Seasonal Variations and Chemical Predictors of Oxidative Potential (OP) of Particulate Matter (PM), for Seven Urban French Sites
Abstract
1. Introduction
2. Experiments
2.1. Samplings
2.2. Chemical Composition of PM10
2.3. OP Measurements of PM10
2.4. Quality of the Data
2.5. Data Treatment Procedures
3. Results
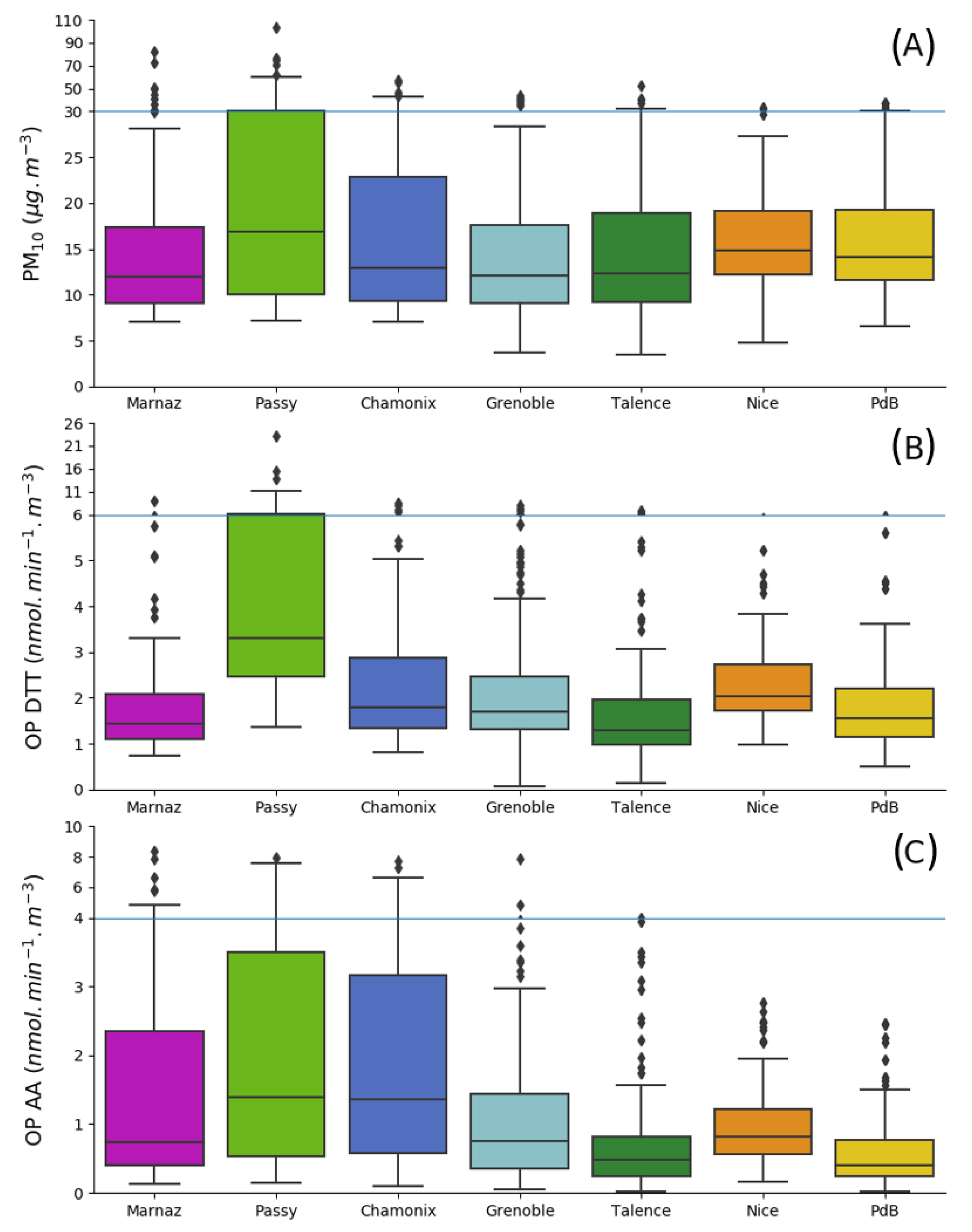
3.1. OP and PM10 Annual Average Concentrations
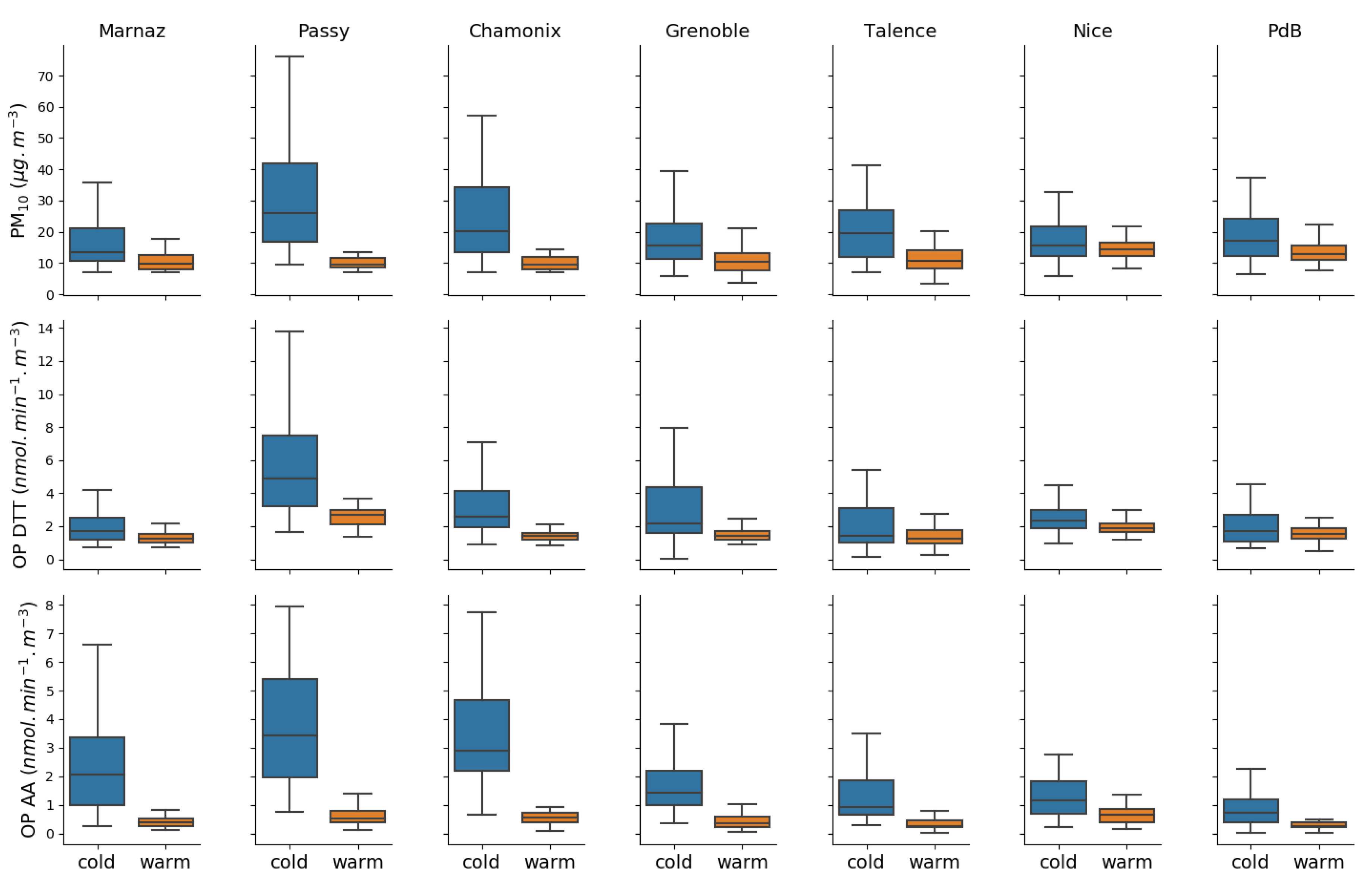
3.2. OP and PM Seasonality
3.3. OP and PM10 Associations
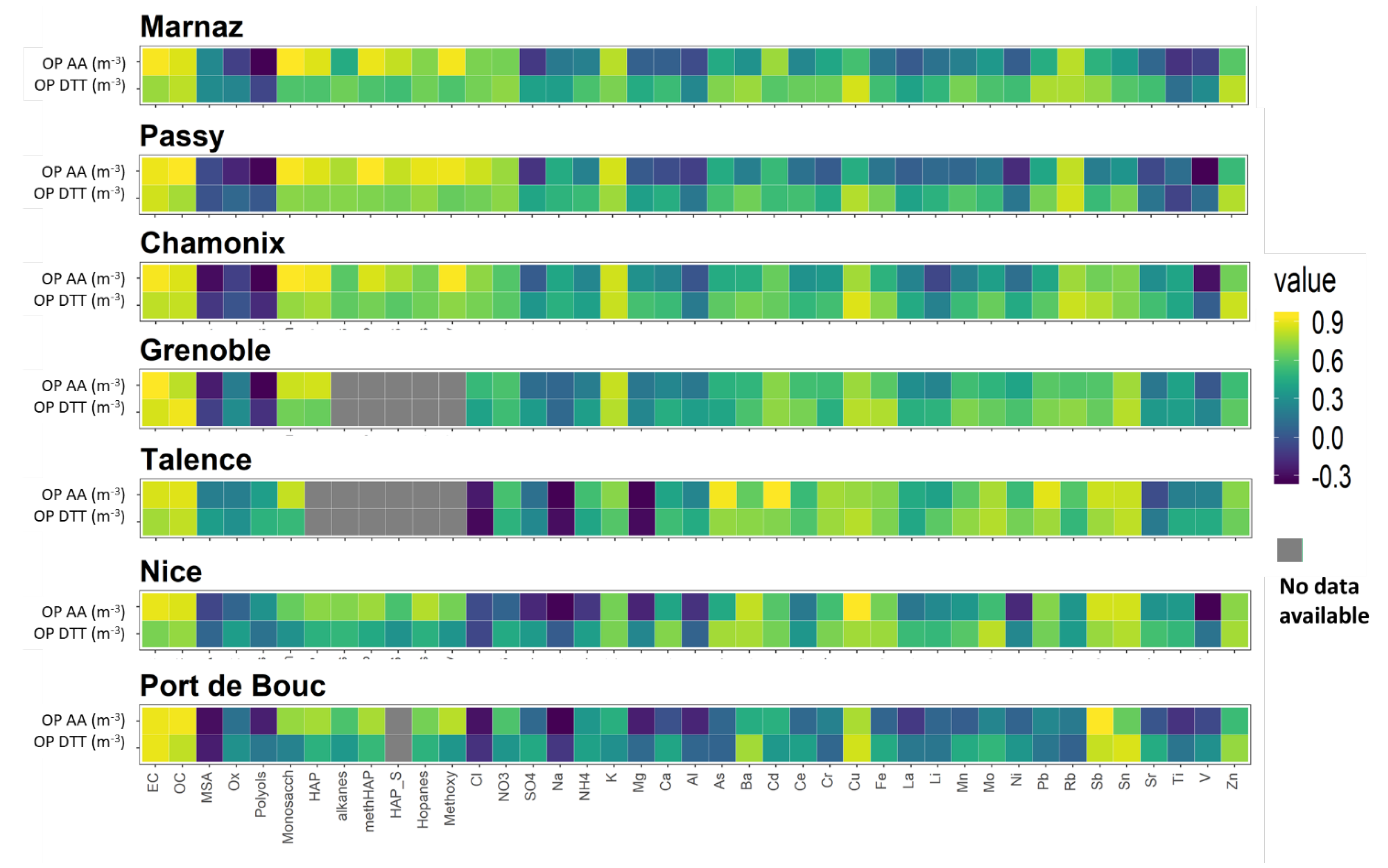
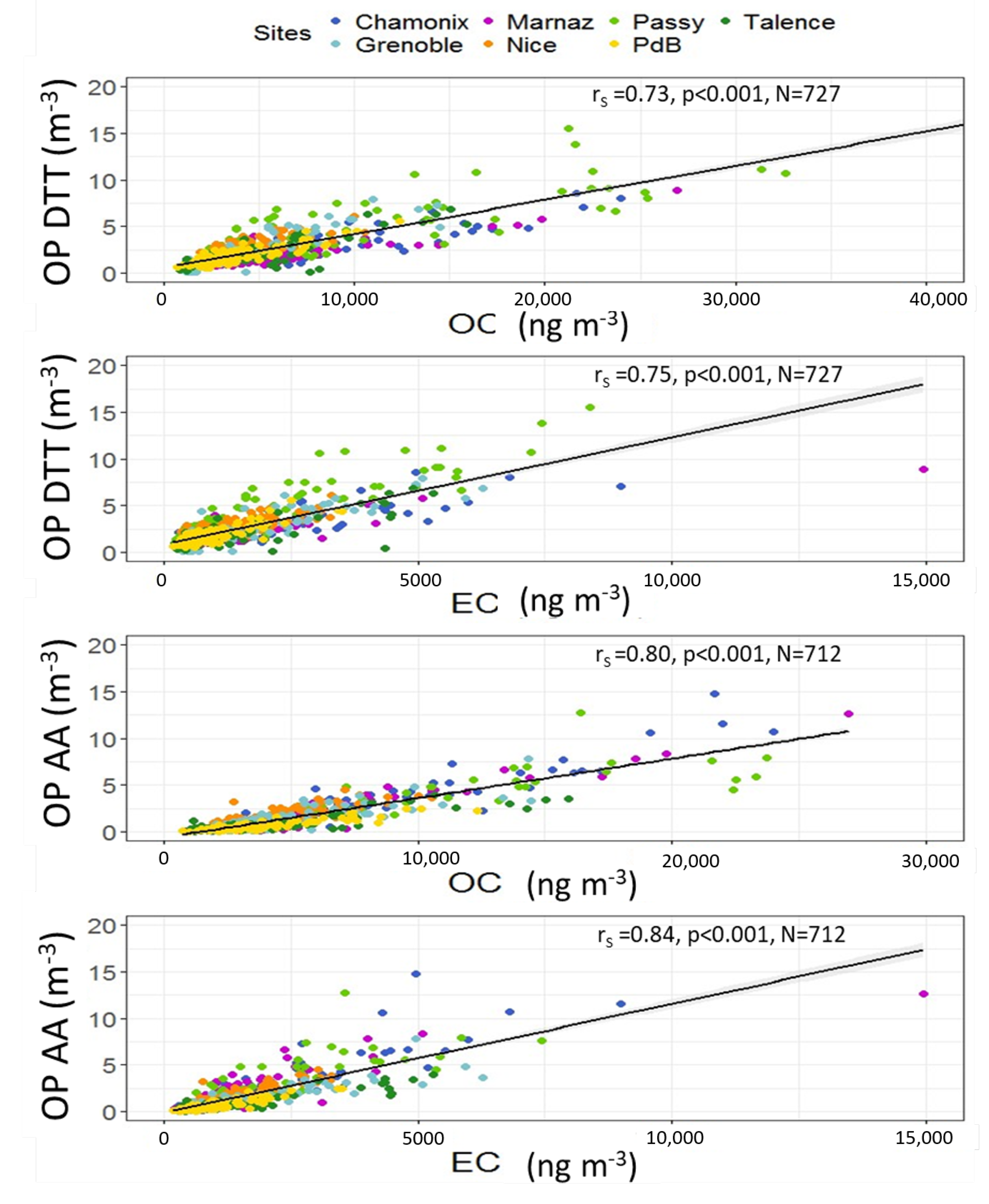
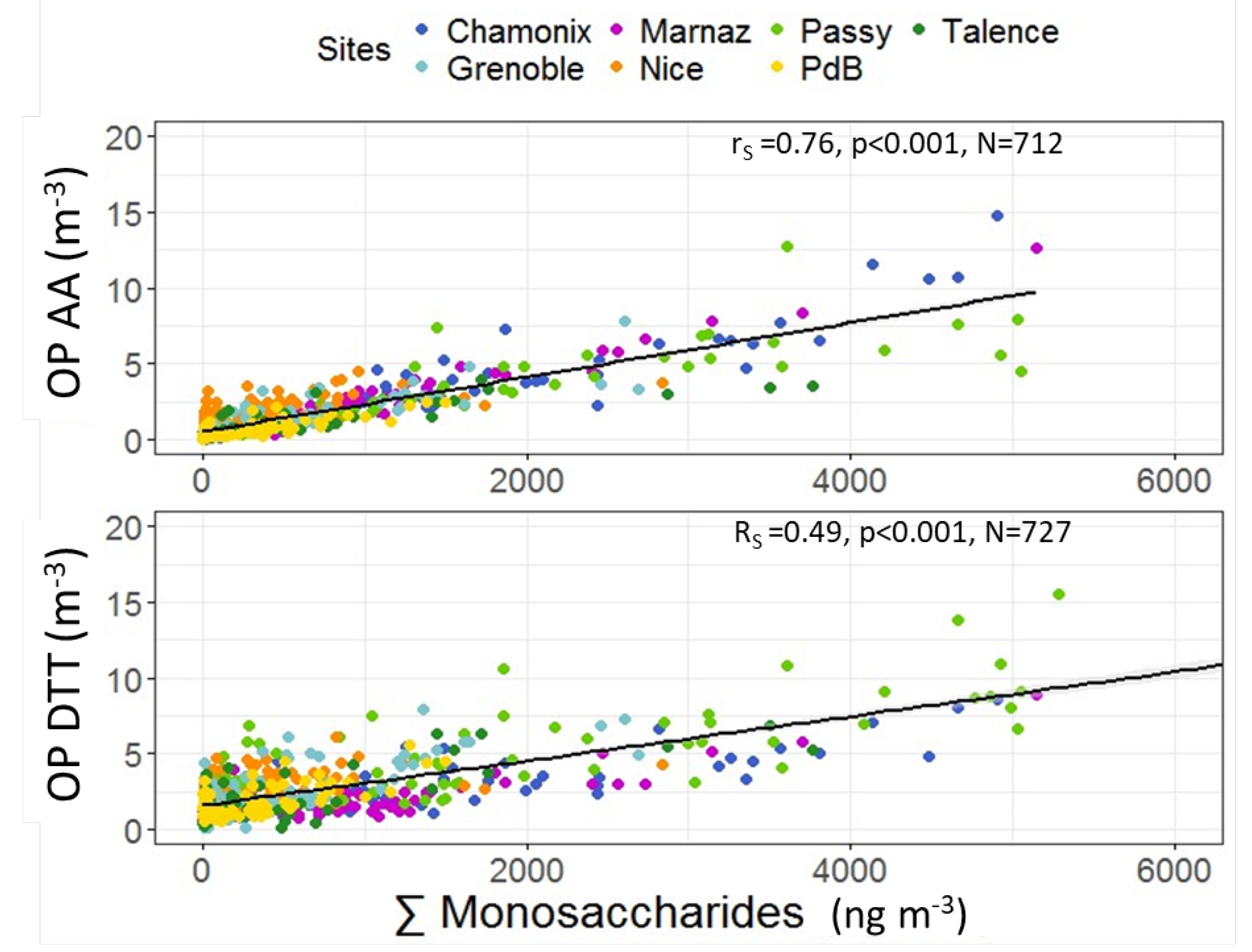
3.4. Correlations between OP and PM Chemistry
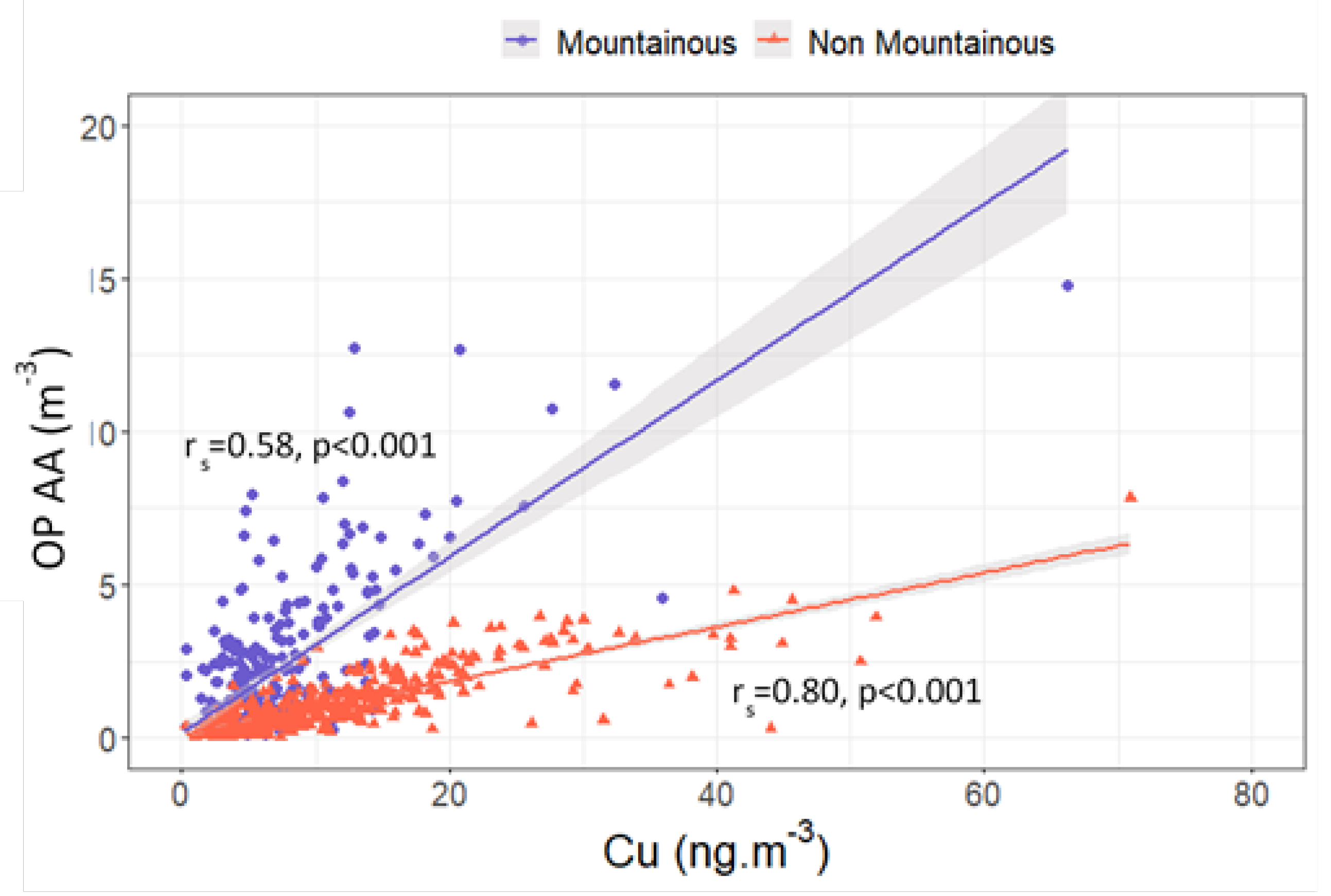
3.4.1. Contrasts between Sites
3.4.2. OP Predictors
3.5. Limitations of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PM10 | Particulate matter with aerodynamic diameter lower than 10 . |
| Oxidative potential of PM measured by the dithiothreitol, normalized by cubic meter of air. | |
| Oxidative potential of PM measured by the ascordbic acid, normalized by cubic meter of air. | |
| AASQA | Agence Agréée de Surveillance de la Qualité de l’Air. |
References
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; Technical Report; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Harari, S.; Martinelli, I.; Franchini, M. Effects on health of air pollution: A narrative review. Intern. Emerg. Med. 2015, 10, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Kelly, F.; Künzli, N.; Schins, R.P.F.; Donaldson, K. Oxidant generation by particulate matter: From biologically effective dose to a promising, novel metric. Occup. Environ. Med. 2007, 64, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Sauvain, J.J.; Rossi, M.J.; Riediker, M. Comparison of three acellular tests for assessing the oxidation potential of nanomaterials. Aerosol Sci. Technol. 2013, 47, 218–227. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential - the RAPTES project. Part. Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hao, M.; Phalen, R.F.; Hinds, W.C.; Nel, A.E. Particulate air pollutants and asthma: A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 2003, 109, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Uzu, G.; Sauvain, J.J.; Baeza-Squiban, A.; Riediker, M.; Sánchez Sandoval Hohl, M.; Val, S.; Tack, K.; Denys, S.; Pradère, P.; Dumat, C. In vitro assessment of the pulmonary toxicity and gastric availability of lead-rich particles from a lead recycling plant. Environ. Sci. Technol. 2011, 45, 7888–7895. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.J.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M.; et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2015, 72, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Staimer, N.; Gillen, D.L.; Tjoa, T.; Schauer, J.J.; Shafer, M.M.; Hasheminassab, S.; Pakbin, P.; Vaziri, N.D.; Sioutas, C.; et al. Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environ. Res. 2016, 150, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Weichenthal, S.A.; Lavigne, E.; Evans, G.J.; Godri Pollitt, K.J.; Burnett, R.T. Fine Particulate Matter and Emergency Room Visits for Respiratory Illness. Effect Modification by Oxidative Potential. Am. J. Respir. Crit. Care Med. 2016, 194, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM 2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Yang, A.; Janssen, N.A.H.; Brunekreef, B.; Cassee, F.R.; Hoek, G.; Gehring, U. Children’s respiratory health and oxidative potential of PM2.5: The PIAMA birth cohort study. Occup. Environ. Med. 2016, 73, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Strak, M.; Janssen, N.; Beelen, R.; Schmitz, O.; Vaartjes, I.; Karssenberg, D.; van den Brink, C.; Bots, M.L.; Dijst, M.; Brunekreef, B.; et al. Long-term exposure to particulate matter, NO2 and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ. Intern. 2017, 108, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Weber, R.J.; Abrams, J.; Verma, V.; Fang, T.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; et al. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ. Sci. Technol. 2015, 49, 13605–13612. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Weber, R.J.; Klein, M.; Samat, S.E.; Chang, H.H.; Strickland, M.J.; Verma, V.; Fang, T.; Bates, J.T.; Mulholland, J.A.; et al. Associations between Ambient Fine Particulate Oxidative Potential and Cardiorespiratory Emergency Department Visits. Environ. Health Perspect. 2017, 125. [Google Scholar] [CrossRef]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Harrison, R.M.; Hider, R.; Kelly, F.; Kooter, I.M.; et al. Evaluating the Toxicity of Airborne Particulate Matter and Nanoparticles by Measuring Oxidative Stress Potential—A Workshop Report and Consensus Statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Baeza Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. Discuss. 2012, 12, 11317–11350. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Richards-Henderson, N.K.; Bein, K.J.; McFall, A.S.; Wexler, A.S.; Anastasio, C. Oxidant production from source-oriented particulate matter – Part 1: Oxidative potential using the dithiothreitol (DTT) assay. Atmos. Chem. Phys. 2015, 15, 2327–2340. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Martins, J.M.F.; Voisin, D.; Spadini, L.; Lacroix, T.; Jaffrezo, J.L. The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter. Sci. Rep. 2017, 7, 11617. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Fang, T.; Xu, L.; Peltier, R.E.; Russell, A.G.; Ng, N.L.; Weber, R.J. Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM 2.5. Environ. Sci. Technol. 2015, 49, 4646–4656. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yu, J.Z. Generation of Reactive Oxygen Species Mediated by Humic-like Substances in Atmospheric Aerosols. Environ. Sci. Technol. 2011, 45, 10362–10368. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.J.; Rattanavaraha, W.; Zhang, Z.; Gold, A.; Surratt, J.D.; Lin, Y.H. Assessing the oxidative potential of isoprene-derived epoxides and secondary organic aerosol. Atmos. Environ. 2016, 130, 211–218. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Kelly, F.J.; Houdier, S.; Martins, J.M.F.; Thomas, F.; Molton, F.; Charron, A.; Dunster, C.; Oliete, A.; et al. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos. Chem. Phys. 2018, 18, 7863–7875. [Google Scholar] [CrossRef]
- Szigeti, T.; Óvári, M.; Dunster, C.; Kelly, F.J.; Lucarelli, F.; Záray, G. Changes in chemical composition and oxidative potential of urban PM2.5 between 2010 and 2013 in Hungary. Sci. Total Environ. 2015, 518-519, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Jedynska, A.; Hellack, B.; Kooter, I.; Hoek, G.; Brunekreef, B.; Kuhlbusch, T.A.; Cassee, F.R.; Janssen, N.A. Measurement of the oxidative potential of PM2.5 and its constituents: The effect of extraction solvent and filter type. Atmos. Environ. 2014, 83, 35–42. [Google Scholar] [CrossRef]
- Perrone, M.G.; Zhou, J.; Malandrino, M.; Sangiorgi, G.; Rizzi, C.; Ferrero, L.; Dommen, J.; Bolzacchini, E. PM chemical composition and oxidative potential of the soluble fraction of particles at two sites in the urban area of Milan, Northern Italy. Atmos. Environ. 2016, 128, 104–113. [Google Scholar] [CrossRef]
- Charrier, J.G.; McFall, A.S.; Richards-Henderson, N.K.; Anastasio, C. Hydrogen Peroxide Formation in a Surrogate Lung Fluid by Transition Metals and Quinones Present in Particulate Matter. Environ. Sci. Technol. 2014, 48, 7010–7017. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, F.; Hasheminassab, S.; Saffari, A.; Schauer, J.J.; Delfino, R.J.; Sioutas, C. Fine and ultrafine particulate organic carbon in the Los Angeles basin: Trends in sources and composition. Sci. Total Environ. 2016, 541, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, L.; Froines, J.R.; Cho, A.K.; Sioutas, C. Relationship between redox activity and chemical speciation of size-fractionated particulate matter. Part. Fibre Toxicol. 2007, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Nalin, F.; Golly, B.; Besombes, J.L.; Pelletier, C.; Aujay-Plouzeau, R.; Verlhac, S.; Dermigny, A.; Fievet, A.; Karoski, N.; Dubois, P.; et al. Fast oxidation processes from emission to ambient air introduction of aerosol emitted by residential log wood stoves. Atmos. Environ. 2016, 143, 15–26. [Google Scholar] [CrossRef]
- Verma, V.; Fang, T.; Guo, H.; King, L.; Bates, J.T.; Peltier, R.E.; Edgerton, E.; Russell, A.G.; Weber, R.J. Reactive oxygen species associated with water-soluble PM2 in the southeastern United States: Spatiotemporal trends and source apportionment. Atmos. Chem. Phys. 2014, 14, 12915–12930. [Google Scholar] [CrossRef]
- Shafer, M.M.; Hemming, J.D.C.; Antkiewicz, D.S.; Schauer, J.J. Oxidative potential of size-fractionated atmospheric aerosol in urban and rural sites across Europe. Faraday Discuss. 2016, 189, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, F.; Stevanovic, S.; Miljevic, B.; Bottle, S.; Ristovski, Z. Review-evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem. Ind. Chem. Eng. Q. 2015, 21, 201–210. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Seasonal and spatial variation in dithiothreitol (DTT) activity of quasi-ultrafine particles in the Los Angeles Basin and its association with chemical species. J. Environ. Sci. Health Part A 2014, 49, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Favez, O.; Salameh, D.; Jaffrezo, J.L. Traitement Harmonisé de Jeux de Données Multi-sites Pour l’étude de Sources de PM par Positive Matrix Factorization (PMF); Technical Report; LCSQA: Verneuil-en-Halatte, France, 2017. [Google Scholar]
- Chevrier, F. Chauffage au Bois et Qualité de l’air en Vallée de l’Arve : Définition d’un Système de Surveillance et Impact d’une Politique de RéNovation du parc des Appareils Anciens. Ph.D. Thesis, Université Grenoble Alpes, Grenoble, France, 2016. [Google Scholar]
- Favez, O.; El Haddad, I.; Piot, C.; Boréave, A.; Abidi, E.; Marchand, N.; Jaffrezo, J.L.; Besombes, J.L.; Personnaz, M.B.; Sciare, J.; et al. Inter-comparison of source apportionment models for the estimation of wood burning aerosols during wintertime in an Alpine city (Grenoble, France). Atmos. Chem. Phys. 2010, 10, 5295–5314. [Google Scholar] [CrossRef]
- Srivastava, D.; Tomaz, S.; Favez, O.; Lanzafame, G.M.; Golly, B.; Besombes, J.L.; Alleman, L.Y.; Jaffrezo, J.L.; Jacob, V.; Perraudin, E.; et al. Speciation of organic fraction does matter for source apportionment. Part 1: A one-year campaign in Grenoble (France). Sci. Total Environ. 2018, 624, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, S.; Jaffrezo, J.L.; Favez, O.; Perraudin, E.; Villenave, E.; Albinet, A. Sources and atmospheric chemistry of oxy- and nitro-PAHs in the ambient air of Grenoble (France). Atmos. Environ. 2017, 161, 144–154. [Google Scholar] [CrossRef]
- Tomaz, S.; Shahpoury, P.; Jaffrezo, J.L.; Lammel, G.; Perraudin, E.; Villenave, E.; Albinet, A. One-year study of polycyclic aromatic compounds at an urban site in Grenoble (France): Seasonal variations, gas/particle partitioning and cancer risk estimation. Sci. Total Environ. 2016, 565, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, A.; Mizzi, A.; Mathiot, S.; Masson, F.; Jaffrezo, J.L.; Dron, J.; Mesbah, B.; Wortham, H.; Marchand, N. Comprehensive chemical characterization of industrial PM 2.5 from steel industry activities. Atmos. Environ. 2017, 152, 180–190. [Google Scholar] [CrossRef]
- Cavalli, F.; Alastuey, A.; Areskoug, H.; Ceburnis, D.; Čech, J.; Genberg, J.; Harrison, R.; Jaffrezo, J.L.; Kiss, G.; Laj, P.; et al. A European aerosol phenomenology—4: Harmonized concentrations of carbonaceous aerosol at 10 regional background sites across Europe. Atmos. Environ. 2016, 144, 133–145. [Google Scholar] [CrossRef]
- Waked, A.; Favez, O.; Alleman, L.Y.; Piot, C.; Petit, J.E.; Delaunay, T.; Verlinden, E.; Golly, B.; Besombes, J.L.; Jaffrezo, J.L.; et al. Source apportionment of PM10 in a north-western Europe regional urban background site (Lens, France) using positive matrix factorization and including primary biogenic emissions. Atmos. Chem. Phys. 2014, 14, 3325–3346. [Google Scholar] [CrossRef]
- Golly, B.; Waked, A.; Weber, S.; Samake, A.; Jacob, V.; Conil, S.; Rangognio, J.; Chrétien, E.; Vagnot, M.P.; Robic, P.Y.; et al. Organic markers and OC source apportionment for seasonal variations of PM2.5 at 5 rural sites in France. Atmos. Environ. 2019, 198, 142–157. [Google Scholar] [CrossRef]
- Piot, C.; Jaffrezo, J.L.; Cozic, J.; Pissot, N.; Haddad, I.E.; Marchand, N.; Besombes, J.L. Quantification of levoglucosan and its isomers by High Performance Liquid Chromatography – Electrospray Ionization tandem Mass Spectrometry and its applications to atmospheric and soil samples. Atmos. Meas. Tech. 2012, 5, 141–148. [Google Scholar] [CrossRef]
- Golly, B.; Brulfert, G.; Berlioux, G.; Jaffrezo, J.L.; Besombes, J.L. Large chemical characterisation of PM10 emitted from graphite material production: Application in source apportionment. Sci. Total Environ. 2015, 538, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; McFall, A.S.; Vu, K.K.T.; Baroi, J.; Olea, C.; Hasson, A.; Anastasio, C. A bias in the “mass-normalized” DTT response – An effect of non-linear concentration-response curves for copper and manganese. Atmos. Environ. 2016, 144, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Mudway, I.S. Protein oxidation at the air-lung interface. Amino Acids 2003, 25, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Velali, E.; Papachristou, E.; Pantazaki, A.; Choli-Papadopoulou, T.; Planou, S.; Kouras, A.; Manoli, E.; Besis, A.; Voutsa, D.; Samara, C. Redox activity and in vitro bioactivity of the water-soluble fraction of urban particulate matter in relation to particle size and chemical composition. Environ. Pollut. 2016, 208, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Constantini, S.; Loukia, C. Mutagenicity and redox activity of size segregated airborne particulate matter in Thessaloniki, Northern Greece, in relation to aerosol chemical composition. Front. Pharmacol. 2010, 1. [Google Scholar] [CrossRef]
- Künzli, N.; Mudway, I.S.; Götschi, T.; Shi, T.; Kelly, F.J.; Cook, S.; Burney, P.; Forsberg, B.; Gauderman, J.W.; Hazenkamp, M.E.; et al. Comparison of Oxidative Properties, Light Absorbance, and Total and Elemental Mass Concentration of Ambient PM2.5 Collected at 20 European Sites. Environ. Health Perspect. 2006, 114, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Kuenzli, N.; Sunyer, J.; Shi, T.; Moreno, T.; Viana, M.; Heinrich, J.; Forsberg, B.; Kelly, F.J.; Sughis, M.; et al. Oxidative properties of ambient PM2.5 and elemental composition: Heterogeneous associations in 19 European cities. Atmos. Environ. 2009, 43, 4595–4602. [Google Scholar] [CrossRef]
- Orru, H.; Kimmel, V.; Kikas, Ü.; Soon, A.; Künzli, N.; Schins, R.P.F.; Borm, P.J.A.; Forsberg, B. Elemental composition and oxidative properties of PM2.5 in Estonia in relation to origin of air masses—Results from the ECRHS II in Tartu. Sci. Total Environ. 2010, 408, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Uzu, G.; Calas, A.; Chevrier, F.; Besombes, J.L.; Charron, A.; Salameh, D.; Ježek, I.; Močnik, G.; Jaffrezo, J.L. An apportionment method for the oxidative potential of atmospheric particulate matter sources: Application to a one-year study in Chamonix, France. Atmos. Chem. Phys. 2018, 18, 9617–9629. [Google Scholar] [CrossRef]
- Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E.; Jaffrezo, J.L. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys Part 2: Particle size distribution. Atmos. Environ. 2008, 42, 55–64. [Google Scholar] [CrossRef][Green Version]
- Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E.; Jaffrezo, J.L. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleysPart 1: Concentrations, sources and gas/particle partitioning. Atmos. Environ. 2008, 42, 43–54. [Google Scholar] [CrossRef]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons—A review. Chem. Soc. Rev. 2013, 42, 9333. [Google Scholar] [CrossRef] [PubMed]
- Tuet, W.Y.; Liu, F.; de Oliveira Alves, N.; Fok, S.; Artaxo, P.; Vasconcellos, P.; Champion, J.A.; Ng, N.L. Chemical Oxidative Potential and Cellular Oxidative Stress from Open Biomass Burning Aerosol. Environ. Sci. Technol. Lett. 2019, 6, 126–132. [Google Scholar] [CrossRef]
- Singh, V.; Ravindra, K.; Sahu, L.; Sokhi, R. Trends of atmospheric black carbon concentration over the United Kingdom. Atmos. Environ. 2018, 178, 148–157. [Google Scholar] [CrossRef]
- Keyte, I.J.; Albinet, A.; Harrison, R.M. On-road traffic emissions of polycyclic aromatic hydrocarbons and their oxy- and nitro- derivative compounds measured in road tunnel environments. Sci. Total Environ. 2016, 566-567, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, B.; Sagebiel, J.; McDonald, J.D.; Whitney, K.; Lawson, D.R. Emission Rates and Comparative Chemical Composition from Selected In-Use Diesel and Gasoline-Fueled Vehicles. J. Air Waste Manag. Assoc. 2004, 54, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Bruns, E.A.; Krapf, M.; Orasche, J.; Huang, Y.; Zimmermann, R.; Drinovec, L.; Močnik, G.; El-Haddad, I.; Slowik, J.G.; Dommen, J.; et al. Characterization of primary and secondary wood combustion products generated under different burner loads. Atmos. Chem. Phys. 2015, 15, 2825–2841. [Google Scholar] [CrossRef]
- Marchand, N.; Besombes, J.L.; Masclet, P.; Jaffrezo, J.L. Atmospheric Polycyclic Aromatic Hydrocarbons (PAHs) in Two French Alpine Valleys. In Environmental Chemistry; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 409–417. [Google Scholar] [CrossRef]
- Salameh, D.; Pey, J.; Bozzetti, C.; El Haddad, I.; Detournay, A.; Sylvestre, A.; Canonaco, F.; Armengaud, A.; Piga, D.; Robin, D.; et al. Sources of PM2.5 at an urban-industrial Mediterranean city, Marseille (France): Application of the ME-2 solver to inorganic and organic markers. Atmos. Res. 2018, 214, 263–274. [Google Scholar] [CrossRef]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Police, S.; Sahu, S.K.; Pandit, G.G. Chemical characterization of atmospheric Particulate Matter in Delhi, India, Part II: Source apportionment studies using PMF3. Sustain. Environ. Res. 2013, 23, 295–306. [Google Scholar]
- Moreno, T.; Karanasiou, A.; Amato, F.; Lucarelli, F.; Nava, S.; Calzolai, G.; Chiari, M.; Coz, E.; Artíñano, B.; Lumbreras, J.; et al. Daily and hourly sourcing of metallic and mineral dust in urban air contaminated by traffic and coal-burning emissions. Atmos. Environ. 2013, 68, 33–44. [Google Scholar] [CrossRef]
- Aymoz, G.; Godefroy, F. Caractérisation Chimique des Particules; Technical Report DRC-08-94285-15186A; LCSQA: Verneuil-en-Halatte, France, 2008. [Google Scholar]
- Zhou, J.; Bruns, E.A.; Zotter, P.; Stefenelli, G.; Prévôt, A.S.H.; Baltensperger, U.; El-Haddad, I.; Dommen, J. Development, characterization and first deployment of an improved online reactive oxygen species analyzer. Atmos. Meas. Tech. 2018, 11, 65–80. [Google Scholar] [CrossRef]
- Constantini, S. On the Redox Activity of Urban Aerosol Particles: Implications for Size Distribution and Relationships with Organic Aerosol Components. Atmosphere 2017, 8, 205. [Google Scholar] [CrossRef]
- Szigeti, T.; Dunster, C.; Cattaneo, A.; Cavallo, D.; Spinazzè, A.; Saraga, D.E.; Sakellaris, I.A.; de Kluizenaar, Y.; Cornelissen, E.J.; Hänninen, O.; et al. Oxidative potential and chemical composition of PM2.5 in office buildings across Europe—The OFFICAIR study. Environ. Intern. 2016, 92–93, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, T.; Dunster, C.; Cattaneo, A.; Spinazzè, A.; Mandin, C.; Le Ponner, E.; de Oliveira Fernandes, E.; Ventura, G.; Saraga, D.E.; Sakellaris, I.A.; et al. Spatial and temporal variation of particulate matter characteristics within office buildings—The OFFICAIR study. Sci. Total Environ. 2017, 587–588, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Beck-Speier, I.; Karg, E.; Behrendt, H.; Stoeger, T.; Alessandrini, F. Ultrafine particles affect the balance of endogenous pro- and anti-inflammatory lipid mediators in the lung: In-vitro and in-vivo studies. Part. Fibre Toxicol. 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, R.; De Berardis, B.; Paoletti, L.; Guastadisegni, C. Inflammatory mediators induced by coarse (PM2.5–10) and fine (PM2.5) urban air particles in RAW 264.7 cells. Toxicology 2003, 183, 243–254. [Google Scholar] [CrossRef]
- Hetland, R.B.; Cassee, F.R.; Låg, M.; Refsnes, M.; Dybing, E.; Schwarze, P.E. Cytokine release from alveolar macrophages exposed to ambient particulate matter: Heterogeneity in relation to size, city and season. Part. Fibre Toxicol. 2005, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Gerlofs-Nijland, M.E.; Lanki, T.; Salonen, R.O.; Cassee, F.R.; Hoek, G.; Fischer, P.; Brunekreef, B.; Krzyzanowski, M. Health Effects of Black Carbon; OCLC: 930804705; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2012. [Google Scholar]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016, 219, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gietl, J.K.; Lawrence, R.; Thorpe, A.J.; Harrison, R.M. Identification of brake wear particles and derivation of a quantitative tracer for brake dust at a major road. Atmos. Environ. 2010, 44, 141–146. [Google Scholar] [CrossRef]
- Amato, F. (Ed.) Non-Exhaust Emissions: An Urban Air Quality Problem for Public Health: Impact and Mitigation Measures; OCLC: on1023047184; Academic Press: London, UK; San Diego, CA, USA, 2018. [Google Scholar]
- Samaké, A.; Jaffrezo, J.L.; Favez, O.; Weber, S.; Jacob, V.; Canete, T.; Albinet, A.; Charron, A.; Riffault, V.; Perdrix, E.; et al. Arabitol, mannitol and glucose as tracers of primary biogenic organic aerosol: Influence of environmental factors on ambient air concentrations and spatial distribution over France. Atmos. Chem. Phys. Discuss. 2019, 1–24. [Google Scholar] [CrossRef]
- Samaké, A.; Jaffrezo, J.L.; Favez, O.; Weber, S.; Jacob, V.; Albinet, A.; Riffault, V.; Perdrix, E.; Waked, A.; Golly, B.; et al. Polyols and glucose particulate species as tracers of primary biogenic organic aerosols at 28 French sites. Atmos. Chem. Phys. 2019, 19, 3357–3374. [Google Scholar] [CrossRef]
- Samaké, A.; Uzu, G.; Martins, J.M.F.; Calas, A.; Vince, E.; Parat, S.; Jaffrezo, J.L. The unexpected role of bioaerosols in the Oxidative Potential of PM. Sci. Rep. 2017, 7, 10978. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Weber, R.J.; Verma, V.; Fang, T.; Ivey, C.; Liu, C.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Russell, A. Source impact modeling of spatiotemporal trends in PM2.5 oxidative potential across the eastern United States. Atmos. Environ. 2018, 193, 158–167. [Google Scholar] [CrossRef]
- Hussain, S.; Boland, S.; Baeza-Squiban, A.; Hamel, R.; Thomassen, L.C.J.; Martens, J.A.; Billon-Galland, M.A.; Fleury-Feith, J.; Moisan, F.; Pairon, J.C.; et al. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: Role of particle surface area and internalized amount. Toxicology 2009, 260, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Vranic, S.; Boland, S.; Moreau, K.; Baeza-Squiban, A.; Gaiser, B.K.; Andrzejczuk, L.A.; Stone, V. An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: Cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity. BMC Nephrol. 2013, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Surratt, J.D.; Lin, Y.H.; Arashiro, M.; Vizuete, W.G.; Zhang, Z.; Gold, A.; Jaspers, I.; Fry, R.C. Understanding the Early Biological Effects of Isoprene-Derived Particulate Matter Enhanced by Anthropogenic Pollutants; Technical Report 198; Health Effects Institute: Bostan, MA, USA, 2019. [Google Scholar]
| Sites | Sampling Period | Coordinates | Altitude (m) | Typology | Climate | N |
|---|---|---|---|---|---|---|
| Marnaz | November 2013 October 2014 | 46°3′27.78″ N, 6°32′0.29″ E | 505 | Urban background | Alpine | 94 |
| Passy | November 2013 October 2014 | 46°55′24.58″ N, 6°42′49.15″ E | 583 | Urban background | Alpine | 95 |
| Chamonix | November 2013 October 2014 | 45°55′21.53″ N, 6°52′11.68″ E | 1035 | Urban background | Alpine | 98 |
| Grenoble | January 2014 December 2014 | 45°9′42.84″ N, 5°44′8.16″ E | 214 | Urban background | Alpine | 121 |
| Talence | March 2012 December 2012 | 44°48′2.016″ N, 0°35′17.016″ W | 20 | Urban background | Oceanic | 92 |
| Nice | June 2014 May 2015 | 43°42′7.48 N, 7°17′10.49″ E | 11 | Urban background | Mediterranean | 115 |
| Port-de-Bouc | June 2014 May 2015 | 43°24′7.03″ N, 4°58′54.91″ E | 3 | Urban background | Mediterranean | 113 |
| Marnaz | Passy | Chamonix | Grenoble | Talence | Nice | PdB | |
|---|---|---|---|---|---|---|---|
| Anions/Cations | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Metals and trace elements | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| EC/OC | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Polyols | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Anhydrous monosacch. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| PAHs | ✓ | ✓ | ✓ | ✓ | Ana | ✓ | ✓ |
| Alkanes | ✓ | ✓ | ✓ | Ana | Ana | ✓ | ✓ |
| Methyl-PAHs | ✓ | ✓ | ✓ | Ana | Ana | ✓ | ✓ |
| PASHs | ✓ | ✓ | ✓ | Ana | Ana | ✓ | ✓ |
| Hopanes | ✓ | ✓ | ✓ | Ana | Ana | ✓ | ✓ |
| Methoxyphenols | ✓ | ✓ | ✓ | Ana | Ana | ✓ | ✓ |
| Station | PM10 | ||
|---|---|---|---|
| Marnaz | 1.7 *** [1.3, 1.8] | 1.5 ** [1.2, 1.6] | 5.6 *** [4.1, 6.6] |
| Passy | 2.8 *** [1.9, 3.2] | 2.1 *** [1.5, 2.4] | 4.6 *** [4.8, 5.4] |
| Chamonix | 2.2 *** [1.7, 2.9] | 2.1 *** [1.5, 2.5] | 6.1 *** [5.4, 6.5] |
| Grenoble | 1.6 *** [1.5, 1.7] | 2.1 *** [1.5, 2.6] | 3.9 *** [4.3, 2.6] |
| Talence | 1.8 *** [1.3, 2.1] | 1.8 [1.1, 2.1] | 3.0 *** [2.3, 3.3] |
| Nice | 1.1 [1.0, 1.2] | 1.2 ** [1.1, 1.4] | 1.8 *** [1.5, 1.9] |
| Port-de-Bouc | 1.3 ** [1.2, 1.6] | 1.2 [0.9, 1.4] | 2.6 *** [2.1, 3.1] |
| Cold Period | Warm Period | |||||
|---|---|---|---|---|---|---|
| – PM10 | – PM10 | – | – PM10 | – PM10 | – | |
| Marnaz | 0.86 *** | 0.77 *** | 0.65 *** | 0.77 *** | 0.41 ** | 0.47 ** |
| Passy | 0.84 *** | 0.85 *** | 0.70 *** | 0.54 *** | 0.53 *** | 0.55 *** |
| Chamonix | 0.90 *** | 0.89 *** | 0.87 *** | 0.67 *** | 0.37 * | 0.46 ** |
| Grenoble | 0.88 *** | 0.78 *** | 0.84 *** | 0.78 *** | 0.54 *** | 0.65 *** |
| Talence | 0.60 *** | 0.84 *** | 0.66 *** | 0.77 *** | 0.44 *** | 0.51 *** |
| Nice | 0.91 *** | 0.75 *** | 0.82 *** | 0.75 *** | 0.35 ** | 0.55 *** |
| PdB | 0.75 *** | 0.69 *** | 0.89 *** | 0.74 *** | 0.36 ** | 0.54 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calas, A.; Uzu, G.; Besombes, J.-L.; Martins, J.M.F.; Redaelli, M.; Weber, S.; Charron, A.; Albinet, A.; Chevrier, F.; Brulfert, G.; et al. Seasonal Variations and Chemical Predictors of Oxidative Potential (OP) of Particulate Matter (PM), for Seven Urban French Sites. Atmosphere 2019, 10, 698. https://doi.org/10.3390/atmos10110698
Calas A, Uzu G, Besombes J-L, Martins JMF, Redaelli M, Weber S, Charron A, Albinet A, Chevrier F, Brulfert G, et al. Seasonal Variations and Chemical Predictors of Oxidative Potential (OP) of Particulate Matter (PM), for Seven Urban French Sites. Atmosphere. 2019; 10(11):698. https://doi.org/10.3390/atmos10110698
Chicago/Turabian StyleCalas, Aude, Gaëlle Uzu, Jean-Luc Besombes, Jean M.F. Martins, Matteo Redaelli, Samuël Weber, Aurelie Charron, Alexandre Albinet, Florie Chevrier, Guillaume Brulfert, and et al. 2019. "Seasonal Variations and Chemical Predictors of Oxidative Potential (OP) of Particulate Matter (PM), for Seven Urban French Sites" Atmosphere 10, no. 11: 698. https://doi.org/10.3390/atmos10110698
APA StyleCalas, A., Uzu, G., Besombes, J.-L., Martins, J. M. F., Redaelli, M., Weber, S., Charron, A., Albinet, A., Chevrier, F., Brulfert, G., Mesbah, B., Favez, O., & Jaffrezo, J.-L. (2019). Seasonal Variations and Chemical Predictors of Oxidative Potential (OP) of Particulate Matter (PM), for Seven Urban French Sites. Atmosphere, 10(11), 698. https://doi.org/10.3390/atmos10110698








