Abstract
The paper presents the results of a numerical evaluation of limiting sensitivity of the method for detecting vapors of nitrocompounds in the atmosphere based on one-color laser fragmentation (LF)/laser-induced fluorescence (LIF) of NO fragments via A2Σ+ (v′ = 0) ← X2Π (v″ = 2) transition. The calculations were performed using the developed kinetic model of the one-color LF/LIF process under consideration. The calculations take into account the influence of ambient nitrogen dioxide as a limiter of the sensitivity of the method when operating in a real atmosphere. It is shown that if the nitrogen dioxide concentration in the atmosphere does not exceed a value of 10 ppb, the maximum detectable vapor concentrations of nitrobenzene and o-nitrotoluene are several ppb. It is also shown that the method of single-frequency one-color excitation usually used for the detection of nitrocompounds does not allow achieving the maximum efficiency of the LF/LIF process.
1. Introduction
The laser fragmentation/laser-induced fluorescence (LF/LIF) method considered in this work was first proposed by Rodgers et al. [1] for the in situ detection of atmospheric trace gases. The essence of the method is to use the photodissociation effect of optically inactive molecules to form characteristic fragments that are highly efficient in the LIF process. The method has found wide application for remote detection of various nitro-based compounds [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21].
Schematically, the LF/LIF process for any nitrocompound (Nc) can be represented as a sequence of photochemical reactions:
where ki are the reaction rate constants.
Electronically excited Nc* molecules that arise during reaction (1) after absorption of a photon with an energy hν, significantly exceeding the dissociation threshold, have enough, or excess, energy to dissociate into fragments. For an excited polyatomic molecule, there are several paths (channels) of fragmentation. For example, in case of photodissociation of nitrobenzene (NB) under excitation at wavelengths between 220 nm and 280 nm, the most probable are the following channels of dissociation with the formation of NO2, NO, and O [4]:
It is known that in the excited state in the nitrobenzene molecule, a break and a redistribution of bonds between atoms occur, leading to the isomerization of the molecule and its transformation into phenyl nitrite, followed by breaking of the C–ONO, O–NO, and NO–O bonds and formation of the NO2, NO, and O fragments [4,6]. An alternative channel for the formation of NO2 competing with the nitro-nitrite rearrangement in the nitrobenzene molecule is the process of radical separation of the NO2 group by direct breaking of the C–N bond [22]. The nitro–nitrite rearrangement mechanism has a much lower activation enthalpy than the energy of the C–N bond breaking, which allows us to suggest that nitro–nitrite rearrangement can be one of the main channels for the decomposition of aromatic nitrocompounds.
Studies of laser-stimulated fragmentation of nitrotoluene (NT) and dinitrotoluene (DNT) [23,24] showed that all isomers of these molecules have the same main fragmentation channels as nitrobenzene. However, in the case of ortho-isomers, an additional channel with the formation of OH was observed [22,24,25]:
For DNT, the maximum yield of OH is observed when both NO2 groups are in the ortho position relative to the methyl group (2,6-DNT). The appearance of the hydroxyl radical is explained by the intramolecular migration of the hydrogen atom from the methyl group to the oxygen atom of the nitro-group, followed by the breaking of the N–OH bond [22].
Reaction (3), in turn, describes the process of photodissociation of NO2 fragments formed during reaction (2), and their decomposition into NO (X2Π) and O (3P) fragments. Dissociation of the NO2 molecule becomes energetically possible for the fragmentation radiation wavelengths shorter than 398 nm [26]. In this case, the shape of the absorption spectrum of the NO2 molecule in this wavelength range acquires characteristic diffuseness, indicating the presence of predissociation.
The NO fragments that appeared during reactions (1) and (3) of the fragmentation process can obviously serve as markers for nitrocompounds and form the basis for constructing a method for detecting molecules of nitrocompounds in the atmosphere. Several approaches are known in the implementation of the LF/LIF method for detecting molecules of nitrocompounds that differ in the ways of excitation of fluorescence of NO fragments (4). However, since the fragmentation process is not selective, the same radiation source is often used to fragment the molecules and to excite fluorescence of NO fragments (one-color LF/LIF). The choice of a specific excitation scheme is substantiated mainly by the need for selective excitation of fluorescence of NO fragments, without involving ambient nitrogen oxide in the interaction. Otherwise, the sensitivity of the method in a real atmosphere will be significantly limited.
A variety of spectroscopic effects associated with the violation of thermodynamic equilibrium in the fragmentation process and a significant redistribution of populations of vibrational-rotational states of NO fragments allows use of several schemes for the excitation of fluorescence of NO fragments. It is assumed that the excess energy of the electronic excited state over the R—NO bond dissociation energy, that arises after the bond is broken, partially goes to the excitation of vibrational states of the NO molecule. According to the data of [10], the ratio of the populations of vibrational levels v″ = 0, 1, 2, 3 of the ground state of NO fragments resulting from the photolysis of nitrobenzene by radiation in the 226–259 nm range, is approximately (v″ = 0):(v″ = 1):(v″ = 2):(v″ = 3) = 1:0.3:0.1:0.02. In the photolysis of o-nitrotoluene in the 220–250 nm range, the relative vibrational populations are (v″ = 0):(v″ = 1):(v″ = 2) = 1:0.6:0.06 [27]. This ratio is similar to those obtained for DNB [11] and DNT [13] photodissociation, indicating generation of significant numbers of NO fragments in vibrationally excited states.
Note that for ambient NO in thermodynamic equilibrium, the population of vibrational levels obeys the Boltzmann distribution and at a temperature of 300 K, the populations of the four lower levels have the following relation (v″ = 0):(v″ = 1):(v″ = 2):(v″ = 3) = 1:10−4:10−8:10−12. Obviously, the distribution function of the population of NO fragments over the vibrational levels of the ground state differs significantly from the Boltzmann one, and this difference can be used for the selective excitation of NO fragments. The presence of excited vibrational states in NO fragments is manifested in their absorption spectra, where additional bands appear, the intensity of which is several orders of magnitude higher than that for ambient NO. Under unfavorable conditions, the concentration of NO molecules in the atmosphere can reach 100 ppb, and it is obvious that this will determine the sensitivity limit of the LF/LIF method upon excitation of fluorescence of NO fragments from the zero vibrational level X2Π (v″ = 0). When fluorescence is excited from overlying vibrational levels X2Π (v″ > 0) and taking into account the ratio of the populations of vibrational states of atmospheric NO ((v″ = 0):(v″ = 1):(v″ = 2):(v″ = 3) = 1:10−4:10−8:10−12), it becomes possible to significantly increase the sensitivity of the detection method and ensure its noise immunity.
On the other hand, one should not forget that in the real atmosphere, in addition to nitrogen monoxide, nitrogen dioxide is present, which, depending on the situation, can have a rather high concentration (1–100 ppb). During photofragmentation, NO2 molecules, as well as other nitrocompounds, will supply NO fragments that can participate in the LIF process (4), being a source of noise that reduces the sensitivity of the LF/LIF method:
The results of studies of the photodissociation of a NO2 molecule in the ultraviolet wavelength range were considered in [28,29]. It was shown that the distribution of NO fragments over vibrational states during fragmentation of the NO2 molecule is inverted. The population of vibrational levels is significant up to the value of the vibrational quantum number v″ = 8. From the point of view of participation in the LF/LIF process, atmospheric nitrogen dioxide is an analog of the nitrocompound supplying NO fragments, which in the detection method based on the LF/LIF, are indicators of the presence of nitrocompound vapors in the atmosphere. Obviously, the concentration of atmospheric nitrogen dioxide will largely determine the sensitivity threshold of the LF/LIF method. That is why the task of determining the value of this threshold and finding ways to reduce it is a key task in the development of the LF/LIF method.
In order to determine the potential of the one-color LF/LIF method for detecting nitrocompound vapors in a real atmosphere, a limiting sensitivity of the method was numerically evaluated taking into account the process of fragmentation of atmospheric nitrogen dioxide (12). Nitrobenzene and ortho-nitrotoluene were chosen as the studied nitro-based compounds. These molecules are of interest as the simplest models of more complex nitrocompounds (e.g., nitro-group containing explosive molecules). The calculations were performed using a mathematical model of the process of the one-color laser fragmentation of nitrocompounds and laser-induced fluorescence of NO fragments via A2Σ+ (v′ = 0) ← X2Π (v″ = 2) transition.
2. Model of the One-Color LF/LIF Process
The features of the photodissociation of nitrobenzene and nitrotoluene discussed above were taken into account when constructing the kinetic model of the one-color LF/LIF process. To visualize the mechanism of laser fragmentation of nitrocompound molecules followed by laser excitation of fluorescence of the vibrationally excited NO (X2Π, v″ = 2) fragments, we will depict all possible processes as a sequence of transitions in a generalized energy level diagram of the nitrocompound molecule, its main fragments—NO and NO2 molecules—as well as molecules of ambient NO2 (Figure 1).
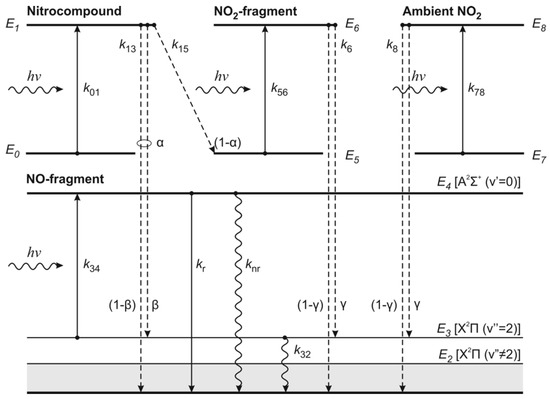
Figure 1.
Schematic diagram of energy levels of molecules involved in the laser fragmentation/laser-induced fluorescence (LF/LIF) process.
The model considers a set of molecules involved in the LF/LIF process as a nine-level system with the ground (terms E0, E5, and E7) and dissociative (terms E1, E6, and E8) states for the molecules of nitrocompound, NO2 fragments, and ambient NO2, respectively, and with vibrational levels of the molecule NO—E2 (X2Π(v″ ≠ 2)), E3 (X2Π(v″ = 2)), and E4 (A2Σ+(v′ = 0)). The LF/LIF process is represented by a sequence of inter- and intramolecular transitions with the corresponding rate constants ki.
We take into account that according to [4,22], the main channels for the dissociation of nitrobenzene and ortho-nitrotoluene under the action of radiation with a wavelength of 248 nm are channels with the formation of NO2− and NO fragments:
A part of the NO fragments for both nitrobenzene (14) and nitrotoluene (16) is formed rapidly, while the remaining part is formed more slowly [22]. According to the estimates for both nitrocompounds, the approximate fraction of “fast” (φ) and “slow” (1 − φ) fragments of their total number is 0.7 and 0.3, respectively.
According to [22], the ratio of the number of “informative” NO and NO2 fragments is [NO]/[NO2] = 0.26 ± 0.12 for nitrobenzene and [NO]/[NO2] = 0.3 ± 0.12 for o-nitrotoluene. We also take into account the participation in the LF/LIF process of atmospheric nitrogen dioxide molecules, which are the source of “false” NO fragments.
According to the diagram (Figure 1), in the process of radiation absorption, the nitrocompound molecule transfers from the ground state E0 to the dissociative state E1 and then, it decomposes into NO and NO2 fragments. Here, α is the fraction of NO fragments of the total number of fragments formed and (1 − α) is the fraction of NO2 fragments. In turn, as a result of the radiation absorption, NO2 fragments and ambient NO2 molecules undergo transitions E5 → E6 and E7 → E8, respectively, followed by dissociation into NO fragments. The quantum yield of the NO2 photodissociation is assumed to be unity [26].
In this case, a nonequilibrium population of vibrational levels of NO fragments arises. Let us denote the relative population of NO fragments formed during fragmentation of the nitrocompound and nitrogen dioxide molecules with respect to the E3 level by the constants β and γ, respectively. Then, for the relative population with respect to other vibrational levels, conditionally combined into one level E2, we have the values (1 − β) and (1 − γ), respectively.
Depletion of the E3 level occurs both due to collisional quenching of vibrational excitation by atmospheric components with a rate constant k32 (transition E3 → E2), and due to the transition of the NO molecule to the electronically excited state E4 as a result of radiation absorption. Relaxation of the E4 level to the ground state is possible both as a result of a radiative transition with a rate constant kr, and due to a nonradiative transition with a rate constant knr, which determines the fluorescence quenching factor.
In view of the foregoing, we write the system of kinetic equations describing the LF/LIF process in the framework of the nine-level model under consideration in the following form:
The values of coefficients in the system of equations are given in Table 1.

Table 1.
Input parameters of the kinetic model.
The solution of the system of kinetic equations was found numerically using the MATLAB software package. The solutions found make it possible to trace the dynamics of the LF/LIF process and evaluate the efficiency of pulsed excitation.
The function allows estimation of the total number of photons spontaneously emitted by a unit volume of a model medium per unit solid angle as:
Here, is the detection time and τ is the laser pulse duration.
3. Results and Discussion
The fluorescence signals (the center wavelength is 226.5 nm, and the FWHM is 1 nm) of informative NO fragments of nitrocompounds SNC and the fluorescence of noise NO fragments of ambient nitrogen dioxide SNO2 were calculated according to Equation (26). Figure 2 shows the family of lines corresponding to the ratio SNC/SNO2 = 1 and determining the maximum detectable concentration of the nitrocompounds [Nc]th as a function of the concentration of atmospheric nitrogen dioxide [NO2] at given radiation energy density w. The calculations were performed for a rectangular laser pulse duration of τ = 20 ns.
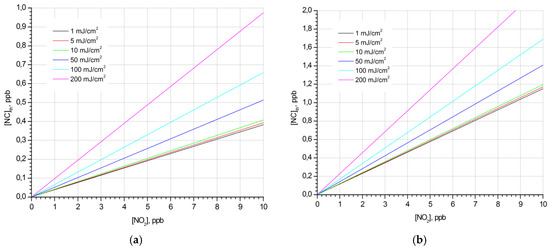
Figure 2.
Dependence of the maximum detectable concentrations of nitrobenzene (a) and o-nitrotoluene (b) vapors [Nc]th on the concentration of atmospheric nitrogen dioxide [NO2] at given values of the radiation energy density.
Analysis of the dependences of [Nc]th([NO2]) at w = const and [Nc]th(w) at [NO2] = const showed that [Nc]th increases linearly with the growth of [NO2] and has a weak quadratic dependence on w. To estimate [Nc]th at given values of [NO2] and w, the following approximation function was obtained:
where [Nc]th and [NO2] are expressed in ppb; w—mJ/cm2; the dimensionless parameters A, B1, B2, and C are presented in Table 2.

Table 2.
Constants in Equation (27) for estimating the maximum detectable concentration of nitrobenzene and o-nitrotoluene vapors.
As can be seen from the figures, in the range of concentrations [NO2] up to 10 ppb, the maximum detectable concentrations of nitrobenzene and o-nitrotoluene vapors [Nc]th are of parts-per-billion-level, while in the case of o-NT, the value of [Nc]th is about three times higher than that for NB under the same conditions. A study of the dynamics of the photodissociation process showed that this difference is due to the different rates of reaction (1) for the selected nitrocompounds. As a result, the total number N3(t) of vibrationally excited NO(X2Π, v″ = 2) fragments formed during the laser pulse will also be different (Figure 3).
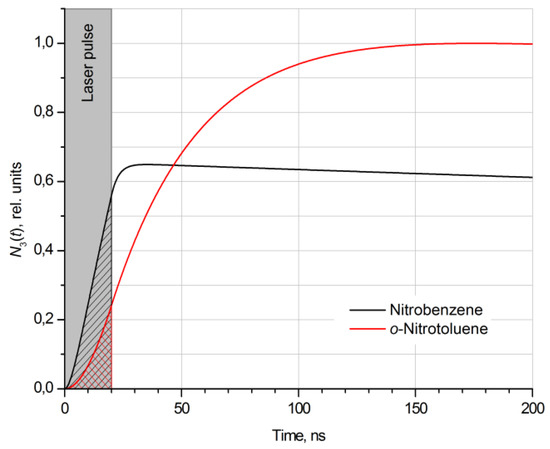
Figure 3.
Time dependence of the normalized population of the second vibrational level (X2П(v″ = 2)) for NO fragments resulting from the laser pulse fragmentation of nitrobenzene and o-nitrotoluene molecules by a 20 ns rectangular laser pulse at an energy density of 10 mJ/cm2. The radiation wavelength is 248 nm.
For a quantitative assessment, we use the ratio of the total number of NO fragments formed during the pulsed photolysis of nitrobenzene and o-nitrotoluene (shaded areas in Figure 3):
which is 2.96 at τ = 20 ns and w = 10 mJ/cm2 and agrees well with the ratio [NB]th/[NT]th = 3.09 (see Figure 2).
As can be seen from Figure 3, in case of pulsed photolysis of nitrocompound molecules, the process of fragmentation continues even after the removal of optical excitation. The maximum concentration of NO fragments is achieved over a time tmax several times greater than the fragmentation pulse duration of 20 ns. This is explained by the fact that complex organic molecules usually do not directly dissociate when light is absorbed in the region of spectral maxima. A large number of closely located electronic states, and many vibrational modes, leads to an increase in the probability of nonradiative transitions: internal conversion and intersystem crossing. Time required for the energy concentration on the breaking bond (bonds) can be several orders of magnitude longer than, for example, the interval 10–13 s required for the dissociation of a diatomic or triatomic molecule [22,32].
It is quite obvious that the one-color approach of implementing the LF/LIF method, in which the fragmentation of the nitrocompound molecules and excitation of their NO fragments occurs in a single laser pulse of duration τ < tmax, does not allow achievement of the maximum efficiency of the LF/LIF process, although it has some attractiveness due to the relative ease of implementation. From the point of view of increasing the overall efficiency of the LF/LIF process and achieving the maximum sensitivity of the method, the two-pulse method of LF/LIF excitation is of interest [35,36].
4. Conclusions
The results obtained in the course of the work indicate a significant effect of ambient nitrogen dioxide as a limiter of the sensitivity of the LF/LIF method when detecting nitrocompound vapors in a real atmosphere. Using nitrobenzene and o-nitrotoluene as an example, it is shown that if the concentration of nitrogen dioxide in the atmosphere does not exceed 10 ppb, the maximum detectable vapor concentrations of nitrobenzene and o-nitrotoluene are several ppb.
As the calculation results show, due to the inertia of the decomposition mechanism of the nitrocompound molecules into fragments, the process of formation of the latter under pulsed action continues after the removal of optical excitation. The maximum concentration of NO fragments is achieved over a time several times greater than the standard fragmentation pulse duration of 10–20 ns. This allows us to conclude that the one-color method does not allow maximum efficiency of the LF/LIF process to be achieved. From the point of view of increasing the efficiency of the LF/LIF method, the time-separated two-pulse laser action on the nitrocompound molecules and their NO fragments is undoubtedly attractive. The results of the experimental studies [35,36] show that this approach allows one to increase the sensitivity of the method by about an order of magnitude.
Author Contributions
Conceptualization, S.B., E.G. and V.Z.; methodology, E.G. and S.B.; validation, V.Z.; formal analysis, E.G.; resources, E.G. and V.Z.; data curation, E.G. and V.Z.; writing—original draft preparation, E.G.; writing—review and editing, S.B.; visualization, E.G.; supervision, S.B.; project administration, S.B.
Funding
This research was funded by the Russian Science Foundation (RSF), grant number 17-19-01229.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodgers, M.O.; Asai, K.; Davis, D.D. Photofragmentation-laser induced fluorescence: A new method for detecting atmospheric trace gases. Appl. Opt. 1980, 19, 3597–3605. [Google Scholar] [CrossRef]
- Rodgers, M.O.; Davis, D.D. A UV-Photofragmentation/Laser-Induced Fluorescence Sensor for the Atmospheric Detection of HONO. Environ. Sci. Technol. 1989, 23, 1106–1112. [Google Scholar] [CrossRef]
- Sandholm, S.T.; Bradshaw, J.D.; Dorris, K.S.; Rodgers, M.O.; Davis, D.D. An Airborne Compatible Photofragmentation Two-Photon Laser-Induced Fluorescence Instrument for Measuring Background Tropospheric Levels of NO, NOx, and NO2. J. Geophys. Res. 1990, 95, 10155–10161. [Google Scholar] [CrossRef]
- Galloway, D.B.; Bartz, J.A.; Huey, L.G.; Crim, F.F. Pathways and kinetic energy disposal in the photodissociation of nitrobenzene. J. Chem. Phys. 1993, 98, 2107–2114. [Google Scholar] [CrossRef]
- Lemire, G.W.; Simeonsson, J.B.; Sausa, R.C. Monitoring of vapor-phase nitro compounds using 226-nm radiation: Fragmentation with subsequent NO resonance-enhanced multiphoton ionization detection. Anal. Chem. 1993, 65, 529–533. [Google Scholar] [CrossRef]
- Galloway, D.B.; Glenewinkel-Meyer, T.; Bartz, J.A.; Huey, L.G.; Crim, F.F. The Kinetic and Internal Energy of NO from the Photodissociation of Nitrobenzene. J. Chem. Phys. 1994, 100, 1946–1952. [Google Scholar] [CrossRef]
- Wu, D.D.; Singh, J.P.; Yueh, F.Y.; Monts, D.L. 2,4,6-Trinitrotoluene detection by laser-photofragmentation–laser-induced fluorescence. Appl. Opt. 1996, 35, 3998–4003. [Google Scholar] [CrossRef]
- Simeonsson, J.B.; Sausa, R.C. A critical review of laser photofragmentation/fragment detection techniques for gas phase chemical analysis. Appl. Spectrosc. Rev. 1996, 31, 1–72. [Google Scholar] [CrossRef]
- Swayambunathan, V.; Singh, G.; Sausa, R.C. Laser photofragmentation–fragment detection and pyrolysis–laser-induced fluorescence studies on energetic materials. Appl. Opt. 1999, 38, 6447–6454. [Google Scholar] [CrossRef]
- Daugey, N.; Shu, J.; Bar, I.; Rosenwaks, S. Nitrobenzene detection by one-color laser photolysis/laser induced fluorescence of NO (v = 0-3). Appl. Spectrosc. 1999, 53, 57–64. [Google Scholar] [CrossRef]
- Shu, J.; Bar, I.; Rosenwaks, S. Dinitrobenzene Detection by Use of One-color Laser Photolysis and Laser-Induced Fluorescence of Vibrationally Excited NO. Appl. Opt. 1999, 38, 4705–4710. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Bar, I.; Rosenwaks, S. NO and PO photofragments as trace analyte indicators of nitrocompounds and organophosphonates. Appl. Phys. B 2000, 71, 665–672. [Google Scholar] [CrossRef]
- Shu, J.; Bar, I.; Rosenwaks, S. The use of rovibrationally excited NO photofragments as trace nitrocompounds indicators. Appl. Phys. B 2000, 70, 621–625. [Google Scholar] [CrossRef]
- Arusi-Parpar, T.; Heflinger, D.; Lavi, R. Photodissociation Followed by Laser-Induced Fluorescence at Atmospheric Pressure and 24 °C: A Unique Scheme for Remote Detection of Explosives. J. Appl. Opt. 2001, 40, 6677–6681. [Google Scholar] [CrossRef] [PubMed]
- Heflinger, D.; Arusi-Parpar, T.; Ron, Y.; Lavi, R. Application of a unique scheme for remote detection of explosives. Opt. Commun. 2002, 204, 327–331. [Google Scholar] [CrossRef]
- Wynn, C.M.; Palmacci, S.; Kunz, R.R.; Zayhowski, J.J.; Edwards, B.; Rothschild, M. Experimental demonstration of remote optical detection of trace explosives. Proc. SPIE 2008. [Google Scholar] [CrossRef]
- Arusi-Parpar, T.; Fastig, S.; Shapira, J.; Shwartzman, B.; Rubin, D.; Ben-Hamo, Y.; Englander, A. Standoff Detection of Explosives in Open Environment Using Enhanced Photodissociation Fluorescence. Proc. SPIE 2010. [Google Scholar] [CrossRef]
- Wynn, C.M.; Palmacci, S.; Kunz, R.R.; Rothschild, M. Noncontact detection of homemade explosive constituents via photodissociation followed by laser-induced fluorescence. Opt. Express 2010, 18, 5399–5406. [Google Scholar] [CrossRef]
- Wynn, C.M.; Palmacci, S.; Kunz, R.R.; Aernecke, M. Noncontact optical detection of explosive particles via photodissociation followed by laser-induced fluorescence. Opt. Express 2011, 19, 18671–18677. [Google Scholar] [CrossRef]
- Bobrovnikov, S.M.; Gorlov, E.V. Lidar method for remote detection of vapors of explosives in the atmosphere. Atmos. Ocean. Opt. 2011, 24, 235–241. [Google Scholar] [CrossRef]
- Bobrovnikov, S.M.; Vorozhtsov, A.B.; Gorlov, E.V.; Zharkov, V.I.; Maksimov, E.M.; Panchenko, Y.N.; Sakovich, G.V. Lidar detection of explosive vapors in the atmosphere. Russ. Phys. J. 2016, 58, 1217–1225. [Google Scholar] [CrossRef]
- Lin, M.-F.; Lee, Y.T.; Ni, C.-K.; Xu, S.; Lin, M.C. Photodissociation dynamics of nitrobenzene and o-nitrotoluene. J. Chem. Phys. 2007, 126, 064310-1–064310-11. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Marshall, A.; Clark, A.; Deas, R.M.; Ledingham, K.W.D.; Singhal, R.P. Multiphoton Ionization and Dissociation of Nitrotoluene Isomers by UV Laser Light. Rapid Commun. Mass Spectrom. 1994, 8, 607–614. [Google Scholar] [CrossRef]
- Weickhardt, C.; Tonnies, K. Short pulse laser mass spectrometry of nitrotoluenes: Ionization and fragmentation behavior. Rapid Commun. Mass Spectrom. 2002, 16, 442–446. [Google Scholar] [CrossRef]
- SenGupta, S.; Upadhyaya, H.P.; Kumar, A.; Dhanya, S.; Naik, P.D.; Bajaj, P. Photodissociation dynamics of nitrotoluene at 193 and 248 nm: Direct observation of OH formation. Chem. Phys. Lett. 2008, 452, 239–244. [Google Scholar] [CrossRef]
- Okabe, H. Photochemistry of Small Molecules; John Wiley and Sons: New York, NY, USA, 1978; pp. 227–235. [Google Scholar]
- Castle, K.J.; Abbott, J.E.; Peng, X.; Kong, W. Photodissociation of o-Nitrotoluene between 220 and 250 nm in a Uniform Electric Field. J. Phys. Chem. A 2000, 104, 10419–10425. [Google Scholar] [CrossRef]
- Morrell, C.; Breheny, C.; Haverd, V.; Cawley, A.; Hancock, G. The 248 nm photolysis of NO2/N2O4: Time-resolved Fourier transform infrared emission from NO and NO2, and quenching of NO (v = 5–8). J. Chem. Phys. 2002, 117, 11121–11130. [Google Scholar] [CrossRef]
- McFarlane, J.; Polanyi, J.C.; Shapter, J.G. Photodissociation dynamics of NO2 at 248 nm. J. Photochem. Photobiol. A Chem. 1991, 58, 139–172. [Google Scholar] [CrossRef]
- Keller-Rudek, H.; Moortgat, G.K.; Sander, R.; Sörensen, R. The MPI-Mainz UV/VIS spectral atlas of gaseous molecules of atmospheric interest. Earth Syst. Sci. Data 2013, 5, 365–373. [Google Scholar] [CrossRef]
- Hancock, G.; Morrison, M.; Saunders, M. Vibrational relaxation of NO (v = 1–16) in collisions with O2 studied by time resolved Fourier transform infrared emission. Chem. Phys. Lett. 2006, 425, 216–220. [Google Scholar] [CrossRef]
- Hallin, K.-E.J.; Merer, A.J. The 2491 A band system of NO2. Rotational structure and evidence for predissociation in the zero-point level. Can. J. Phys. 1976, 54, 1157–1171. [Google Scholar] [CrossRef]
- Luque, J.; Crosley, D.R. Radiative and predissociative rates for NO A2Σ+ v′ = 0–5 and D2Σ+ v′ = 0–3. J. Chem. Phys. 2000, 112, 9411–9416. [Google Scholar] [CrossRef]
- Nee, J.B.; Juan, C.Y.; Hsu, J.Y.; Yang, J.C.; Chen, W.J. The electronic quenching rates of NO (A2Σ+, v′ = 0–2). J. Chem. Phys. 2004, 300, 85–92. [Google Scholar]
- Bobrovnikov, S.M.; Gorlov, E.V.; Zharkov, V.I.; Panchenko, Y.N.; Puchikin, A.V. Dynamics of the laser fragmentation/laser-induced fluorescence process in nitrobenzene vapors. Appl. Opt. 2018, 57, 9381–9387. [Google Scholar] [CrossRef]
- Bobrovnikov, S.M.; Gorlov, E.V.; Zharkov, V.I.; Panchenko, Y.N.; Puchikin, A.V. Two-pulse laser fragmentation/laser-induced fluorescence of nitrobenzene and nitrotoluene vapors. Appl. Opt. 2019, 58, 7497–7502. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).