Abstract
As revealed by the recent resurgence of yellow fever virus (YFV) activity in the tropical regions of Africa and South America, YFV control measures need urgent rethinking. Over the last decade, most reported outbreaks occurred in, or eventually reached, areas with low vaccination coverage but that are suitable for virus transmission, with an unprecedented risk of expansion to densely populated territories in Africa, South America and Asia. As reflected in the World Health Organization’s initiative launched in 2017, it is high time to strengthen epidemiological surveillance to monitor accurately viral dissemination, and redefine vaccination recommendation areas. Vector-control and immunisation measures need to be adapted and vaccine manufacturing must be reconciled with an increasing demand. We will have to face more yellow fever (YF) cases in the upcoming years. Hence, improving disease management through the development of efficient treatments will prove most beneficial. Undoubtedly, these developments will require in-depth descriptions of YFV biology at molecular, physiological and ecological levels. This second section of a two-part review describes the current state of knowledge and gaps regarding the molecular biology of YFV, along with an overview of the tools that can be used to manage the disease at the individual, local and global levels.
2. Molecular Biology of Yellow Fever Virus: State of Play
In 1985, the first complete sequence of YFV was determined from complementary DNA (cDNA) clones of the 17D vaccine strain by C. Rice [44]. Thanks to the combination of these sequencing data with an NH2-terminal sequence analysis of all structural and some of the non-structural (NS) proteins, the amino acid sequence of the virus was inferred and the flavivirus gene organisation was described [45]. Despite its historical importance, only a small amount of genetic data are available for YFV species and our understanding of the virus biology is thus partly based on the data obtained for closely related flaviviruses such as dengue virus (DENV).
As for all flaviviruses, the YFV genome consists of a positive single-stranded RNA molecule ((+)ssRNA) comprising about 11,000 nucleotides (nts) with a type I cap at the 5′ terminus (m7GpppAm) [46,47] but lacking a polyA tail at the 3′ terminus [48,49]. The cap structure of flaviviral genomes is thought to be important for cap-dependent translation and to protect the genome from degradation by cellular 5′-3′exonucleases [48]. This (+)ssRNA corresponds to one large open reading frame (ORF), flanked at its 5′ and 3′ termini by untranslated regions (UTRs) that are required for RNA replication and translation [44,50,51]. Yellow fewer virus ORF spans 10,233 to 10,236 nts and encodes a polypeptide of 3411 to 3412 amino acids. The amino-terminal residues correspond to the three structural proteins while the remainder of the ORF encodes the seven NS proteins with the following organisation: 5′cap-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-2k-NS4B-NS5-3′. The 5′UTR of YFV (~110 nts) is much shorter than the 3′UTR (~400–650 nts), the size of which varies among YF strains [28,31].
2.1. A Highly Structured and Slowly Evolving Positive Single-Stranded RNA Genome
The cis-acting replication elements (CREs) are RNA sequence motifs and secondary structures that take part in the replication of the virus. Most of the CREs that have been identified in flaviviral genomes are located within the 5′ and 3′ UTRs. The functions ensured by CREs in the viral replication cycle have been extensively studied for DENV and West Nile virus (WNV) [52,53,54,55,56]. Several CREs are conserved across numerous flaviviral species and are thus likely to perform similar functions, as comprehensively reviewed by several authors [51,57].
2.1.1. Promoters
Some CREs are crucial protagonists of the genome replication cycle and are therefore called “promoters”. During the replication of YFV genomic (+)ssRNA, the interaction between the 5′ end of the genome and the 3′UTR is critical for the recruitment and correct positioning of the NS5, a viral RNA-dependent RNA polymerase (RdRp), for the initiation of the (−)ssRNA synthesis [50,52,58,59]. This circularization mechanism is probably ubiquitous among flaviviruses [60,61,62] and involves several sequence motifs that ensure long-distance RNA-RNA interactions across the genome (see Figure 1a).

Figure 1.
Functional sequences and secondary structures within Yellow fever virus (YFV) genome: diversity among genotypes (Modified from [57] and [87]). (Upper part) Functional sequences and secondary structures within YFV genome. Promoter and enhancer elements are highlighted in light blue and grey, respectively. At the 5′ end: Cap-1 and functional RNA structures SLA and SSL. The translation start (AUG) and stop (UGA) codons encompassing the complete coding sequence (CDS) are indicated in italics. Sequence motifs involved in viral genome cyclization are shown in blue: UAR, DAR and CYC sequences. The PN is shown in dark yellow. The 3′UTR is organized into three domains (I, II, and III). Domain I: functional RNA structures RYF1, 2 and 3 (RYFs). Domain II: functional RNA structures SL-E, SL-D, ΨDB and DB. The pseudoknot elements (PK1, PK2, and PK3) are indicated by dashed, maroon lines, and the corresponding interacting sequences, by solid lines. The 5′ end of YFV the subgenomic flavivirus RNA sequence is indicated by a triangle. Domain III: functional RNA structures sHP and 3′SL. The PN sequence “CACAG” is indicated by a dark yellow line. (Lower part) Schematic representation of the seven patterns described for the untranslated regions (UTRs) of YFV strains. (Ia) Full “native” sequence corresponding to pattern “I” corresponding to West African genotypes I and II. (Ib) Only two RYFs (1/3) are present in pattern “Ib”, which is associated with the Angolan, East and East/Central African genotypes. (II) Only one RYF (3) is present in pattern “III” (deletion YFVSADM1), it is associated with the South American II genotype. Patterns “III”, “IV”, “V” and “VI” correspond to sequences from South American I genotype. (III) Pattern “III” involves the deletion YFVSADM1 and 2 and the insertions YFVSACM1 and YFVSAUM. (IV) Pattern “IV” involves the deletions YFVSADM1 and 2 and the insertion YFVSACM1. (V) Pattern “V” involves the deletion YFVSADM1 and the insertion YFVSACM1. (VI) Pattern “VI” involves the deletion YFVSADM1 and the insertions YFVSACM1 and 2. Motifs: YFVSADM1: deletion of RYF1 and RYF2; YFVSADM2: partial deletion of 3′SL (incl. disruption of the PN); YFVSACM1: insertion disrupting the CYC sequence and including an imperfect cyclization sequence (imp-CYC); YFVSACM2: insertion upstream CYC sequence; YFVSAUM: insertion of a unique motif (YFVSAUM) disrupting the DAR sequence and the sHP structure, partly deleting the 3′SL (=YFVSADM2). Abbreviations: CS1/2: conserved sequences 1/2, CYC: cyclization motif, DAR: Downstream of AUG region motif, DB: dumbbell, imp-CYC: imperfect CYC, imp-DAR: imperfect DAR, PN: pentanucleotide sequence, RYF: imperfectly repeated sequences, YFVSAUM: South American unique motif, YFVSACM1/2: South American conserved motif, YFVSADM1/2: South American deleted motif, sHP: small hairpin structure, SL: stem-loop, SSL: side stem-loop, UAR: Upstream of AUG region.
The first conserved sequence is located at the 5′ end of the YFV genome (5′CS) and its complementary sequence lies at the 3′ end (CS1). Their interaction was shown to be critical for YFV replication in cell culture [63]. The 5′CS includes the 5′ cyclisation (CYC) and downstream initiation AUG region (DAR) motifs and the CS1 involves the 3′ complementary CYC and DAR motifs (see Figure 1). The most distal promoter motif at the 5′ end is the upstream initiation AUG region (UAR) motif, which interacts through base-pairing with the 3′UAR. 5′ and 3′ UAR elements are not part of the 5′CS/CS1 motifs. The interaction between the 5′ and 3′ CYC motifs is essential to the formation and stability of the circular, replication-competent conformation of the RNA [64]. In YFV the genome, CYC motifs are 18 nts long and are found in the capsid coding sequence (nt positions 146–163) and in the 3′UTR (nt positions 10752–10765) [50]. Of note, in the 3′UTR of some Brazilian isolates from the South America I (SAI) genotype, the CYC sequence is deleted and circularization is preserved through an alternate long-range RNA-RNA interaction between the 5′ CYC and an imperfect 3′ CYC motif, as shown in Figure 1b. Finally, base pairing between the 5′ and 3′ elements of the UAR and DAR favours the initiation of the (−)ssRNA synthesis during WNV and DENV replication [64,65,66,67,68,69,70].
2.1.2. Enhancers
The replication enhancer elements are not essential for viral replication in vitro but increase its efficiency. However, they may be far more important for viral replication and transmission in vivo [71,72]. At the 5′ end of the YFV genome (see Figure 1a), the stem loop A (5′ SLA) folds into a main stem loop from which emerges a smaller side stem-loop (SSL) [72,73]. This structure enables the binding and activity of the NS5 protein that ensures genome replication [58,66,74,75]. The 5′ SLA is also involved in directing the addition of the cap-1 structure at the 5′ end of the genomic RNA [47,70].
The 3′UTR is constituted of three domains (I to III), from the most proximal to the most distal (see Figure 1a) and varies importantly in length among YF strains, notably from the SAI genotype. Domains I and II include secondary structures that likely modulate the efficiency of genome replication but may not be essential to the viral replication while several elements of the 3rd domain (DIII) are crucial to YFV viability.
The first domain diverges significantly among flaviviruses and in the YFV genome, it includes imperfectly repeated sequences (RYFs) of 41 to 44 nts [76,77], the biological function of which remains to be elucidated. They include two hairpin structures that are not necessary for viral replication, either in vertebrate or invertebrate host cells [31,63,76]. In YFV species, the number of RYF varies according to the genotype (see Figure 1). Sequences from the West African genotype include three copies of the RYF (RYF1/2/3) whereas Angolan, East and Central African involve two copies (RYF1/3) [76] and those from South America I and II genotypes only contain a single—and in some cases partial—RYF (RYF3) [31]. Although the scenarios differ regarding the evolutionary history of RYFs within the ancestor of South American lineages, they converge on the view that South American genotypes of YFV evolved from West African genotypes and that this evolution involved the deletion of some RYF(s) [31,76,77].
Although the second domain (DII) is less variable across flaviviruses than the DI, YFV DII differs from that of Japanese encephalitis virus (JEV) and DENV groups. Folding analyses revealed that this domain involves two stem-loops (SLE and D), a hairpin, a dumbbell (DB) and a pseudo-DB (ψ-DB) (see Figure 1a). Sequence similarities between the linear and folded regions of DII enables the formation of pseudoknots (PK1, 2 and 3) which may increase the stability of the secondary structures [78,79]. Comparisons between wild-type and vaccinal YF strains suggest a strong association between the integrity of the SLE and virulence, as most genomes of YFV vaccine strains exhibit altered SL and PK structures [79]. The DB structure and the conserved sequence it includes are common to JEV and DENV groups and may participate in viral translation [80] and RNA synthesis [81,82]. Finally, SLE is involved in subgenomic flavivirus RNA (sfRNA) formation during YFV infection [81]. Subgenomic flavivirus RNA are found in mosquito-borne flaviviruses and result from the incomplete degradation of the viral genome by the host 5′-3′ exonuclease Xrn1 that stalls at the level of SLE [83,84]. They play an important role in virus-vector interactions, replication, cytopathology and pathogenesis [85]. It has been shown that sfRNAs interfere with the cellular RNA decay machinery and the interferon type I pathway, but they may act through other mechanisms, as comprehensively reviewed elsewhere [86]. Finally, insertions of specific sequences, now referred to as the South American conserved motifs 1 and 2 (SACM1 and 2), can be found in the DII of some genomes from the SAI genotype, in a lineage-independent manner [31,87]. Most likely, these insertions were generated through the duplication of a conserved stem-loop structure. The SACM1 insertion disrupts the CYC sequence but includes an imperfect CYC sequence (imp-CYC) that ensures the circularization of the genome through an alternate interaction with the 5′ CYC sequence (see Figure 1b) [87].
Domain III is highly similar between flaviviruses and contains a small hairpin (sHP), a large terminal stem-loop (3′SL) structure, as well as the 3′ cyclization motifs (CYC, DAR and UAR). The 3′SL was found to be essential for YFV replication in cellulo [63]. Furthermore, studies on WNV, DENV and Kunjin (KUNV) virus demonstrated that both sHP and 3′SL are necessary for the completion of the viral cycle and may establish interactions with viral and non-viral proteic processes [52,60,88,89,90,91,92,93,94,95,96,97,98]. However, a deletion, the South American deleted motif 2 (SADM2), has been reported in some Brazilian strains from genotype SAI, and has been predicted to—at least partially—disrupt the sHP and 3′SL structures [87]. Hence, as viruses with minimal versions of the sHP/3′SL are viable, the dependence of YFV cycle on the sHP and 3′SL structures may not be strict [87]. Finally, a conserved pentanucleotide (PN) sequence 5′-CACAG-3′ is located within the loop of the 3′SL structure, the most distal part of the 3′UTR. The conservation of this PN sequence was shown to be essential to KUNV and WNV replication but for YFV, the dependence is likely to be significantly looser and the role of this PN sequence within the viral replication cycle remains to be determined [93,99]. In vitro experiments demonstrated that YFV 17D-derived mutants with altered PN sequences were viable under experimental conditions [100]. As the SADM2 deletion that has been identified in Brazilian isolates encompasses the PN sequence [87], the latter is not likely to be involved in RNA-RNA interactions for such a mechanism would require a much tighter conservation. Rather, the PN sequence could be part of a host or viral protein binding site [100].
Overall, the biological importance of duplications and deletions within the 3′UTR of YFV strains (RYFs, YFVSACM1/2, YFVSADM2) remains to be elucidated and should provide most valuable insights regarding the regulatory role of the RNA secondary structures and motifs they involve. There are several experimental demonstrations that mutations within flavivirus 3′UTRs have a differential impact depending on the host system (insect or mammalian) (e.g., [92,101,102]). Villordo and colleagues have comprehensively reviewed the present knowledge of the relationships between secondary structure RNA elements and host specialization in flaviviruses [101]. Elucidating the role of the structured elements of the YFV genome in the interactions between the virus and its hosts/vectors will be most useful to understand how virus-vector-host networks can shape viral evolution and may open new research avenues for tuning host-vector preference within flaviviral genomes.
2.1.3. A Genome That Evolves Relatively Slowly
RNA viruses typically have higher mutation rates than DNA viruses because their replication involves low-fidelity viral RdRps that frequently do not have proofreading activity. Hence, while for most DNA viruses, the mutation rate ranges between 10−8 and 10−6 substitutions/site/generation, for most RNA viruses it is much higher, with 10−5 to 10−3 substitutions/site/generation [103]. Since the nucleotide substitution rate (or evolutionary rate) depends on the mutation rate, the replication rate and the subsequent selection, an elevated mutation rate is not necessarily indicative of fast evolution. As dual-host pathogens, arboviruses and, notably, flaviviruses exhibit low evolutionary rates (<10−3 substitutions/site/year) [104] when compared with other RNA viruses such as avian influenza A virus, or the human immunodeficiency virus (HIV) (2 to 8 × 10−3 and 3 to 8 × 10−3 substitutions/site/year, respectively) [105,106,107]. Yellow fever virus appears to have a relatively low evolutionary rate, with 2 to 5 × 10−4 substitutions/site/year, as reported by several studies of the prM/E junction coding sequence [87,108,109,110]. In terms of evolutionary behaviour, marked differences have been observed between YFV and other flaviviruses with close ecological features such as DENV, which replicates in similar hosts and shares common vector species but exhibits evolutionary rates ranging from 7 to 9 × 10−4 substitutions/site/year according to the serotype [108,111]. Several hypotheses have been put forward by Sall and colleagues to explain the differences in evolutionary dynamics between YFV and DENV. They suggest that a preponderant role of transovarial transmission (TOT) within mosquitoes in the maintenance of the virus could be implicated as such a mechanism, where the virus may remain quiescent in mosquito eggs for many months, would decrease the replication rate of the virus [108]. As detailed in the first part of this review, several elements indicate that the TOT mechanism is not likely to play a significant role in YFV maintenance, neither in Africa nor in South America [112]. Notably, the rates of infection of the progeny observed under laboratory conditions are too low to enable the long-term survival of the virus through a TOT mechanism [113]. Hence, a TOT mode of survival is not likely to be preponderant in YF maintenance and would thus have a minor impact on the evolutionary rate of the virus. Finally, YFV and DENV differ importantly in terms of epidemiology, as DENV can establish sustained chains of transmission in humans while YFV does not (see first part of this review [112]). These distinct epidemiological trends could be the source of differences in evolutionary dynamics as these can be modulated according to the dynamics of transmission and between intra and inter-epidemic periods (e.g., [114]). Further investigations and comparisons using larger sequence datasets are thus needed to identify the possible mechanisms resulting in the apparently slow evolution of YFV. These could be intrinsic to the virus (mode of replication, polymerase fidelity) or extrinsic (ecology, epidemiology, immune response). Finally, in addition to mutations, recombination between viruses can further participate in sequence diversity within a given species. Recombination has been reported for some tick and mosquito-borne flaviviruses [115,116,117,118,119,120,121] but this has never been the case with YFV and experimental data further suggest that recombination between YFV strains is unlikely [122]. However, the mechanism by which the insertions and deletions identified within YFV 3′UTR have been generated remains to be described and could imply intra or inter-molecular reactions close to a recombination mechanism [31].
Originally defined on a serological basis, the YFV species corresponds to a single serotype and includes seven genotypes (West I, West II, East/Central and East Africa, Angola and South America I and II). The first genotypes were identified as topotypes, using RNAase T1 oligonucleotide fingerprinting techniques [123,124]. Genotype definition has progressively emerged and was formally outlined by Mutebi and colleagues in 2001 [125]. They defined genotypes as “distinct lineages which differ by greater than 9% at the nucleotide sequence level”, a criterion that had previously been used in several studies including their own [125,126,127]. It is commonly considered that the evolutionary rate is homogeneous across YFV genomes, with no significant variation across specific coding regions (e.g., structural versus NS proteins), as comprehensively detailed by Beasley and colleagues [28]. Hence, in the 1990s and 2000s, YFV molecular phylogenies have been performed using a large variety of sequences, including structural and NS protein coding sequences, as well as UTRs [31,76,77,125,127,128,129,130]. Over the last decade, YFV molecular phylogenies have primarily been performed on the complete genome of the virus or on a partial sequence of ca. 670 nt, called the “prM/E junction” [125], that encompasses 108 to 334 of the 3′ nts of the prM coding sequence, the full-length M protein coding sequence and the 337 5′ nts of the E coding sequence [30,87,109,110,131,132,133,134,135,136,137,138,139,140]. However, the NS5 coding sequence and the 3′UTR are still used on some occasions [137,141]. As detailed in the first section of this review, there is a strong association between the phylogenetic and the geographic clustering of YFV strains so genotypes correspond to specific geographic areas that rarely overlap [112]. This is suggestive of a predominant role of in situ evolution in shaping YFV diversity, consistent with the maintenance of YFV in a primarily “sylvatic” mode of transmission [142]. As comprehensively reviewed by Beasley and colleagues, and as it may appear in the above paragraphs, the association between specific genotypes or lineages and phenotypic features such as virulence or adaptation to specific vector(s) and/or host(s) is severely understudied [28] and much remains to be learned regarding the biological significance and determinants of YFV genetic diversity.
2.2. Structure and Replication of the Viral Particle
Yellow fever virus is an enveloped virus that replicates in the cell cytoplasm throughout a cycle which is usually defined in seven contiguous stages: (i) attachment to the cell surface, (ii) internalisation into the host cell, (iii) fusion and transfer of the viral RNA genome into the cytoplasm, (iv) translation of the viral proteins, (v) replication of the genomic RNA, (vi) assembly and maturation of the virions, and (vii) release of progeny viruses from the cell [143,144]. Most knowledge of the YFV replication cycle arose from studies achieved with other flaviviruses and the YFV 17D strain. As the flavivirus replication cycle has been described elsewhere [144,145,146], this section will be limited to a brief overview of the respective roles of YFV proteins within the YFV life cycle. While only the structural proteins become part of the mature infectious virion, the NS proteins play multiple roles in polyprotein processing, viral RNA synthesis, and virus morphogenesis.
Yellow fever virus is a small, enveloped virus of 50 nm diameter. The capsid (C), the membrane (prM/M) and the envelope (E) are the three structural proteins of YFV. The virion structure involves a nucleocapsid core (NC), surrounded by a host-derived lipid bilayer with an outer glycoprotein shell composed of two glycoproteins, M and E, of which 180 copies are assembled following an icosahedral symmetry [147].
The capsid is involved in genomic RNA packaging and in the formation of the NC [44,148,149]. In addition, it was recently shown that YFV C protein plays a role in inhibiting RNA silencing in A. aegypti mosquitoes by binding long dsRNAs with high affinity, thereby impeding their efficient processing by the Dicer protein [149]. The membrane protein exists under both an immature (prM) and a mature (M) form and may act as a chaperone to ensure the proper folding and assembly of the E protein [150,151]. At the surface of immature virions, prM proteins form heterodimers together with E proteins, giving the viral particles a spiky aspect [147] and preventing the adventitious fusion of the virus during egress [152]. Upon maturation, prM and E proteins successively reorganise as (prM/E) and finally as E homodimers giving a smoother aspect to the viral particles. However, most virions retain an important degree of structural heterogeneity [153] due to both incomplete furine cleavage of the prM protein [154,155,156] and the dynamic “breathing” behaviour of the metastable E dimers [157,158].
The E protein mediates the entry of the virus into the cell and interacts with cellular receptor molecules at the cell surface [143]. First, low-affinity interactions with a range of surface molecules such as heparan sulphate [24,159] and subsequently through high-affinity interactions with specific—and yet unknown—receptor molecules such as integrins, as proposed in the case of YFV 17D [160]. After recognition and attachment, the virus enters the host cell by clathrin-dependent endocytosis. The low pH environment inside the endosomes triggers a rearrangement of the E proteins in fusion-active homotrimers that facilitate fusion by bringing viral and cell membranes in close proximity [161,162,163,164,165] to form a fusion pore through which the nucleocapsid is released into the cytoplasm [166,167]. Of note, the vaccine strain YFV 17D has recently been reported to enter the cell through a clathrin-independent pathway. This switch in entry mechanism relies essentially on the 12 mutations differentiating YFV 17D E protein from that of its parental (Asibi) strain [23]. Other alterations of YFV E protein have been reported to impact viral tropism and virulence [23,27,35,168,169,170,171].
After its dissociation from the capsid protein through a still undefined uncoating mechanism [172], the viral genomic RNA is translated and replicated in the cytoplasmic compartment, in close association to the endoplasmic reticulum membranes, as a prelude to viral particle assembly. The translation gives rise to the polyprotein precursor which is glycosylated by cellular glycosyltransferases and finally cleaved, post- and co-translationally, by host and viral proteases, into three structural and seven NS proteins along with the 2K peptide. The virus-encoded serine protease (NS3-NS2B complex), ensures the cleavage between NS2A/NS2B, NS2B/NS3, NS3/NS4A and NS4B/NS5 [173,174,175]. The 2K peptide is thought to act as a signal sequence and is removed after cleavage [176].
NS5 and NS3 are major elements of the replication complex and achieve the synthesis and capping of the viral genomic progeny (flaviviral RNA replication is comprehensively reviewed in [177]). Genome replication takes place in vesicle packets that emerge through ER membrane rearrangements, a mechanism that may be induced by the NS4A protein [178,179]. During genome replication, regions of the transmembrane NS2B, NS4A, and NS4B proteins interact with the NS3 and anchor the replication complex to the ER membrane. The NS1 protein [180,181] and cellular factors may also be involved in the replication step [182], but their roles have not yet been defined. The viral genomic progeny are then used for further translation or associated with the structural proteins before being incorporated into immature virions. In addition to their role in the replication process, the NS1 protein takes part in the immune evasion of the virus (reviewed in [183]), the NS4A and NS4B are allosteric cofactors for the NS3 helicase domain [184,185] and the NS4B blocks interferon α/β signalling [186].
Immature, fusion-incompetent, viral particles are first assembled through the combination of newly synthetized viral proteins and nucleic acids [187] through non-specific, electrostatic, C-RNA interactions facilitated by the close association of replication and genome encapsidation [188,189]. Immature virions acquire a host-derived lipid envelope by budding into the lumen of the endoplasmic reticulum (ER) [190] and progress through protease- and pH-dependent maturation while they transit through the trans-Golgi network to the cell surface [152]. This maturation involves the reorganization of the E proteins and the cleavage of the prM protein by cellular endoproteinase furin proteins in to the “pr” peptide and the M protein [191,192], a mechanism which is tightly constrained by calcium concentration [193]. Once cleaved, the N-terminal pr fragment remains virion-associated until it is exocytosed, when it is then shed from the virion during mature viral particle release [194]. In this metastable structural state, virions are “fusion-competent” (i.e., able to undergo the low-pH triggered fusion events during the upcoming stage of cell entry) [195].
Flavivirus replication is considered to be primarily cytoplasmic however, some flaviviral proteins, namely the C, NS4B and NS5, can translocate to the nucleus, a mechanism that may participate in replication and/or immune suppression. The current understanding of the modalities and function(s) of the nuclear localisation of flaviviral effectors during the viral replication cycle is limited for flaviviruses in general and for YFV in particular, as comprehensively reviewed by Lopez-Denman and Jason M. Mackenzie [196]. Although the viral cycle of YFV—and other flaviviruses—has been partly elucidated, many gaps remain in our understanding of the mechanisms and of the viral/host factors that are involved in YFV replication. Improving our grasp on the molecular biology of the virus will pinpoint specific steps and components of the viral life cycle that could be targeted to develop safe and efficient antiviral strategies against YFV and possibly, other flaviviruses.
3. How to Mitigate and Manage YFV Infections?
3.1. Virus Tracking: Diagnostic Tools Inventory
The recent YFV outbreaks in Latin America [197,198] and Africa [199] demonstrated the need for reliable YFV diagnostics as a part of global YFV control. Outbreak management is largely dependent on rapid diagnosis of cases to establish appropriate mitigation measures (e.g., medical care, emergency vaccination, vector control). In addition, efficient diagnostic tools are necessary to identify the areas where the virus circulates and, on this basis, implement adequate long-term immunisation plans. The World Health Organization (WHO) estimates that YFV cases are still massively under-reported, with a true number of cases estimated to be 10 to 250 times those now being reported. Important reasons for under-reporting are asymptomatic infections and mild infections with nonspecific symptoms that are not identified by differential diagnostics [200]. To improve case-reporting, the WHO recommends that every at-risk country ensures that YF blood tests can be performed in at least one national laboratory [201]. In the past, virus isolation was a common tool in arbovirus laboratory diagnostics. Nowadays, time-saving molecular and serological methods provide the basis for arbovirus diagnostics [202]. Nonetheless, sensitivity and specificity of these methods are constant challenges to virological diagnostics and on some occasions, virologists and clinicians need to accept that unambiguous diagnoses cannot be achieved despite the usage of multiple tests. In the following, we provide details on the different diagnostic methods, discuss official recommendations and new trends, and point at gaps in our current knowledge of YFV laboratory diagnostics.
3.1.1. Molecular YFV Diagnostics
Until the beginning of the 21st century, molecular YFV diagnostics was achieved partly by conventional reverse transcription PCR (RT-PCR) [203,204,205]. Subsequently, YFV-specific reverse transcription loop-mediated isothermal amplification (RT-LAMP) [206,207], real-time recombinase polymerase amplification (RT-RPA) [208] and real-time RT-PCR assays using either intercalant dyes such as SYBR Green I [209,210] or TaqMan (hydrolysis) probes [211,212,213,214,215,216,217,218,219,220] were developed. Very recently, a Specific High Sensitivity Enzymatic Reporter UnLOCKing (SHERLOCK) was developed for DENV and Zika virus (ZIKV) detection by using the RNA-targeting CRISPR-associated enzyme Cas13 [221,222]. This innovative method could be adapted for YFV detection, but its robustness in clinical use remains to be demonstrated. Nowadays, TaqMan assays are most commonly used due to their specificity and ease of use, notably because the probe format is available across western and resource-limited regions at affordable prices. However, molecular protocols apply to a short timeframe of acute infection due to short-lived viremia in arbovirus infections [223,224]. Although potentially extended in severe cases [225,226,227], viraemia is frequently short and peaks around 2–3 days following YFV infection and in most cases, the viral RNA can only be detected in the blood for 3–6 days post-infection. In addition, viral loads are often low and vary significantly, ranging between 102 and 107 genome copies per mL [228,229,230,231,232,233,234]. As for other flaviviruses [235,236], YFV RNA can be detected in urine and semen but unfortunately, we lack reliable data on the frequency of detection of the virus in urine/semen samples. The closely related ZIKV is detected in urine with a 50–95% frequency [237,238,239,240] and in semen in up to 33% [236] of confirmed male cases. Noteworthy, prolonged detection of ZIKV RNA was reported in urine/semen as compared to serum [237,238,240,241], with semen being tested positive up to six months after the onset of symptoms [242]. On this basis, it has been suggested that testing urine might improve molecular ZIKV detection [237,238]. Nonetheless, efficiency may vary depending on cohorts and with regard to the type of test that is used [223]. Overall, most information on YFV viraemia comes from single case reports or vaccine studies and more reliable data from the field are urgently needed. Notably, it would be worthwhile determining if higher YFV loads or prolonged viraemia correlate with disease severity as reported for other arboviruses [243]. Due to the short time-span of YFV presence in blood, official WHO [244] and Pan American Health Organization (PAHO) [245] guidelines advise molecular YFV testing for up to 6–10 days following the onset of symptoms, followed and complemented by serological methods. The complexity of molecular YFV diagnostic was also revealed by external quality assessments (EQA) published in 2012 and very recently in 2018 [246]. In 2012, among participating laboratories from Europe, the Americas, Middle-East, Asia and Africa, 84% needed to improve their testing procedures. The major problems that were reported were lack of sensitivity relative to wild-type viruses and lack of specificity [247]. Within the 2018 EQA, 47% of the participants revealed major problems [246].
3.1.2. Serological YFV Diagnostics
Serological flavivirus diagnostics are commonly recommended from day six post-infection onwards [244,245] and are usually based on the detection of specific immunoglobulin M (IgM) or immunoglobulin G (IgG) antibodies. IgMs are developed a few days after infection and can be detected for up to six months [248] whereas IgGs are developed during convalescence but can usually be detected for decades [249]. In both cases, seroconversion using paired sera can confirm acute infections, but such samples are not always available from remote areas. The most commonly used biological specimens for YFV serology are serum and plasma. Although other specimens, including cerebrospinal fluid (CSF), are used for tick-borne encephalitis virus (TBEV) serology [250], YFV IgM detection in CSF was only reported for vaccine-associated adverse events (YF-VAAE) [251,252].
A variety of enzyme-linked immunosorbent assays (ELISA) [253,254], indirect immunofluorescence assays (IFA) [255,256], microsphere immunoassays (MIA) [257] or the plaque reduction neutralization test (PRNT) [258,259,260] procedures are operable for YFV serology. PRNT50 and PRNT90 are used to detect neutralizing antibodies [259,261,262,263] and are considered the gold standard for arbovirus serology [244,264]. However, largely for technical reasons, including the need for biosafety level 3 (BSL-3) laboratory facilities (or in some Asian countries, BSL-4), PRNT is used less frequently than other methods. Recently, a non-infectious virus-like particle (VLP)-based YFV PRNT was developed, partly to overcome these problems, that enables final analysis by flow cytometry [265].
Finally, while the PRNT still remains an extremely valuable diagnostic tool, flavivirus serology can be challenged at different levels. First, the kinetics of antibody responses can be influenced by former flavivirus infections [266] or pregnancy [267] and might vary greatly between individuals [268]. Second, serological diagnostics are hampered by broad cross-reactive anti-flaviviral antibodies that may cause false-positive test results [253,268,269]. Due to the complexity of arbovirus serology, precise guidelines for serologic YFV diagnostics were provided by the WHO. Suspected YFV cases are confirmed serologically by (i) the detection of YFV-specific IgM antibodies in the absence of IgM against DENV, WNV and ZIKV without recent YFV vaccination history, (ii) by a fourfold increase in YFV IgM/IgG titres between acute and convalescent blood specimens, (iii) by the presence of YFV neutralizing antibodies without YFV vaccination history, or (iv) by detection of YFV antigens using an immunoassay without recent YFV vaccination history [244]. Unfortunately, there is only limited knowledge concerning the performance of diagnostic laboratories ensuring the quality of YFV serology. The latest EQA on YFV diagnostics was published in 2012 and revealed problems of specificity and, more importantly, sensitivity, as almost 50% of the participants failed to detect YFV 17D IgM [247]. These problems are not unique to YFV diagnostics, as reported for other arboviruses such as DENV [270] or WNV [271], but are of concern given the medical relevance of YFV. New EQAs are suitable to assess the actual capacity of diagnostic laboratories and might be the key to optimizing YFV diagnostics.
3.1.3. Changing YFV Diagnostics in Times of Mass Vaccination Campaigns
Recently, the demand for new molecular YFV diagnostics became evident. In December 2016, Brazil reported the largest YFV outbreak for decades and launched an extensive vaccination programme. During the past 80 years, live attenuated YFV 17D and YFV 17DD vaccines have proven to be safe and efficacious for use in humans >6 months of age and many millions of doses have been administered globally. However, even using the existing carefully regulated live-attenuated YFV vaccines, approximately 0.4/100,000 cases of YF-VAAE are estimated to occur [272] and mass YFV vaccination thus raises the need to differentiate between YF-VAAE and wild-type infections [273]. Discrimination of vaccine and wild-type YFV strains is hindered by low genetic divergence within YFV species. This is particularly important in the case of Western African genotypes, which are closely related to the Asibi strain, the parental strain of modern YFV vaccines. Accordingly, new real-time RT-PCR assays discriminating between wild-type and vaccine strains [218] or specifically detecting vaccinal strains [219] were recently published. Molecular YFV diagnostics are further challenged by the application of the new chimeric, live- attenuated DENV vaccine Dengvaxia® in countries that are endemic for YFV such as Brazil [274,275,276]. This vaccine is based on a YFV 17D-backbone expressing the prM and E regions of the four DENV serotypes [277]. Most RT-PCR assays listed above would falsely detect this vaccine as a wild-type YFV hence new, discriminant assays are needed. Noteworthy, DENV-based DENV vaccines are currently in phase three clinical trials [278]. Their application should overcome problems in differentiation between DENV vaccination and YFV infection.
3.1.4. Limited Information about Genetic YFV Diversity
The target regions of published molecular assays for YFV detection are not evenly spread across the viral genome. Of at least 20 published assays for molecular YFV diagnostics, six target the 5′UTR, five the NS5 coding region and four the NS1 coding region. Remarkably, the information about the genetic variation of YFV is relatively small and is limiting reliable oligonucleotide design for YFV detection. As of 15 May, only 88 complete coding sequences of YFV isolates are available at GenBank, NCBI. This number is surprisingly low given the long time-span since the discovery of YFV and given its medical relevance. In comparison, the genetic information available for other arboviruses is considerably greater, including for the bird-associated Usutu virus (USUV), that causes only sporadic human infections [279] (Figure 2). This underrepresentation of YFV in terms of genetic data might be a major problem for public health since existing real-time RT-PCR assays are designed based on limited knowledge about genetic YFV diversity and nucleotide mismatches at oligonucleotide binding sites may affect test performance [223,280]. Despite the relatively low evolutionary rate of YFV [28,108,133], larger YFV genomic datasets are urgently required to ensure reliability of molecular YFV diagnostics.
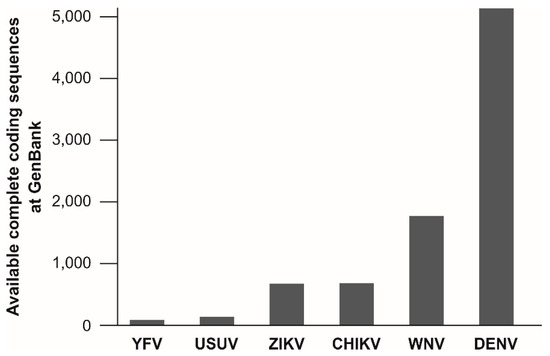
Figure 2.
Number of available complete coding sequences at GenBank for selected arboviruses. Sequences were accessed on 15 May 2018. Viruses are ordered by the number of available sequences. Chikungunya virus was included for comparison. CHIKV, Chikungunya virus; DENV, dengue virus; USUV, Usutu virus; WNV, West Nile virus; YFV, Yellow fever virus; ZIKV, Zika virus. YFV, n = 88; USUV, n = 142; ZIKV, n = 677; CHIKV, n = 684; WNV, n = 1778; DENV, n = 5145. For YFV, 49 sequences originate from the Americas (including 40 from Brazil), 28 from Africa and 11 from Asia (including five cases imported from Africa).
3.2. Infection Prevention in Endemic and At-Risk Areas
3.2.1. Vaccination Policies
YFV vaccination is based on the use of the attenuated YFV 17D strain, which was originally elaborated by M. Theiler in 1935 [38]. This live-attenuated vaccine is commonly recognized as one of the most effective ever created and vaccines are still being manufactured using its substrains, YFV 17D-204 and YFV 17DD, as seeds [2]. Although differences in antigenicity between YF strains have been reported on some occasions [281,282,283,284,285,286,287], the view that the YFV species corresponds to a single serotype is commonly accepted and it is considered that immunisation using either YFV 17D-204 or YFV 17DD-derived vaccines confers protection against all circulating YFV strains. YFV vaccine and vaccination have been comprehensively reviewed by several authors over the past decade [2,145,272,288,289]. For the sake of parsimony, we will thus focus on the major issues regarding YFV vaccination practices.
Currently, population immunisation is ensured through both routine and emergency vaccination efforts.
Routine vaccination campaigns are common in endemic areas but vary in width and with regard to the target population according to the regions of the world. Notably, the low vaccination coverage in the endemic areas of East Africa which historically recorded weaker YF activity is likely to have contributed to the advent of important outbreaks in these regions [145] as in 2010 in Uganda [290,291], in 2012–2013 in Ethiopia and Sudan [292], and more recently in Central Africa, with several thousand suspected cases reported between 2016 and 2017 in Angola and DRC [199,293]. In addition, the recent outbreak in Brazil reached areas with no vaccination recommendation hence, with a potentially low proportion of immunised inhabitants [198] and similarly, the ongoing outbreak in Nigeria takes place in an area with presumably low vaccination coverage [43]. A map recapitulating YFV outbreaks over past decades, with estimated vaccination coverage, can be found in Figure 3. Such events are blatant indications that routine vaccination should be taken to a larger scale, regarding both the target populations and the areas encompassed.
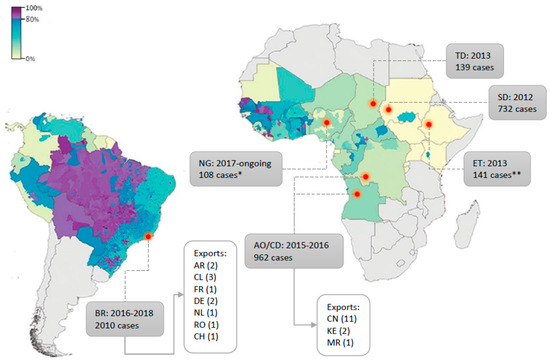
Figure 3.
Yellow fever (YF) outbreaks since 2010 with regard to vaccination coverage at the time (modified from [299]). Estimated proportion of the population (across all ages, in %) to have ever received a YF vaccine at the beginning of 2010 for countries at risk of YFV transmission, based on the untargeted, unbiased vaccination-targeting scenario [299]. Countries where YFV outbreaks occurred since 2010 are indicated by a red dot. Outbreak time-span and total number of cases are detailed in grey boxes. Export cases are detailed when applicable. All country names’ abbreviations are defined according to international ISO country codes. * Presumptive positive cases ** reported cases.
Emergency vaccination efforts can be mounted during outbreaks, notably when they occur outside endemic areas or reach uncommonly large magnitude, as illustrated by the large campaign that was established to contain the 2016–2018 outbreak in Brazil [198]. In 2016–2017, the virus reached the South-Eastern part of the country, notably the metropolitan areas of Belo Horizonte, Rio de Janeiro and Sao Paulo, which are densely populated and include numerous localities where the vaccine was not recommended. This immunisation initiative was unprecedented in that it aimed to reach an extremely large population of ca. 26 million people [42] notably thanks to the adoption of dose-fractioning strategies [294,295]. The first large use of the dose sparing approach dates back to August 2016, in Kinshasa, when more than seven million people, including children from two years of age, were immunised using 1/5 of the YFV 17DD vaccine to contain the 2015–2016 outbreak in a context of vaccine shortage [296]. This procedure was established again in 2017–2018 in Brazil, and more than seven million people have been vaccinated using 1/5 of the standard dose as of epidemiological week 18 (1st week of May) [42,297].
The WHO currently recommends that fractional YF vaccine dosing should be used as a short-term response to outbreaks during periods of shortage of full-dose YF vaccine. In practice, the lowest dose that can be used is of 1000 IU and should be administered subcutaneously or intramuscularly. Finally, the WHO recommends the use of the YF-17DD vaccine for dose-sparing procedures, as the available immunogenicity and safety data were obtained for this specific vaccinal strain [298].
Recent results from a dose-response study in which young adult males were immunised using dose-tapered YFV 17DD vaccine [295] demonstrated that all seroconverters remained seropositive eight years later, for doses ranging from 31 to 27,476 UI [297]. Such insights are crucial to evaluate the necessity and timing for re-vaccinating the increasingly large populations that have been immunised using fractionated doses over the past years in DRC and Brazil (>12 million people). Further investigations are needed, as the dose-response study included exclusively young adult males while the vaccine is recommended for individuals of nine months of age or older. Indeed, differences in immune response among age groups were reported during the dose-fractioning campaign in Kinshasa [296], so there is a risk that the duration of immunity may be reduced in young children and adults over 50 years [297].
Intradermal administration of the vaccine has been proposed as an additional means of reducing the dose necessary for effective immunisation against YFV [29,298,300,301,302,303]. In the past, this approach has proved to be fully efficient in the context of vaccination against variola, tuberculosis and more recently, influenza virus [294,301]. This procedure offers the advantage of mimicking the natural infection better than intramuscular and subcutaneous routes and is believed to elicit cell-mediated immunity [298,301,304]. Overall, intradermal immunisation appears to be a relevant strategy for ensuring efficient YF vaccination in the context of limited vaccine stocks and there is first evidence of a similar effectiveness between the intradermal administration of a low dose YFV 17D vaccine (Stamaril) and standard vaccination [303]. Further investigations are needed to provide full evidence of superior or equal efficacy of the use of the intradermal immunisation when compared to conventional routes, for all clinically available vaccines, to enable this strategy to be taken to the next level.
3.2.2. Vector-Control Plans: Past, Present and Future Strategies
Despite the existence of an effective vaccine for YFV, preventing and reducing (during epidemics) viral transmission also depends on controlling the mosquito vectors. The sylvatic transmission of YFV has no possibility of vector control because of the multiplicity and/or the inaccessibility of natural breeding sites (mainly tree holes) used by sylvatic mosquito species in Africa or in the Americas (see the first part of the review for more details). Due to potential adverse effects of insecticides on non-targeted organisms, the use of insecticides against both immature and adult stages is also inapplicable in natural ecosystems. The only way to prevent the contact between human and sylvatic vectors remains limited to personal protection using topical repellents or insecticide-treated clothes.
The urban transmission of YF is mainly supported by A. aegypti, which also ensures the epidemic transmission of emerging Aedes-borne viruses that pose growing global threats. All vector control programmes developed during the past decades to control dengue, chikungunya or Zika epidemics and A. aegypti (and Aedes albopictus to a smaller extent), are transposable to YFV. Top-down vertical Aedes control programmes have been successfully applied to control YF in the Americas (1900–60s) and more recently dengue, in Singapore (1970–80s) and Cuba (1980–90s) [305,306,307]. However, these large, paramilitary and vertically structured programmes, that include thousands of inspectors and other public health staff with enforcing laws that prohibit owners from allowing mosquito production in their dwellings (top-down campaigns) have become impossible to implement in the modern era. Nowadays, community participation programmes (bottom-up approaches) such as the use of communication for behavioural impact [308] in cities, could be efficient to control Aedes [308,309]. To curtail YFV transmission in urban environments, Integrated Vector Control Management (IVM) approaches can combine several methods including personal protection, larval (source reduction, larviciding and biological control) and adult (adulticiding) vector control measures [310].
Among relevant control methods, those targeting adult mosquitoes with insecticides can be deployed as an ‘emergency’ measure for preventing transmission as peri-domestic treatments carried out in and around households where human infection has been reported. Adulticiding can be done with different methodologies, space spraying versus residual spraying, indoor versus outdoor, house-to-house application using portable equipment versus vehicle-mounted fogging and cold versus thermal fogging. Insecticides can be useful to curtail transmission when applied properly but they have several constraints as insecticide resistance, low societal acceptability, environmental impact and potential impact on non-target organisms. In relation to personal protection measures, the use of insecticide-treated materials and house screening has been shown to be effective [310]. Topical repellents can be useful for individual protection and can be distributed during epidemics. Larval control, such as environmental management, source reduction, larviciding or biological control is more effective when it is consistent and routinely used rather than a haphazard emergency response. Source reduction should primarily target artificial containers in private and public spaces and may also include some natural containers such as bamboos and bromeliads, that can also harbor Aedes larvae. Community-based campaigns of source reduction have proved to be efficient for controlling disease transmission [309]. Several larvicides (Bti, temephos, pyryproxifen or diflubenzuron) and biological control methods (fish or copepods) can be used for the treatment of large and permanent breeding sites such as drinking water containers. Other control methods, such as sterile and transgenic mosquitoes, mass trapping or Wolbachia-based strategies are being developed but there is insufficient evidence to recommend their application at present [311].
These control measures should be applied in an integrated manner, with community mobilization and adequate inter-sectoral collaboration. Epidemiological and entomological surveillance may guide their implementation in areas with high transmission risk (e.g., neighbourhoods). Integrated Vector Control Management must be conducted in a precise, sustainable and proactive manner, targeting both larvae and adults, and if possible, in combination with vaccination campaigns increasing the effectiveness for controlling and reducing YFV transmission.
3.3. Patient Care: Perspectives for Treatments against Yellow Fever Virus Infection
To date, no specific drug is available for YF treatment, which remains limited to symptomatic and supportive care. Although different molecules have shown in vitro and/or in vivo activity against YFV, none is available clinically. The specifications for antiviral compounds depend on the purpose of their intended use. In the case of an outbreak or VAAE, an ideal curative anti-YFV molecule should be effective if administered directly after the onset of symptoms associated with YF disease. On the other hand, for a preventive use (e.g., in travellers), requirements may be looser in terms of efficiency, as the drug can be administrated ahead of the infection. However, higher standards are required in terms of safety, for the benefit/risk ratio will depend on the probability of infection. Efficient antiviral drugs can target effectors involved in the viral cycle or in pathogenesis, whether viral or cellular.
Both structural and NS proteins of YFV have been proposed as targets for antiviral drug design.
Targeting the structural proteins and notably the envelope of YFV is used to interfere with the early stages of the viral cycle by blocking viral entry or through neutralization of virus infectivity. Several candidate molecules have been identified based on modelling and in silico screening studies, some of which have proven effective in inhibiting YFV replication in vitro [312,313,314,315,316,317,318,319]. So far, passive immunisation using sera from vaccinated hamsters is the only procedure that has proved to ensure effective YFV neutralisation in vivo [320]. Finally, another option is to suppress E protein expression using RNA interference. Thanks to this approach, adult BALB/C mice were protected against YFV following intra-cranial injection with the YFV 17DD strain [321].
Inhibition of the activity of YFV NS proteins has been widely investigated in the last decade, with compounds targeting the NS1 protein [321], the NS2B-NS3 complex [322], the NS3 [322,323], the NS4B [324,325] and the NS5 proteins [326,327,328,329]. Among all the types of molecule that have been tested for anti-polymerase activity, some exhibited significant efficiency in vivo and notably, compounds corresponding to the group of nucleoside analogues. The pyrazine carboxamide T-1106 has shown activity against YFV in the hamster model [330] and treatment with the T-705 (Favipiravir), an FDA-approved chemically related compound, significantly improved disease parameters in YFV-infected hamsters when beginning administration up to three days post-infection [331]. Of note, T-705 in vitro activity against YFV was synergistically enhanced when combined with the ER α-glucosidase inhibitor IHVR-19029 [332]. Finally, a novel adenosine analogue, the pyridine carboxamide BCX4430, offered complete protection from mortality with significant improvement of other disease parameters in a hamster model, and remained effective even when treatment was initiated at the peak of viral replication (i.e., four days post infection) [333]. Overall, targeting the viral polymerase (NS5) presents several advantages. First, the absence of a human host equivalent to this enzyme makes likely that inhibition of the NS5 RdRp activity is only associated with low levels of toxicity [334]. Second, because of the uniqueness and importance of the NS5 polymerase activity, there is a high degree of structural conservation of the NS5 polymerase domain among Flaviviridae family members [335]. This opens avenues for the evaluation of the anti-YFV activity of molecules that have been clinically approved for treatment against other Flaviviridae and notably, the hepatitis C virus (HCV). For instance, Sofosbuvir, a uridine nucleotide prodrug which targets the viral RNA polymerase and is clinically approved for use against HCV, has been shown to inhibit ZIKV replication efficiently in vivo, and prevented death in ZIKV-infected mice [336,337]. In addition, Sofosbuvir showed antiviral activity against DENV in vitro [338], so its potential as an anti-YFV compound is currently being investigated.
Host proteins that take part in the viral replication cycle constitute another pool of potential drug targets for anti-YFV molecule development. The purine nucleoside Ribavirin inhibits (among other potential modes of action) the inosine monophosphate dehydrogenase (IMPDH) protein and induces a reduction in the GTP pool, a mechanism that has been shown to correlate with its antiviral activity against several RNA viruses [339]. Ribavirin has shown anti-YFV activity in vitro and in vivo in hamsters, but not in NHPs [1,340,341]. The inhibition of the host caseine kinase 1, an NS5-interactant [342], and of both NTRK1 and MAPKAPK5 kinases, necessary for virus assembly, efficiently precluded YFV replication in vitro but none of these strategies has been tested in vivo yet [343].
Proteins involved in the host immunological response are also relevant targets for YF disease management as its most critical part, the shock (or intoxication) phase, has been suggested to have an immunopathological basis and is thought to involve a “cytokine storm” (i.e., unbalanced cytokine response). Several therapies have thus been developed to modulate this inflammatory response. Treatment with interferon-α significantly improved survival and reduced serum alanine aminotransferase levels in hamsters and African green monkeys when administered within 24 h following infection [1,340], showing a good potential for post-exposure prophylactic administration. The use of a recombinant adenovirus expressing interferon-α effected protection of hamsters against challenge with the hamster-virulent YF Jimenez strain up to two days post-infection [344]. Moreover, data from a retrospective analysis in patients having YF VAAE accompanied by shock, showed that treatment with stress-dose corticosteroid improved survival in humans [272,345]. These experimental and clinical insights strongly suggest that strategies counteracting cytokine storm and shock are efficient therapies for YF disease and should be further investigated.
4. Discussion
Control over YFV infections is a multifaceted issue to which no miraculous solution can be proposed for the time being. Indeed, curbing the circulation of a virus which circulates in the forest, in multiple non-human primate species, and which can be transmitted to humans through the bite of numerous mosquito species is a tremendous challenge. The fight against YFV—and numerous other zoonotic viruses—is like an arm-wrestling match during which every drop in effort, no matter how slight, constitutes, for the adversary, a chance to get the upper hand. This phenomenon can be appreciated in the pattern of shrinkage and expansion of YFV areas of endemicity according to vaccination and vector control campaigns across the last century of the fight against YFV [145,272].
Yellow fever activity has importantly increased in both Africa and South America over the past decades, as the result of insufficient vaccination coverage, mosquito reinfestation, deforestation and urbanisation. The fear of large urban YFV outbreaks has resurfaced in South America and with equal concern, numerous territories in the world that, until now, have apparently remained free of the virus, appear to bring together host(s), vector(s) and climatic conditions that are suitable for YFV dissemination [346]. In response to this resurgence, and to prevent the risk of expansion of YFV outbreaks to urban areas with dense non-immune populations, it is necessary to strengthen and refine surveillance, increase prevention through vaccination and vector-control and finally, improve patient care. Notably, from this perspective in 2017, the WHO initiated the “EYE” (Eliminate Yellow fever Epidemics) initiative in order to “protect the populations most at risk, ensure a ready supply of YF vaccine, build resilience in urban centres and prevent international spread” [347].
If we refer to the most recent outbreaks in Brazil and in Africa (e.g., in Nigeria, Angola, DRC, Chad, Sudan, Ethiopia), a likely explanation for the large number of cases that were reported is that the virus circulated in regions with low vaccination coverage [145]. Hence, we need to rethink how areas with vaccination recommendation are defined. In this prospect, it is necessary to improve our understanding of how the virus spreads and evolves.
Epidemiological surveillance is the main approach for tracing virus circulation and is primarily dependent on the availability of sensitive and specific diagnostic tools. As discussed in Section 3.1, there is still room for improvement in terms of sensitivity and specificity regarding diagnostic tools for YFV detection. In the case of YFV, these requirements come in a particularly intricate context as flaviviruses close to YFV in terms of serological and clinical features circulate in YFV endemic areas (e.g., Zika, Dengue) and as wild-type and vaccinal strains elaborated from YFV 17D (e.g., YFV 17DD or Dengvaxia®) should be accurately distinguished. Finally, in an outbreak context, diagnostic tools should be operable under field conditions.
Given the limited amount of genetic data available for YFV, it is necessary to constitute comprehensive catalogues of genomes of wild-type YF strains, notably during outbreaks, to better appraise YFV genetic diversity and develop comprehensive molecular detection systems. During the last Brazilian outbreak, efforts have been made to achieve genome sequencing using conventional PCR methods [133,139,348] and in the field, using portable sequencing tools, resulting in more than 50 additional genomic sequences now being available on Genbank for YFV [197]. To take full advantage of viral genetic data, they should be constantly confronted with the clinical and phenotypical features of the viruses in order to identify potential causal relationships and whenever possible, investigate them. It has been proposed that divergence between YFV genotypes would contribute to differential adaptation to mosquito vectors [113]. In the same vein, specific South American lineages have been suggested to be associated with increased epidemic potential (e.g., South American I modern lineage) [132,137]. However, no causal link has been demonstrated in either case.
Finally, it has been postulated that differences in transmission dynamics (e.g., sylvatic versus sylvatic/savannah cycle) or specific interactions with mosquito vectors (e.g., maintenance through a TOT mechanism) may result in different evolutionary dynamics among YFV genotypes [113]. Indeed, differences in maintenance mechanisms may modulate the selective pressures and the replication rate that dictate the evolution of the viral genome [108]. As its natural upkeep importantly relies on a sylvatic transmission cycle, YFV is considered to be a “sylvatic” virus. By contrast, DENV, the maintenance of which largely relies on an urban transmission cycle [349], is considered to be an “urban” virus, with a greater epidemic potential than YFV [350]. As discussed in Section 2.1.3, in terms of evolutionary dynamics, YFV exhibits a slower evolutionary trend when compared to DENV. As reported by Sall and colleagues [108], the evolutionary rate of DENV2 sylvatic genotype is the one which is closest to that of YFV and thus the lowest when compared to other DENV2 genotypes, with ~6 × 10−4 substitutions/site/year. Although this could be pure chance and if not, would possibly involve multiple factors, it could be interpreted as additional evidence that “sylvatic” behaviour may result in a lower evolutionary rate than an “urban” one. Overall, we need additional genetic and phenotypic data to properly determine whether there is a significant difference in evolutionary rate between sylvatic and non-sylvatic isolates of DENV and if an analogy cannot reliably be drawn between sylvatic DENV and YFV [351,352]. Further investigating the impact of specific maintenance patterns on the evolutionary behaviour of YFV may allow us to gauge the changing landscape within its reach and thereby, the epidemic potential of this virus.
In recent decades, increasing efforts have been implemented to expand vaccination coverage in at-risk areas and outbreak occurrence in densely populated areas have additionally increased the demand for YF vaccine. Hence, reconciling vaccine supply and demand is more than ever a central issue for YF prevention. For instance, the EYE initiative has planned to use 1.4 billion doses throughout the next eight years. This target would require the production of 175 million vaccine doses each year [353] but currently, the maximum yearly production of vaccine has never exceeded 80 million doses [353]. This challenge comes in a particularly difficult context of expansion of YFV-endemic areas and in which the connection between these regions and territories that are free of YFV—but suitable for virus transmission—is strengthening.
As evoked in this manuscript, several strategies have been proposed to increase the number of available vaccine doses by using reduced amount of vaccine through dose-fractioning and intradermal administration. To overcome the issue of supply it is necessary to increase gross vaccine production. The easiest option would be to have more doses produced each year, as planned by the EYE strategy, but this would not solve the problem of timeliness in replenishment in a context of successive large outbreaks, as observed during the past three years. To refine the vaccine manufacturing process, procedures could be revisited, as virus production technologies have evolved tremendously during the last decades. DENV (Dengvaxia) and JEV (Imojev) vaccines are cultured in cellulo rather than in embryonated chicken eggs, which is a much more efficient procedure [29]. Given that these vaccines have a YFV 17D backbone, it is likely that these protocols could successfully be adapted to overcome the difficulties previously encountered in the production of YFV 17D in cell culture [29,145]. Although revisiting manufacturing protocols is a laborious process, it will result in a more convenient, faster and more reliable production pipeline that will certainly benefit the overall disease prevention process. Furthermore, expertise from such developments should prove most useful for the implementation of more efficient and convenient vaccine manufacturing procedures in general.
Widening the use of YFV vaccine would also imply to improve management of VAAE. Although their occurrence can be considered as rare (~0.4 cases/100,000 cases), if 1.4 billion doses of the vaccine are to be used in the next eight years this would consequently lead to ~5600 adverse events that will have to be taken care of. In addition, if the EYE reaches its ambitious goal by 2026, until then, it is possible that other YFV outbreaks will occur. Therefore, it is still crucial to work on new antiviral strategies against YFV. Additional investigations should be made to identify the host factors that are necessary to the different steps of the YFV replication cycle (e.g., entry, replication, assembly, and egress) and to identify precisely, the host and viral factors that are involved in pathogenesis (e.g., immune response, entry pathway).
General questions deserve to be raised as the YFV vaccination campaigns that are to be implemented—notably through the EYE initiative—will be unprecedented in scale and may have several side effects that it would be worth anticipating as far as possible (e.g., exposure of immunocompromised individuals, impact on the circulation of other antigenically closely related flaviviruses, etc.). There is also an urgent need to improve our understanding of the vaccine itself (e.g., attenuation mechanism, efficient priming of a long-term immune response), the latter considerations have been concisely discussed in the recent work of Douam and Ploss [145].
Finally, increasing our knowledge of the molecular biology of wild-type YFV should benefit greatly to the research on other zoonotic viruses and more specifically, arboviruses. Furthermore, working on tools and strategies to contain YF outbreaks will be most useful to the development of countermeasures against other arboviral diseases notably flaviviruses, including pathogens of importance for Public Health at the moment such as DENV or ZIKV.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Monath, T.P. Treatment of yellow fever. Antivir. Res. 2008, 78, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.G.S. Yellow fever vaccine. In Vaccines, 6th ed.; Elsevier: New York, NY, USA, 2012; pp. 870–968. [Google Scholar]
- Beeuwkes, H. Clinical manifestations of yellow fever in the West African native as observed during four extensive epidemics of the disease in the Gold Coast and Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1936, 30, 61–86. [Google Scholar] [CrossRef]
- Berry, G.P.; Kitchen, S.F. Yellow fever accidentally contracted in the laboratory. Am. J. Trop. Med. Hyg. 1931, s1-11, 365–434. [Google Scholar] [CrossRef]
- Monath, T.P. Yellow fever: A medically neglected disease. Report on a seminar. Rev. Infect. Dis. 1987, 9, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Barrett, A.D. Pathogenesis and pathophysiology of yellow fever. Adv. Virus Res. 2003, 60, 343–395. [Google Scholar] [PubMed]
- Klotz, O.; Belt, T.H. Regeneration of liver and kidney following yellow fever. Am. J. Pathol. 1930, 6, 689–697. [Google Scholar] [PubMed]
- Quaresma, J.A.; Pagliari, C.; Medeiros, D.B.; Duarte, M.I.; Vasconcelos, P.F. Immunity and immune response, pathology and pathologic changes: Progress and challenges in the immunopathology of yellow fever. Rev. Med. Virol. 2013, 23, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Klotz, O.; Belt, T.H. The pathology of the liver in yellow fiver. Am. J. Pathol. 1930, 6, 663–688. [Google Scholar] [PubMed]
- Klotz, O.; Belt, T.H. The pathology of the spleen in yellow fever. Am. J. Pathol. 1930, 6, 655–662. [Google Scholar] [PubMed]
- Engelmann, F.; Josset, L.; Girke, T.; Park, B.; Barron, A.; Dewane, J.; Hammarlund, E.; Lewis, A.; Axthelm, M.K.; Slifka, M.K.; et al. Pathophysiologic and transcriptomic analyses of viscerotropic yellow fever in a rhesus macaque model. PLoS Negl. Trop. Dis. 2014, 8, e3295. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; McArthur, M.A.; Cohen, M.; Jahrling, P.B.; Janosko, K.B.; Josleyn, N.; Kang, K.; Zhang, T.; Holbrook, M.R. Characterization of yellow fever virus infection of human and non-human primate antigen presenting cells and their interaction with CD4+ T cells. PLoS Negl. Trop. Dis. 2016, 10, e0004709. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.F.; Rizvanov, A.A.; Holbrook, M.R.; St Jeor, S. Yellow fever virus strains asibi and 17d-204 infect human umbilical cord endothelial cells and induce novel changes in gene expression. Virology 2005, 342, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, A.; Contamin, H.; Decelle, T.; Fournier, C.; Lang, J.; Deubel, V.; Marianneau, P. Host-cell interaction of attenuated and wild-type strains of yellow fever virus can be differentiated at early stages of hepatocyte infection. Microbes Infect. 2006, 8, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.E.; Freiberg, A.N.; Holbrook, M.R. Differential cytokine responses from primary human Kupffer cells following infection with wild-type or vaccine strain yellow fever virus. Virology 2011, 412, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.E.; Holbrook, M.R. Infection of hepatocytes with 17-D vaccine-strain yellow fever virus induces a strong pro-inflammatory host response. J. Gen. Virol. 2011, 92, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- McLinden, J.H.; Bhattarai, N.; Stapleton, J.T.; Chang, Q.; Kaufman, T.M.; Cassel, S.L.; Sutterwala, F.S.; Haim, H.; Houtman, J.C.; Xiang, J. Yellow fever virus, but not Zika virus or dengue virus, inhibits T-cell receptor-mediated T-cell function by an RNA-based mechanism. J. Infect. Dis. 2017, 216, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Ter Meulen, J.; Sakho, M.; Koulemou, K.; Magassouba, N.; Bah, A.; Preiser, W.; Daffis, S.; Klewitz, C.; Bae, H.G.; Niedrig, M.; et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J. Infect. Dis. 2004, 190, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.; Barros, V.L.; Pagliari, C.; Fernandes, E.R.; Guedes, F.; Takakura, C.F.; Andrade, H.F., Jr.; Vasconcelos, P.F.; Duarte, M.I. Revisiting the liver in human yellow fever: Virus-induced apoptosis in hepatocytes associated with TGF-β, TNF-α and NK cells activity. Virology 2006, 345, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.; Duarte, M.I.; Vasconcelos, P.F. Midzonal lesions in yellow fever: A specific pattern of liver injury caused by direct virus action and in situ inflammatory response. Med. Hypotheses 2006, 67, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.; Barros, V.L.; Fernandes, E.R.; Pagliari, C.; Guedes, F.; da Costa Vasconcelos, P.F.; de Andrade Junior, H.F.; Duarte, M.I. Immunohistochemical examination of the role of Fas ligand and lymphocytes in the pathogenesis of human liver yellow fever. Virus Res. 2006, 116, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.; Barros, V.L.; Pagliari, C.; Fernandes, E.R.; Andrade, H.F., Jr.; Vasconcelos, P.F.; Duarte, M.I. Hepatocyte lesions and cellular immune response in yellow fever infection. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, M.D.; Meertens, L.; Chazal, M.; Hafirassou, M.L.; Dejarnac, O.; Zamborlini, A.; Despres, P.; Sauvonnet, N.; Arenzana-Seisdedos, F.; Jouvenet, N.; et al. Vaccine and wild-type strains of yellow fever virus engage distinct entry mechanisms and differentially stimulate antiviral immune responses. MBio 2016, 7, e01956-15. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lobigs, M. E protein domain III determinants of yellow fever virus 17D vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J. Virol. 2008, 82, 6024–6033. [Google Scholar] [CrossRef] [PubMed]
- McElroy, K.L.; Girard, Y.A.; McGee, C.E.; Tsetsarkin, K.A.; Vanlandingham, D.L.; Higgs, S. Characterization of the antigen distribution and tissue tropisms of three phenotypically distinct yellow fever virus variants in orally infected Aedes aegypti mosquitoes. Vector Borne Zoonotic Dis. 2008, 8, 675–687. [Google Scholar] [CrossRef] [PubMed]
- McElroy, K.L.; Tsetsarkin, K.A.; Vanlandingham, D.L.; Higgs, S. Role of the yellow fever virus structural protein genes in viral dissemination from the Aedes aegypti mosquito midgut. J. Gen. Virol. 2006, 87, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Sil, B.K.; Dunster, L.M.; Ledger, T.N.; Wills, M.R.; Minor, P.D.; Barrett, A.D. Identification of envelope protein epitopes that are important in the attenuation process of wild-type yellow fever virus. J. Virol. 1992, 66, 4265–4270. [Google Scholar] [PubMed]
- Beasley, D.W.; McAuley, A.J.; Bente, D.A. Yellow fever virus: Genetic and phenotypic diversity and implications for detection, prevention and therapy. Antivir. Res. 2015, 115, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D. Yellow fever in Angola and beyond—The problem of vaccine supply and demand. N. Engl. J. Med. 2016, 375, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.K.; Laraway, H.; Faye, O.; Diallo, M.; Niedrig, M.; Sall, A.A. Biological and phylogenetic characteristics of yellow fever virus lineages from West Africa. J. Virol. 2013, 87, 2895–2907. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.E.; Vasconcelos, P.F.; Rijnbrand, R.C.; Mutebi, J.P.; Higgs, S.; Barrett, A.D. Size heterogeneity in the 3’ noncoding region of South American isolates of yellow fever virus. J. Virol. 2005, 79, 3807–3821. [Google Scholar] [CrossRef] [PubMed]
- Laemmert, H.W. Susceptibility of marmosets to different strains of yellow fever virus1. Am. J. Trop. Med. Hyg. 1944, s1-24, 71–81. [Google Scholar] [CrossRef]
- Liprandi, F.; Walder, R. Replication of virulent and attenuated strains of yellow fever virus in human monocytes and macrophage-like cells (U937). Arch. Virol. 1983, 76, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.; Gould, E.A. Comparison of neurovirulence of different strains of yellow fever virus in mice. J. Gen. Virol. 1986, 67, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Klitting, R.; Roth, L.; Rey, F.A.; de Lamballerie, X. Molecular determinants of yellow fever virus pathogenicity in Syrian golden hamsters: One mutation away from virulence. Emerg. Microbes Infect. 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Tesh, R.B.; Wood, T.G.; Widen, S.G.; Ryman, K.D.; Barrett, A.D. Comparison of the live attenuated yellow fever vaccine 17D-204 strain to its virulent parental strain Asibi by deep sequencing. J. Infect. Dis. 2014, 209, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.; Carroll, J.; Agramonte, A.; Lazear, J.W. The etiology of yellow fever-a preliminary note. Public Health Pap. Rep. 1900, 26, 37–53. [Google Scholar] [PubMed]
- Theiler, M.; Smith, H.H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 1937, 65, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M.; Yellow Fever Expert Committee. Yellow fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef] [PubMed]
- Le Prince, J.A.; Orenstein, A.J. Mosquito Control in Panama: The Eradication of Malaria and Yellow Fever in Cuba and Panama; Putnam: New York, NY, USA, 1916. [Google Scholar]
- WHO. Winning the War against Yellow Fever. Available online: http://www.who.int/features/2016/winning-the-war-against-yellow-fever/en/ (accessed on 1 June 2018).
- MS Brasil. Monitoramento do Período Sazonal da Febre Amarela. Brasil—2017/2018: Informe nº 25; Ministério da Saúde: Brasília, Distrito Federal, Brasil, 2018. [Google Scholar]
- NCDC. Yellow Fever Outbreak in Nigeria; Nigerian Center for Disease Control: Abuja, Nigeria, 2018. [Google Scholar]
- Rice, C.M.; Lenches, E.M.; Eddy, S.R.; Shin, S.J.; Sheets, R.L.; Strauss, J.H. Nucleotide sequence of yellow fever virus: Implications for flavivirus gene expression and evolution. Science 1985, 229, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.R.; Kinney, R.M.; Trent, D.W.; Lenches, E.M.; Dalgarno, L.; Strauss, J.H. Amino-terminal amino acid sequences of structural proteins of three flaviviruses. Virology 1985, 143, 224–229. [Google Scholar] [CrossRef]
- Ray, D.; Shah, A.; Tilgner, M.; Guo, Y.; Zhao, Y.; Dong, H.; Deas, T.S.; Zhou, Y.; Li, H.; Shi, P.Y. West Nile virus 5’-cap structure is formed by sequential guanine N-7 and ribose 2’-O methylations by nonstructural protein 5. J. Virol. 2006, 80, 8362–8370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ray, D.; Zhao, Y.; Dong, H.; Ren, S.; Li, Z.; Guo, Y.; Bernard, K.A.; Shi, P.Y.; Li, H. Structure and function of flavivirus ns5 methyltransferase. J. Virol. 2007, 81, 3891–3903. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, G.R.; Dubin, D.T. Methylation status of intracellular dengue type 2 40 S RNA. Virology 1979, 96, 159–165. [Google Scholar] [CrossRef]
- Wengler, G.; Wengler, G.; Gross, H.J. Studies on virus-specific nucleic acids synthesized in vertebrate and mosquito cells infected with flaviviruses. Virology 1978, 89, 423–437. [Google Scholar] [CrossRef]
- Corver, J.; Lenches, E.; Smith, K.; Robison, R.A.; Sando, T.; Strauss, E.G.; Strauss, J.H. Fine mapping of a cis-acting sequence element in yellow fever virus RNA that is required for RNA replication and cyclization. J. Virol. 2003, 77, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.C.; Soto-Acosta, R.; Bradrick, S.S.; Garcia-Blanco, M.A.; Ooi, E.E. The 5’ and 3’ untranslated regions of the flaviviral genome. Viruses 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.E.; De Lella Ezcurra, A.L.; Fucito, S.; Gamarnik, A.V. Role of RNA structures present at the 3’UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 2005, 339, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Holden, K.L.; Harris, E. Enhancement of dengue virus translation: Role of the 3’ untranslated region and the terminal 3’ stem-loop domain. Virology 2004, 329, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Holden, K.L.; Stein, D.A.; Pierson, T.C.; Ahmed, A.A.; Clyde, K.; Iversen, P.L.; Harris, E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3’ stem-loop structure. Virology 2006, 344, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Brinton, M.A. The 3’ stem loop of the West Nile virus genomic RNA can suppress translation of chimeric mRNAs. Virology 2001, 287, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Qin, C.; Jiang, T.; Li, X.; Zhao, H.; Liu, Z.; Deng, Y.; Liu, R.; Chen, S.; Yu, M.; et al. Translational regulation by the 3’ untranslated region of the dengue type 2 virus genome. Am. J. Trop Med. Hyg. 2009, 81, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanles, A.; Rios-Marco, P.; Romero-Lopez, C.; Berzal-Herranz, A. Functional information stored in the conserved structural RNA domains of flavivirus genomes. Front. Microbiol. 2017, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Filomatori, C.V.; Lodeiro, M.F.; Alvarez, D.E.; Samsa, M.M.; Pietrasanta, L.; Gamarnik, A.V. A 5’ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev. 2006, 20, 2238–2249. [Google Scholar] [CrossRef] [PubMed]
- Lodeiro, M.F.; Filomatori, C.V.; Gamarnik, A.V. Structural and functional studies of the promoter element for dengue virus RNA replication. J. Virol. 2009, 83, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Khromykh, A.A.; Meka, H.; Guyatt, K.J.; Westaway, E.G. Essential role of cyclization sequences in flavivirus RNA replication. J. Virol. 2001, 75, 6719–6728. [Google Scholar] [CrossRef] [PubMed]
- Song, B.H.; Yun, S.I.; Choi, Y.J.; Kim, J.M.; Lee, C.H.; Lee, Y.M. A complex RNA motif defined by three discontinuous 5-nucleotide-long strands is essential for flavivirus RNA replication. RNA 2008, 14, 1791–1813. [Google Scholar] [CrossRef] [PubMed]
- Villordo, S.M.; Gamarnik, A.V. Genome cyclization as strategy for flavivirus RNA replication. Virus Res. 2009, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Bredenbeek, P.J.; Kooi, E.A.; Lindenbach, B.; Huijkman, N.; Rice, C.M.; Spaan, W.J. A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J. Gen. Virol. 2003, 84, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Polacek, C.; Foley, J.E.; Harris, E. Conformational changes in the solution structure of the dengue virus 5’ end in the presence and absence of the 3’ untranslated region. J. Virol. 2009, 83, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.G.; Basu, M.; Elrod, E.J.; Germann, M.W.; Brinton, M.A. Identification of cis-acting nucleotides and a structural feature in West Nile virus 3’-terminus RNA that facilitate viral minus strand RNA synthesis. J. Virol. 2013, 87, 7622–7636. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, B.; Shi, P.Y. Terminal structures of West Nile virus genomic RNA and their interactions with viral NS5 protein. Virology 2008, 381, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Filomatori, C.V.; Iglesias, N.G.; Villordo, S.M.; Alvarez, D.E.; Gamarnik, A.V. RNA sequences and structures required for the recruitment and activity of the dengue virus polymerase. J. Boil. Chem. 2011, 286, 6929–6939. [Google Scholar] [CrossRef] [PubMed]
- Friebe, P.; Harris, E. Interplay of RNA elements in the dengue virus 5’ and 3’ ends required for viral RNA replication. J. Virol. 2010, 84, 6103–6118. [Google Scholar] [CrossRef] [PubMed]
- Friebe, P.; Shi, P.Y.; Harris, E. The 5’ and 3’ downstream AUG region elements are required for mosquito-borne flavivirus RNA replication. J. Virol. 2011, 85, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, H.; Zhou, Y.; Shi, P.Y. Genetic interactions among the west nile virus methyltransferase, the RNA-dependent RNA polymerase, and the 5’ stem-loop of genomic RNA. J. Virol. 2008, 82, 7047–7058. [Google Scholar] [CrossRef] [PubMed]
- Gritsun, T.S.; Gould, E.A. Origin and evolution of flavivirus 5’UTRs and panhandles: Trans-terminal duplications? Virology 2007, 366, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gritsun, T.S.; Gould, E.A. Direct repeats in the flavivirus 3’ untranslated region; a strategy for survival in the environment? Virology 2007, 358, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Thurner, C.; Witwer, C.; Hofacker, I.L.; Stadler, P.F. Conserved RNA secondary structures in Flaviviridae genomes. J. Gen. Virol. 2004, 85, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Gritsun, T.S.; Gould, E.A. Direct repeats in the 3’ untranslated regions of mosquito-borne flaviviruses: Possible implications for virus transmission. J. Gen. Virol. 2006, 87, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Jiang, T.; Yu, X.D.; Deng, Y.Q.; Zhao, H.; Zhu, Q.Y.; Qin, E.D.; Qin, C.F. RNA elements within the 5’ untranslated region of the West Nile virus genome are critical for RNA synthesis and virus replication. J. Gen. Virol. 2010, 91, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, J.P.; Rijnbrand, R.C.; Wang, H.; Ryman, K.D.; Wang, E.; Fulop, L.D.; Titball, R.; Barrett, A.D. Genetic relationships and evolution of genotypes of yellow fever virus and other members of the yellow fever virus group within the flavivirus genus based on the 3’ noncoding region. J. Virol. 2004, 78, 9652–9665. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Weaver, S.C.; Shope, R.E.; Tesh, R.B.; Watts, D.M.; Barrett, A.D. Genetic variation in yellow fever virus: Duplication in the 3’ noncoding region of strains from africa. Virology 1996, 225, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Olsthoorn, R.C.; Bol, J.F. Sequence comparison and secondary structure analysis of the 3’ noncoding region of flavivirus genomes reveals multiple pseudoknots. RNA 2001, 7, 1370–1377. [Google Scholar] [PubMed]
- Proutski, V.; Gould, E.A.; Holmes, E.C. Secondary structure of the 3’ untranslated region of flaviviruses: Similarities and differences. Nucleic Acids Res. 1997, 25, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Reichert, E.D.; Polo, S.; Falgout, B.; Kasprzak, W.; Shapiro, B.A.; Padmanabhan, R. Identification of cis-acting elements in the 3’-untranslated region of the dengue virus type 2 RNA that modulate translation and replication. J. Boil. Chem. 2011, 286, 22521–22534. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.; Truong, K.; Nagasaki, T.; Torres, S.; Floden, N.; Balmori Melian, E.; Edmonds, J.; Dong, H.; Shi, P.Y.; Khromykh, A.A. RNA structures required for production of subgenomic flavivirus RNA. J. Virol. 2010, 84, 11407–11417. [Google Scholar] [CrossRef] [PubMed]
- Sztuba-Solinska, J.; Teramoto, T.; Rausch, J.W.; Shapiro, B.A.; Padmanabhan, R.; Le Grice, S.F. Structural complexity of dengue virus untranslated regions: Cis-acting RNA motifs and pseudoknot interactions modulating functionality of the viral genome. Nucleic Acids Res. 2013, 41, 5075–5089. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.G.; Costantino, D.A.; Rabe, J.L.; Moon, S.L.; Wilusz, J.; Nix, J.C.; Kieft, J.S. The structural basis of pathogenic subgenomic flavivirus RNA (sfRNA) production. Science 2014, 344, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.G.; Moon, S.L.; Wilusz, J.; Kieft, J.S. RNA structures that resist degradation by Xrn1 produce a pathogenic dengue virus RNA. Elife 2014, 3, e01892. [Google Scholar] [CrossRef] [PubMed]
- Pijlman, G.P.; Funk, A.; Kondratieva, N.; Leung, J.; Torres, S.; van der Aa, L.; Liu, W.J.; Palmenberg, A.C.; Shi, P.Y.; Hall, R.A.; et al. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe 2008, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Charley, P.A.; Wilusz, J. Standing your ground to exoribonucleases: Function of flavivirus long non-coding rnas. Virus Res. 2016, 212, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Palacios, G.; Cardoso, J.F.; Martins, L.C.; Sousa, E.C., Jr.; de Lima, C.P.; Medeiros, D.B.; Savji, N.; Desai, A.; Rodrigues, S.G.; et al. Genomic and phylogenetic characterization of Brazilian yellow fever virus strains. J. Virol. 2012, 86, 13263–13271. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.L.; Brinton, M.A. BHK cell proteins that bind to the 3’ stem-loop structure of the West Nile virus genome RNA. J. Virol. 1995, 69, 5650–5658. [Google Scholar] [PubMed]
- Brinton, M.A.; Fernandez, A.V.; Dispoto, J.H. The 3’-nucleotides of flavivirus genomic RNA form a conserved secondary structure. Virology 1986, 153, 113–121. [Google Scholar] [CrossRef]
- Hahn, C.S.; Hahn, Y.S.; Rice, C.M.; Lee, E.; Dalgarno, L.; Strauss, E.G.; Strauss, J.H. Conserved elements in the 3’ untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol 1987, 198, 33–41. [Google Scholar] [CrossRef]
- Khromykh, A.A.; Westaway, E.G. Subgenomic replicons of the flavivirus Kunjin: Construction and applications. J. Virol. 1997, 71, 1497–1505. [Google Scholar] [PubMed]
- Men, R.; Bray, M.; Clark, D.; Chanock, R.M.; Lai, C.J. Dengue type 4 virus mutants containing deletions in the 3’ noncoding region of the RNA genome: Analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J. Virol. 1996, 70, 3930–3937. [Google Scholar] [PubMed]
- Tilgner, M.; Deas, T.S.; Shi, P.Y. The flavivirus-conserved penta-nucleotide in the 3’ stem-loop of the west nile virus genome requires a specific sequence and structure for RNA synthesis, but not for viral translation. Virology 2005, 331, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Villordo, S.M.; Alvarez, D.E.; Gamarnik, A.V. A balance between circular and linear forms of the dengue virus genome is crucial for viral replication. RNA 2010, 16, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Villordo, S.M.; Gamarnik, A.V. Differential RNA sequence requirement for dengue virus replication in mosquito and mammalian cells. J. Virol. 2013, 87, 9365–9372. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Markoff, L. The topology of bulges in the long stem of the flavivirus 3’ stem-loop is a major determinant of RNA replication competence. J. Virol. 2005, 79, 2309–2324. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Nomaguchi, M.; Padmanabhan, R.; Markoff, L. Specific requirements for elements of the 5’ and 3’ terminal regions in flavivirus RNA synthesis and viral replication. Virology 2008, 374, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Falgout, B.; Markoff, L. Identification of specific nucleotide sequences within the conserved 3’-SL in the dengue type 2 virus genome required for replication. J. Virol. 1998, 72, 7510–7522. [Google Scholar] [PubMed]
- Khromykh, A.A.; Kondratieva, N.; Sgro, J.Y.; Palmenberg, A.; Westaway, E.G. Significance in replication of the terminal nucleotides of the flavivirus genome. J. Virol. 2003, 77, 10623–10629. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.; Molenkamp, R.; Dalebout, T.J.; Charlier, N.; Neyts, J.H.; Spaan, W.J.; Bredenbeek, P.J. Conservation of the pentanucleotide motif at the top of the yellow fever virus 17D 3’ stem-loop structure is not required for replication. J. Gen. Virol. 2007, 88, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Villordo, S.M.; Carballeda, J.M.; Filomatori, C.V.; Gamarnik, A.V. RNA structure duplications and flavivirus host adaptation. Trends Microbiol. 2016, 24, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.E., Jr.; Sathe, N.S.; Goddard, L.; Hanson, C.T.; Romero, T.A.; Hanley, K.A.; Murphy, B.R.; Whitehead, S.S. Dengue virus type 3 vaccine candidates generated by introduction of deletions in the 3’ untranslated region (3’-UTR) or by exchange of the DENV-3 3’-UTR with that of DENV-4. Vaccine 2008, 26, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.W.; Charlesworth, B.; Charlesworth, D.; Crow, J.F. Rates of spontaneous mutation. Genetics 1998, 148, 1667–1686. [Google Scholar] [PubMed]
- Jenkins, G.M.; Rambaut, A.; Pybus, O.G.; Holmes, E.C. Rates of molecular evolution in RNA viruses: A quantitative phylogenetic analysis. J. Mol. Evol. 2002, 54, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Worobey, M.; Han, G.Z.; Rambaut, A. A synchronized global sweep of the internal genes of modern avian influenza virus. Nature 2014, 508, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Holmes, E.C. Avian influenza virus exhibits rapid evolutionary dynamics. Mol. Boil. Evol. 2006, 23, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Lemey, P.; Rambaut, A.; Pybus, O.G. HIV evolutionary dynamics within and among hosts. AIDS Rev. 2006, 8, 125–140. [Google Scholar] [PubMed]
- Sall, A.A.; Faye, O.; Diallo, M.; Firth, C.; Kitchen, A.; Holmes, E.C. Yellow fever virus exhibits slower evolutionary dynamics than dengue virus. J. Virol. 2010, 84, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.E.; Holmes, E.C.; Barrett, A.D. Out of Africa: A molecular perspective on the introduction of yellow fever virus into the americas. PLoS Pathog. 2007, 3, e75. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.J.; Lemey, P.; Pybus, O.G.; Suchard, M.A.; Salas, R.A.; Adesiyun, A.A.; Barrett, A.D.; Tesh, R.B.; Weaver, S.C.; Carrington, C.V. Yellow fever virus maintenance in Trinidad and its dispersal throughout the Americas. J. Virol. 2010, 84, 9967–9977. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.; Voloch, C.M.; Schrago, C.G. Comparative evolutionary epidemiology of dengue virus serotypes. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012, 12, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Klitting, R.; Gould, E.A.; Paupy, C.; de Lamballerie, X. What does the future hold for yellow fever virus? (I). Genes 2018, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.R.; Barrett, A.D. The enigma of yellow fever in east africa. Rev. Med. Virol. 2008, 18, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Descloux, E.; Cao-Lormeau, V.M.; Roche, C.; De Lamballerie, X. Dengue 1 diversity and microevolution, french polynesia 2001–2006: Connection with epidemiology and clinics. PLoS Negl. Trop. Dis. 2009, 3, e493. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.; Thu, H.M.; Lowry, K.; Wang, X.F.; Holmes, E.C.; Aaskov, J. Diverse dengue type 2 virus populations contain recombinant and both parental viruses in a single mosquito host. J. Virol. 2003, 77, 4463–4467. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, Y.; Topel, M.; Elvang, A.; Melik, W.; Johansson, M. First dating of a recombination event in mammalian tick-borne flaviviruses. PLoS ONE 2012, 7, e31981. [Google Scholar] [CrossRef] [PubMed]
- Aaskov, J.; Buzacott, K.; Field, E.; Lowry, K.; Berlioz-Arthaud, A.; Holmes, E.C. Multiple recombinant dengue type 1 viruses in an isolate from a dengue patient. J. Gen. Virol. 2007, 88, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Worobey, M.; Rambaut, A.; Holmes, E.C. Widespread intra-serotype recombination in natural populations of dengue virus. Proc. Natl. Acad. Sci. USA 1999, 96, 7352–7357. [Google Scholar] [CrossRef] [PubMed]
- Uzcategui, N.Y.; Camacho, D.; Comach, G.; Cuello de Uzcategui, R.; Holmes, E.C.; Gould, E.A. Molecular epidemiology of dengue type 2 virus in Venezuela: Evidence for in situ virus evolution and recombination. J. Gen. Virol. 2001, 82, 2945–2953. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Chen, W.J. Experimental evidence that RNA recombination occurs in the Japanese encephalitis virus. Virology 2009, 394, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.; Daly, J.M.; Nisalak, A.; Solomon, T. Recombination and positive selection identified in complete genome sequences of Japanese encephalitis virus. Arch. Virol. 2012, 157, 75–83. [Google Scholar] [CrossRef] [PubMed]
- McGee, C.E.; Tsetsarkin, K.A.; Guy, B.; Lang, J.; Plante, K.; Vanlandingham, D.L.; Higgs, S. Stability of yellow fever virus under recombinatory pressure as compared with Chikungunya virus. PLoS ONE 2011, 6, e23247. [Google Scholar] [CrossRef] [PubMed]
- De Wachter, R.; Fiers, W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 p-labeled RNA. Anal. Biochem. 1972, 49, 184–197. [Google Scholar] [CrossRef]
- Deubel, V.; Digoutte, J.P.; Monath, T.P.; Girard, M. Genetic heterogeneity of yellow fever virus strains from Africa and the Americas. J. Gen. Virol. 1986, 67, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, J.P.; Wang, H.; Li, L.; Bryant, J.E.; Barrett, A.D. Phylogenetic and evolutionary relationships among yellow fever virus isolates in Africa. J. Virol. 2001, 75, 6999–7008. [Google Scholar] [CrossRef] [PubMed]
- Lepiniec, L.; Dalgarno, L.; Huong, V.T.; Monath, T.P.; Digoutte, J.P.; Deubel, V. Geographic distribution and evolution of yellow fever viruses based on direct sequencing of genomic cDNA fragments. J. Gen. Virol. 1994, 75, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.J.; Cropp, B.C.; Kinney, R.M.; Trent, D.W.; Gubler, D.J. Nucleotide sequence variation of the envelope protein gene identifies two distinct genotypes of yellow fever virus. J. Virol. 1995, 69, 5773–5780. [Google Scholar] [PubMed]
- Wang, H.; Jennings, A.D.; Ryman, K.D.; Late, C.M.; Wang, E.; Ni, H.; Minor, P.D.; Barrett, A.D. Genetic variation among strains of wild-type yellow fever virus from Senegal. J. Gen. Virol. 1997, 78, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.E.; Barrett, A.D. Comparative phylogenies of yellow fever isolates from peru and Brazil. FEMS Immunol. Med. Microbiol. 2003, 39, 103–118. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.; Bryant, J.E.; da Rosa, T.P.; Tesh, R.B.; Rodrigues, S.G.; Barrett, A.D. Genetic divergence and dispersal of yellow fever virus, Brazil. Emerg. Infect. Dis. 2004, 10, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.J.; Lemey, P.; Bergren, N.A.; Giambalvo, D.; Moncada, M.; Moron, D.; Hernandez, R.; Navarro, J.C.; Weaver, S.C. Enzootic transmission of yellow fever virus, venezuela. Emerg. Infect. Dis. 2015, 21, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Mir, D.; Delatorre, E.; Bonaldo, M.; Lourenco-de-Oliveira, R.; Vicente, A.C.; Bello, G. Phylodynamics of yellow fever virus in the americas: New insights into the origin of the 2017 Brazilian outbreak. Sci. Rep. 2017, 7, 7385. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Soto, A.; Torres, M.C.; Lima de Mendonça, M.C.; Mares-Guia, M.A.; Dos Santos Rodrigues, C.D.; Fabri, A.A.; Dos Santos, C.C.; Machado Araújo, E.S.; Fischer, C.; Ribeiro Nogueira, R.M.; et al. Evidence for multiple sylvatic transmission cycles during the 2016–2017 yellow fever virus outbreak, Brazil. Clin. Microbiol. Infect. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.R.; Kayiwa, J.; Mossel, E.C.; Lutwama, J.; Staples, J.E.; Lambert, A.J. Phylogeny of yellow fever virus, Uganda, 2016. Emerg. Infect. Dis. 2018, 24, 1598. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.K.; Boschetti, N.; Herzog, C.; Appelhans, M.S.; Niedrig, M. The phylogeny of yellow fever virus 17D vaccines. Vaccine 2012, 30, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Goenaga, S.; Fabbri, C.; Duenas, J.C.; Gardenal, C.N.; Rossi, G.C.; Calderon, G.; Morales, M.A.; Garcia, J.B.; Enria, D.A.; Levis, S. Isolation of yellow fever virus from mosquitoes in Misiones province, Argentina. Vector Borne Zoonotic Dis. 2012, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.P.; Foster, P.G.; Sallum, M.A.; Coimbra, T.L.; Maeda, A.Y.; Silveira, V.R.; Moreno, E.S.; da Silva, F.G.; Rocco, I.M.; Ferreira, I.B.; et al. Detection of a new yellow fever virus lineage within the south american genotype I in Brazil. J. Med. Virol. 2010, 82, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Pan, Y.; Lyu, Y.; Liang, Z.; Li, J.; Sun, Y.; Dou, X.; Tian, L.; Huo, D.; Chen, L.; et al. Detection of yellow fever virus genomes from four imported cases in China. Int. J. Infect. Dis. 2017, 60, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, M.C.; Gomez, M.M.; Dos Santos, A.A.; Abreu, F.V.S.; Ferreira-de-Brito, A.; Miranda, R.M.; Castro, M.G.; Lourenco-de-Oliveira, R. Genome analysis of yellow fever virus of the ongoing outbreak in Brazil reveals polymorphisms. Mem. Do Inst. Oswaldo Cruz 2017, 112, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Baronti, C.; Goitia, N.J.; Cook, S.; Roca, Y.; Revollo, J.; Flores, J.V.; de Lamballerie, X. Molecular epidemiology of yellow fever in Bolivia from 1999 to 2008. Vector Borne Zoonotic Dis. 2011, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z. Adaptive diversification between yellow fever virus west African and south American lineages: A genome-wide study. Am. J. Trop. Med. Hyg. 2017, 96, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Carrington, C.V.; Auguste, A.J. Evolutionary and ecological factors underlying the tempo and distribution of yellow fever virus activity. Infect. Genet. Evol. 2013, 13, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Douam, F.; Ploss, A. Yellow fever virus: Knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Klema, V.J.; Padmanabhan, R.; Choi, K.H. Flaviviral replication complex: Coordination between RNA synthesis and 5’-RNA capping. Viruses 2015, 7, 4640–4656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Corver, J.; Chipman, P.R.; Zhang, W.; Pletnev, S.V.; Sedlak, D.; Baker, T.S.; Strauss, J.H.; Kuhn, R.J.; Rossmann, M.G. Structures of immature flavivirus particles. EMBO J. 2003, 22, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef]
- Samuel, G.H.; Wiley, M.R.; Badawi, A.; Adelman, Z.N.; Myles, K.M. Yellow fever virus capsid protein is a potent suppressor of RNA silencing that binds double-stranded RNA. Proc. Natl. Acad. Sci. USA 2016, 113, 13863–13868. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, I.C.; Allison, S.L.; Heinz, F.X.; Helenius, A. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J. Virol. 2002, 76, 5480–5491. [Google Scholar] [CrossRef] [PubMed]
- Konishi, E.; Mason, P.W. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J. Virol. 1993, 67, 1672–1675. [Google Scholar] [PubMed]
- Li, L.; Lok, S.M.; Yu, I.M.; Zhang, Y.; Kuhn, R.J.; Chen, J.; Rossmann, M.G. The flavivirus precursor membrane-envelope protein complex: Structure and maturation. Science 2008, 319, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.A.; Stiasny, K.; Heinz, F.X. Flavivirus structural heterogeneity: Implications for cell entry. Curr. Opin. Virol. 2017, 24, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Battisti, A.J.; Junjhon, J.; Winkler, D.C.; Holdaway, H.A.; Keelapang, P.; Sittisombut, N.; Kuhn, R.J.; Steven, A.C.; Rossmann, M.G. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO Rep. 2011, 12, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Lin, T.Y.; Dowd, K.A.; Manhart, C.J.; Pierson, T.C. The infectivity of prM-containing partially mature west nile virus does not require the activity of cellular furin-like proteases. J. Virol. 2011, 85, 12067–12072. [Google Scholar] [CrossRef] [PubMed]
- Junjhon, J.; Lausumpao, M.; Supasa, S.; Noisakran, S.; Songjaeng, A.; Saraithong, P.; Chaichoun, K.; Utaipat, U.; Keelapang, P.; Kanjanahaluethai, A.; et al. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. J. Virol. 2008, 82, 10776–10791. [Google Scholar] [CrossRef] [PubMed]
- Fibriansah, G.; Ng, T.S.; Kostyuchenko, V.A.; Lee, J.; Lee, S.; Wang, J.; Lok, S.M. Structural changes in dengue virus when exposed to a temperature of 37 °C. J. Virol. 2013, 87, 7585–7592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sheng, J.; Plevka, P.; Kuhn, R.J.; Diamond, M.S.; Rossmann, M.G. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc. Natl. Acad. Sci. USA 2013, 110, 6795–6799. [Google Scholar] [CrossRef] [PubMed]
- Germi, R.; Crance, J.M.; Garin, D.; Guimet, J.; Lortat-Jacob, H.; Ruigrok, R.W.; Zarski, J.P.; Drouet, E. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology 2002, 292, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Van der Most, R.G.; Corver, J.; Strauss, J.H. Mutagenesis of the RGD motif in the yellow fever virus 17D envelope protein. Virology 1999, 265, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.L.; Schalich, J.; Stiasny, K.; Mandl, C.W.; Kunz, C.; Heinz, F.X. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J. Virol. 1995, 69, 695–700. [Google Scholar] [PubMed]
- Stiasny, K.; Allison, S.L.; Marchler-Bauer, A.; Kunz, C.; Heinz, F.X. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J. Virol. 1996, 70, 8142–8147. [Google Scholar] [PubMed]
- Stiasny, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J. Virol. 2002, 76, 3784–3790. [Google Scholar] [CrossRef] [PubMed]
- Bressanelli, S.; Stiasny, K.; Allison, S.L.; Stura, E.A.; Duquerroy, S.; Lescar, J.; Heinz, F.X.; Rey, F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004, 23, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Gollins, S.W.; Porterfield, J.S. Flavivirus infection enhancement in macrophages: An electron microscopic study of viral cellular entry. J. Gen. Virol. 1985, 66 Pt 9, 1969–1982. [Google Scholar] [CrossRef]
- Gollins, S.W.; Porterfield, J.S. The uncoating and infectivity of the flavivirus West Nile on interaction with cells: Effects of pH and ammonium chloride. J. Gen. Virol. 1986, 67 Pt 9, 1941–1950. [Google Scholar] [CrossRef]
- Jennings, A.D.; Whitby, J.E.; Minor, P.D.; Barrett, A.D. Comparison of the nucleotide and deduced amino acid sequences of the structural protein genes of the yellow fever 17DD vaccine strain from senegal with those of other yellow fever vaccine viruses. Vaccine 1993, 11, 679–681. [Google Scholar] [CrossRef]
- Ryman, K.D.; Ledger, T.N.; Campbell, G.A.; Watowich, S.J.; Barrett, A.D. Mutation in a 17D-204 vaccine substrain-specific envelope protein epitope alters the pathogenesis of yellow fever virus in mice. Virology 1998, 244, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Arroyo, J.; Levenbook, I.; Zhang, Z.X.; Catalan, J.; Draper, K.; Guirakhoo, F. Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: Relevance to development and safety testing of live, attenuated vaccines. J. Virol. 2002, 76, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- McArthur, M.A.; Suderman, M.T.; Mutebi, J.P.; Xiao, S.Y.; Barrett, A.D. Molecular characterization of a hamster viscerotropic strain of yellow fever virus. J. Virol. 2003, 77, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Blanco, M.A.; Vasudevan, S.G.; Bradrick, S.S.; Nicchitta, C. Flavivirus RNA transactions from viral entry to genome replication. Antivir. Res. 2016, 134, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Grakoui, A.; Rice, C.M. Processing of the yellow fever virus nonstructural polyprotein: A catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J. Virol. 1991, 65, 6042–6050. [Google Scholar] [PubMed]
- Chambers, T.J.; Droll, D.A.; Tang, Y.; Liang, Y.; Ganesh, V.K.; Murthy, K.H.; Nickells, M. Yellow fever virus NS2B-NS3 protease: Characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J. Gen. Virol. 2005, 86, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Nestorowicz, A.; Rice, C.M. Mutagenesis of the yellow fever virus NS2B/3 cleavage site: Determinants of cleavage site specificity and effects on polyprotein processing and viral replication. J. Virol. 1995, 69, 1600–1605. [Google Scholar] [PubMed]
- Lin, C.; Amberg, S.M.; Chambers, T.J.; Rice, C.M. Cleavage at a novel site in the NS4A region by the yellow fever virus NS2B-3 proteinase is a prerequisite for processing at the downstream 4a/4b signalase site. J. Virol. 1993, 67, 2327–2335. [Google Scholar] [PubMed]
- Saeedi, B.J.; Geiss, B.J. Regulation of flavivirus RNA synthesis and capping. Wiley Interdiscip. Rev. RNA 2013, 4, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, J.; Westaway, E.G.; Khromykh, A.; Mackenzie, J.M. Regulated cleavages at the west nile virus NS4A-2k-NS4B junctions play a major role in rearranging cytoplasmic membranes and golgi trafficking of the NS4A protein. J. Virol. 2006, 80, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Kastner, S.; Krijnse-Locker, J.; Buhler, S.; Bartenschlager, R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2k-regulated manner. J. Boil. Chem. 2007, 282, 8873–8882. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Trans-complementation of yellow fever virus ns1 reveals a role in early RNA replication. J. Virol. 1997, 71, 9608–9617. [Google Scholar] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J. Virol. 1999, 73, 4611–4621. [Google Scholar] [PubMed]
- Le Sommer, C.; Barrows, N.J.; Bradrick, S.S.; Pearson, J.L.; Garcia-Blanco, M.A. G protein-coupled receptor kinase 2 promotes Flaviviridae entry and replication. PLoS Negl. Trop. Dis. 2012, 6, e1820. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Sharma, N.; Singh, S.K. Flavivirus NS1: A multifaceted enigmatic viral protein. Virol. J. 2016, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Umareddy, I.; Chao, A.; Sampath, A.; Gu, F.; Vasudevan, S.G. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 2006, 87, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, S.A.; Chernov, A.V.; Aleshin, A.E.; Shiryaeva, T.N.; Strongin, A.Y. NS4A regulates the atpase activity of the NS3 helicase: A novel cofactor role of the non-structural protein NS4A from West Nile virus. J. Gen. Virol. 2009, 90, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Jordan, J.L.; Laurent-Rolle, M.; Ashour, J.; Martinez-Sobrido, L.; Ashok, M.; Lipkin, W.I.; Garcia-Sastre, A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005, 79, 8004–8013. [Google Scholar] [CrossRef] [PubMed]
- Apte-Sengupta, S.; Sirohi, D.; Kuhn, R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Pong, W.L.; Huang, Z.S.; Teoh, P.G.; Wang, C.C.; Wu, H.N. RNA binding property and RNA chaperone activity of dengue virus core protein and other viral RNA-interacting proteins. FEBS Lett. 2011, 585, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Teoh, P.G.; Huang, Z.S.; Pong, W.L.; Chen, P.C.; Wu, H.N. Maintenance of dimer conformation by the dengue virus core protein α4-α4’ helix pair is critical for nucleocapsid formation and virus production. J. Virol. 2014, 88, 7998–8015. [Google Scholar] [CrossRef] [PubMed]
- Deubel, V.; Digoutte, J.P.; Mattei, X.; Pandare, D. Morphogenesis of yellow fever virus in Aedes aegypti cultured cells. II. An ultrastructural study. Am. J. Trop. Med. Hyg. 1981, 30, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Elshuber, S.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J. Gen. Virol. 2003, 84, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997, 71, 8475–8481. [Google Scholar] [PubMed]
- Hoffmann, H.H.; Schneider, W.M.; Blomen, V.A.; Scull, M.A.; Hovnanian, A.; Brummelkamp, T.R.; Rice, C.M. Diverse viruses require the calcium transporter SPCA1 for maturation and spread. Cell Host Microbe 2017, 22, 460–470.e5. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.M.; Zhang, W.; Holdaway, H.A.; Li, L.; Kostyuchenko, V.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 2008, 319, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Stiasny, K.; Heinz, F.X. Flavivirus membrane fusion. J. Gen. Virol. 2006, 87, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Denman, A.J.; Mackenzie, J.M. The importance of the nucleus during flavivirus replication. Viruses 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.; de Jesus, J.G.; Aguiar, R.S.D.; Iani, F.C.M.; Xavier, J.; Quick, J.; Plessis, L.D.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. bioRxiv 2018. [Google Scholar] [CrossRef]
- Chaves, T.D.S.S.; Orduna, T.; Lepetic, A.; Macchi, A.; Verbanaz, S.; Risquez, A.; Perret, C.; Echazarreta, S.; Rodríguez-Morales, A.J.; Lloveras, S.C. Yellow fever in Brazil: Epidemiological aspects and implications for travelers. Travel Med. Infect. Dis. 2018, 23, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Faria, N.R.; Reiner, R.C., Jr.; Golding, N.; Nikolay, B.; Stasse, S.; Johansson, M.A.; Salje, H.; Faye, O.; Wint, G.R.; et al. Spread of yellow fever virus outbreak in angola and the democratic republic of the congo 2015–16: A modelling study. Lancet Infect. Dis. 2017, 17, 330–338. [Google Scholar] [CrossRef]
- Johansson, M.A.; Vasconcelos, P.F.; Staples, J.E. The whole iceberg: Estimating the incidence of yellow fever virus infection from the number of severe cases. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 482–487. [Google Scholar] [CrossRef] [PubMed]
- WHO. Yellow Fever. Available online: http://www.who.int/mediacentre/factsheets/fs100/en/ (accessed on 1 July 2018).
- Cleton, N.; Koopmans, M.; Reimerink, J.; Godeke, G.J.; Reusken, C. Come fly with me: Review of clinically important arboviruses for global travelers. J. Clin. Virol. 2012, 55, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Chang, G.J.; Cropp, C.B.; Robbins, K.E.; Tsai, T.F. Detection of yellow fever virus by polymerase chain reaction. Clin. Diagn. Virol. 1994, 2, 41–51. [Google Scholar] [CrossRef]
- Pierre, V.; Drouet, M.T.; Deubel, V. Identification of mosquito-borne flavivirus sequences using universal primers and reverse transcription/polymerase chain reaction. Res. Virol. 1994, 145, 93–104. [Google Scholar] [CrossRef]
- Sanchez-Seco, M.P.; Rosario, D.; Hernandez, L.; Domingo, C.; Valdes, K.; Guzman, M.G.; Tenorio, A. Detection and subtyping of dengue 1–4 and yellow fever viruses by means of a multiplex RT-nested-PCR using degenerated primers. Trop. Med. Int. Health 2006, 11, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Vianez, J.L., Jr.; Nunes, K.N.; da Silva, S.P.; Lima, C.P.; Guzman, H.; Martins, L.C.; Carvalho, V.L.; Tesh, R.B.; Vasconcelos, P.F. Analysis of a reverse transcription loop-mediated isothermal amplification (RT-LAMP) for yellow fever diagnostic. J. Virol. Methods 2015, 226, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kwallah, A.; Inoue, S.; Muigai, A.W.; Kubo, T.; Sang, R.; Morita, K.; Mwau, M. A real-time reverse transcription loop-mediated isothermal amplification assay for the rapid detection of yellow fever virus. J. Virol. Methods 2013, 193, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Escadafal, C.; Faye, O.; Sall, A.A.; Faye, O.; Weidmann, M.; Strohmeier, O.; von Stetten, F.; Drexler, J.; Eberhard, M.; Niedrig, M.; et al. Rapid molecular assays for the detection of yellow fever virus in low-resource settings. PLoS Negl. Trop. Dis. 2014, 8, e2730. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Palacios, G.; Nunes, K.N.; Casseb, S.M.; Martins, L.C.; Quaresma, J.A.; Savji, N.; Lipkin, W.I.; Vasconcelos, P.F. Evaluation of two molecular methods for the detection of yellow fever virus genome. J. Virol. Methods 2011, 174, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Boutonnier, A.; Prina, E.; Sharma, S.; Reiter, P. Development of a SYBR green I based RT-PCR assay for yellow fever virus: Application in assessment of YFV infection in Aedes aegypti. Virol. J. 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Mendez, J.A.; Nakoune, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Gottig, S.; Schilling, S.; Asper, M.; Panning, M.; Schmitz, H.; Gunther, S. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 2002, 40, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, M.; Faye, O.; Faye, O.; Kranaster, R.; Marx, A.; Nunes, M.R.; Vasconcelos, P.F.; Hufert, F.T.; Sall, A.A. Improved LNA probe-based assay for the detection of African and South American yellow fever virus strains. J. Clin. Virol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N.; Aguirre, M.; Gulia, S.; Girerd-Chambaz, Y.; Colombani, S.; Moste, C.; Barban, V. Standardized quantitative RT-PCR assays for quantitation of yellow fever and chimeric yellow fever-dengue vaccines. J. Virol. Methods 2008, 151, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Monteiro, A.G.; Trindade, G.F.; Yamamura, A.M.; Moreira, O.C.; de Paula, V.S.; Duarte, A.C.; Britto, C.; Lima, S.M. New approaches for the standardization and validation of a real-time qPCR assay using taqman probes for quantification of yellow fever virus on clinical samples with high quality parameters. Hum. Vaccines Immunother. 2015, 11, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.G.; Nitsche, A.; Teichmann, A.; Biel, S.S.; Niedrig, M. Detection of yellow fever virus: A comparison of quantitative real-time PCR and plaque assay. J. Virol. Methods 2003, 110, 185–191. [Google Scholar] [CrossRef]
- Chao, D.Y.; Davis, B.S.; Chang, G.J. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medically important flaviviruses in mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Torres, M.C.; Patel, P.; Moreira-Soto, A.; Gould, E.A.; Charrel, R.N.; de Lamballerie, X.; Nogueira, R.M.R.; Sequeira, P.C.; Rodrigues, C.D.S.; et al. Lineage-specific real-time RT-PCR for yellow fever virus outbreak surveillance, Brazil. Emerg. Infect. Dis. 2017, 23, 1867. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.R.; Russell, B.J.; Mossel, E.C.; Kayiwa, J.; Lutwama, J.; Lambert, A.J. Development of a real-time RT-PCR assay for the global differentiation of yellow fever virus vaccine adverse events from natural infections. J. Clin. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Diagne, C.T.; Stittleburg, V.D.; Mohamed-Hadley, A.; de Guillen, Y.A.; Balmaseda, A.; Faye, O.; Faye, O.; Sall, A.A.; Harris, E.; et al. Internally controlled, multiplex real-time reverse transcription PCR for dengue virus and yellow fever virus detection. Am. J. Trop. Med. Hyg. 2018, 98, 1833–1836. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, And Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Rasche, A.; Baronti, C.; Aldabbagh, S.; Cadar, D.; Reusken, C.B.; Pas, S.D.; Goorhuis, A.; Schinkel, J.; Molenkamp, R.; et al. Assay optimization for molecular detection of Zika virus. Bull. World Health Organ. 2016, 94, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Cleton, N.B.; Reusken, C.B.; Wagenaar, J.F.; van der Vaart, E.E.; Reimerink, J.; van der Eijk, A.A.; Koopmans, M.P. Syndromic approach to arboviral diagnostics for global travelers as a basis for infectious disease surveillance. PLoS Negl. Trop. Dis. 2015, 9, e0004073. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.M.; Di Paola, N.; Cunha, M.P.; Rodrigues-Jesus, M.J.; Araujo, D.B.; Silveira, V.B.; Leal, F.B.; Mesquita, F.S.; Botosso, V.F.; Zanotto, P.M.A.; et al. Yellow fever virus RNA in urine and semen of convalescent patient, Brazil. Emerg Infect. Dis. 2018, 24, 176. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Yactayo, S.; Agbenu, E.; Demanou, M.; Schulz, A.R.; Daskalow, K.; Niedrig, M. Detection of yellow fever 17D genome in urine. J. Clin. Microbiol. 2011, 49, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Hamer, D.H.; Angelo, K.; Caumes, E.; van Genderen, P.J.J.; Florescu, S.A.; Popescu, C.P.; Perret, C.; McBride, A.; Checkley, A.; Ryan, J.; et al. Fatal yellow fever in travelers to Brazil, 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Yellow fever: An update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- Gardner, C.L.; Ryman, K.D. Yellow fever: A reemerging threat. Clin. Lab. Med. 2010, 30, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, L.; Lv, Y.; Zhang, W.; Li, J.; Zhang, Y.; Di, T.; Zhang, S.; Liu, J.; Li, J.; et al. A fatal yellow fever virus infection in China: Description and lessons. Emerg. Microbes Infect. 2016, 5, e69. [Google Scholar] [CrossRef] [PubMed]
- Wouthuyzen-Bakker, M.; Knoester, M.; van den Berg, A.P.; GeurtsvanKessel, C.H.; Koopmans, M.P.; Van Leer-Buter, C.; Oude Velthuis, B.; Pas, S.D.; Ruijs, W.L.; Schmidt-Chanasit, J.; et al. Yellow fever in a traveller returning from suriname to The Netherlands, March 2017. Euro Surveill. 2017, 22, 30488. [Google Scholar] [CrossRef] [PubMed]
- Barnett, E.D. Yellow fever: Epidemiology and prevention. Clin. Infect. Dis. 2007, 44, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Colebunders, R.; Mariage, J.L.; Coche, J.C.; Pirenne, B.; Kempinaire, S.; Hantson, P.; Van Gompel, A.; Niedrig, M.; Van Esbroeck, M.; Bailey, R.; et al. A Belgian traveler who acquired yellow fever in the Gambia. Clin. Infect. Dis. 2002, 35, e113–e116. [Google Scholar] [CrossRef] [PubMed]
- Akondy, R.S.; Johnson, P.L.; Nakaya, H.I.; Edupuganti, S.; Mulligan, M.J.; Lawson, B.; Miller, J.D.; Pulendran, B.; Antia, R.; Ahmed, R. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc. Natl. Acad. Sci. USA 2015, 112, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, M.C.; Lee, T.H.; Wen, L.; Kaidarova, Z.; Bravo, M.D.; Kiely, N.E.; Kamel, H.T.; Tobler, L.H.; Norris, P.J.; Busch, M.P. West Nile virus nucleic acid persistence in whole blood months after clearance in plasma: Implication for transfusion and transplantation safety. Transfusion 2014, 54, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S.; Duggal, N.K.; Hook, S.A.; Delorey, M.; Fischer, M.; Olzenak McGuire, D.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika virus shedding in semen of symptomatic infected men. N. Engl. J. Med. 2018, 378, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- St George, K.; Sohi, I.S.; Dufort, E.M.; Dean, A.B.; White, J.L.; Limberger, R.; Sommer, J.N.; Ostrowski, S.; Wong, S.J.; Backenson, P.B.; et al. Zika virus testing considerations: Lessons learned from the first 80 real-time reverse transcription-PCR-positive cases diagnosed in new york state. J. Clin. Microbiol. 2017, 55, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A.M.; Cone, M.; Mock, V.; Heberlein-Larson, L.; Stanek, D.; Blackmore, C.; Likos, A. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease—Florida, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.; Patriota, J.V.; de Souza Mde, L.; Abd El Wahed, A.; Sanabani, S.S. Detection of Zika virus in Brazilian patients during the first five days of infection—Urine versus plasma. Euro Surveill. 2016, 21, 30302. [Google Scholar] [CrossRef] [PubMed]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Gubern, C.G.; et al. Persistence of Zika virus in body fluids—Preliminary report. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Joguet, G.; Mansuy, J.M.; Matusali, G.; Hamdi, S.; Walschaerts, M.; Pavili, L.; Guyomard, S.; Prisant, N.; Lamarre, P.; Dejucq-Rainsford, N.; et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: A prospective observational study. Lancet Infect. Dis. 2017, 17, 1200–1208. [Google Scholar] [CrossRef]
- Nicastri, E.; Castilletti, C.; Liuzzi, G.; Iannetta, M.; Capobianchi, M.R.; Ippolito, G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016, 21, 30314. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef] [PubMed]
- WHO. Yellow Fever Laboratory Diagnostic Testing in Africa; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- PAHO. Laboratory Diagnosis of Yellow Fever Virus Infection February 2018; Pan American Health Organization: Washington, DC, USA, 2018. [Google Scholar]
- Domingo, C.; Ellerbrok, H.; Koopmans, M.; Nitsche, A.; Leitmeyer, K.; Charrel, R.N.; Reusken, C. Need for additional capacity and improved capability for molecular detection of yellow fever virus in European expert laboratories: External quality assessment, March 2018. Euro Surveill. 2018, 23, 1800341. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Escadafal, C.; Rumer, L.; Mendez, J.A.; Garcia, P.; Sall, A.A.; Teichmann, A.; Donoso-Mantke, O.; Niedrig, M. First international external quality assessment study on molecular and serological methods for yellow fever diagnosis. PLoS ONE 2012, 7, e36291. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.E.; Matud, J.L. Estimation of dengue virus IGM persistence using regression analysis. Clin. Vaccine Immunol. 2011, 18, 2183–2185. [Google Scholar] [CrossRef] [PubMed]
- Andries, A.C.; Duong, V.; Ly, S.; Cappelle, J.; Kim, K.S.; Lorn Try, P.; Ros, S.; Ong, S.; Huy, R.; Horwood, P.; et al. Value of routine dengue diagnostic tests in urine and saliva specimens. PLoS Negl. Trop. Dis. 2015, 9, e0004100. [Google Scholar] [CrossRef] [PubMed]
- Veje, M.; Studahl, M.; Johansson, M.; Johansson, P.; Nolskog, P.; Bergstrom, T. Diagnosing tick-borne encephalitis: A re-evaluation of notified cases. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Pires-Marczeski, F.C.; Martinez, V.P.; Nemirovsky, C.; Padula, P.J. Intrathecal antibody production in two cases of yellow fever vaccine associated neurotropic disease in argentina. J. Med. Virol. 2011, 83, 2208–2212. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Riccio, P.; Patrucco, L.; Rojas, J.I.; Cristiano, E. Longitudinal myelitis associated with yellow fever vaccination. J. Neurovirol. 2009, 15, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.J.; Goodman, C.; Horiuchi, K.; Laven, J.; Panella, A.J.; Kosoy, O.; Lanciotti, R.S.; Johnson, B.W. Development and validation of an ELISA kit (YF MAC-HD) to detect igm to yellow fever virus. J. Virol. Methods 2015, 225, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Adungo, F.; Yu, F.; Kamau, D.; Inoue, S.; Hayasaka, D.; Posadas-Herrera, G.; Sang, R.; Mwau, M.; Morita, K. Development and characterization of monoclonal antibodies to yellow fever virus and application in antigen detection and igm capture enzyme-linked immunosorbent assay. Clin. Vaccine Immunol. 2016, 23, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Niedrig, M.; Kursteiner, O.; Herzog, C.; Sonnenberg, K. Evaluation of an indirect immunofluorescence assay for detection of immunoglobulin M (IgM) and IgG antibodies against yellow fever virus. Clin. Vaccine Immunol. 2008, 15, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Cropp, C.B.; Muth, D.J.; Calisher, C.H. Indirect fluorescent antibody test for the diagnosis of yellow fever. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 282–286. [Google Scholar] [CrossRef]
- Cleton, N.B.; Godeke, G.J.; Reimerink, J.; Beersma, M.F.; Doorn, H.R.; Franco, L.; Goeijenbier, M.; Jimenez-Clavero, M.A.; Johnson, B.W.; Niedrig, M.; et al. Spot the difference-development of a syndrome based protein microarray for specific serological detection of multiple flavivirus infections in travelers. PLoS Negl. Trop. Dis. 2015, 9, e0003580. [Google Scholar] [CrossRef] [PubMed]
- Spector, S.; Tauraso, N.M. Yellow fever virus. I. Development and evaluation of a plaque neutralization test. Appl. Microbiol. 1968, 16, 1770–1775. [Google Scholar] [PubMed]
- Simoes, M.; Camacho, L.A.; Yamamura, A.M.; Miranda, E.H.; Cajaraville, A.C.; da Silva Freire, M. Evaluation of accuracy and reliability of the plaque reduction neutralization test (micro-PRNT) in detection of yellow fever virus antibodies. Biologicals 2012, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.L.; Horton, D.L.; Johnson, N.; Li, L.; Barrett, A.D.; Smith, D.J.; Galbraith, S.E.; Solomon, T.; Fooks, A.R. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011, 92, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Halstead, S.B.; Larsen, L.K. Comparison of dengue virus plaque reduction neutralization by macro and “semi-micro’ methods in LLC-MK2 cells. Microbiol. Immunol. 1985, 29, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Halstead, S.B.; Repik, P.M.; Putvatana, R.; Raybourne, N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: Comparison of the BHK suspension test with standard plaque reduction neutralization. J. Clin. Microbiol. 1985, 22, 250–254. [Google Scholar] [PubMed]
- Buckley, A.; Dawson, A.; Moss, S.R.; Hinsley, S.A.; Bellamy, P.E.; Gould, E.A. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J. Gen. Virol. 2003, 84, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Mardekian, S.K.; Roberts, A.L. Diagnostic options and challenges for dengue and chikungunya viruses. BioMed Res. Int. 2015, 2015, 834371. [Google Scholar] [CrossRef] [PubMed]
- Mercier-Delarue, S.; Durier, C.; Colin de Verdiere, N.; Poveda, J.D.; Meiffredy, V.; Fernandez Garcia, M.D.; Lastere, S.; Cesaire, R.; Manuggera, J.C.; Molina, J.M.; et al. Screening test for neutralizing antibodies against yellow fever virus, based on a flavivirus pseudotype. PLoS ONE 2017, 12, e0177882. [Google Scholar] [CrossRef] [PubMed]
- Houghton-Trivino, N.; Montana, D.; Castellanos, J. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Rev. Salud Publica (Bogota) 2008, 10, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.J.; Theiler, R.N.; Rasmussen, S.A. Emerging infections and pregnancy. Emerg. Infect. Dis. 2006, 12, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N. Diagnosis of arboviral infections—A quagmire of cross reactions and complexities. Travel Med. Infect. Dis. 2016, 14, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Gabor, J.J.; Schwarz, N.G.; Esen, M.; Kremsner, P.G.; Grobusch, M.P. Dengue and chikungunya seroprevalence in Gabonese infants prior to major outbreaks in 2007 and 2010: A sero-epidemiological study. Travel Med. Infect. Dis. 2016, 14, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Alves, M.J.; de Ory, F.; Teichmann, A.; Schmitz, H.; Muller, R.; Niedrig, M. International external quality control assessment for the serological diagnosis of dengue infections. BMC Infect. Dis. 2015, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Sanchini, A.; Donoso-Mantke, O.; Papa, A.; Sambri, V.; Teichmann, A.; Niedrig, M. Second international diagnostic accuracy study for the serological detection of West Nile virus infection. PLoS Negl. Trop. Dis. 2013, 7, e2184. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E. Yellow fever vaccine-associated viscerotropic disease: Current perspectives. Drug Des. Dev. Ther. 2016, 10, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dengue vaccine: WHO position paper—July 2016. Vaccine 2017, 35, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Skipetrova, A.; Wartel, T.A.; Gailhardou, S. Dengue vaccination during pregnancy—An overview of clinical trials data. Vaccine 2018, 36, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Halasa-Rappel, Y.A.; Baurin, N.; Coudeville, L.; Shepard, D.S. Cost-effectiveness of dengue vaccination in ten endemic countries. Vaccine 2018, 36, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Briand, O.; Lang, J.; Saville, M.; Jackson, N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015, 33, 7100–7111. [Google Scholar] [CrossRef] [PubMed]
- Vannice, K.S.; Durbin, A.; Hombach, J. Status of vaccine research and development of vaccines for dengue. Vaccine 2016, 34, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Rossini, G. An overview of Usutu virus. Microbes Infect. 2017, 19, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; de Souza Luna, L.K.; Pedroso, C.; Pedral-Sampaio, D.B.; Queiroz, A.T.; Brites, C.; Netto, E.M.; Drosten, C. Rates of and reasons for failure of commercial human immunodeficiency virus type 1 viral load assays in Brazil. J. Clin. Microbiol. 2007, 45, 2061–2063. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, J.J.; Brandriss, M.W.; Monath, T.P. Monoclonal antibodies distinguish between wild and vaccine strains of yellow fever virus by neutralization, hemagglutination inhibition, and immune precipitation of the virus envelope protein. Virology 1983, 125, 8–17. [Google Scholar] [CrossRef]
- Ryman, K.D.; Ledger, T.N.; Weir, R.C.; Schlesinger, J.J.; Barrett, A.D. Yellow fever virus envelope protein has two discrete type-specific neutralizing epitopes. J. Gen. Virol. 1997, 78 Pt 6, 1353–1356. [Google Scholar] [CrossRef]
- Ledger, T.N.; Sil, B.K.; Wills, M.R.; Lewis, G.; Kinney, R.M.; Jennings, A.D.; Stephenson, J.R.; Barrett, A.D. Variation in the biological function of envelope protein epitopes of yellow fever vaccine viruses detected with monoclonal antibodies. Boil. J. Int. Assoc. Boil. Stand. 1992, 20, 117–128. [Google Scholar] [CrossRef]
- Daffis, S.; Kontermann, R.E.; Korimbocus, J.; Zeller, H.; Klenk, H.D.; Ter Meulen, J. Antibody responses against wild-type yellow fever virus and the 17D vaccine strain: Characterization with human monoclonal antibody fragments and neutralization escape variants. Virology 2005, 337, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.H. Antigenic analysis of certain group B arthropodborne viruses by antibody absorption. J. Exp. Med. 1960, 111, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.; Pryde, A.; Medlen, A.R.; Ledger, T.N.; Whitby, J.E.; Gibson, C.A.; DeSilva, M.; Groves, D.J.; Langley, D.J.; Minor, P.D. Examination of the envelope glycoprotein of yellow fever vaccine viruses with monoclonal antibodies. Vaccine 1989, 7, 333–336. [Google Scholar] [CrossRef]
- Barrett, A.D.; Mathews, J.H.; Miller, B.R.; Medlen, A.R.; Ledger, T.N.; Roehrig, J.T. Identification of monoclonal antibodies that distinguish between 17D-204 and other strains of yellow fever virus. J. Gen. Virol. 1990, 71 Pt 1, 13–18. [Google Scholar] [CrossRef]
- Monath, T.P. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev. Vaccines 2012, 11, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. 17D yellow fever virus vaccine. Am. J. Trop. Med. Hyg. 2013, 89, 1225. [Google Scholar] [CrossRef] [PubMed]
- Lilay, A.; Asamene, N.; Bekele, A.; Mengesha, M.; Wendabeku, M.; Tareke, I.; Girmay, A.; Wuletaw, Y.; Adossa, A.; Ba, Y.; et al. Reemergence of yellow fever in Ethiopia after 50 years, 2013: Epidemiological and entomological investigations. BMC Infect. Dis. 2017, 17, 343. [Google Scholar] [CrossRef] [PubMed]
- Wamala, J.F.; Malimbo, M.; Okot, C.L.; Atai-Omoruto, A.D.; Tenywa, E.; Miller, J.R.; Balinandi, S.; Shoemaker, T.; Oyoo, C.; Omony, E.O.; et al. Epidemiological and laboratory characterization of a yellow fever outbreak in Northern Uganda, October 2010-January 2011. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2012, 16, e536–e542. [Google Scholar] [CrossRef] [PubMed]
- Markoff, L. Yellow fever outbreak in Sudan. N. Engl. J. Med. 2013, 368, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Q.A.; Memish, Z.A. Yellow fever from Angola and Congo: A storm gathers. Trop. Doct. 2017, 47, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Campi-Azevedo, A.C.; de Almeida Estevam, P.; Coelho-Dos-Reis, J.G.; Peruhype-Magalhaes, V.; Villela-Rezende, G.; Quaresma, P.F.; Maia Mde, L.; Farias, R.H.; Camacho, L.A.; Freire Mda, S.; et al. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Infect. Dis. 2014, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Maia Mde, L.; Farias, R.H.; Camacho, L.A.; Freire, M.S.; Galler, R.; Yamamura, A.M.; Almeida, L.F.; Lima, S.M.; Nogueira, R.M.; et al. 17DD yellow fever vaccine: A double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum. Vaccines Immunother. 2013, 9, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Ahuka-Mundeke, S.; Casey, R.M.; Harris, J.B.; Dixon, M.G.; Nsele, P.M.; Kizito, G.M.; Umutesi, G.; Laven, J.; Paluku, G.; Gueye, A.S.; et al. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak—Preliminary report. N. Engl. J. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Menezes Martins, R.; Maia, M.L.S.; de Lima, S.M.B.; de Noronha, T.G.; Xavier, J.R.; Camacho, L.A.B.; de Albuquerque, E.M.; Farias, R.H.G.; da Matta de Castro, T.; Homma, A.; et al. Duration of post-vaccination immunity to yellow fever in volunteers eight years after a dose-response study. Vaccine 2018, 36, 4112–4117. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Hamer, D.H. Vaccination strategies during shortages of yellow fever vaccine—Reply. JAMA 2018, 319, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Shearer, F.M.; Moyes, C.L.; Pigott, D.M.; Brady, O.J.; Marinho, F.; Deshpande, A.; Longbottom, J.; Browne, A.J.; Kraemer, M.U.G.; O’Reilly, K.M.; et al. Global yellow fever vaccination coverage from 1970 to 2016: An adjusted retrospective analysis. Lancet Infect. Dis. 2017, 17, 1209–1217. [Google Scholar] [CrossRef]
- Chen, L.H.; Hamer, D.H. Vaccination challenges in confronting the resurgent threat from yellow fever. JAMA 2017, 318, 1651–1652. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.A.; Morens, D.M. Vaccination strategies during shortages of yellow fever vaccine. JAMA 2018, 319, 1280. [Google Scholar] [CrossRef] [PubMed]
- Roukens, A.H.; Gelinck, L.B.; Visser, L.G. Intradermal vaccination to protect against yellow fever and influenza. Curr. Top. Microbiol. Immunol. 2012, 351, 159–179. [Google Scholar] [PubMed]
- Roukens, A.H.; Vossen, A.C.; Bredenbeek, P.J.; van Dissel, J.T.; Visser, L.G. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: A randomized controlled non-inferiority trial. PLoS ONE 2008, 3, e1993. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.M.; Lam, L.K.; Klimstra, W.B.; Ryman, K.D. The 17d-204 vaccine strain-induced protection against virulent yellow fever virus is mediated by humoral immunity and CD4+ but not CD8+ T cells. PLoS Pathog. 2016, 12, e1005786. [Google Scholar] [CrossRef] [PubMed]
- Lok. Singapore’s Dengue Haemorraghic Fever Control Programme: A Case Study on the Succesful Control of Aedes Aegypti and Aedes Albopictus Using Mainly Environmental Measures as a Part of an Integrated Vector Control; Southeast Asian Medical Information Center: Tokyo, Japan, 1985. [Google Scholar]
- Armada Gessa, J.A.; Figueredo González, R. Application of environmental management principles in the program for eradication of Aedes (Stegomyia) aegypti (Linneus, 1762) in the Republic of Cuba, 1984. Bull. Pan Am. Health Organ. 1986, 20, 186–193. [Google Scholar] [PubMed]
- Morrison, A.C.; Zielinski-Gutierrez, E.; Scott, T.W.; Rosenberg, R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008, 5, e68. [Google Scholar] [CrossRef] [PubMed]
- Renganathan, E.; Parks, W.; LIoyd, L.; Nathan, M.B.; Hosein, E.; Odugleh, A.; Clark, G.G.; Gubler, D.J.; Prasittisuk, C.; Palmer, K.; et al. Towards sustaining behavioural impact in dengue prevention and control. Dengue Bull. 2003, 27, 6–12. [Google Scholar]
- Alvarado-Castro, V.; Paredes-Solís, S.; Nava-Aguilera, E.; Morales-Pérez, A.; Alarcón-Morales, L.; Balderas-Vargas, N.A.; Andersson, N. Assessing the effects of interventions for Aedes aegypti control: Systematic review and meta-analysis of cluster randomised controlled trials. BMC Public Health 2017, 17, 384. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.R.; Donegan, S.; McCall, P.J. Is dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004551. [Google Scholar] [CrossRef] [PubMed]
- Achee, N.L.; Gould, F.; Perkins, T.A.; Reiner, R.C., Jr.; Morrison, A.C.; Ritchie, S.A.; Gubler, D.J.; Teyssou, R.; Scott, T.W. A critical assessment of vector control for dengue prevention. PLoS Negl. Trop. Dis. 2015, 9, e0003655. [Google Scholar] [CrossRef] [PubMed]
- Ono, L.; Wollinger, W.; Rocco, I.M.; Coimbra, T.L.; Gorin, P.A.; Sierakowski, M.R. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain). Antivir. Res. 2003, 60, 201–208. [Google Scholar] [CrossRef]
- Zhou, Z.; Khaliq, M.; Suk, J.E.; Patkar, C.; Li, L.; Kuhn, R.J.; Post, C.B. Antiviral compounds discovered by virtual screening of small-molecule libraries against dengue virus e protein. ACS Chem. Biol. 2008, 3, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, T.; Yennamalli, R.; Campbell, P.; Stoermer, M.J.; Fairlie, D.P.; Kobe, B.; Young, P.R. In silico screening of small molecule libraries using the dengue virus envelope E protein has identified compounds with antiviral activity against multiple flaviviruses. Antivir. Res. 2009, 84, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Mayhoub, A.S.; Khaliq, M.; Kuhn, R.J.; Cushman, M. Design, synthesis, and biological evaluation of thiazoles targeting flavivirus envelope proteins. J. Med. Chem. 2011, 54, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Dai, J.X.; Ji, G.H.; Jiang, T.; Wang, H.J.; Yang, H.O.; Tan, W.L.; Liu, R.; Yu, M.; Ge, B.X.; et al. A broadly flavivirus cross-neutralizing monoclonal antibody that recognizes a novel epitope within the fusion loop of e protein. PLoS ONE 2011, 6, e16059. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, A.; Kumar, M.M.; Pradhan, D.; Marisetty, H. Docking studies towards exploring antiviral compounds against envelope protein of yellow fever virus. Interdiscip. Sci. 2011, 3, 64–77. [Google Scholar] [CrossRef] [PubMed]
- De Burghgraeve, T.; Kaptein, S.J.; Ayala-Nunez, N.V.; Mondotte, J.A.; Pastorino, B.; Printsevskaya, S.S.; de Lamballerie, X.; Jacobs, M.; Preobrazhenskaya, M.; Gamarnik, A.V.; et al. An analogue of the antibiotic teicoplanin prevents flavivirus entry in vitro. PLoS ONE 2012, 7, e37244. [Google Scholar] [CrossRef] [PubMed]
- Assuncao-Miranda, I.; Cruz-Oliveira, C.; Neris, R.L.; Figueiredo, C.M.; Pereira, L.P.; Rodrigues, D.; Araujo, D.F.; Da Poian, A.T.; Bozza, M.T. Inactivation of Dengue and Yellow Fever viruses by heme, cobalt-protoporphyrin IX and tin-protoporphyrin IX. J. Appl. Microbiol. 2016, 120, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Trent, D.W.; Monath, T.P. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine 2011, 29, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Pacca, C.C.; Severino, A.A.; Mondini, A.; Rahal, P.; D’Avila, S.G.; Cordeiro, J.A.; Nogueira, M.C.; Bronzoni, R.V.; Nogueira, M.L. RNA interference inhibits yellow fever virus replication in vitro and in vivo. Virus Genes 2009, 38, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sakamuru, S.; Huang, R.; Brecher, M.; Koetzner, C.A.; Zhang, J.; Chen, H.; Qin, C.F.; Zhang, Q.Y.; Zhou, J.; et al. Erythrosin B is a potent and broad-spectrum orthosteric inhibitor of the flavivirus NS2B-NS3 protease. Antivir. Res. 2018, 150, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Patkar, C.G.; Larsen, M.; Owston, M.; Smith, J.L.; Kuhn, R.J. Identification of inhibitors of yellow fever virus replication using a replicon-based high-throughput assay. Antimicrob. Agents Chemother. 2009, 53, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wu, S.; Julander, J.; Ma, J.; Zhang, X.; Kulp, J.; Cuconati, A.; Block, T.M.; Du, Y.; Guo, J.T.; et al. A novel benzodiazepine compound inhibits yellow fever virus infection by specifically targeting NS4B protein. J. Virol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pierra, C.; Amador, A.; Benzaria, S.; Cretton-Scott, E.; D’Amours, M.; Mao, J.; Mathieu, S.; Moussa, A.; Bridges, E.G.; Standring, D.N.; et al. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2’-C-methylcytidine. J. Med. Chem. 2006, 49, 6614–6620. [Google Scholar] [CrossRef] [PubMed]
- Stuyver, L.J.; McBrayer, T.R.; Tharnish, P.M.; Clark, J.; Hollecker, L.; Lostia, S.; Nachman, T.; Grier, J.; Bennett, M.A.; Xie, M.Y.; et al. Inhibition of hepatitis C replicon RNA synthesis by β-D-2’-deoxy-2’-fluoro-2’-C-methylcytidine: A specific inhibitor of hepatitis C virus replication. Antivir. Chem. Chemother. 2006, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fogt, J.; Januszczyk, P.; Framski, G.; Onishi, T.; Izawa, K.; De Clercq, E.; Neyts, J.; Boryski, J. Synthesis and antiviral activity of novel derivatives of 2’-β-C-methylcytidine. Nucleic Acids Symp. Ser. (Oxf.) 2008, 52, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Jha, A.K.; Choi, J.A.; Jung, K.H.; Smee, D.F.; Morrey, J.D.; Chu, C.K. Efficacy of 2’-C-methylcytidine against yellow fever virus in cell culture and in a hamster model. Antivir. Res. 2010, 86, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Furuta, Y.; Shafer, K.; Sidwell, R.W. Activity of T-1106 in a hamster model of yellow fever virus infection. Antimicrob. Agents Chemother. 2007, 51, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Shafer, K.; Smee, D.F.; Morrey, J.D.; Furuta, Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob. Agents Chemother. 2009, 53, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, X.; Soloveva, V.; Warren, T.; Guo, F.; Wu, S.; Lu, H.; Guo, J.; Su, Q.; Shen, H.; et al. Enhancing the antiviral potency of ER α-glucosidase inhibitor IHVR-19029 against hemorrhagic fever viruses in vitro and in vivo. Antivir. Res. 2018, 150, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Bantia, S.; Taubenheim, B.R.; Minning, D.M.; Kotian, P.; Morrey, J.D.; Smee, D.F.; Sheridan, W.P.; Babu, Y.S. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a hamster model. Antimicrob. Agents Chemother. 2014, 58, 6607–6614. [Google Scholar] [CrossRef] [PubMed]
- Malet, H.; Masse, N.; Selisko, B.; Romette, J.L.; Alvarez, K.; Guillemot, J.C.; Tolou, H.; Yap, T.L.; Vasudevan, S.; Lescar, J.; et al. The flavivirus polymerase as a target for drug discovery. Antivir. Res. 2008, 80, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Noble, C.G.; Shi, P.Y. The dengue virus NS5 protein as a target for drug discovery. Antivir. Res. 2015, 119, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Bullard-Feibelman, K.M.; Govero, J.; Zhu, Z.; Salazar, V.; Veselinovic, M.; Diamond, M.S.; Geiss, B.J. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir. Res. 2017, 137, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Zaverucha-do-Valle, C.; Reis, P.A.; Barbosa-Lima, G.; Vieira, Y.R.; Mattos, M.; Silva, P.P.; Sacramento, C.; de Castro Faria Neto, H.C.; Campanati, L.; et al. Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci. Rep. 2017, 7, 9409. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.T.; Colby-Germinario, S.P.; Hassounah, S.A.; Fogarty, C.; Osman, N.; Palanisamy, N.; Han, Y.; Oliveira, M.; Quan, Y.; Wainberg, M.A. Evaluation of sofosbuvir (β-D-2’-deoxy-2’-α-fluoro-2’-β-C-methyluridine) as an inhibitor of Dengue virus replication. Sci. Rep. 2017, 7, 6345. [Google Scholar] [CrossRef] [PubMed]
- Leyssen, P.; Balzarini, J.; De Clercq, E.; Neyts, J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 2005, 79, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Morrey, J.D.; Blatt, L.M.; Shafer, K.; Sidwell, R.W. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antivir. Res. 2007, 73, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, E.; Xiao, S.Y.; Guzman, H.; Ye, M.; Travassos da Rosa, A.P.; Tesh, R.B. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am. J. Trop. Med. Hyg. 2004, 71, 306–312. [Google Scholar] [PubMed]
- Bhattacharya, D.; Ansari, I.H.; Striker, R. The flaviviral methyltransferase is a substrate of Casein Kinase 1. Virus Res. 2009, 141, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Hosoya, T.; Leong, K.M.; Onogi, H.; Okuno, Y.; Hiramatsu, T.; Koyama, H.; Suzuki, M.; Hagiwara, M.; Garcia-Blanco, M.A. The kinase inhibitor SFV785 dislocates dengue virus envelope protein from the replication complex and blocks virus assembly. PLoS ONE 2011, 6, e23246. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Ennis, J.; Turner, J.; Morrey, J.D. Treatment of yellow fever virus with an adenovirus-vectored interferon, DEF201, in a hamster model. Antimicrob. Agents Chemother. 2011, 55, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Vellozzi, C.; Mitchell, T.; Miller, E.; Casey, C.G.; Eidex, R.B.; Hayes, E.B. Yellow fever vaccine-associated viscerotropic disease (YEL-AVD) and corticosteroid therapy: Eleven united states cases, 1996–2004. Am. J. Trop. Med. Hyg. 2006, 75, 333–336. [Google Scholar] [PubMed]
- Shearer, F.M.; Longbottom, J.; Browne, A.J.; Pigott, D.M.; Brady, O.J.; Kraemer, M.U.G.; Marinho, F.; Yactayo, S.; de Araujo, V.E.M.; da Nobrega, A.A.; et al. Existing and potential infection risk zones of yellow fever worldwide: A modelling analysis. Lancet Glob. Health 2018, 6, e270–e278. [Google Scholar] [CrossRef]
- WHO. Eliminate Yellow Fever Epidemics (EYE): A Global Strategy, 2017–2026; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Gómez, M.M.; Abreu, F.V.S.; Santos, A.A.C.D.; Mello, I.S.; Santos, M.P.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Miranda, R.M.; Castro, M.G.; Ribeiro, M.S.; et al. Genomic and structural features of the yellow fever virus from the 2016–2017 Brazilian outbreak. J. Gen. Virol. 2018, 99, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Vasilakis, N. Dengue—Quo tu et quo vadis? Viruses 2011, 3, 1562–1608. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2013, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pickering, P.; Duchêne, S.; Holmes, E.C.; Aaskov, J.G. Highly divergent dengue virus type 2 in traveler returning from Borneo to Australia. Emerg. Infect. Dis. 2016, 22, 2146–2148. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Cardosa, J.; Diallo, M.; Sall, A.A.; Holmes, E.C.; Hanley, K.A.; Weaver, S.C.; Mota, J.; Rico-Hesse, R. Sylvatic dengue viruses share the pathogenic potential of urban/endemic dengue viruses. J. Virol. 2010, 84, 3726–3727; author reply 3727–3728. [Google Scholar] [CrossRef] [PubMed]
- WHO. Eliminating Yellow Fever Epidemics (EYE) Strategy: Meeting Demand for Yellow Fever Vaccines. Available online: http://www.who.int/csr/disease/yellowfev/meeting-demand-for-vaccines/en/ (accessed on 1 July 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).