans, G.; Fleming, Z.L.; Bandy, B. Ambient black carbon particle hygroscopic properties controlled by mixing state and composition. Atmos. Chem. Phys. 2013, 13, 2015–2029. [Google Scholar] [CrossRef]
- Hitzenberger, R.; Berner, A.; Glebl, H.; Drobesch, K.; Kasper-Giebl, A.; Loeflund, M.; Urban, H.; Puxbaum, H. Black carbon (BC) in alpine aerosols and cloud water—Concentrations and scavenging efficiencies. Atmos. Environ. 2001, 35, 5135–5141. [Google Scholar] [CrossRef]
- Browse, J.; Carslaw, K.S.; Arnold, S.R.; Pringle, K.; Boucher, O. The scavenging processes controlling the seasonal cycle in Arctic sulphate and black carbon aerosol. Atmos. Chem. Phys. 2012, 12, 6775–6798. [Google Scholar] [CrossRef]
- Ohata, S.; Moteki, N.; Mori, T.; Koike, M.; Kondo, Y. A key process controlling the wet removal of aerosols: New observational evidence. Sci. Rep. 2016, 6, 34113. [Google Scholar] [CrossRef]
- Vignati, E.; Karl, M.; Krol, M.; Wilson, J.; Stier, P.; Cavalli, F. Sources of uncertainties in modelling black carbon at a global scale. Atmos. Chem. Phys. 2010, 10, 2595–2611. [Google Scholar] [CrossRef]
- Johnson, K.S.; Zuberi, B.; Molina, L.T.; Molina, M.J.; Cowin, J.P.; Gaspar, D.J.; Wang, C.; Laskin, A. Processing of soot in an urban environment: Case study from the Mexico City Metropolitan Area. Atmos. Chem. Phys. 2005, 5, 3033–3043. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.F.; Pagels, J.; Zhang, D.; Xue, H.; McMurry, P.H. Variability in morphology, hygroscopicity, and optical properties of soot aerosols during atmospheric processing. Proc. Natl. Acad. Sci. USA 2008, 105, 10291–10296. [Google Scholar] [CrossRef]
- Zaveri, R.A.; Barnard, J.C.; Easter, R.C.; Riemer, N.; West, M. Particle-resolved simulation of aerosol size, composition, mixing state, and the associated optical and cloud condensation nuclei activation properties in an evolving urban plume. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
- Henning, S.; Ziese, M.; Kiselev, A.; Saathoff, H.; Mohler, O.; Mentel, T.F.; Buchholz, A.; Spindler, C.; Michaud, V.; Monier, M.; et al. Hygroscopic growth and droplet activation of soot particles: Uncoated, succinic or sulfuric acid coated. Atmos. Chem. Phys. 2012, 12, 4525–4537. [Google Scholar] [CrossRef]
- Hoose, C.; Lohmann, U.; Bennartz, R.; Croft, B.; Lesins, G. Global simulations of aerosol processing in clouds. Atmos. Chem. Phys. 2008, 8, 6939–6963. [Google Scholar] [CrossRef]
- Ervens, B. Modeling the processing of aerosol and trace gases in clouds and fogs. Chem. Rev. 2015, 115, 4157–4198. [Google Scholar] [CrossRef]
- Mertes, S.; Verheggen, B.; Walter, S.; Connolly, P.; Ebert, M.; Schneider, J.; Bower, K.N.; Cozic, J.; Weinbruch, S.; Baltensperger, U.; et al. Counterflow Virtual Impactor Based Collection of Small Ice Particles in Mixed-Phase Clouds for the Physico-Chemical Characterization of Tropospheric Ice Nuclei: Sampler Description and First Case Study. Aerosol Sci. Technol. 2007, 41, 848–864. [Google Scholar] [CrossRef]
- Shingler, T.; Dey, S.; Sorooshian, A.; Brechtel, F.J.; Wang, Z.; Metcalf, A.; Coggon, M.; Mulmenstadt, J.; Russell, L.M.; Jonsson, H.H.; et al. Characterisation and airborne deployment of a new counterflow virtual impactor inlet. Atmos. Meas. Tech. 2012, 5, 1259–1269. [Google Scholar] [CrossRef]
- Sorooshian, A.; Wang, Z.; Coggon, M.M.; Jonsson, H.H.; Ervens, B. Observations of sharp oxalate reductions in stratocumulus clouds at variable altitudes: Organic acid and metal measurements during the 2011 E-PEACE campaign. Environ. Sci. Technol. 2013, 47, 7747–7756. [Google Scholar] [CrossRef]
- Petzold, A.; Ogren, J.A.; Fiebig, M.; Laj, P.; Li, S.; Baltensperger, U.; Holzer-Popp, T.; Kinne, S.; Pappalardo, G.; Sugimoto, N.; et al. Recommendations for reporting “black carbon” measurements. Atmos. Chem. Phys. 2013, 13, 8365–8379. [Google Scholar] [CrossRef]
- Drinovec, L.; Močnik, G.; Zotter, P.; Prévôt, A.S.H.; Ruckstuhl, C.; Coz, E.; Rupakheti, M.; Sciare, J.; Müller, T.; Wiedensohler, A.; et al. The “dual-spot” Aethalometer: An improved measurement of aerosol black carbon with real-time loading compensation. Atmos. Meas. Tech. 2015, 8, 1965–1979. [Google Scholar] [CrossRef]
- Mori, T.; Kondo, Y.; Ohata, S.; Moteki, N.; Matsui, H.; Oshima, N.; Iwasaki, A. Wet deposition of black carbon at a remote site in the East China Sea. J. Geophys. Res. Atmos. 2014, 119, 10485–10498. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Chen, M.; Collier, S.; Zhou, S.; Ge, X.; Xu, J.; Shi, J.; Xie, C.; Hu, J.; et al. First Chemical Characterization of Refractory Black Carbon Aerosols and Associated Coatings over the Tibetan Plateau (4730 m a.s.l). Environ. Sci. Technol. 2017, 51, 14072–14082. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.R.; Kondo, Y.; Koike, M.; Ogren, J.A.; Jefferson, A.; Barrett, T.E.; Sheesley, R.J.; Ohata, S.; Moteki, N.; Coe, H.; et al. Evaluation of ground-based black carbon measurements by filter-based photometers at two Arctic sites. J. Geophys. Res. Atmos. 2017, 122, 3544–3572. [Google Scholar] [CrossRef]
- Bae, S.Y.; Jung, C.H.; Kim, Y.P. Relative contributions of individual phoretic effect in the below-cloud scavenging process. J. Aerosol Sci. 2009, 40, 621–632. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Guo, J.; Gao, R.; Xue, L.; Zhang, J.; Zhou, Y.; Zhou, X.; Zhang, Q.; Wang, W. Formation of secondary organic carbon and cloud impact on carbonaceous aerosols at Mount Tai, North China. Atmos. Environ. 2012, 46, 516–527. [Google Scholar] [CrossRef]
- Liu, J.; Fan, S.; Horowitz, L.W.; Levy, H. Evaluation of factors controlling long-range transport of black carbon to the Arctic. J. Geophys. Res. Atmos. 2011, 116. [Google Scholar] [CrossRef]
- Qi, L.; Li, Q.; He, C.; Wang, X.; Huang, J. Effects of the Wegener–Bergeron–Findeisen process on global black carbon distribution. Atmos. Chem. Phys. 2017, 17, 7459–7479. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Yu, F.; Pan, X.; Li, J.; Ge, B.; Wang, Z.; Hu, M.; Yang, W.; Chen, H. Estimation of atmospheric aging time of black carbon particles in the polluted atmosphere over central-eastern China using microphysical process analysis in regional chemical transport model. Atmos. Environ. 2017, 163, 44–56. [Google Scholar] [CrossRef]
- Wang, Q.; Jacob, D.J.; Spackman, J.R.; Perring, A.E.; Schwarz, J.P.; Moteki, N.; Marais, E.A.; Ge, C.; Wang, J.; Barrett, S.R.H. Global budget and radiative forcing of black carbon aerosol: Constraints from pole-to-pole (HIPPO) observations across the Pacific. J. Geophys. Res. Atmos. 2014, 119, 195–206. [Google Scholar] [CrossRef]
- Xu, D.; Ge, B.; Wang, Z.; Sun, Y.; Chen, Y.; Ji, D.; Yang, T.; Ma, Z.; Cheng, N.; Hao, J.; et al. Below-cloud wet scavenging of soluble inorganic ions by rain in Beijing during the summer of 2014. Environ. Pollut. 2017, 230, 963–973. [Google Scholar] [CrossRef]
- Aikawa, M.; Kajino, M.; Hiraki, T.; Mukai, H. The contribution of site to washout and rainout: Precipitation chemistry based on sample analysis from 0.5 mm precipitation increments and numerical simulation. Atmos. Environ. 2014, 95, 165–174. [Google Scholar] [CrossRef]
- Hadley, O.L.; Corrigan, C.E.; Kirchstetter, T.W. Modified Thermal-Optical Analysis Using Spectral Absorption Selectivity to Distinguish Black Carbon from Pyrolized Organic Carbon. Environ. Sci. Technol. 2008, 42, 8459–8464. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, L.; Yan, J.; Shi, P.; Li, Y.; Li, W. Characteristics of Particulate Carbon in Precipitation during the Rainy Season in Xiamen Island, China. Atmosphere 2016, 7, 140. [Google Scholar] [CrossRef]
- Torres, A.; Bond, T.C.; Lehmann, C.M.B.; Subramanian, R.; Hadley, O.L. Measuring Organic Carbon and Black Carbon in Rainwater: Evaluation of Methods. Aerosol Sci. Tech. 2013, 48, 239–250. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Gao, X.; Xue, L.; Wang, X.; Wang, Z.; Gao, J.; Zhang, Q.; Wang, W. Continuous observations of water-soluble ions in PM2.5 at Mount Tai (1534 m a.s.l.) in central-eastern China. J. Atmos. Chem. 2009, 64, 107–127. [Google Scholar] [CrossRef]
- Blanco-Alegre, C.; Calvo, A.I.; Coz, E.; Castro, A.; Oduber, F.; Prevot, A.S.H.; Mocnik, G.; Fraile, R. Quantification of source specific black carbon scavenging using an aethalometer and a disdrometer. Environ. Pollut. 2019, 246, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gallet, J.C.; Pedersen, C.A.; Zhang, X.; Strom, J.; Ci, Z. Elemental carbon in snow at Changbai Mountain, northeastern China: Concentrations, scavenging ratios, and dry deposition velocities. Atmos. Chem. Phys. 2014, 14, 629–640. [Google Scholar] [CrossRef]
- Hegg, D.A.; Clarke, A.D.; Doherty, S.J.; Strom, J. Measurements of black carbon aerosol washout ratio on Svalbard. Tellus B 2011, 63, 891–900. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Liu, J.; Yi, K.; Xiang, S.; Hu, X.; Wang, Y.; Tao, S.; Ban-Weiss, G. Influence of cloud microphysical processes on black carbon wet removal, global distributions, and radiative forcing. Atmos. Chem. Phys. 2019, 19, 1587–1603. [Google Scholar] [CrossRef]
- Gundel, L.A.; Benner, W.H.; Hansen, A.D.A. Chemical-Composition of Fog Water and Interstitial Aerosol in Berkeley, California. Atmos. Environ. 1994, 28, 2715–2725. [Google Scholar] [CrossRef]
- Hallberg, A.; Organ, J.A.; Noone, K.J.; Heintenberg, J. Phase partitioning for different aerosol spcies in fog. Tellus B 1992, 44, 545–555. [Google Scholar] [CrossRef]
- Gilardoni, S.; Massoli, P.; Giulianelli, L.; Rinaldi, M.; Paglione, M.; Pollini, F.; Lanconelli, C.; Poluzzi, V.; Carbone, S.; Hillamo, R.; et al. Fog scavenging of organic and inorganic aerosol in the Po Valley. Atmos. Chem. Phys. 2014, 14, 6967–6981. [Google Scholar] [CrossRef]
- Hitzenberger, R.; Berner, A.; Kromp, R.; Kasper-Giebl, A.; Limbeck, A.; Tscherwenka, W.; Puxbaum, H. Black carbon and other species at a high-elevation European site (Mount Sonnblick, 3106 m, Austria): Concentrations and scavenging efficiencies. J. Geophys. Res. Atmos. 2000, 105, 24637–24645. [Google Scholar] [CrossRef]
- Heintzenberg, J.; Leck, C. Seasonal variation of the atmospheric aerosol near the top of the marine boundary layer over Spitsbergen related to the Arctic sulphur cycle. Tellus B 1994, 46, 52–67. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Sun, J.; Li, W.; Yang, L.; Wen, L.; Wang, W.; Wang, X.; Collett, J.L., Jr.; Shi, Y.; et al. Severe haze episodes and seriously polluted fog water in Ji’nan, China. Sci. Total Environ. 2014, 493, 133–137. [Google Scholar] [CrossRef]
- Gieray, R.; Wieser, P.; Engelhardt, T. Phase partitioning of aerosol constitutents in cloud based on single-particle and bulk analysis. Atmos. Environ. 1997, 31, 2491–2502. [Google Scholar] [CrossRef]
- Motos, G.; Schmale, J.; Corbin, J.C.; Modini, R.; Karlen, N.; Bertò, M.; Baltensperger, U.; Gysel, M. Cloud droplet activation properties and scavenged fraction of black carbon in liquid-phase clouds at the high-alpine research station Jungfraujoch (3580 m a.s.l.). Atmos. Chem. Phys. Discuss. 2018. [Google Scholar] [CrossRef]
- Dlugi, R. Chemistry and Deposition of Soot Particles in Moist Air and Fog. Aerosol Sci. Tech. 1989, 10, 93–105. [Google Scholar] [CrossRef]
- Collett, J.L., Jr.; Herckes, P.; Youngster, S.; Lee, T. Processing of atmospheric organic matter by California radiation fogs. Atmos. Res. 2008, 87, 232–241. [Google Scholar] [CrossRef]
- Motos, G.; Schmale, J.; Corbin, J.C.; Zanatta, M.; Baltensperger, U.; Gysel, M. Droplet activation behaviour of atmospheric black carbon particles in fog as a function of their size and mixing state. Atmos. Chem. Phys. 2019, 19, 2183–2207. [Google Scholar] [CrossRef]
- Hansen, A.D.A.; Novakov, T. Real-Time Measurements of the Size Fractionation of Ambient Black Carbon Aerosols at Elevated Humidities. Aerosol Sci. Tech. 1989, 10, 106–110. [Google Scholar] [CrossRef]
- Chylek, P.; Banic, C.M.; Johnson, B.; Damiano, P.A.; Isaac, G.A.; Leaitch, W.R.; Liu, P.S.K.; Boudala, F.S.; Winter, B.; Ngo, D. Black carbon: Atmospheric concentrations and cloud water content measurements over southern Nova Scotia. J. Geophys. Res. Atmos. 1996, 101, 29105–29110. [Google Scholar] [CrossRef]
- Hallberg, A.; Ogren, J.A.; Noone, K.J.; Okada, K.; Heintzenberg, J.; Svenningsson, I.B. The Influence of Aerosol-Particle Composition on Cloud Droplet Formation. J. Atmos. Chem. 1994, 19, 153–171. [Google Scholar] [CrossRef]
- Schneider, J.; Mertes, S.; van Pinxteren, D.; Herrmann, H.; Borrmann, S. Uptake of nitric acid, ammonia, and organics in orographic clouds: mass spectrometric analyses of droplet residual and interstitial aerosol particles. Atmos. Chem. Phys. 2017, 17, 1571–1593. [Google Scholar] [CrossRef]
- Acker, K.; Mertes, S.; Moller, D.; Wieprecht, W.; Auel, R.; Kalass, D. Case study of cloud physical and chemical processes in low clouds at Mt. Brocken. Atmos. Res. 2002, 64, 41–51. [Google Scholar] [CrossRef]
- Laj, P.; Flossmann, A.I.; Wobrock, W.; Fuzzi, S.; Orsi, G.; Ricci, L.; Mertes, S.; Schwarzenbock, A.; Heintzenberg, J.; Ten Brink, H. Behaviour of H2O2, NH3, and black carbon in mixed-phase clouds during CIME. Atmos. Res. 2001, 58, 315–336. [Google Scholar] [CrossRef]
- Kasper-Giebl, A.; Koch, A.; Hitzenberger, R.; Puxbaum, H. Scavenging efficiency of ‘aerosol carbon’ and sulfate in supercooled clouds at Mt. Sonnblick (3106m a.s.l., Austria). J. Atmos. Chem. 2000, 35, 33–46. [Google Scholar] [CrossRef]
- Moteki, N.; Kondo, Y.; Oshima, N.; Takegawa, N.; Koike, M.; Kita, K.; Matsui, H.; Kajino, M. Size dependence of wet removal of black carbon aerosols during transport from the boundary layer to the free troposphere. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Matsui, H. Black carbon simulations using a size- and mixing-state-resolved three-dimensional model: 2. Aging timescale and its impact over East Asia. J. Geophys. Res. Atmos. 2016, 121, 1808–1821. [Google Scholar] [CrossRef]
- Dusek, U.; Reischl, G.P.; Hitzenberger, R. CCN activation of pure and coated carbon black particles. Environ. Sci. Technol. 2006, 40, 1223–1230. [Google Scholar] [CrossRef]
- Ching, J.; West, M.; Riemer, N. Quantifying Impacts of Aerosol Mixing State on Nucleation-Scavenging of Black Carbon Aerosol Particles. Atmosphere 2018, 9, 17. [Google Scholar] [CrossRef]
- Dusek, U.; Frank, G.P.; Hildebrandt, L.; Curtius, J.; Schneider, J.; Walter, S.; Chand, D.; Drewnick, F.; Hings, S.; Jung, D.; et al. Size matters more than chemistry for cloud-nucleating ability of aerosol particles. Science 2006, 312, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liu, J.; Horowitz, L.W.; Henze, D.K.; Fan, S.; Levy, H.; Mauzerall, D.L.; Lin, J.; Tao, S. Analysis of transpacific transport of black carbon during HIPPO-3: Implications for black carbon aging. Atmos. Chem. Phys. 2014, 14, 6315–6327. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Tao, S.; Ban-Weiss, G.A. Long-range transport of black carbon to the Pacific Ocean and its dependence on aging timescale. Atmos. Chem. Phys. 2015, 15, 11521–11535. [Google Scholar] [CrossRef]
- He, C.; Li, Q.; Liou, K.N.; Qi, L.; Tao, S.; Schwarz, J.P. Microphysics-based black carbon aging in a global CTM: Constraints from HIPPO observations and implications for global black carbon budget. Atmos. Chem. Phys. 2016, 16, 3077–3098. [Google Scholar] [CrossRef]
- Li, K.; Ye, X.; Pang, H.; Lu, X.; Chen, H.; Wang, X.; Yang, X.; Chen, J.; Chen, Y. Temporal variations in the hygroscopicity and mixing state of black carbon aerosols in a polluted megacity area. Atmos. Chem. Phys. 2018, 18, 15201–15218. [Google Scholar] [CrossRef]
- Sarangi, B.; Ramachandran, S.; Rajesh, T.A.; Dhaker, V.K. Black carbon linked aerosol hygroscopic growth: Size and mixing state are crucial. Atmos. Environ. 2019, 200, 110–118. [Google Scholar] [CrossRef]
- Wittbom, C.; Eriksson, A.C.; Rissler, J.; Carlsson, J.E.; Roldin, P.; Nordin, E.Z.; Nilsson, P.T.; Swietlicki, E.; Pagels, J.H.; Svenningsson, B. Cloud droplet activity changes of soot aerosol upon smog chamber ageing. Atmos. Chem. Phys. 2014, 14, 9831–9854. [Google Scholar] [CrossRef]
- Kupiszewski, P.; Zanatta, M.; Mertes, S.; Vochezer, P.; Lloyd, G.; Schneider, J.; Schenk, L.; Schnaiter, M.; Baltensperger, U.; Weingartner, E.; et al. Ice residual properties in mixed-phase clouds at the high-alpine Jungfraujoch site. J. Geophys. Res. Atmos. 2016, 121, 12343–12362. [Google Scholar] [CrossRef] [PubMed]
- Cziczo, D.J.; Froyd, K.D.; Hoose, C.; Jensen, E.J.; Diao, M.; Zondlo, M.A.; Smith, J.B.; Twohy, C.H.; Murphy, D.M. Clarifying the dominant sources and mechanisms of cirrus cloud formation. Science 2013, 340, 1320–1324. [Google Scholar] [CrossRef]
- Cozic, J.; Mertes, S.; Verheggen, B.; Cziczo, D.J.; Gallavardin, S.J.; Walter, S.; Baltensperger, U.; Weingartner, E. Black carbon enrichment in atmospheric ice particle residuals observed in lower tropospheric mixed phase clouds. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Targino, A.C.; Coe, H.; Cozic, J.; Crosier, J.; Crawford, I.; Bower, K.; Flynn, M.; Gallagher, M.; Allan, J.; Verheggen, B.; et al. Influence of particle chemical composition on the phase of cold clouds at a high-alpine site in Switzerland. J. Geophys. Res. Atmos. 2009, 114. [Google Scholar] [CrossRef]
- Kulkarni, G.; China, S.; Liu, S.; Nandasiri, M.; Sharma, N.; Wilson, J.; Aiken, A.C.; Chand, D.; Laskin, A.; Mazzoleni, C.; et al. Ice nucleation activity of diesel soot particles at cirrus relevant temperature conditions: Effects of hydration, secondary organics coating, soot morphology, and coagulation. Geophys. Res. Lett. 2016, 43, 3580–3588. [Google Scholar] [CrossRef]
- Mahmood, R.; von Salzen, K.; Flanner, M.; Sand, M.; Langner, J.; Wang, H.L.; Huang, L. Seasonality of global and Arctic black carbon processes in the Arctic Monitoring and Assessment Programme models. J. Geophys. Res. Atmos. 2016, 121, 7100–7116. [Google Scholar] [CrossRef]
- Liu, D.; Flynn, M.; Gysel, M.; Targino, A.; Crawford, I.; Bower, K.; Choularton, T.; Juranyi, Z.; Steinbacher, M.; Huglin, C.; et al. Single particle characterization of black carbon aerosols at a tropospheric alpine site in Switzerland. Atmos. Chem. Phys. 2010, 10, 7389–7407. [Google Scholar] [CrossRef]
- Ducret, J.; Cachier, H. Particulate Carbon Content in Rain at Various Temperate and Tropical Locations. J. Atmos. Chem. 1992, 15, 55–67. [Google Scholar] [CrossRef]
- Cerqueira, M.; Pio, C.; Legrand, M.; Puxbaum, H.; Kasper-Giebl, A.; Afonso, J.; Preunkert, S.; Gelencser, A.; Fialho, P. Particulate carbon in precipitation at European background sites. J. Aerosol Sci. 2010, 41, 51–61. [Google Scholar] [CrossRef]
- Tsyro, S.; Simpson, D.; Tarrason, L.; Klimont, Z.; Kupiainen, K.; Pio, C.; Yttri, K.E. Modeling of elemental carbon over Europe. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Gogoi, M.M.; Babu, S.S.; Moorthy, K.K.; Thakur, R.C.; Chaubey, J.P.; Nair, V.S. Aerosol black carbon over Svalbard regions of Arctic. Polar Sci. 2016, 10, 60–70. [Google Scholar] [CrossRef]
- Gogoi, M.M.; Babu, S.S.; Pandey, S.K.; Nair, V.S.; Vaishya, A.; Girach, I.A.; Koushik, N. Scavenging ratio of black carbon in the Arctic and the Antarctic. Polar Sci. 2018, 16, 10–22. [Google Scholar] [CrossRef]
- Grythe, H.; Kristiansen, N.I.; Zwaaftink, C.D.G.; Eckhardt, S.; Strom, J.; Tunved, P.; Krejci, R.; Stohl, A. A new aerosol wet removal scheme for the Lagrangian particle model FLEXPART v10. Geosci. Model Dev. 2017, 10, 1447–1466. [Google Scholar] [CrossRef]
- Zhang, L.; Michelangeli, D.V.; Taylor, P.A. Numerical studies of aerosol scavenging by low-level, warm stratiform clouds and precipitation. Atmos. Environ. 2004, 38, 4653–4665. [Google Scholar] [CrossRef]
- Levin, Z.; Teller, A.; Ganor, E.; Graham, B.; Andreae, M.O.; Maenhaut, W.; Falkovich, A.H.; Rudich, Y. Role of aerosol size and composition in nucleation scavenging within clouds in a shallow cold front. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Andronache, C. Estimated variability of below-cloud aerosol removal by rainfall for observed aerosol size distributions. Atmos. Chem. Phys. 2003, 3, 131–143. [Google Scholar] [CrossRef]
- Kondo, Y.; Moteki, N.; Oshima, N.; Ohata, S.; Koike, M.; Shibano, Y.; Takegawa, N.; Kita, K. Effects of wet deposition on the abundance and size distribution of black carbon in East Asia. J. Geophys. Res. Atmos. 2016, 121, 4691–4712. [Google Scholar] [CrossRef]
- Emerson, E.W.; Katich, J.M.; Schwarz, J.P.; McMeeking, G.R.; Farmer, D.K. Direct Measurements of Dry and Wet Deposition of Black Carbon Over a Grassland. J. Geophys. Res. Atmos. 2018, 123, 12277–12290. [Google Scholar] [CrossRef]
- Budhavant, K.B.; Rao, P.S.P.; Safai, P.D.; Leck, C.; Rodhe, H. Black carbon in cloud-water and rain water during monsoon season at a high altitude station in India. Atmos. Environ. 2016, 129, 256–264. [Google Scholar] [CrossRef]
- Kanaya, Y.; Pan, X.; Miyakawa, T.; Komazaki, Y.; Taketani, F.; Uno, I.; Kondo, Y. Long-term observations of black carbon mass concentrations at Fukue Island, western Japan, during 2009–2015: Constraining wet removal rates and emission strengths from East Asia. Atmos. Chem. Phys. 2016, 16, 10689–10705. [Google Scholar] [CrossRef]
- Huo, M.Q.; Sato, K.; Ohizumi, T.; Akimoto, H.; Takahashi, K. Characteristics of carbonaceous components in precipitation and atmospheric particle at Japanese sites. Atmos. Environ. 2016, 146, 164–173. [Google Scholar] [CrossRef]
- Andronache, C.; Gronholm, T.; Laakso, L.; Phillips, V.; Venalainen, A. Scavenging of ultrafine particles by rainfall at a boreal site: observations and model estimations. Atmos. Chem. Phys. 2006, 6, 4739–4754. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, M.; Lin, P.; Liu, S.; Wehner, B.; Wiedensohler, A. Particle number size distribution in the urban atmosphere of Beijing, China. Atmos. Environ. 2008, 42, 7967–7980. [Google Scholar] [CrossRef]
- Bae, S.Y.; Park, R.J.; Kim, Y.P.; Woo, J.H. Effects of below-cloud scavenging on the regional aerosol budget in East Asia. Atmos. Environ. 2012, 58, 14–22. [Google Scholar] [CrossRef]
- Querel, A.; Monier, M.; Flossmann, A.I.; Lemaitre, P.; Porcheron, E. The importance of new collection efficiency values including the effect of rear capture for the below-cloud scavenging of aerosol particles. Atmos. Res. 2014, 142, 57–66. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, Y.; He, J.; Yin, D.; Wang, B. Below-cloud scavenging of aerosol particles by precipitation in a typical valley city, northwestern China. Atmos. Environ. 2015, 102, 70–78. [Google Scholar] [CrossRef]
- Seland, O.; Iversen, T. A scheme for black carbon and sulphate aerosols tested in a hemispheric scale, Eulerian dispersion model. Atmos. Environ. 1999, 33, 2853–2879. [Google Scholar] [CrossRef]
- Hou, P.; Wu, S.; McCarty, J.L.; Gao, Y. Sensitivity of atmospheric aerosol scavenging to precipitation intensity and frequency in the context of global climate change. Atmos. Chem. Phys. 2018, 18, 8173–8182. [Google Scholar] [CrossRef]
- Witkowska, A.; Lewandowska, A.; Falkowska, L.M. Parallel measurements of organic and elemental carbon dry (PM1, PM2.5) and wet (rain, snow, mixed) deposition into the Baltic Sea. Mar. Pollut. Bull. 2016, 104, 303–312. [Google Scholar] [CrossRef]
- Oshima, N.; Kondo, Y.; Moteki, N.; Takegawa, N.; Koike, M.; Kita, K.; Matsui, H.; Kajino, M.; Nakamura, H.; Jung, J.S.; et al. Wet removal of black carbon in Asian outflow: Aerosol Radiative Forcing in East Asia (A-FORCE) aircraft campaign. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Girach, I.A.; Nair, V.S.; Babu, S.S.; Nair, P.R. Black carbon and carbon monoxide over Bay of Bengal during W_ICARB: Source characteristics. Atmos. Environ. 2014, 94, 508–517. [Google Scholar] [CrossRef]
- Latha, K.M.; Badarinath, K.V.S.; Reddy, P.M. Scavenging efficiency of rainfall on black carbon aerosols over an urban environment. Atmos. Sci. Lett. 2005, 6, 148–151. [Google Scholar] [CrossRef]
- Hoose, C.; Lohmann, U.; Stier, P.; Verheggen, B.; Weingartner, E. Aerosol processing in mixed-phase clouds in ECHAM5-HAM: Model description and comparison to observations. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Koch, D.; Schulz, M.; Kinne, S.; McNaughton, C.; Spackman, J.R.; Balkanski, Y.; Bauer, S.; Berntsen, T.; Bond, T.C.; Boucher, O.; et al. Evaluation of black carbon estimations in global aerosol models. Atmos. Chem. Phys. 2009, 9, 9001–9026. [Google Scholar] [CrossRef]
- Samset, B.H.; Myhre, G.; Herber, A.; Kondo, Y.; Li, S.; Moteki, N.; Koike, M.; Oshima, N.; Schwarz, J.P.; Balkanski, Y.; et al. Modelled black carbon radiative forcing and atmospheric lifetime in AeroCom Phase II constrained by aircraft observations. Atmos. Chem. Phys. 2014, 14, 12465–12477. [Google Scholar] [CrossRef]
- Iversen, T. A scheme for process-tagged SO4 and BC aerosols in NCAR CCM3: Validation and sensitivity to cloud processes. J. Geophys. Res. Atmos. 2002, 107. [Google Scholar] [CrossRef]
- Srivastava, R.; Bran, S.H. Impact of dynamical and microphysical schemes on black carbon prediction in a regional climate model over India. Environ. Sci. Pollut. Res. Int. 2018, 25, 14844–14855. [Google Scholar] [CrossRef]
- Huang, Y.; Chameides, W.L.; Tan, Q.; Dickinson, R.E. Characteristics of Anthropogenic Sulfate and Carbonaceous Aerosols over East Asia: Regional Modeling and Observation. Adv. Atmos. Sci. 2008, 25, 946–959. [Google Scholar] [CrossRef]
- Koch, D. Transport and direct radiative forcing of carbonaceous and sulfate aerosols in the GISS GCM. J. Geophys. Res. Atmos. 2001, 106, 20311–20332. [Google Scholar] [CrossRef]
- Kipling, Z.; Stier, P.; Schwarz, J.P.; Perring, A.E.; Spackman, J.R.; Mann, G.W.; Johnson, C.E.; Telford, P.J. Constraints on aerosol processes in climate models from vertically-resolved aircraft observations of black carbon. Atmos. Chem. Phys. 2013, 13, 5969–5986. [Google Scholar] [CrossRef]
- Pierce, J.R.; Croft, B.; Kodros, J.K.; D’Andrea, S.D.; Martin, R.V. The importance of interstitial particle scavenging by cloud droplets in shaping the remote aerosol size distribution and global aerosol-climate effects. Atmos. Chem. Phys. 2015, 15, 6147–6158. [Google Scholar] [CrossRef]
- Croft, B.; Lohmann, U.; Martin, R.V.; Stier, P.; Wurzler, S.; Feichter, J.; Hoose, C.; Heikkila, U.; van Donkelaar, A.; Ferrachat, S. Influences of in-cloud aerosol scavenging parameterizations on aerosol concentrations and wet deposition in ECHAM5-HAM. Atmos. Chem. Phys. 2010, 10, 1511–1543. [Google Scholar] [CrossRef]
- Yang, Q.; Gustafson, W.I.; Fast, J.D.; Wang, H.; Easter, R.C.; Morrison, H.; Lee, Y.N.; Chapman, E.G.; Spak, S.N.; Mena-Carrasco, M.A. Assessing regional scale predictions of aerosols, marine stratocumulus, and their interactions during VOCALS-REx using WRF-Chem. Atmos. Chem. Phys. 2011, 11, 11951–11975. [Google Scholar] [CrossRef]
- Thomas, J.L.; Polashenski, C.M.; Soja, A.J.; Marelle, L.; Casey, K.A.; Choi, H.D.; Raut, J.C.; Wiedinmyer, C.; Emmons, L.K.; Fast, J.D.; et al. Quantifying black carbon deposition over the Greenland ice sheet from forest fires in Canada. Geophys. Res. Lett. 2017, 44, 7965–7974. [Google Scholar] [CrossRef]
- Hienola, A.I.; Pietikäinen, J.P.; Jacob, D.; Pozdun, R.; Petäjä, T.; Hyvärinen, A.P.; Sogacheva, L.; Kerminen, V.M.; Kulmala, M.; Laaksonen, A. Black carbon concentration and deposition estimations in Finland by the regional aerosol–climate model REMO-HAM. Atmos. Chem. Phys. 2013, 13, 4033–4055. [Google Scholar] [CrossRef]
- Stier, P.; Feichter, J.; Kinne, S.; Kloster, S.; Vignati, E.; Wilson, J.; Ganzeveld, L.; Tegen, I.; Werner, M.; Balkanski, Y.; et al. The aerosol-climate model ECHAM5-HAM. Atmos. Chem. Phys. 2005, 5, 1125–1156. [Google Scholar] [CrossRef]
- Yang, Q.; Gustafson, W.I.; Fast, J.D.; Wang, H.; Easter, R.C.; Wang, M.; Ghan, S.J.; Berg, L.K.; Leung, L.R.; Morrison, H. Impact of natural and anthropogenic aerosols on stratocumulus and precipitation in the Southeast Pacific: A regional modelling study using WRF-Chem. Atmos. Chem. Phys. 2012, 12, 8777–8796. [Google Scholar] [CrossRef]
- Fan, S.-M.; Schwarz, J.P.; Liu, J.; Fahey, D.W.; Ginoux, P.; Horowitz, L.W.; Levy, H.; Ming, Y.; Spackman, J.R. Inferring ice formation processes from global-scale black carbon profiles observed in the remote atmosphere and model simulations. J. Geophys. Res. Atmos. 2012, 117, D23205. [Google Scholar] [CrossRef]
- Ching, J.; Riemer, N.; West, M. Impacts of black carbon mixing state on black carbon nucleation scavenging: Insights from a particle-resolved model. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Lund, M.T.; Berntsen, T.K.; Samset, B.H. Sensitivity of black carbon concentrations and climate impact to aging and scavenging in OsloCTM2–M7. Atmos. Chem. Phys. 2017, 17, 6003–6022. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, C.; Chen, H.; Nizkorodov, S.A.; Chen, J.; Yang, X. Size distribution and mixing state of black carbon particles during a heavy air pollution episode in Shanghai. Atmos. Chem. Phys. 2016, 16, 5399–5411. [Google Scholar] [CrossRef]
- Hitzenberger, R.; Tohno, S. Comparison of black carbon (BC) aerosols in two urban areas—Concentrations and size distributions. Atmos. Environ. 2001, 35, 2153–2167. [Google Scholar] [CrossRef]
- Safai, P.D.; Raju, M.P.; Budhavant, K.B.; Rao, P.S.P.; Devara, P.C.S. Long term studies on characteristics of black carbon aerosols over a tropical urban station Pune, India. Atmos. Res. 2013, 132, 173–184. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, T.; Liu, D.; Allan, J.D.; Zheng, L.; Chen, H. Light Absorption Enhancement of Black Carbon Aerosol Constrained by Particle Morphology. Environ. Sci. Technol. 2018, 52, 6912–6919. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Chung, S.H.; Buseck, P.R. Shapes of soot aerosol particles and implications for their effects on climate. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
- Li, W.; Shao, L.; Zhang, D.; Ro, C.; Hu, M.; Bi, X.; Geng, H.; Matsuki, A.; Niu, H.; Chen, J. A review of single aerosol particle studies in the atmosphere of East Asia: Morphology, mixing state, source, and heterogeneous reactions. J. Clean. Prod. 2016, 112, 1330–1349. [Google Scholar] [CrossRef]
- Ueda, S.; Osada, K.; Okada, K. Mixing states of cloud interstitial particles between water-soluble and insoluble materials at Mt. Tateyama, Japan: Effects of meteorological conditions. Atmos. Res. 2011, 99, 325–336. [Google Scholar] [CrossRef]
- Croft, B.; Martin, R.V.; Leaitch, W.R.; Tunved, P.; Breider, T.J.; D’Andrea, S.D.; Pierce, J.R. Processes controlling the annual cycle of Arctic aerosol number and size distributions. Atmos. Chem. Phys. 2016, 16, 3665–3682. [Google Scholar] [CrossRef]
- Yin, J.; Wang, D.; Zhai, G.; Xu, H. An investigation into the relationship between liquid water content and cloud number concentration in the stratiform clouds over north China. Atmos. Res. 2014, 139, 137–143. [Google Scholar] [CrossRef]
- Koracin, D.; Businger, J.; Dorman, C.; Lewis, J. Formation, evolution, and dissipation of coastal sea fog. Bound.-Layer Meteorol. 2005, 117, 447–478. [Google Scholar] [CrossRef]
- Dupont, J.-C.; Haeffelin, M.; Protat, A.; Bouniol, D.; Boyouk, N.; Morille, Y. Stratus–Fog Formation and Dissipation: A 6-Day Case Study. Bound.-Layer Meteorol. 2012, 143, 207–225. [Google Scholar] [CrossRef]
- Targino, A.C.; Noone, K.J.; Drewnick, F.; Schneider, J.; Krejci, R.; Olivares, G.; Hings, S.; Borrmann, S. Microphysical and chemical characteristics of cloud droplet residuals and interstitial particles in continental stratocumulus clouds. Atmos. Res. 2007, 86, 225–240. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Fu, Y.; Lin, Q.; Jiang, F.; Lian, X.; Li, L.; Wang, Z.; Zhang, G.; Bi, X.; Wang, X.; et al. Recent Advances in Quantifying Wet Scavenging Efficiency of Black Carbon Aerosol. Atmosphere 2019, 10, 175. https://doi.org/10.3390/atmos10040175
Yang Y, Fu Y, Lin Q, Jiang F, Lian X, Li L, Wang Z, Zhang G, Bi X, Wang X, et al. Recent Advances in Quantifying Wet Scavenging Efficiency of Black Carbon Aerosol. Atmosphere. 2019; 10(4):175. https://doi.org/10.3390/atmos10040175
Chicago/Turabian StyleYang, Yuxiang, Yuzhen Fu, Qinhao Lin, Feng Jiang, Xiufeng Lian, Lei Li, Zhanyong Wang, Guohua Zhang, Xinhui Bi, Xinming Wang, and et al. 2019. "Recent Advances in Quantifying Wet Scavenging Efficiency of Black Carbon Aerosol" Atmosphere 10, no. 4: 175. https://doi.org/10.3390/atmos10040175
APA StyleYang, Y., Fu, Y., Lin, Q., Jiang, F., Lian, X., Li, L., Wang, Z., Zhang, G., Bi, X., Wang, X., & Sheng, G. (2019). Recent Advances in Quantifying Wet Scavenging Efficiency of Black Carbon Aerosol. Atmosphere, 10(4), 175. https://doi.org/10.3390/atmos10040175





